Molecular Control and Application of Male Fertility for Two-Line Hybrid Rice Breeding
Abstract
1. Introduction
1.1. Development of Hybrid Rice Technologies
1.1.1. Three-Line HR Technology
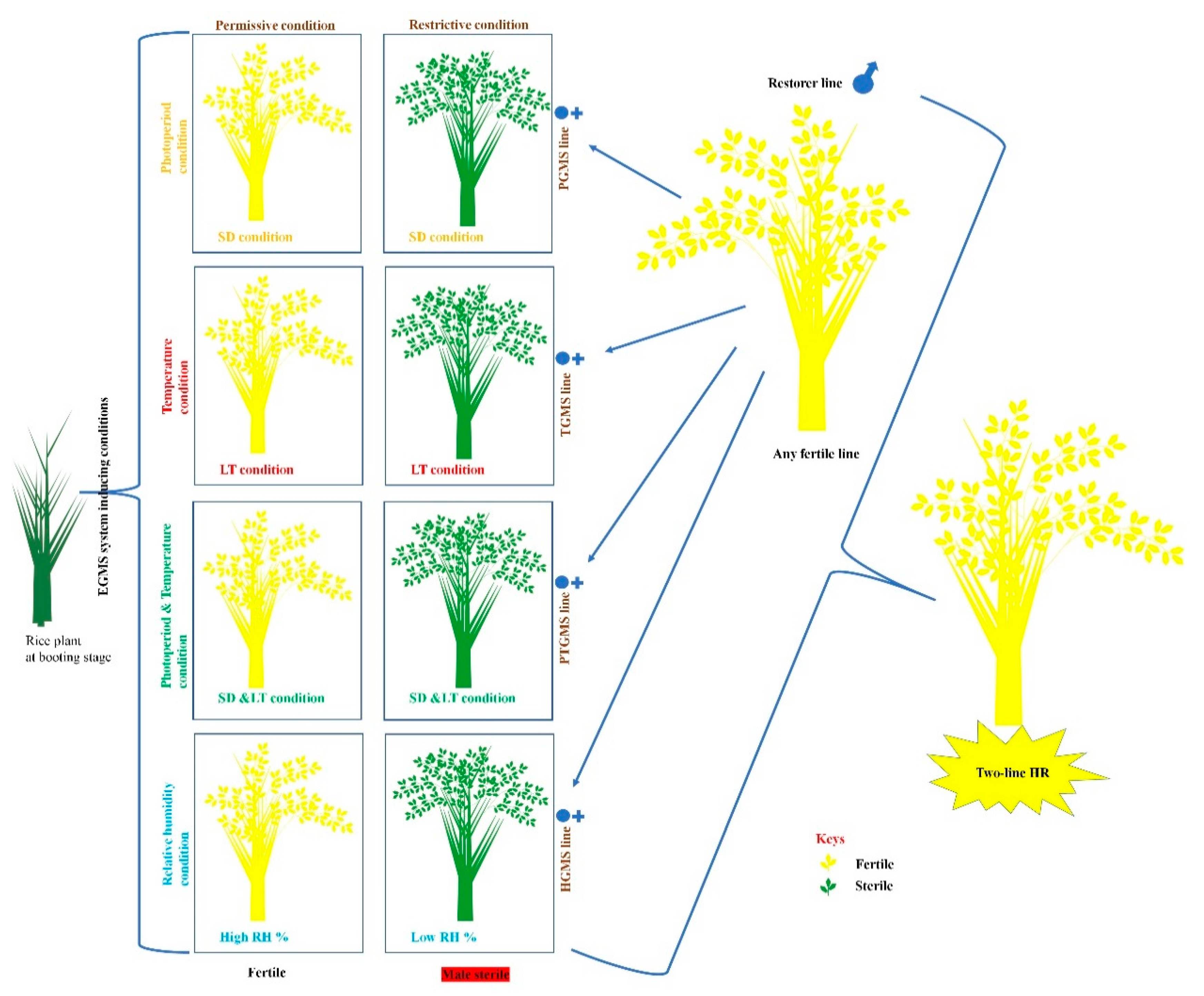
1.1.2. Two-Line HR Technology
2. Environment-Sensitive Genic Male Sterility Systems
2.1. Photoperiod-Sensitive-Genic-Male-Sterility (PGMS) System
2.2. Temperature-Sensitive-Genic-Male-Sterility (TGMS) System
2.3. Simultaneously Photoperiod and Temperature Influence PGMS and TGMS Systems in PTGMS Lines
2.4. Humidity-Sensitive-Genic-Male-Sterility (HGMS) System
3. Importance and Application of Two-Line HR for Seed Production of EGMS-Lines
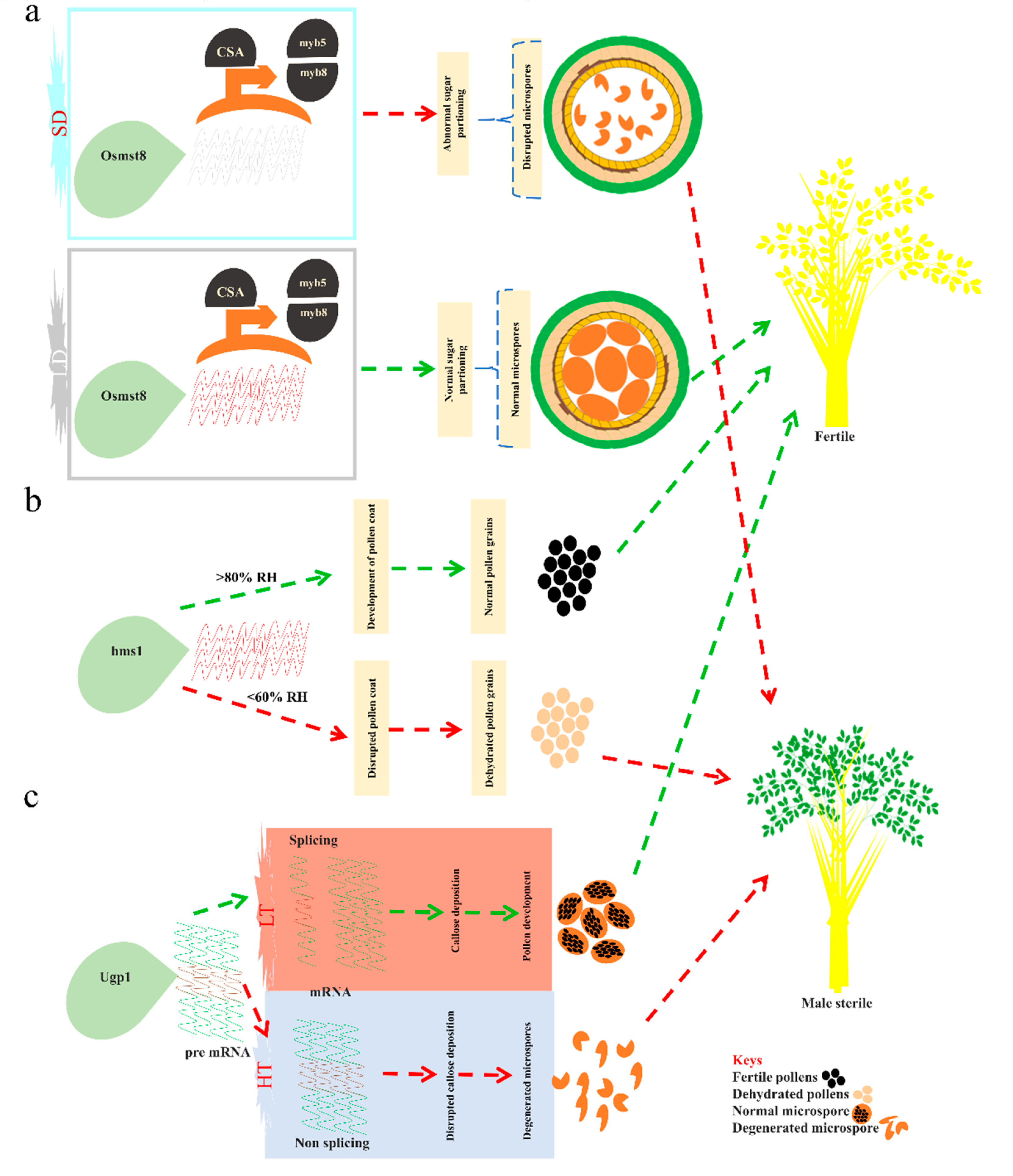
3.1. Molecular Regulation of EGMS Lines
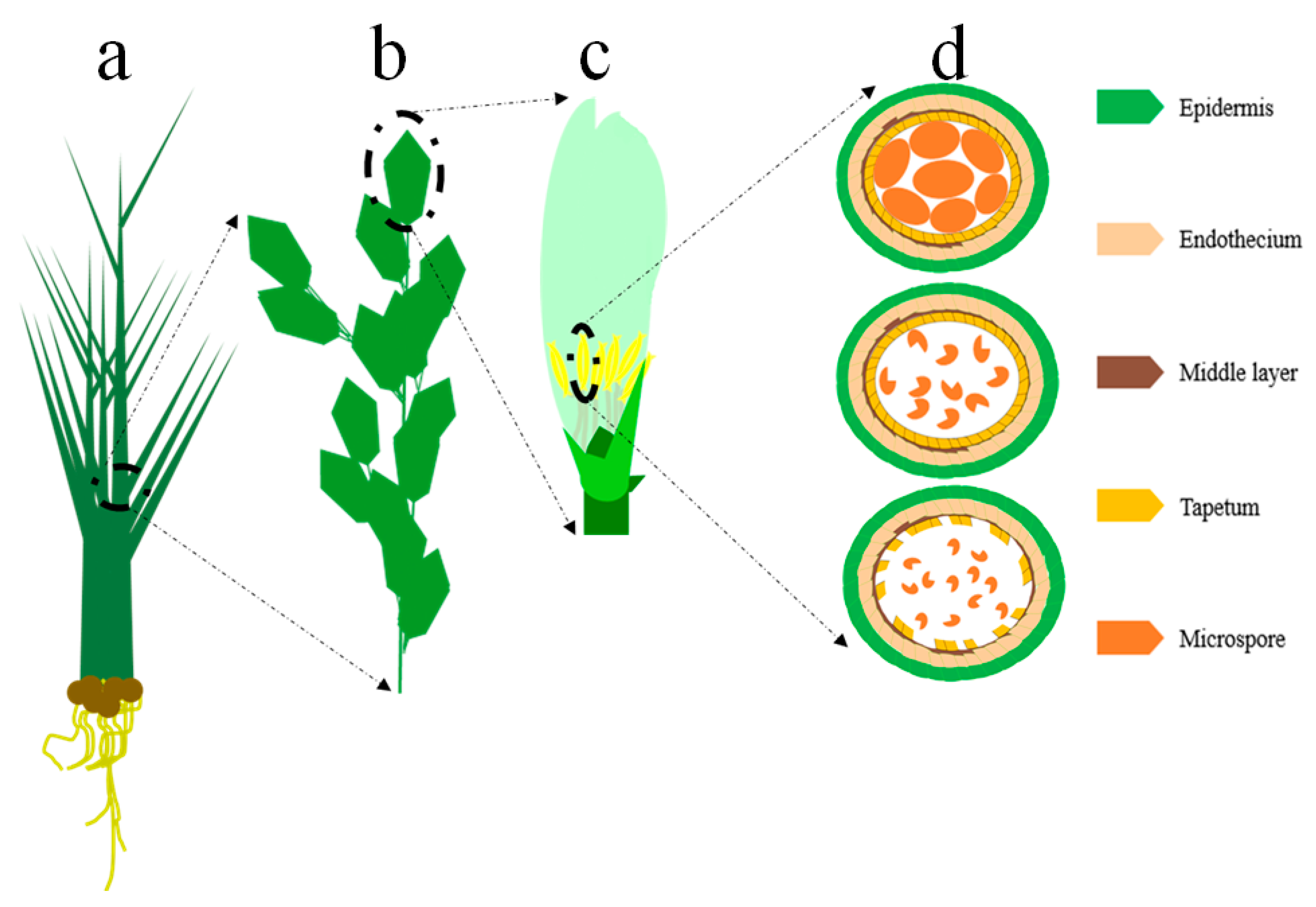
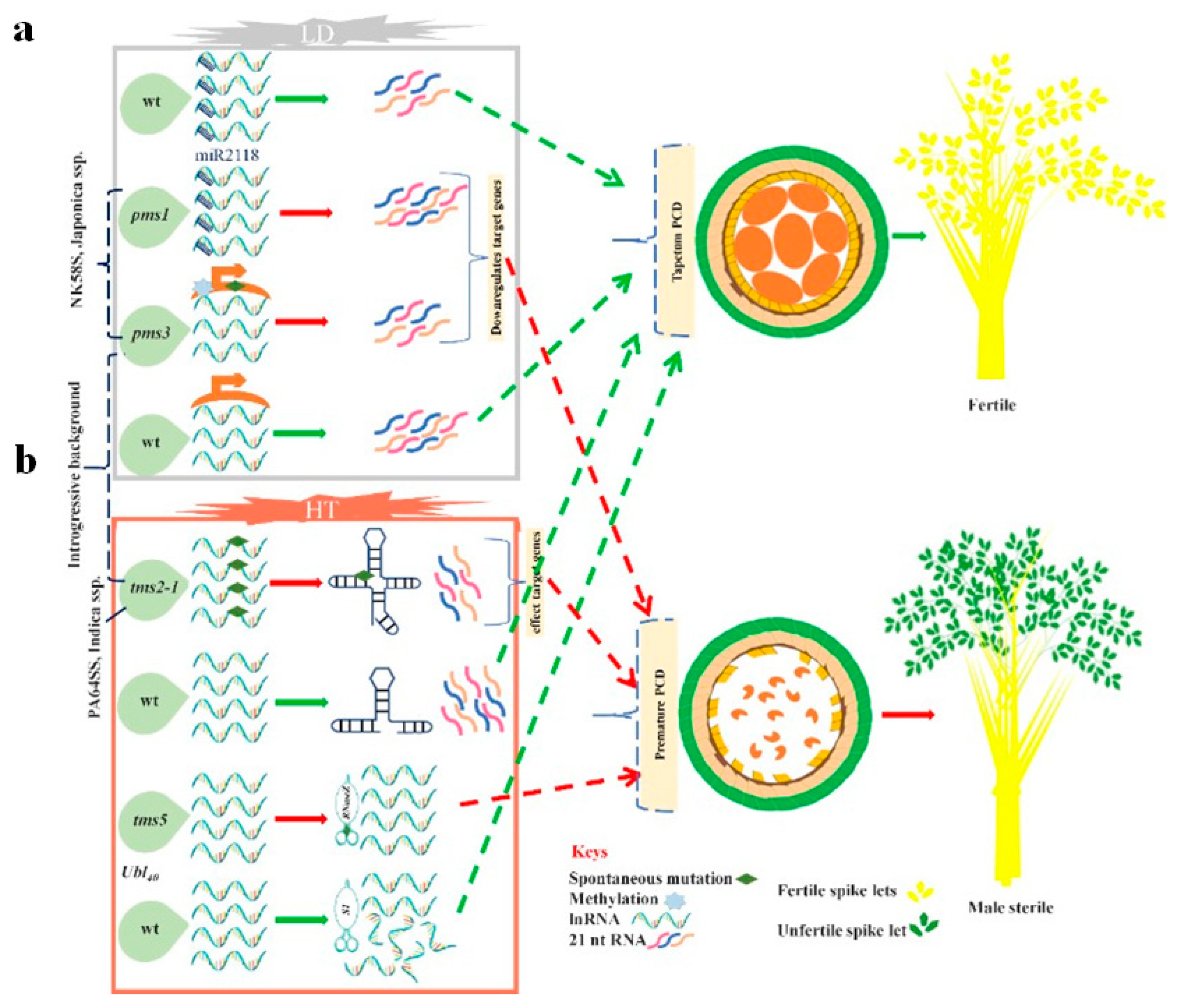
3.1.1. EGMS Lines Are Influenced by Genetic and Epigenetic Alterations
3.1.2. Regulation of the EGMS Lines by Noncoding RNAs and RNA Metabolism
The tms5 Regulates RNA-Metabolism
Transcriptional Regulation of the EGMS Lines via Noncoding RNAs
3.1.3. Post-Transcriptional Regulation of the EGMS Lines via Alterations in RNA Expression
3.1.4. The mRNA Splicing Regulates Fertility Transition in EGMS Lines
3.1.5. Metabolism of the miRNAs and Structural Substances Regulate the EGMS Trait
3.1.6. Transcription Factors Implicated in EGMS-Lines
3.1.7. Thermo-Sensitive-Male-Sterility Is Regulated by LRR-RLK
3.1.8. Development of EGMS Lines via CRISPR/Cas9 Technology
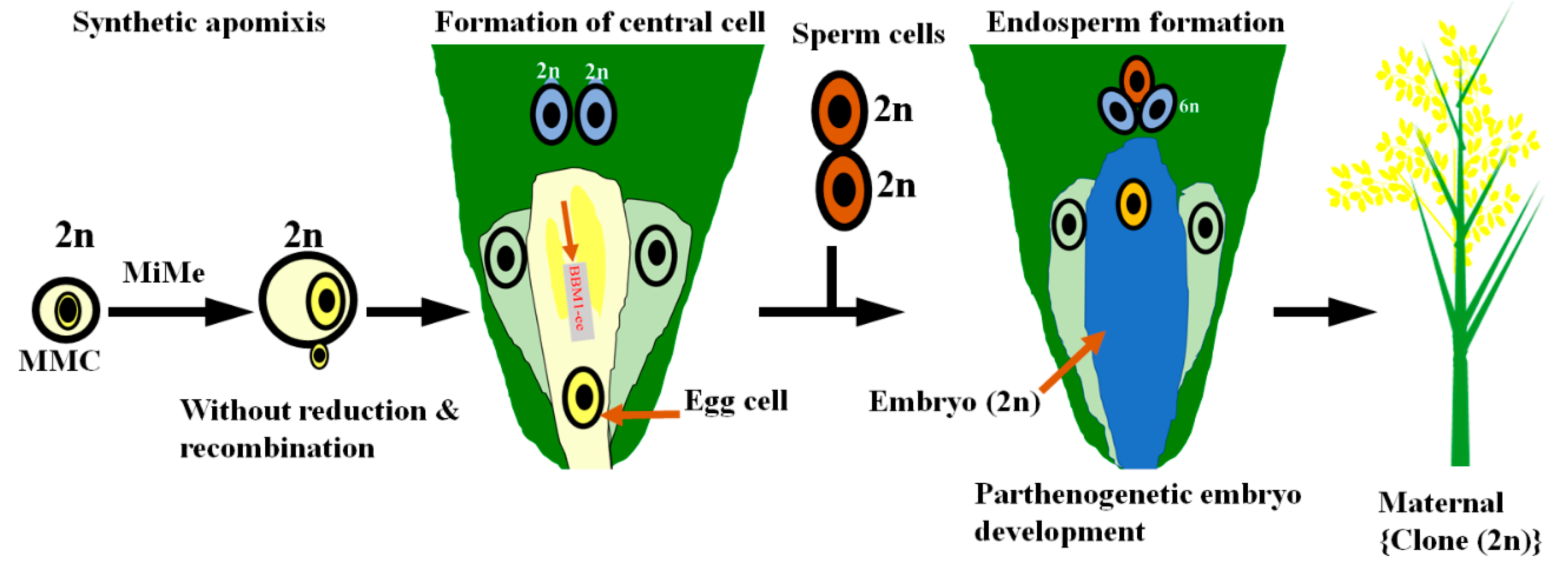
4. Apomixis Technology Could Be the Future of the Single-Line Hybrid Breeding
5. Conclusions
6. Future Perspectives of EGMS Research and Application
Funding
Acknowledgments
Conflicts of Interest
References
- Palanisamy, D.; Marappan, S.; Ponnuswamy, R.D.; Mahalingam, P.S.; Bohar, R.; Vaidyanathan, S. Accelerating hybrid rice breeding through the adoption of doubled haploid technology for R-line development. Biologia 2019, 74, 1259–1269. [Google Scholar] [CrossRef]
- Khush, G.S. Strategies for increasing the yield potential of cereals: Case of rice as an example. Plant Breed. 2013, 132, 433–436. [Google Scholar] [CrossRef]
- Ma, X.; Su, Z.; Ma, H. Molecular Genetic Analyses of Abiotic Stress Responses During plant Reproductive Development; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Eshed, Y.; Lippman, Z.B. Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science 2019, 366, eaax0025. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, F. Semidwarfing genes of high yielding rice varieties in Japan. In Rice Genetics I: (In 2 Parts); World Scientific: Singapore, 1986; pp. 285–295. [Google Scholar]
- Peng, S.; Tang, Q.; Huang, J.; Zou, Y.; Cui, K.; Zhang, Y.; He, F.; Laza, R.; Visperas, R. Yield Attributes and Nitrogen-Use Efficiency of “Super” Hybrid Rice; Accelerating Hybrid Rice Development, International Rice Research Institute: Manila, Philippines, 2010; pp. 419–428. [Google Scholar]
- Khush, G.S. What it will take to Feed 5.0 Billion Rice consumers in 2030. Plant Mol. Biol. 2005, 59, 15950. [Google Scholar] [CrossRef]
- Myers, N. The next green revolution: Its environmental underpinnings. Curr. Sci. 1999, 76, 507–513. [Google Scholar]
- Jones, J.W. Hybrid vigour in rice. Am. Soc. Agron. 1926, 18, 424–428. [Google Scholar]
