Gastrointestinal Nematode-Derived Antigens Alter Colorectal Cancer Cell Proliferation and Migration through Regulation of Cell Cycle and Epithelial-Mesenchymal Transition Proteins
Abstract
1. Introduction
2. Results
2.1. H. polygyrus-Derived Antigens Limit CRC Cell Proliferation and DNA Synthesis
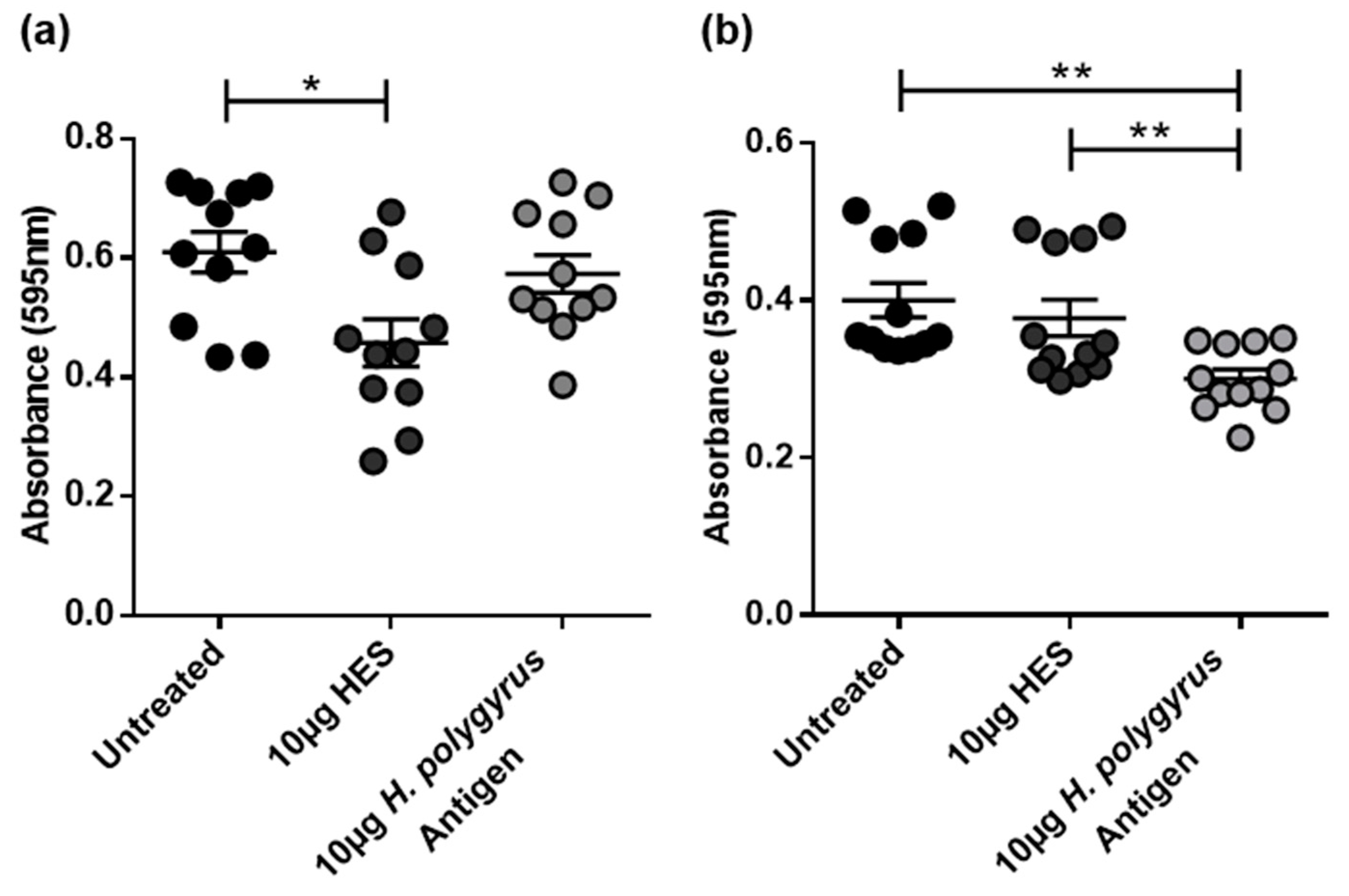
2.2. H. polygyrus Excretory/Secretory Products Significantly Decrease CRC Cell Viability in a Species-Specific Manner
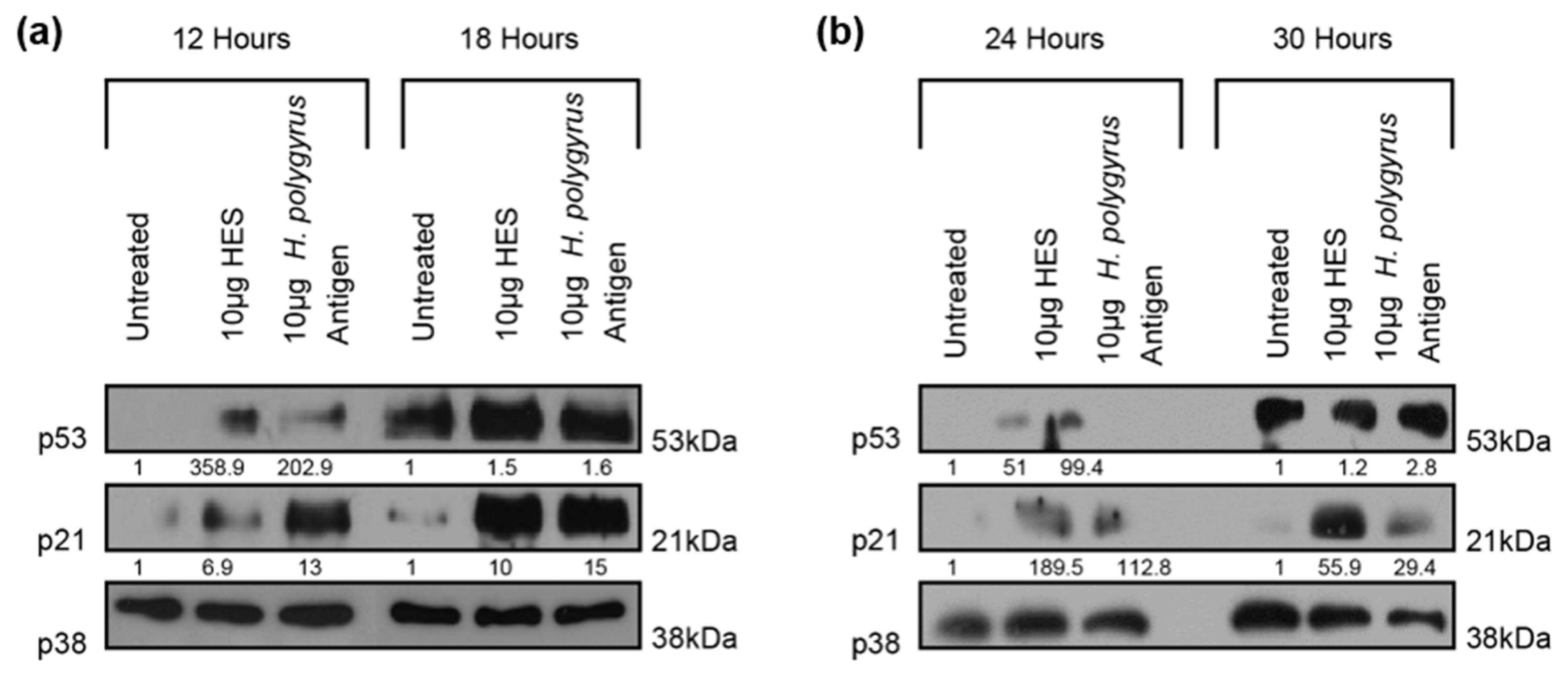
2.3. H. polygyrus-Derived Antigens Increase the Expression of the Cell-Cycle Arrest Proteins p21 and p53 in CRC Cells
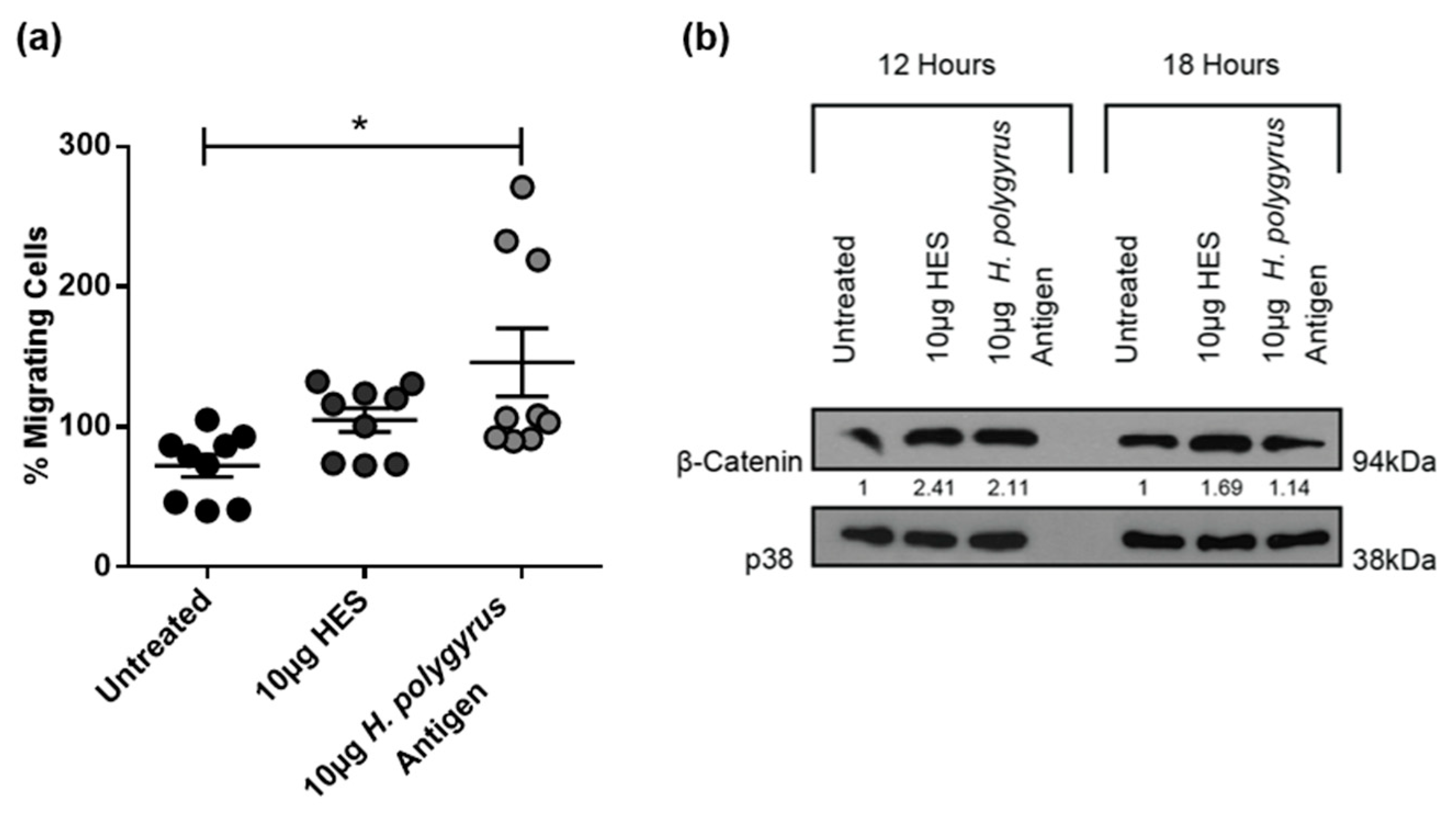
2.4. H. polygyrus-Derived Antigens Increase Murine CRC Cell Migration and β-Catenin Expression
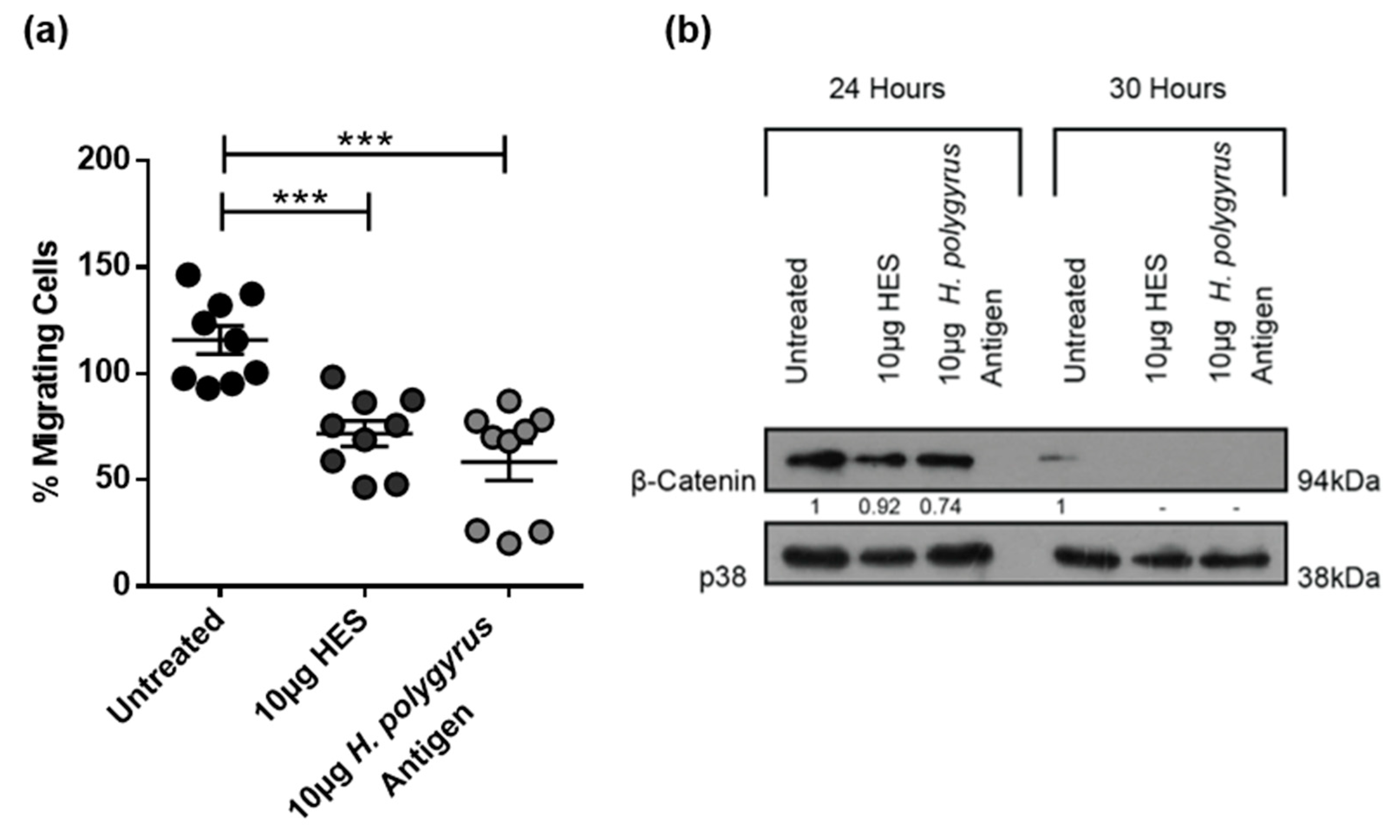
2.5. H. polygyrus-Derived Antigens Influence CRC Cell Migration and β-Catenin Expression in a Species-Specific Manner
3. Discussion
4. Materials and Methods
4.1. Heligmosomoides Polygyrus Antigen and HES Preparation
4.2. Cell Culture
4.3. Growth Curve Assay
4.4. 3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
4.5. Bromodeoxyuridine (BrdU) Incorporation Assay