- Ramaiah, K. Inheritance of flowering duration in rice. Indian J. Agric. Sci. 1933, 3, 377–410. [Google Scholar]
- Yuan, L. The execution and theory of developing hybrid rice. Agric. Sci. China 1977, 1, 27–31. [Google Scholar]
- Weng, J.; Suhai Gu, S.; Wan, X.; Gao, H.; Guo, T.; Su, N.; Lei, C.; Zhang, X.; Cheng, Z.; Guo, X.; et al. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 2008, 18, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Virmani, S.S. Hybrid rice. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1996; Volume 57, pp. 377–462. [Google Scholar]
- Virmani, S.S.; Sun, Z.X.; Mou, T.M.; Jauhar, A.A.; Mao, C.X. Male sterility systems in rice. In Two-Line Hybrid Rice Breeding Manual; International Rice Research Institute: Los Baños, Philippines, 2003; pp. 5–14. [Google Scholar]
- Kropff, M.; Cassman, K.; Peng, S.; Matthews, R.; Setter, T. Quantitative Understanding of Yield Potential. In Breaking the Yield Barrier; International Rice Research Institute: Los Banos, Philippines, 1994; pp. 21–38. [Google Scholar]
- Zhou, G.; Chen, Y.; Yao, W.; Zhang, C.; Xie, W.; Hua, J.; Xing, Y.; Xiao, J.; Zhang, Q. Genetic composition of yield heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. USA 2012, 109, 15847–15852. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Piao, Z.; Wan, C.; Lee, G.; Ruan, X.; Bai, J. Breeding for three-line japonica hybrid rice combinations with high resistant starch content using molecular marker-assisted selection. Breed. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zeng, G.; Hao, M.; Jiang, H.; Xiao, Y. Improvement of rice blast and brown planthopper resistance of PTGMS line C815S in two-line hybrid rice through marker-assisted selection. Mol. Breed. 2020, 40, 21. [Google Scholar] [CrossRef]
- Ansari, M.U.R.; Shaheen, T.; BUKHARI, S.; Husnain, T. Genetic improvement of rice for biotic and abiotic stress tolerance. Turk. J. Bot. 2015, 39, 911–919. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.G. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E. Joseph Gottlieb Kolreuter’s contributions to biology. Osiris 1986, 2, 135–176. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Q. Genetic and molecular characterization of photoperiod and thermo-sensitive male sterility in rice. Plant Reprod. 2018, 31, 3–14. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.-M.; Lin, H.-X.; Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef]
- Eckardt, N.A. Cytoplasmic Male Sterility and Fertility Restoration. Am. Soc. Plant Biol. 2006. [Google Scholar] [CrossRef]
- Feng, T.; Zhu, Q.; Guo, X.; Li, W.; Yu, W.; Li, S.; Wu, W.; Wan, Z.; Huang, D.; Chen, L.; et al. Identification of Loss of Rf1 Gene Function Phenomenon in Restorer Line and its Mutant of Dian-Type Hybrid Rice. Rice Genom. Genet. 2020, 11, 5. [Google Scholar]
- Kim, Y.J.; Zhang, D. Molecular Control of Male Fertility for Crop Hybrid Breeding. Trends Plant Sci. 2018, 23, 53–65. [Google Scholar] [CrossRef]
- Itabashi, E.; Iwata, N.; Fujii, S.; Kazama, T.; Toriyama, K. The fertility restorer gene, Rf2, for Lead Rice-type cytoplasmic male sterility of rice encodes a mitochondrial glycine-rich protein. Plant J. 2011, 65, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, K.; Huang, W.; Liu, G.; Gao, Y.; Wang, J.; Huang, Q.; Ji, Y.; Qin, X.; Wan, L.; et al. The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. Plant J. 2012, 24, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yu, C.; Hu, J.; Wang, L.; Dan, Z.; Zhou, W.; He, C.; Zeng, Y.; Yao, G.; Qi, J.; et al. Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proc. Natl. Acad. Sci. USA 2015, 112, 14984–14989. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Luo, D.; Zhou, D.; Zhang, Q.; Tian, D.; Zheng, X.; Chen, L.; Liu, Y.-G. The rice restorer Rf4 for wild-abortive cytoplasmic male sterility encodes a mitochondrial-localized PPR protein that functions in reduction of WA352 transcripts. Mol. Plant. 2014, 7, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Y.; Li, X.; Zhang, Q.; Chen, L.; Wu, H.; Su, D.; Chen, Y.; Guo, J.; Luo, D.; et al. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 2006, 18, 676–687. [Google Scholar] [CrossRef]
- Komori, T.; Ohta, S.; Murai, N.; Takakura, Y.; Kuraya, Y.; Suzuki, S.; Hiei, Y.; Imaseki, H.; Nitta, N. Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J. 2004, 37, 315–325. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Lu, Y.; Li, C.; Wang, J.; Dong, B.; Liang, B.; Qiu, T.; Zeng, W.; Cao, M. A preliminary identification of Rf*-A619, a novel restorer gene for CMS-C in maize (Zea mays L.). PeerJ 2016, 4, e2719. [Google Scholar] [CrossRef]
- Fujii, S.; Toriyama, K. Suppressed expression of RETROGRADE-REGULATED MALE STERILITY restores pollen fertility in cytoplasmic male sterile rice plants. Proc. Natl. Acad. Sci. USA 2009, 106, 9513–9518. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Q.; Zi, Q.; Lv, S.; Qiu, D.; Zeng, H. Enhanced disease resistance and drought tolerance in transgenic rice plants overexpressing protein elicitors from Magnaporthe oryzae. PLoS ONE 2017, 12, e0175734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Strategies for developing Green Super Rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Omasa, K.; Horie, T. High Temperature at Flowering Inhibits Swelling of Pollen Grains, a Driving Force for Thecae Dehiscence in Rice (Oryza sativa L.). Plant Prod. Sci. 2015, 3, 430–434. [Google Scholar] [CrossRef]
- Yuan, L. Progress in super-hybrid rice breeding. Crop J. 2017, 5, 100–102. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Liu, Y.; Zhaung, C. HMS1 interacts with HMS1I to regulate very-long-chain fatty acid biosynthesis and the humidity-sensitive genic male sterility in rice (Oryza sativa). New Phytol. 2020, 225, 2077–2093. [Google Scholar] [CrossRef]
- Zhou, H.; He, M.; Li, J.; Chen, L.; Huang, Z.; Zheng, S.; Zhu, L.; Ni, E.; Jiang, D.; Zhao, B.; et al. Development of Commercial Thermo-sensitive Genic Male Sterile Rice Accelerates Hybrid Rice Breeding Using the CRISPR/Cas9-mediated TMS5 Editing System. Sci. Rep. 2016, 6, 37395. [Google Scholar] [CrossRef]
- Yu, J.; Han, J.; Kim, Y.-J.; Song, M.; Yang, Z.; He, Y.; Fu, R.; Luo, Z.; Hu, J.; Liang, W.; et al. Two rice receptor-like kinases maintain male fertility under changing temperatures. Proc. Natl. Acad. Sci. USA 2017, 114, 12327–12332. [Google Scholar] [CrossRef]
- Jan, M.; Shah, G.; Yuqing, H.; Xuejiao, L.; Peng, Z.; Hao, C.; Hao, D.; Jumin, T.J. Development of Heat Tolerant Two-Line Hybrid Rice Restorer Line Carrying Dominant Locus of OsHTAS. Rice Sci. Available online: www.sciencedirect.com (accessed on 10 September 2020).