4.6. Schistosoma Mansoni Egg Antigen (SEA) Preparation
4.7. Transwell Migration Assay
4.8. Western blotting
4.9. Ethics
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Adenomatous Polyposis Coli |
| ATCC | American Type Culture Collection |
| BCA | Bicinchoninic Acid |
| BrdU | Bromodeoxyuridine |
| CAC | Colitis Associated Colorectal Cancer |
| CCA | Cholangiocarcinoma |
| CRC | Colorectal Cancer |
| DALY | Disability-Adjusted Life Years |
| DAPI | 4’6-diamidino-2-phenylindole |
| ECL | Enhanced Chemiluminescence |
| EMT | Epithelial-Mesenchymal Transition |
| FBS | Foetal Bovine Serum |
| HES | H. polygyrus Excretory/Secretory Products |
| Hp-TGM | H. polygyrus TGF-β Mimic |
| IBD | Inflammatory Bowel Disease |
| LMIC | Low-and Middle-Income Countries |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NMRI | Naval Medical Research Institute |
| PBS | Phosphate Buffered Saline |
| SD | Standard Deviation |
| SEA | Schistosoma mansoni egg antigen |
| STH | Soil-transmitted helminth |
References
- International Agency for Research on Cancer-Globocan 2018: Cancer Fact Sheets—Colorectal Cancer. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 1 September 2020).