- Si, H.; Liu, W.Z.; Fu, Y.; Sun, Z.; Hu, G. Current situation and suggestions for development of two-line hybrid rice in China. Chin. J. Rice Sci. 2011, 5, 544–552. [Google Scholar]
- Zhou, H.; Zhou, M.; Yang, Y.; Li, J.; Zhu, L.; Jiang, D.; Dong, J.; Liu, Q.; Gu, L.; Zhou, L.; et al. RNase Z(S1) processes UbL40 mRNAs and controls thermosensitive genic male sterility in rice. Nat. Commun. 2014, 5, 4884. [Google Scholar] [CrossRef]
- Lei, D.; Tang, W.; Xie, Z.; Liu, H.; Chen, L. Solutions to insecurity problems in seed production of two-line hybrid rice. Agric. Sci. Technol. 2014, 15, 1160–1166. [Google Scholar]
- Zhu, L.; Chen, Z.; Li, H.; Sun, Y.; Wang, L.; Zeng, H.; He, Y. Lipid metabolism is involved in male fertility regulation of the photoperiod-and thermo sensitive genic male sterile rice line Peiai 64S. Plant Sci. 2020, 299, 110581. [Google Scholar] [CrossRef]
- Zhou, H.; Qinjian Liu, Q.; Li, J.; Jiang, D.; Zhou, L.; Wu, P.; Lu, S.; Li, F.; Zhu, L.; Liu, Z.; et al. Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res. 2012, 22, 649–660. [Google Scholar] [CrossRef]
- Deng, Q.; Fu, X. Studies of fertility stability of p (t) gms rice. III. Drift of critical temperature inducing male sterility and its controlling technology. J. Hum. Agric. Univ. 1998, 24, 8–13. [Google Scholar]
- Shi, M. The discovery and preliminary studies of the photoperiod-sensitive recessive male-sterile rice (Oryza sativa L. subsp. Japonica). Sci. Agric. Sin. 1985, 2, 44–48. [Google Scholar]
- Pan, Y.; Li, Q.; Wang, Z.; Wang, Y.; Ma, R.; Zhu, L.; He, G.; Chen, R. Genes associated with thermosensitive genic male sterility in rice identified by comparative expression profiling. BMC Genom. 2014, 15, 1114. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Xiao, Y.-H.; Lei, D.-Y. Mechanism of Sterility and Breeding Strategies for Photoperiod/Thermo-Sensitive Genic Male Sterile Rice. Rice Sci. 2010, 17, 161–167. [Google Scholar] [CrossRef]
- Xue, Z.; Xu, X.; Zhou, Y.; Wang, X.; Zhang, Y.; Liu, D.; Zhao, B.; Duan, L.; Qi, X. Deficiency of a triterpene pathway results in humidity-sensitive genic male sterility in rice. Nat. Commun. 2018, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, J.; Li, J.; Gao, S.; Zhou, R.; Liu, H.; Chen, Y. Climate change in Urumqi City during 1960–2013. Quat. Int. 2015, 358, 93–100. [Google Scholar] [CrossRef]
- Yuan, L. Purification and Production of Foundation Seed of Rice PGMS and TGMS Lines. Hybrid Rice 1994, 6, 1–3. [Google Scholar]
- Chen, R.; Zhao, X.; Shao, Z.; Wei, Z.; Wang, Y.; Zhu, L.; Zhao, J.; Sun, M.; He, R.; He, G. Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell 2007, 19, 847–861. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, X.; Shao, Z.; Wei, Z.; Wang, Y.; Zhu, L.; Zhao, J.; Sun, M.; He, R.; He, G. A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc. Natl. Acad. Sci. USA 2012, 109, 2654–2659. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, J.; Mathioni, S.M.; Yu, J.; Shen, J.; Yang, X.; Wang, L.; Zhang, Q.; Cai, Z.; Xu, C.; et al. PMS1T, producing phased small-interfering RNAs, regulates photoperiod-sensitive male sterility in rice. Proc. Natl. Acad. Sci. USA 2016, 113, 15144–15149. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Jiang, T.; Berg, H.; Li, C.; Xia, Y. The Arabidopsis U–box/ARM repeat E3 ligase At PUB 4 influences growth and degeneration of tapetal cells, and its mutation leads to conditional male sterility. Plant J. 2013, 74, 511–523. [Google Scholar] [CrossRef]
- Millar, A.A.; Gubler, F. The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 2005, 17, 705–721. [Google Scholar] [CrossRef]
- Liu, N.; Shan, Y.; Wang, F.P.; Xu, C.G.; Peng, K.M.; Li, X.H.; Zhang, Q. Identification of an 85-kb DNA fragment containing pms1, a locus for photoperiod-sensitive genic male sterility in rice. Mol. Genet. Genomics 2001, 266, 271–275. [Google Scholar] [CrossRef]
- Mingsong, S.; Jingyang, D. The discovery, determination and utilization of the Hubei photosensitive genic male-sterile rice (subsp.). Acta Genet. Sin. 1986, 13, 107–112. [Google Scholar]
- He, H.H.; Yuan, S.C. Analyses of plant character in Hubei Photoperiodsensitive Genie Male-sterile Rice (HPGMR) under different light and temperature conditions. Hybrid Rice 1989, 5, 42–44. [Google Scholar]
- Zhang, Q.; Shen, B.; Dai, X.; Mei, M.; Maroof, M.S.; Li, Z. Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male sterility in rice. Proc. Natl. Acad. Sci. USA 1994, 91, 8675–8679. [Google Scholar] [CrossRef]
- Mei, M.; Chen, L.; Zhang, Z.; Li, Z.; Xu, C.; Zhang, Q. pms3 is the locus causing the original photoperiod-sensitive male sterility mutation of ‘Nongken 58S’. Sci. China Ser. C Life Sci. 1999, 42, 316–322. [Google Scholar] [CrossRef]
- Mei, M.; Dai, X.; Xu, C.; Zhang, Q. Mapping and genetic analysis of the genes for photoperiod-sensitive genic male sterility in rice using the original mutant Nongken 58S. Crop Sci. 1999, 39, 1711–1715. [Google Scholar] [CrossRef]
- Li, X.; Lu, Q.; Wang, F.; Xu, C.; Zhang, Q. Separation of the two-locus inheritance of photoperiod sensitive genic male sterility in rice and precise mapping the pms3 locus. Euphytica 2001, 119, 343–348. [Google Scholar] [CrossRef]
- Lu, Q.; Li, X.H.; Guo, D.; Xu, C.G.; Zhang, Q. Localization of pms3, a gene for photoperiod-sensitive genic male sterility, to a 28.4-kb DNA fragment. Mol. Genet. Genom. 2005, 273, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pan, X.; Chen, J.; Yao, Y.; Lu, B.; Tian, X. Out-crossing seed setting rate was seriously reduced by high-temperature in hybrid rice seed production under field conditions. Chin. J. Rice Sci. 2015, 29, 106–110. [Google Scholar]
- Yan, H.; Xu, Z.; Chen, J.; Yao, Y.; Shao, P.; Wang, Y.; Tian, X. Susceptibility of hybrid rice seed production system to high temperature. Chin. Agric. Sci. Bull. 2014, 30, 37–40. [Google Scholar]
- Tian, X.; Huang, Y.; Matsui, T. Characterizing the rice field climatic factors under high temperature stress at anthesis. In Proceedings of the 5th International Crop Science Congress, Goa, India, 19–20 November 2018. [Google Scholar]