- Durko, L.; Malecka-Panas, E. Lifestyle Modifications and Colorectal Cancer. Curr. Colorectal Cancer Rep. 2014, 10, 45–54. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260.e1216. [Google Scholar] [CrossRef]
- Kinugasa, T.; Akagi, Y. Status of colitis-associated cancer in ulcerative colitis. World J. Gastrointest. Oncol. 2016, 8, 351–357. [Google Scholar] [CrossRef]
- Romano, M.; De Francesco, F.; Zarantonello, L.; Ruffolo, C.; Ferraro, G.A.; Zanus, G.; Giordano, A.; Bassi, N.; Cillo, U. From Inflammation to Cancer in Inflammatory Bowel Disease: Molecular Perspectives. Anticancer Res. 2016, 36, 1447–1460. [Google Scholar]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef]
- León-Cabrera, S.; Callejas, B.E.; Ledesma-Soto, Y.; Coronel, J.; Pérez-Plasencia, C.; Gutiarrez-Cirlos, E.B.; Avila-Moreno, F.; Rodraguez-Sosa, M.; Hernandez-Pando, R.; Marquina-Castillo, B.; et al. Extraintestinal Helminth Infection Reduces the Development of Colitis-Associated Tumorigenesis. Int. J. Biol. Sci. 2014, 10, 948–956. [Google Scholar] [CrossRef]
- Leon-Cabrera, S.A.; Molina-Guzman, E.; Delgado-Ramirez, Y.G.; Vazquez-Sandoval, A.; Ledesma-Soto, Y.; Perez-Plasencia, C.G.; Chirino, Y.I.; Delgado-Buenrostro, N.L.; Rodriguez-Sosa, M.; Vaca-Paniagua, F.; et al. Lack of STAT6 Attenuates Inflammation and Drives Protection against Early Steps of Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2017, 5, 385–396. [Google Scholar] [CrossRef]
- Pastille, E.; Frede, A.; McSorley, H.J.; Grab, J.; Adamczyk, A.; Kollenda, S.; Hansen, W.; Epple, M.; Buer, J.; Maizels, R.M.; et al. Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer. PLoS Pathog. 2017, 13, e1006649. [Google Scholar] [CrossRef]
- Terrazas, L.I.; Callejas, B.; Reyes, S.; Ledesma-Soto, Y.; Olguín, E.J.; León-Cabrera, S.; Rodríguez-Sosa, M. Down-modulation of colitis-associated colorectal cancer development by treatment with helminth-derived molecules. J. Immunol. 2017, 198, 66–67. [Google Scholar]
- Elliott, D.E.; Summers, R.W.; Weinstock, J.V. Helminths as governors of immune-mediated inflammation. Int. J. Parasitol. 2007, 37, 457–464. [Google Scholar] [CrossRef]
- WHO/TDR. Research Priorities for Helminth Infections. Available online: https://www.who.int/tdr/publications/helminth_infections/en/ (accessed on 1 September 2020).
- Mathers, C.D.; Ezzati, M.; Lopez, A.D. Measuring the burden of neglected tropical diseases: The global burden of disease framework. PLoS Negl. Trop. Dis. 2007, 1, e114. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention—Parasites—Soil-Transmitted Helminths. Available online: https://www.cdc.gov/parasites/sth/index.html (accessed on 1 September 2020).
- Cooper, P.J.; Sandoval, C.; Chico, M.E.; Griffin, G.E. Geohelminth infections protect against severe inflammatory diarrhoea in children. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 519–521. [Google Scholar] [CrossRef]
- Feary, J.; Britton, J.; Leonardi-Bee, J. Atopy and current intestinal parasite infection: A systematic review and meta-analysis. Allergy 2011, 66, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, V.; Mohan, V.; Surendar, J.; Rao, M.M.; Ranjani, H.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Decreased prevalence of lymphatic filariasis among subjects with type-1 diabetes. Am. J. Trop. Med. Hyg. 2010, 83, 1336–1339. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M. Association between parasite infection and immune responses in multiple sclerosis. Ann. Neurol. 2007, 61, 97–108. [Google Scholar] [CrossRef]
- Cooper, P.J.; Espinel, I.; Paredes, W.; Guderian, R.H.; Nutman, T.B. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: A possible role for interleukin-10. J. Infect. Dis. 1998, 178, 1133–1138. [Google Scholar] [CrossRef]
- Chen, C.C.; Louie, S.; McCormick, B.; Walker, W.A.; Shi, H.N. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect. Immun. 2005, 73, 5468–5481. [Google Scholar] [CrossRef]
- Su, L.; Su, C.W.; Qi, Y.; Yang, G.; Zhang, M.; Cherayil, B.J.; Zhang, X.; Shi, H.N. Coinfection with an intestinal helminth impairs host innate immunity against Salmonella enterica serovar Typhimurium and exacerbates intestinal inflammation in mice. Infect. Immun. 2014, 82, 3855–3866. [Google Scholar] [CrossRef]
- Choi, D.; Lim, J.H.; Lee, K.T.; Lee, J.K.; Choi, S.H.; Heo, J.S.; Jang, K.T.; Lee, N.Y.; Kim, S.; Hong, S.T. Cholangiocarcinoma and Clonorchis sinensis infection: A case-control study in Korea. J. Hepatol. 2006, 44, 1066–1073. [Google Scholar] [CrossRef]
- Sriamporn, S.; Pisani, P.; Pipitgool, V.; Suwanrungruang, K.; Kamsa-ard, S.; Parkin, D.M. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop. Med. Int. Health 2004, 9, 588–594. [Google Scholar] [CrossRef]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Muscheck, M.; Abol-Enein, H.; Chew, K.; Moore, D., 2nd; Bhargava, V.; Ghoneim, M.A.; Carroll, P.R.; Waldman, F.M. Comparison of genetic changes in schistosome-related transitional and squamous bladder cancers using comparative genomic hybridization. Carcinogenesis 2000, 21, 1721–1726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Rifai, W.; Kamel, D.; Larramendy, M.L.; Shoman, S.; Gad, Y.; Baithun, S.; El-Awady, M.; Eissa, S.; Khaled, H.; Soloneski, S.; et al. DNA copy number changes in Schistosoma-associated and non-Schistosoma-associated bladder cancer. Am. J. Pathol. 2000, 156, 871–878. [Google Scholar] [CrossRef]
- Madbouly, K.M.; Senagore, A.J.; Mukerjee, A.; Hussien, A.M.; Shehata, M.A.; Navine, P.; Delaney, C.P.; Fazio, V.W. Colorectal cancer in a population with endemic Schistosoma mansoni: Is this an at-risk population? Int. J. Colorectal Dis. 2007, 22, 175–181. [Google Scholar] [CrossRef]
- Waku, M.; Napolitano, L.; Clementini, E.; Staniscia, T.; Spagnolli, C.; Andama, A.; Kasiriye, P.; Innocenti, P. Risk of cancer onset in sub-Saharan Africans affected with chronic gastrointestinal parasitic diseases. Int. J. Immunopathol. Pharmacol. 2005, 18, 503–511. [Google Scholar] [CrossRef]
- Hayes, K.S.; Cliffe, L.J.; Bancroft, A.J.; Forman, S.P.; Thompson, S.; Booth, C.; Grencis, R.K. Chronic Trichuris muris infection causes neoplastic change in the intestine and exacerbates tumour formation in APC min/+ mice. PLoS Negl. Trop. Dis. 2017, 11, e0005708. [Google Scholar] [CrossRef]
- Biton, M.; Haber, A.L.; Rogel, N.; Burgin, G.; Beyaz, S.; Schnell, A.; Ashenberg, O.; Su, C.W.; Smillie, C.; Shekhar, K.; et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 2018, 175, 1307–1320.e1322. [Google Scholar] [CrossRef]
- Matsuda, K.; Masaki, T.; Ishii, S.; Yamashita, H.; Watanabe, T.; Nagawa, H.; Muto, T.; Hirata, Y.; Kimura, K.; Kojima, S. Possible Associations of Rectal Carcinoma with Schistosoma japonicum Infection and Membranous Nephropathy: A Case Report with a Review. Jpn. J. Clin. Oncol. 1999, 29, 576–581. [Google Scholar] [CrossRef]
- Tuffour, I.; Ayi, I.; Gwira, T.M.; Dumashie, E.; Ashong, Y.; Appiah-Opong, R. Schistosoma Egg Antigen Induces Oncogenic Alterations in Human Prostate Cells. Anal. Cell. Pathol. 2018, 2018, 4675380. [Google Scholar] [CrossRef]
- Brooks, C.L.; Gu, W. New insights into p53 activation. Cell Res. 2010, 20, 614–621. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.P.; Pretlow, T.G.; Rao, J.S.; Pretlow, T.P. Beta-catenin expression is altered in human colonic aberrant crypt foci. Cancer Res 2001, 61, 8085–8088. [Google Scholar] [PubMed]
- Hiendlmeyer, E.; Regus, S.; Wassermann, S.; Hlubek, F.; Haynl, A.; Dimmler, A.; Koch, C.; Knoll, C.; van Beest, M.; Reuning, U.; et al. Beta-catenin up-regulates the expression of the urokinase plasminogen activator in human colorectal tumors. Cancer Res. 2004, 64, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Godkin, A.; Smith, K.A. Chronic infections with viruses or parasites: Breaking bad to make good. Immunology 2017, 150, 389–396. [Google Scholar] [CrossRef]