- Peng, H.; Zhang, Z.; Wu, B.; Chen, X.; Zhang, G.; Zhang, Z.; Wan, B.; Lu, Y.J.T.; Genetics, A. Molecular mapping of two reverse photoperiod-sensitive genic male sterility genes (rpms1 and rpms2) in rice (Oryza sativa L.). Theor. Appl. Genet. 2008, 118, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.A.; Chen, Z.; Ma, D.; Zeng, H.-L. Aanlysis of short photo-periodic sensitive genic male sterility and molecular mapping of rpms3 (t) gene in rice (Oryza sativa L.) using SSR markers. Genes Genomics 2011, 33, 513. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; He, Y.; Zong, J.; Yang, X.; Si, H.; Sun, Z.; Hu, J.; Liang, W.; Zhang, D. Mutation in CSA creates a new photoperiod-sensitive genic male sterile line applicable for hybrid rice seed production. Proc. Natl. Acad. Sci. USA 2013, 110, 76. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, W.; Yang, X.; Luo, X.; Jiang, N.; Ma, H.; Zhang, D. Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 2010, 22, 672–689. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Z.; Hu, Y.; Shi, S.; Peng, T.; Chu, X.; Shi, J.; Xiang, Z.; Liu, D. Genetic Analysis and Primary Mapping of pms4, a Photoperiod-Sensitive Genic Male Sterility Gene in Rice (Oryza sativa L.). Rice Sci. 2008, 15, 153–156. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Zhang, X.Y.; Xue, Q.Z. Fine mapping and candidate gene prediction of the photoperiod and thermo-sensitive genic male sterile gene pms1(t) in rice. J. Zhejiang Univ. Sci. B 2011, 12, 436–447. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Chen, M.; Liang, W.; Wei, J.; Qi, Y.; Yuan, Z. Development of japonica photo-sensitive genic male sterile rice lines by editing carbon starved anther using CRISPR/Cas9. Genet. Genom. 2016, 43, 415. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xiong, Z.; Min, S.; Si, H. Identification of the temperature sensitive male-sterile rice. China J. Rice Sci. 1989, 3, 49–55. [Google Scholar]
- Wang, B.; Xu, W.W.; Wang, J.Z.; Wu, W.; Zheng, H.G.; Yang, Z.Y.; Ray, J.D.; Nguyen, H.T. Tagging and mapping the thermo-sensitive genic male-sterile gene in rice (Oryza sativa L.) with molecular markers. Theor. Appl. Genet. 1995, 91, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, Q.; Zhang, L.; Mao, B.; Yan, D.; Jin, Q.; He, Z. Fine mapping and candidate gene analysis of the novel thermo-sensitive genic male sterility tms9-1 gene in rice. TAG. Theor. Appl. Genet. Theor. Und Angew. Genet. 2014, 127, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.F.; Chen, X.H.; Lu, Y.P.; Peng, Y.F.; Wan, B.H.; Chen, N.D.; Wu, B.; Xin, S.P.; Zhang, G.Q. Fine mapping of a gene for non-pollen type thermosensitive genic male sterility in rice (Oryza sativa L.). Theor. Appl. Genet. 2010, 120, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.H.; Wei, X.; Shao, G.; Chen, M.L.; Song, J.; Tang, S.Q.; Luo, J.; Hu, Y.C.; Hu, P.; Chen, L.Y. Genetic analysis and fine mapping of tms9, a novel thermosensitive genic male-sterile gene in rice (Oryza sativa L.). Plant Breed. 2013, 132, 159–164. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Tang, P.L.; Yang, W.C.; Liu, A.M.; Chen, Y.Q.; Lin, W.B.; Shi, T.B. Breeding and utilization of TGMS line Zhu1S in rice. Hybrid Rice 2000, 15, 6–8. [Google Scholar]
- Yamaguchi, Y.; Hirasawa, H.; Minami, M.; Ujihara, A. Linkage analysis of thermosensitive genic male sterility gene, tms-2 in rice (Oryza sativa L.). Jpn. J. Breed. 1997, 47, 371–373. [Google Scholar] [CrossRef]
- Subudhi, P.K.; Borkakati, R.P.; Virmani, S.S.; Huang, N. Molecular mapping of a thermo-sensitive genetic male sterility gene in rice using bulked segregant analysis. Genome 1997, 40, 188–194. [Google Scholar] [CrossRef]
- Dong, N.V.; Subudhi, P.K.; Luong, P.N.; Quang, V.D.; Quy, T.D.; Zheng, H.G.; Wang, B.; Nguyen, H.T. Molecular mapping of a rice gene conditioning thermosensitive genic male sterility using AFLP, RFLP and SSR techniques. Theor. Appl. Genet. 2000, 100, 727–734. [Google Scholar] [CrossRef]
- Lee, D.S.; Chen, L.J.; Suh, H.S. Genetic characterization and fine mapping of a novel thermo-sensitive genic male-sterile gene tms6 in rice (Oryza sativa L.). Theor. Appl. Genet. 2005, 111, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.U.K.; Siddiq, E.A.; Sarma, N.P.; Ali, J.; Hussain, A.J.; Nimmakayala, P.; Ramasamy, P.; Pammi, S.; Reddy, A.S. Genetic analysis of temperature-sensitive male sterilty in rice. Theor. Appl. Genet. 2000, 100, 794–801. [Google Scholar] [CrossRef]
- Jia, J.H.; Zhang, D.S.; Li, C.Y.; Qu, X.P.; Wang, S.W.; Chamarerk, V.; Nguyen, H.T.; Wang, B. Molecular mapping of the reverse thermo-sensitive genic male-sterile gene (rtms1) in rice. Theor. Appl. Genet. 2001, 103, 607–612. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Zhang, X.; Wang, S. Genetic analysis and mapping of a thermosensitive genic male sterility gene, tms6 (t), in rice (Oryza sativa L.). Genome 2010, 53, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xiao, H.; Lei, D.; Duan, Q. The breeding of indica photosensitive male sterile line. Hunan Acad. Agric. Sci. 1988, 6, 16–18. [Google Scholar]
- Sun, Z. A temperaturesensitive male-sterile line found in rice. Rice Genet. Newslett. 1989, 6, 116–117. [Google Scholar]
- Maruyama, K.; Araki, H.; Kato, H. Thermosensitive genetic male sterility induced by irradiation. In Rice Genetics II: (In 2 Parts); World Scientific: Singapore, 1991; pp. 227–232. [Google Scholar]
- Virmani, S.; Voc, P. Induction of photo-and thermo-sensitive male sterility in indica rice. Agron. Abstr. 1991, 119. [Google Scholar]
- Ali, J.; Siddiq, E.; Zaman, F.; Abraham, M.; Ahmed, I. Identification and characterization of temperature sensitive genic male sterile sources in rice (Oryza sativa L.). Indian J. Genet. Plant Breed. 1995, 55, 243–259. [Google Scholar]
- Pandey, M.; Rongbai, L.; Singh, J.; Mani, S.; Singh, H.; Singh, S. The identification and nature of a new thermosensitive genic male sterility source, UPRI 95-140 TGMS in rice. Cereal Res. Commun. 1998, 26, 265–269. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, G.; Chen, L. Response of fertility of Pei^ ai 64 S to temperature and photoperiod in rice. Zuo Wu Xue Bao 1999, 25, 772–776. [Google Scholar]
- Chuan-gen, L.; Zou, J.-S.; Ning, H.; Yao, K.-M. Plant temperature for sterile alteration of a temperature-sensitive genic male sterile rice, Peiai64S. Agric. Sci. China 2007, 6, 1283–1290. [Google Scholar] [CrossRef]
- Yuan, L. Progress of two-line system hybrid rice breeding. Sci. Agric. 1990, 3, 1–6. [Google Scholar]
- Lang, N.T.; Subudhi, P.K.; Virmani, S.S.; Brar, D.S.; Khush, G.S.; Li, Z.; Huang, N. Development of PCR-based markers for thermosensitive genetic male sterility gene tms3 (t) in rice (Oryza sativa L.). Hereditas 1999, 131, 121–127. [Google Scholar] [CrossRef]
- Wang, Y.G.; Xing, Q.H.; Deng, Q.Y.; Liang, F.S.; Yuan, L.P.; Weng, M.L.; Wang, B. Fine mapping of the rice thermo-sensitive genic male-sterile gene tms5. Theor. Appl. Genet. 2003, 107, 917–921. [Google Scholar] [CrossRef]
- Ku, S.J.; Yoon, H.; Suh, H.S.; Chung, Y.Y. Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 2003, 217, 559–565. [Google Scholar] [CrossRef]
- Guo, G.; Guo, M.; Yin, M.; Yin, J.; Meng, W. Discovery and analysis of new PTGMS lines. Guangxi Agric. Sci. 2004, 35, 187–188. [Google Scholar]
- Peng, H.; Qiu, Z.; Chen, X.; Wan, B.; Zhang, G.; Lu, Y. Pollen fertility and cytological observation of a thermosensitive genic male sterile line of non-pollen type XianS in rice (Oryza sativa L.). Acta Ecol. Sin. 2006, 26, 2322–2327. [Google Scholar]
- Yang, Y.; Fu, C.; Hu, X.; Zhang, Z.; Zhou, Y.; Song, Y. Discovery of thermosensitive genic male sterile gene in Zhu1S and study on super hybrid early rice breeding. J. China Rice 2007, 6, 17–22. [Google Scholar]
- Peng, H.; Wan, B.; Zhang, G.; Lu, Y.; Zhou, G.; Chen, X. Microstructure observations of pollenless abortion in thermo-and photoperiod-sensitive genic male sterile line N28S in rice (Oryza sativa L.). Acta Bot. Yunnanica 2009, 31, 15–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Ju, C.; Xu, G.; Huang, Z.; Zhao, H.; Xie, P.; Gao, M. Breeding and utilization of fine quality indica TGMS line HD9802S in Rice. Hybrid Rice 2008, 23, 7–10. [Google Scholar]
- Yiming, J. Studies on the Effect of High Temperature on Fertility of the Male Sterile Lines in Dian-tpye Hybrid Rice. Yunnan Agric. Univ. 1988, 2. [Google Scholar] [CrossRef]
- Zhang, Z. Preliminary observation of fertility changes in the new type temperature sensitive male sterile lines. IV. A. Hybrid Rice 1991, 1, 31–34. [Google Scholar]
- Shen, Y.; Gao, M.; Cai, Q. A novel environment-induced genic male sterile (EGMS) mutant in indica rice. Euphytica 1994, 76, 89–96. [Google Scholar] [CrossRef]
- Ali, A.J.; Siddiq, E. Isolation and characterization of a reverse temperature sensitive genic male sterile mutant in rice. Indian J. Genet. Plant Breed. 1999, 59, 423–428. [Google Scholar]
- Luo, X.; Qiu, Z.; Li, R. Pei’ai 64S, a dual purpose sterile line whose sterility is induced by low critical temperature. Hybrid Rice 1992, 1, 27–29. [Google Scholar]
- Virmani, S. Heterosis and hybrid rice breeding. Theoretical and Applied Genetics 1994, 22, 1–189. [Google Scholar]
- Ashraf, M.F.; Yang, S.; Wu, R.; Wang, Y.; Hussain, A.; Noman, A.; Khan, M.I.; Liu, Z.; Qiu, A.; Guan, D.; et al. Capsicum annuum HsfB2a positively regulates the response to Ralstonia solanacearum infection or high temperature and high humidity forming transcriptional cascade with CaWRKY6 and CaWRKY40. Plant Cell Physiol. 2018, 59, 2608–2623. [Google Scholar] [CrossRef]
- Abd-Elmabod, S.K.; Muñoz-Rojas, M.; Jordán, A.; Anaya-Romero, M.; Phillips, J.D.; Jones, L.; Zhang, Z.; Pereira, P.; Fleskens, L.; der Ploeg, M.; et al. Climate change impacts on agricultural suitability and yield reduction in a Mediterranean region. Geoderma 2020, 374, 114453. [Google Scholar] [CrossRef]
- Singh, A.S.; Eanes, F.; Prokopy, L.S. Climate change uncertainty among American farmers: An examination of multi-dimensional uncertainty and attitudes towards agricultural adaptation to climate change. Clim. Change 2020. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Wu, Y.; Du, P.; Wang, J.; Wang, M.; Yi, C.; Gu, M.; Liang, G. Fine mapping and candidate gene analysis of ptgms2-1, the photoperiod-thermo-sensitive genic male sterile gene in rice (Oryza sativa L.). Theor. Appl. Genet.USA 2011, 122, 365–372. [Google Scholar] [CrossRef]
- Yu, B.; Liu, L.; Wang, T. Deficiency of very long chain alkanes biosynthesis causes humidity-sensitive male sterility via affecting pollen adhesion and hydration in rice. Plant Cell Environ. 2019, 42, 3340–3354. [Google Scholar] [CrossRef] [PubMed]
- Baklien, K.; Fausa, O.; Brandtzaeg, P.; Froland, S.S.; Gjone, E. Malabsorption, villous atrophy, and excessive serum IgA in a patient with unusual intestinal immunocyte infiltration. Scand. J. Gastroenterol. 1977, 12, 421–432. [Google Scholar] [CrossRef]
- Si, H.-M.; Fu, Y.-P.; Liu, W.-Z.; Sun, Z.-X.; Hu, G.-C. Pedigree Analysis of Photoperiod-thermo Sensitive Genic Male Sterile Rice. Acta Agron. Sin. 2012, 38, 394–407. [Google Scholar] [CrossRef]
- Lu, X. Sterility Ecology of Chinese Photoperiod-/Temperature-Sensitive Genic Male Sterile Rice; Science Press: Beijing, China, 2003. [Google Scholar]
- Zhang, Z.; Zen, Y.; Yang, J.; Yuan, S. Fertility altering conditions and ecological adaptability of photosensitive genic male sterile rice. Chin. J. Rice Sci. 1993, 7, 123–128. [Google Scholar]
- Cheng, S.; Si, H.; Zhuo, L.; Sun, Z. Classification of environmentally induced genetic male sterile lines of rice based on their fertility responses to photoperiod and temperature. J. Agric. Sci. 1996, 127, 161–167. [Google Scholar] [CrossRef]
- Mengchen, Z.; Shan, W.; Jianfang, Y.; Shuiyong, S.; Xin, X.; Qun, X.; Xiaoping, Y.; Xinghua, W.; Yaolong, Y. Classification and Identification of indica P/TGMS Lines in China. Rice Sci. 2019, 26, 195–198. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, X.; Sassenrath, G.F. Current irrigation practices in the central United States reduce drought and extreme heat impacts for maize and soybean, but not for wheat. Sci. Total Environ. 2015, 508, 331–342. [Google Scholar] [CrossRef]
- De Guzman, C.T.; Linscombe, S.D.; Oard, J.H. Genetic analysis of environment-sensitive genic male sterile rice under US environments. Euphytica 2019, 215, 39. [Google Scholar] [CrossRef]
- Nowinszky, L.; Nautiyal, M.K.; Tripathi, A.; Gautam, A. Two Line Hybrid Seed Production in Rice. J. Vacc. Res. 2018, 6. [Google Scholar]
- Nthakanio, P.N.; Kariuki, S.N. Production of Hybrid Rice seeds using environment sensitive genic male sterile (EGMS) and basmati rice lines in Kenya. J. BioRxiv 2019, 755306. [Google Scholar] [CrossRef]
- Hu, X.; Tian, Y.; Xu, Q. Review of extension and analysis on current status of hybrid rice in China. Hybrid Rice 2016, 31, 1–8. [Google Scholar]
- Yuan, L.-P. Development of Hybrid Rice to Ensure Food Security. Rice Sci. 2014, 21, 1–2. [Google Scholar] [CrossRef]
- Huang, J.; Qin, F.; Zang, G.; Kang, Z.; Zou, H.; Hu, F.; Yue, C.; Li, X.; Wang, G. Corrigendum to “Mutation of OsDET1 increases chlorophyll content in rice”. Plant Sci. 2014, 214, 241–249. [Google Scholar] [CrossRef]