- Kishimoto, H.; Momiyama, M.; Aki, R.; Kimura, H.; Suetsugu, A.; Bouvet, M.; Fujiwara, T.; Hoffman, R.M. Development of a clinically-precise mouse model of rectal cancer. PLoS ONE 2013, 8, e79453. [Google Scholar] [CrossRef] [PubMed]
- Callejas, B.E.; Martínez-Saucedo, D.; Terrazas, L.I. Parasites as negative regulators of cancer. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Loke, P.; MacDonald, A.S.; Robb, A.; Maizels, R.M.; Allen, J.E. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur. J. Immunol. 2000, 30, 2669–2678. [Google Scholar] [CrossRef]
- Wang, X.L.; Fu, B.Q.; Yang, S.J.; Wu, X.P.; Cui, G.Z.; Liu, M.F.; Zhao, Y.; Yu, Y.L.; Liu, X.Y.; Deng, H.K.; et al. Trichinella spiralis—A potential anti-tumor agent. Vet. Parasitol. 2009, 159, 249–252. [Google Scholar] [CrossRef]
- Liu, W.-F.; Wen, S.-H.; Zhan, J.-H.; Li, Y.-S.; Shen, J.-T.; Yang, W.-J.; Zhou, X.-W.; Liu, K.-X. Treatment with Recombinant Trichinella spiralis Cathepsin B–like Protein Ameliorates Intestinal Ischemia/Reperfusion Injury in Mice by Promoting a Switch from M1 to M2 Macrophages. J. Immunol. 2015, 195, 317–328. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Harcus, Y.; Murray, J.; van Agtmaal, M.; Filbey, K.J.; Grainger, J.R.; Bridgett, S.; Blaxter, M.L.; Ashton, P.D.; Ashford, D.; et al. Proteomic analysis of secretory products from the model gastrointestinal nematode Heligmosomoides polygyrus reveals dominance of Venom Allergen-Like (VAL) proteins. J. Proteom. 2011, 74, 1573–1594. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Filbey, K.J.; Grainger, J.R.; Dowle, A.A.; Pearson, M.; Murray, J.; Harcus, Y.; Maizels, R.M. Heligmosomoides polygyrus Elicits a Dominant Nonprotective Antibody Response Directed against Restricted Glycan and Peptide Epitopes. J. Immunol. 2011, 187, 4764. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef]
- Boonmars, T.; Wu, Z.; Nagano, I.; Takahashi, Y. What is the role of p53 during the cyst formation of Trichinella spiralis? A comparable study between knockout mice and wild type mice. Parasitology 2005, 131, 705–712. [Google Scholar] [CrossRef]
- Morales-Montor, J.; Rodríguez-Dorantes, M.; Mendoza-Rodríguez, C.A.; Camacho-Arroyo, I.; Cerbón, M.A. Differential expression of the estrogen-regulated proto-oncogenes c-fos, c-jun, and bcl-2 and of the tumor-suppressor p53 gene in the male mouse chronically infected with Taenia crassiceps cysticerci. Parasitol. Res. 1998, 84, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, F.; Zhu, D.; Duan, Y.; Chen, J.; Sun, X.; He, X.; Li, P.; Sun, W.; Feng, J. Schistosoma japonicum soluble egg antigens facilitate hepatic stellate cell apoptosis by downregulating Akt expression and upregulating p53 and DR5 expression. PLoS Negl. Trop. Dis. 2014, 8, e3106. [Google Scholar] [CrossRef] [PubMed]
- Grainger, J.R.; Smith, K.A.; Hewitson, J.P.; McSorley, H.J.; Harcus, Y.; Filbey, K.J.; Finney, C.A.; Greenwood, E.J.; Knox, D.P.; Wilson, M.S.; et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J. Exp. Med. 2010, 207, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular Origins of Cancer: Molecular Basis of Colorectal Cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.; Kido, K.; Yoshitake, H.; Flanigan, A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: A study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis 2000, 21, 757–768. [Google Scholar] [CrossRef]