- Whitford, R.; Fleury, D.; Reif, J.C.; Garcia, M.; Okada, T.; Korzun, V.; Langridge, P. Hybrid breeding in wheat: Technologies to improve hybrid wheat seed production. J. Exp. Bot. 2013, 64, 5411–5428. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.C.; Liang, K.L.; Wang, N.Y.; Chen, S. A recessive gene in indica rice 5460S for thermosensitive genic male sterility. Rice Genet. Newslett. 1992, 9, 56–57. [Google Scholar]
- Lopez, M.T.; Toojinda, T.; Vanavichit, A.; Tragoonrung, S. Microsatellite markers flanking the tms2 gene facilitated tropical TGMS rice line development. Crop Sci. 2003, 43, 2267–2271. [Google Scholar]
- Mei, G.; Wang, X.; Wang, M. Genetic analysis of the photoperiod sensitive male sterility of Nongken 58S and its derivatives. J. Huangzhong Agric. Univ. 1990, 9, 400–406. [Google Scholar]
- Alcochete, A.A.N.D.; Rangel, P.H.N.; Ferreira, M.E. Mapping of quantitative trait loci for thermosensitive genic male sterility in indica rice. Pesqui. Agropecu. Bras. 2005, 40, 1179–1188. [Google Scholar]
- Chen, X.; Hu, J.; Zhang, H.; Ding, Y. DNA methylation changes in photoperiod-thermo-sensitive male sterile rice PA64S under two different conditions. Gene 2014, 537, 143–148. [Google Scholar] [CrossRef]
- Kelen, K.V.D.; Beyaert, R.; Inzé, D.; Veylder, L.D. Translational control of eukaryotic gene expression. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 143–168. [Google Scholar] [CrossRef]
- Shin, C.; Manley, J.L. Cell signalling and the control of pre-mRNA splicing. Nat. Rev. Molecular. Cell Biol. 2004, 5, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Halbeisen, R.E.; Galgano, A.; Scherrer, T.; Gerber, A.P. Post-transcriptional gene regulation: From genome-wide studies to principles. Cell. Mol. Life Sci. 2008, 65, 798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.M.; Chen, J.; Pang, H.B.; Liu, S.; Gao, Q.; Wang, J.R.; Qiao, W.H.; Wang, H.; Liu, J.; Olsen, K.M.; et al. Genome-wide analyses reveal the role of noncoding variation in complex traits during rice domestication. Sci. Adv. 2019, 5, eaax3619. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shen, J.; Mao, H.; Xie, W.; Li, X.; Zhang, Q. RNA-directed DNA methylation is involved in regulating photoperiod-sensitive male sterility in rice. Mol. Plant 2012, 5, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Teotia, S.; Tang, G. To bloom or not to bloom: Role of microRNAs in plant flowering. Mol. Plant Pathol. 2015, 8, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Zhang, H.; Arikit, S.; Huang, K.; Nan, G.-L.; Walbot, V.; Meyers, B.C. Spatiotemporally dynamic, cell-type–dependent premeiotic and meiotic phasiRNAs in maize anthers. Proc. Natl. Acad. Sci. USA 2015, 112, 3146–3151. [Google Scholar] [CrossRef]
- Wu, S.; Tan, H.; Hao, X.; Xie, Z.; Wang, X.; Li, D.; Tian, L. Profiling miRNA expression in photo-thermo-sensitive male genic sterility line (PTGMS) PA64S under high and low temperature. Plant Signal. Behav. 2019, 14, 1679015. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Cai, M.; Ramachandran, S. ORYZA SATIVA MYOSIN XI B controls pollen development by photoperiod-sensitive protein localizations. Dev. Biol. 2007, 304, 579–592. [Google Scholar] [CrossRef]
- Noman, A.; Fahad, S.; Aqeel, M.; Ali, U.; Anwar, S.; Baloch, S.K.; Zainab, M.J.B.L. miRNAs: Major modulators for crop growth and development under abiotic stresses. Biotechnol. Lett. 2017, 39, 685–700. [Google Scholar] [CrossRef]
- Chueasiri, C.; Chunthong, K.; Pitnjam, K.; Chakhonkaen, S.; Sangarwut, N.; Sangsawang, K.; Suksangpanomrung, M.; Michaelson, L.V.; Napier, J.A.; Muangprom, A. Rice ORMDL controls sphingolipid homeostasis affecting fertility resulting from abnormal pollen development. PLoS ONE 2014, 9, e106386. [Google Scholar] [CrossRef]
- Jimmy, J.L.; Babu, S. Role of OsWRKY transcription factors in rice disease resistance. Trop. Plant Pathol. 2015, 40, 355–361. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Z.; Vizcay-Barrena, G.; Yang, C.; Liang, W.; Zong, J.; Wilson, Z.A.; Zhang, D. PERSISTENT TAPETAL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice. Plant Physiol. 2011, 156, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.R.; Davis, S.J.; Bastow, R.M.; McWatters, H.G.; Kozma-Bognár, L.; Nagy, F.; Millar, A.J.; Amasino, R.M. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature 2002, 419, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, X.; Zhang, H.; Ding, Y. Genome-wide analysis of DNA methylation in photoperiod-and thermo-sensitive male sterile rice Peiai 64S. BMC Genom. 2015, 16, 102. [Google Scholar] [CrossRef]
- He, J.-X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.-Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190. [Google Scholar] [CrossRef]
- Nonomura, K.-I.; Nakano, M.; Murata, K.; Miyoshi, K.; Eiguchi, M.; Miyao, A.; Hirochika, H.; Kurata, N. An insertional mutation in the rice PAIR2 gene, the ortholog of Arabidopsis ASY1, results in a defect in homologous chromosome pairing during meiosis. Mol. Genet. Genom. 2004, 271, 121–129. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xia, L. Precise Genome Modification via Sequence-Specific Nucleases-Mediated Gene Targeting for Crop Improvement. Front. Plant Sci. 2016, 7, 1928. [Google Scholar] [CrossRef]
- Feng, Z.; Botao Zhang, B.; Ding, W.; Liu, X.; Yang, D.-L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y.; et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; He, S. CRISPR-Cas9: Tool for Qualitative and Quantitative Plant Genome Editing. Front. Plant Sci. 2016, 7, 1740. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Fiaz, S.; Ahmad, S.; Noor, M.A.; Wang, X.; Younas, A.; Riaz, A.; Riaz, A.; Ali, F. Applications of the CRISPR/Cas9 System for Rice Grain Quality Improvement: Perspectives and Opportunities. Int. J. Mol. Sci. 2019, 20, 888. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhang, H.; Lou, D.; Yu, D. Selection of highly efficient sgRNAs for CRISPR/Cas9-based plant genome editing. Sci. Rep. 2016, 6, 21451. [Google Scholar] [CrossRef] [PubMed]
- Kuyek, D.; Zamora, O.; Quijano, R. Hybrid rice in Asia: An unfolding threat. Curr. Trends Agric. Res. and Dev. Biothai Grain Kmp Masipag Pan Indones. Philipp. Greens Ub. 2000, 3, 1–20. [Google Scholar]
- Mishra, R.; Rao, G.J.N.; Rao, R.N.; Kaushal, P. Development and Characterization of Elite Doubled Haploid Lines from Two Indica Rice Hybrids. Rice Sci. 2015, 22, 290–299. [Google Scholar] [CrossRef]
- Niizeki, H.; Oono, K. Induction of haploid rice plant from anther culture. Proc. Jpn. Acad. 1968, 44, 554–557. [Google Scholar] [CrossRef]
- Zapata-Arias, F. Laboratory protocol for anther culture technique in rice. In Doubled Haploid Production in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2003; pp. 109–116. [Google Scholar]
- Lee, J.H.; Lee, S.Y. Selection of stable mutants from cultured rice anthers treated with ethyl methane sulfonic acid. Plant Cell Tissue And Organ Cult. 2002, 71, 165–171. [Google Scholar] [CrossRef]
- Grewal, R.K.; Lulsdorf, M.; Croser, J.; Ochatt, S.; Vandenberg, A.; Warkentin, T.D. Doubled-haploid production in chickpea (Cicer arietinum L.): Role of stress treatments. Plant Cell Rep. 2009, 28, 1289–1299. [Google Scholar] [CrossRef]
- Hooghvorst, I.; Nogués, S. Chromosome doubling methods in doubled haploid and haploid inducer-mediated genome-editing systems in major crops. Plant Cell Rep. 2020. [Google Scholar] [CrossRef]
- Priyadarshan, P. Backcross Breeding; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Spillane, C.; Curtis, M.D.; Grossniklaus, U. Apomixis technology development-virgin births in farmers’ fields? Nat. Biotechnol. 2004, 22, 687–691. [Google Scholar] [CrossRef]
- Hand, M.L.; Koltunow, A.M. The genetic control of apomixis: Asexual seed formation. Genetics 2014, 197, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Barcaccia, G.; Albertini, E. Apomixis in plant reproduction: A novel perspective on an old dilemma. Plant Reprod. 2013, 26, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.A.; Mookkan, M.; Huo, H.; Chae, K.; Ozias-Akins, P. A parthenogenesis gene of apomict origin elicits embryo formation from unfertilized eggs in a sexual plant. Proc. Natl. Acad. Sci. USA 2015, 112, 11205–11210. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.; Willemsen, V.; Boutilier, K.; Heidstra, R. AINTEGUMENTA-LIKE proteins: Hubs in a plethora of networks. Trends Plant Sci. 2014, 19, 146–157. [Google Scholar] [CrossRef]
- Horstman, A.; Li, M.; Heidmann, I.; Weemen, M.; Chen, B.; Muino, J.M.; Angenent, G.C.; Boutilier, K. The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis. Plant Physiol. 2017, 175, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Khanday, I.; Skinner, D.; Yang, B.; Mercier, R.; Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 2019, 565, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, P.; Dwivedi, K.K.; Radhakrishna, A.; Srivastava, M.K.; Kumar, V.; Roy, A.K.; Malaviya, D.R. Partitioning apomixis components to understand and utilize gametophytic apomixis. Front. Plant Sci. 2019, 10, 256. [Google Scholar] [CrossRef] [PubMed]
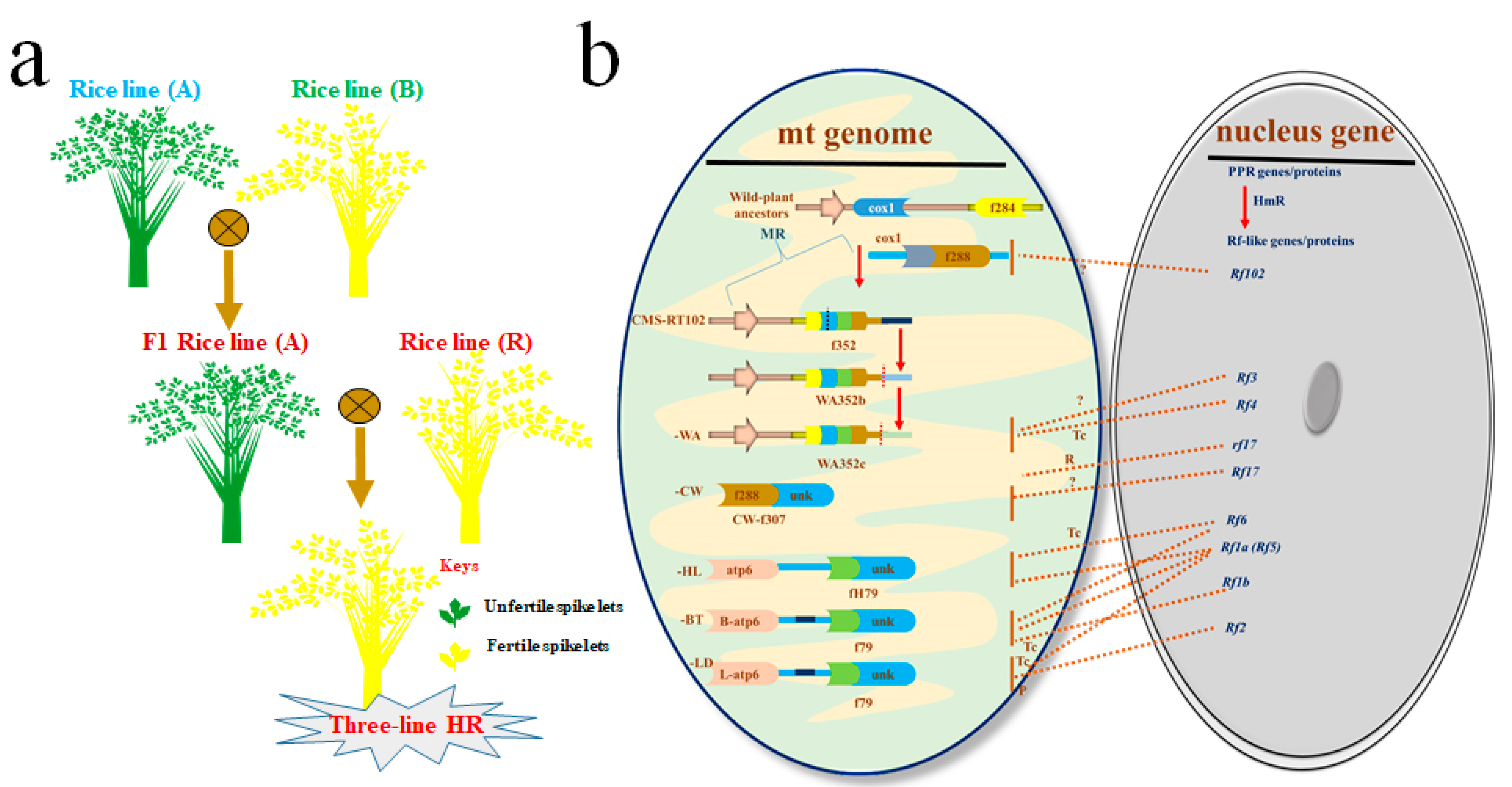
| Sr. # | Rf Locus in CMS Line for Three-Line HR Technology | References |
|---|---|---|
| 1 | Rf4 identified in CMS-wild-abortive (WA) and classified as PPR protein | [30,32] |
| 2 | Rf1a, Rf1b (Rf5) identified in CMS-Chinsurah-Boro II/Taichung 65 (BT) and classified as PPR protein | [33,34] |
| 3 | Rf2 identified in CMS Lead-rice (LD) and classified as non-PPR protein with glycine-rich domain | [29] |
| 4 | Rf-A619 region identified in CMS-Charrua (CMS-C) with unknown protein character | [35] |
| 5 | Rf5 (Rf1b), Rf6 identified in CMS-Honglian (HL) and classified as PPR protein | [30,31] |
| 6 | Rf17 identified in CMS Chinese wild-type rice (CW) and classified as Acyl-synthase a carrier protein | [36] |
| Sr. # | EGMS Line | Locus/Genes Responsive for EGMS Lines in Rice | References |
|---|---|---|---|
| 1 | NK58S | PMS1, PMS2, PMS3 generate PGMS 1 in Japonica | [51,65,66] |
| 2 | Mian9S | PMS4 generates PGMS 1 in Indica | [77] |
| 3 | Yi D1S | RPMS1 and RPMS2 generate rPGMS 2 in Indica | [73,78] |
| 4 | 9522csa | CSA generates rPGMS 2 in Japonica | [79] |
| 5 | 5460S | TMS1 generates TGMS 3 in Indica | [80,81] |
| 6 | AnnongS-1 | TMS5 generates TGMS 3 in Indica | [46] |
| 7 | HengnongS-1 | TMS9-1 generates TGMS 3 in Indica | [82] |
| 8 | Zhu1S | TMS9 generates TGMS 3 in Indica | [83,84,85] |
| 9 | NorinPL12 | TMS2 generates TGMS 3 in Japonica | [86] |
| 10 | IR32364 | TMS3(t) generates TGMS 3 in Indica | [87] |
| 11 | TGMS-VN1 | TMS4(t) generates TGMS 3 in Indica | [88] |
| 12 | Sokcho-MS | TMS6 generates TGMS 3 in Japonica | [89] |
| 13 | SA2 | TGMS generates TGMS 3 in Indica | [90] |
| 14 | J207S | RTMS1 generates TGMS 3 in Indica | [91] |
| 15 | G20S | TMS6(t) generates TGMS 3 in Japonica | [92] |
| Sr. # | Regulation Point of EGMS System in EGMS Line | References |
|---|---|---|
| 1 | PGMS 1, DL ≤ 13 h (MF), ≥ 13.75 h (MS) in NK58S | [68,69,74] |
| 2 | TGMS 2, LT ≤ 23.5 °C (MF), HT ≥ 27 °C (MS) in PA64S | [78,119] |
| 3 | rPGMS 3, HT ≥ 13.5 h (MF), LT ≤ 12.5 (MS) in CSA | [75] |
| 4 | TGMS 2, LT ≤ 21 °C (MF), HT ≥ 28 °C (MS) in Ugp1 | [57] |
| 5 | TGMS, LT ≤ 23.5 °C (MF), HT ≥ 27 °C (MS) in 93-11s | [42] |
| 6 | HGMS 4, RH > 80% (MF), RH < 60% (MS) in E157 and S4928 | [54] |
| 7 | HGMS 4, RH > 80%(MF), RH 30–60% (MS) in osgl1-4 | [120] |
| 8 | HGMS 4, RH > 75%(MF), RH = 45% (MS) in hms1 | [41] |
| 9 | TGMS 2, TGMS = 22–24 °C (MF), >24 °C (MS) in tms10 | [43] |
| 10 | PTGMS 5, LD (14 h) and SD (12 h) conditions or HT (27–30 °C) and LT (21–23 °C) in p/tms12-1 | [42] |
| 11 | PGMS 1 ≤ 13 h (MF), ≥13.75 h (MS) in YiD1S | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.F.; Peng, G.; Liu, Z.; Noman, A.; Alamri, S.; Hashem, M.; Qari, S.H.; Mahmoud al Zoubi, O. Molecular Control and Application of Male Fertility for Two-Line Hybrid Rice Breeding. Int. J. Mol. Sci. 2020, 21, 7868. https://doi.org/10.3390/ijms21217868
Ashraf MF, Peng G, Liu Z, Noman A, Alamri S, Hashem M, Qari SH, Mahmoud al Zoubi O. Molecular Control and Application of Male Fertility for Two-Line Hybrid Rice Breeding. International Journal of Molecular Sciences. 2020; 21(21):7868. https://doi.org/10.3390/ijms21217868
Chicago/Turabian StyleAshraf, Muhammad Furqan, Guoqing Peng, Zhenlan Liu, Ali Noman, Saad Alamri, Mohamed Hashem, Sameer H. Qari, and Omar Mahmoud al Zoubi. 2020. "Molecular Control and Application of Male Fertility for Two-Line Hybrid Rice Breeding" International Journal of Molecular Sciences 21, no. 21: 7868. https://doi.org/10.3390/ijms21217868
APA StyleAshraf, M. F., Peng, G., Liu, Z., Noman, A., Alamri, S., Hashem, M., Qari, S. H., & Mahmoud al Zoubi, O. (2020). Molecular Control and Application of Male Fertility for Two-Line Hybrid Rice Breeding. International Journal of Molecular Sciences, 21(21), 7868. https://doi.org/10.3390/ijms21217868