- Wang, C.; Lei, H.; Tian, Y.; Shang, M.; Wu, Y.; Li, Y.; Zhao, L.; Shi, M.; Tang, X.; Chen, T.; et al. Clonorchis sinensis granulin: Identification, immunolocalization, and function in promoting the metastasis of cholangiocarcinoma and hepatocellular carcinoma. Parasites Vectors 2017, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.-A.; Chetty, A.; Horsnell, W.G.C.; Schäfer, G.; Prince, S.; Smith, K.A. Hookworm exposure decreases human papillomavirus uptake and cervical cancer cell migration through systemic regulation of epithelial-mesenchymal transition marker expression. Sci. Rep. 2018, 8, 11547. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.J.C.; Smyth, D.J.; Kodali, R.B.; White, M.P.J.; Harcus, Y.; Filbey, K.J.; Hewitson, J.P.; Hinck, C.S.; Ivens, A.; Kemter, A.M.; et al. A structurally distinct TGF-beta mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 2017, 8, 1741. [Google Scholar] [CrossRef] [PubMed]
- Datto, M.B.; Li, Y.; Panus, J.F.; Howe, D.J.; Xiong, Y.; Wang, X.F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl. Acad. Sci. USA 1995, 92, 5545–5549. [Google Scholar] [CrossRef]
- Zaccone, P.; Burton, O.; Miller, N.; Jones, F.M.; Dunne, D.W.; Cooke, A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur. J. Immunol. 2009, 39, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.S.; Kikuchi, H.; Zeng, M.; Herraiz, M.T.; Sperduti, I.; Berger, D.; Park, D.Y.; Iafrate, A.J.; Zukerberg, L.R.; Chung, D.C. Epithelial to mesenchymal transition is impaired in colon cancer cells with microsatellite instability. Gastroenterology 2010, 138, 1406–1417. [Google Scholar] [CrossRef]
- Hawinkels, L.J.A.C.; Paauwe, M.; Verspaget, H.W.; Wiercinska, E.; van der Zon, J.M.; van der Ploeg, K.; Koelink, P.J.; Lindeman, J.H.N.; Mesker, W.; ten Dijke, P.; et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene 2014, 33, 97–107. [Google Scholar] [CrossRef]
- Johnston, C.J.C.; Robertson, E.; Harcus, Y.; Grainger, J.R.; Coakley, G.; Smyth, D.J.; McSorley, H.J.; Maizels, R. Cultivation of Heligmosomoides Polygyrus: An Immunomodulatory Nematode Parasite and its Secreted Products. J. Vis. Exp. Jove 2015. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Nguyen, D.L.; van Diepen, A.; Smit, C.H.; Koeleman, C.A.; McSorley, H.J.; Murray, J.; Maizels, R.M.; Hokke, C.H. Novel O-linked methylated glycan antigens decorate secreted immunodominant glycoproteins from the intestinal nematode Heligmosomoides polygyrus. Int. J. Parasitol. 2016, 46, 157–170. [Google Scholar] [CrossRef]
- Willmer, T.; Hare, S.; Peres, J.; Prince, S. The T-box transcription factor TBX3 drives proliferation by direct repression of the p21WAF1 cyclin-dependent kinase inhibitor. Cell Div. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Abrahams, A.; Mowla, S.; Parker, M.I.; Goding, C.R.; Prince, S. UV-mediated regulation of the anti-senescence factor Tbx2. J. Biol. Chem. 2008, 283, 2223–2230. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, B.-A.; Prince, S.; Smith, K.A. Gastrointestinal Nematode-Derived Antigens Alter Colorectal Cancer Cell Proliferation and Migration through Regulation of Cell Cycle and Epithelial-Mesenchymal Transition Proteins. Int. J. Mol. Sci. 2020, 21, 7845. https://doi.org/10.3390/ijms21217845
Jacobs B-A, Prince S, Smith KA. Gastrointestinal Nematode-Derived Antigens Alter Colorectal Cancer Cell Proliferation and Migration through Regulation of Cell Cycle and Epithelial-Mesenchymal Transition Proteins. International Journal of Molecular Sciences. 2020; 21(21):7845. https://doi.org/10.3390/ijms21217845
Chicago/Turabian StyleJacobs, Brittany-Amber, Sharon Prince, and Katherine Ann Smith. 2020. "Gastrointestinal Nematode-Derived Antigens Alter Colorectal Cancer Cell Proliferation and Migration through Regulation of Cell Cycle and Epithelial-Mesenchymal Transition Proteins" International Journal of Molecular Sciences 21, no. 21: 7845. https://doi.org/10.3390/ijms21217845
APA StyleJacobs, B.-A., Prince, S., & Smith, K. A. (2020). Gastrointestinal Nematode-Derived Antigens Alter Colorectal Cancer Cell Proliferation and Migration through Regulation of Cell Cycle and Epithelial-Mesenchymal Transition Proteins. International Journal of Molecular Sciences, 21(21), 7845. https://doi.org/10.3390/ijms21217845





