Abstract
Cancer represents one of the most pernicious public health problems with a high mortality rate among patients worldwide. Chemotherapy is one of the major therapeutic approaches for the treatment of various malignancies. Platinum-based drugs (cisplatin, oxaliplatin, carboplatin, etc.) are highly effective chemotherapeutic drugs used for the treatment of several types of malignancies, but their application and dosage are limited by their toxic effects on various systems, including neurotoxicity. Simultaneously, researchers have tried to improve the survival rate and quality of life of cancer patients and decrease the toxicity of platinum-containing drugs by combining them with non-chemotherapy-based drugs, dietary supplements and/or antioxidants. Additionally, recent studies have shown that the root cause for the many side effects of platinum chemotherapeutics involves the production of reactive oxygen species (ROS) in naive cells. Therefore, suppression of ROS generation and their inactivation with antioxidants represents an appropriate approach for platinum drug-induced toxicities. The aim of this paper is to present an updated review of the protective effects of different antioxidant agents (vitamins, dietary antioxidants and supplements, medicaments, medicinal plants and their bioactive compounds) against the neurotoxicity induced by platinum-based chemotherapeutics. This review highlights the high potential of plant antioxidants as adjuvant strategies in chemotherapy with platinum drugs.
1. Introduction
A “honeymoon” with platinum-based compounds in chemotherapy that officially started by pronouncing cisplatin as “the drug of the century” more than 50 years ago has gradually been overshadowed by very serious therapeutic limitations of those drugs. Besides the inherited resistance to treatment with any of the currently approved platinum agents, each of them has a number of clinically confirmed side-effects, ranging from minor to dose-limiting. The fact that almost half of all the patients who receive anticancer chemotherapy are treated with a platinum drug [1] gives a good insight into broad-spectrum platinum-based chemotherapeutics adverse effects.
2. Therapeutic Indications for Platinum-Based Chemotherapy
For decades various platinum-based compounds were employed as an important part of the combination chemotherapy regimens used to treat different types of solid tumors. However, there are only three platinum-based medications that are used throughout the world for the cancer treatment: cisplatin (cis-diamminedichloridoplatinum II), carboplatin (cis-diammine-1,1-cyclobutanedicarboxylateplatinum), and oxaliplatin (trans-R,R-cyclohexane-1,2-diamineoxalato- platinum II), while some other platinum-based therapeutics are approved only in individual countries, such as heptaplatin (Korea), lobaplatin (China), miriplatin (Japan), and nedaplatin (Japan) [2].
Since 1979 cisplatin has been widely used (along with other antineoplastic drugs) in the treatment of various malignancies: lung [3], ovarian [4], testicular [5], breast [6] and brain cancer [7], sarcomas [8], and lymphomas [9]. Starting in 1989, carboplatin confirmed clinical relevance as an antineoplastic agent (in combination with other chemotherapeutics) for advanced ovarian carcinoma [10], head and neck cancer [11], and lung cancer [12]. The latest worldwide accepted platinum-based chemotherapeutic (2002), oxaliplatin, is used as a part of the therapeutic protocols for metastatic colorectal cancer [13], advanced gastric [14] and ovarian cancer [15].
3. Side Effects of Platinum-Based Compounds in Clinical Practice
Like for the other chemotherapeutic drugs, the basic cytotoxic effect of platinum-based compounds (DNA damage) is not restricted only to target tissue (tumor cells), but is also affecting numerous organ systems in patients receiving chemotherapy, resulting in a variety of side effects. Based on a similar pathophysiological background, the adverse effects of platinum-based chemotherapeutics may be classified in certain categories of toxicities according to their clinical manifestations. The most commonly described types of side effects associated with platinum-based treatment are usually classified as nephrotoxicity, hepatotoxicity, neuro- and ototoxicity, cardiotoxicity, hematological toxicity, and gastrointestinal toxicity. However, patients’ responses to chemotherapy toxicity, including adverse effects of platinum-based compounds, are significantly determined by several factors, such as age, gender, drug administration schedule and performance status [16].
Nausea and vomiting are considered as the most common clinical manifestations of side effects following cisplatin administration. Strongly depending on the applied dose, this effect of cisplatin, which can be successfully abolished by antiemetic action of 5-HT3 antagonists [16], is found more often than in chemotherapeutic protocols with oxaliplatin and carboplatin [17].
Nephrotoxicity represents the main limitation for chemotherapeutic protocols that involved platinum-based compounds which is not surprising due to the fact that kidneys are the main route for platinum compounds excretion. Although the platinum-based drugs affect all three key kidney functions (filtration, reabsorption, and excretion), the two most common nephrotoxic side effects of cisplatin are acute kidney injury (also known as acute renal failure) and hypomagnesemia, which is reported to affect up to 90% of cisplatin-treated patients [2]. However, the comparison of toxicities for three platinum-containing chemotherapy regimens confirms the lower nephrotoxicity manifestations of both carbo- and oxaliplatin when compared to cisplatin [18].
Hepatotoxicity is also considered as one of the most frequent adverse effects of platinum-based compounds administration in clinical practice. Morphological alterations accompanying platinum-based compounds application are manifested as necrosis, degeneration of hepatocytes, and increased inflammatory response [19], as well as a consequent increase in hepatic enzymes and bilirubin [20]. Long-term survival analysis of platinum-based compounds-induced hepatotoxicity showed that liver damage was more pronounced following cisplatin administration when compared to carboplatin [21], while drug-induced hepatotoxicity manifestations accompanying oxaliplatin therapy were predominantly restricted to hepatic vascular injury [22].
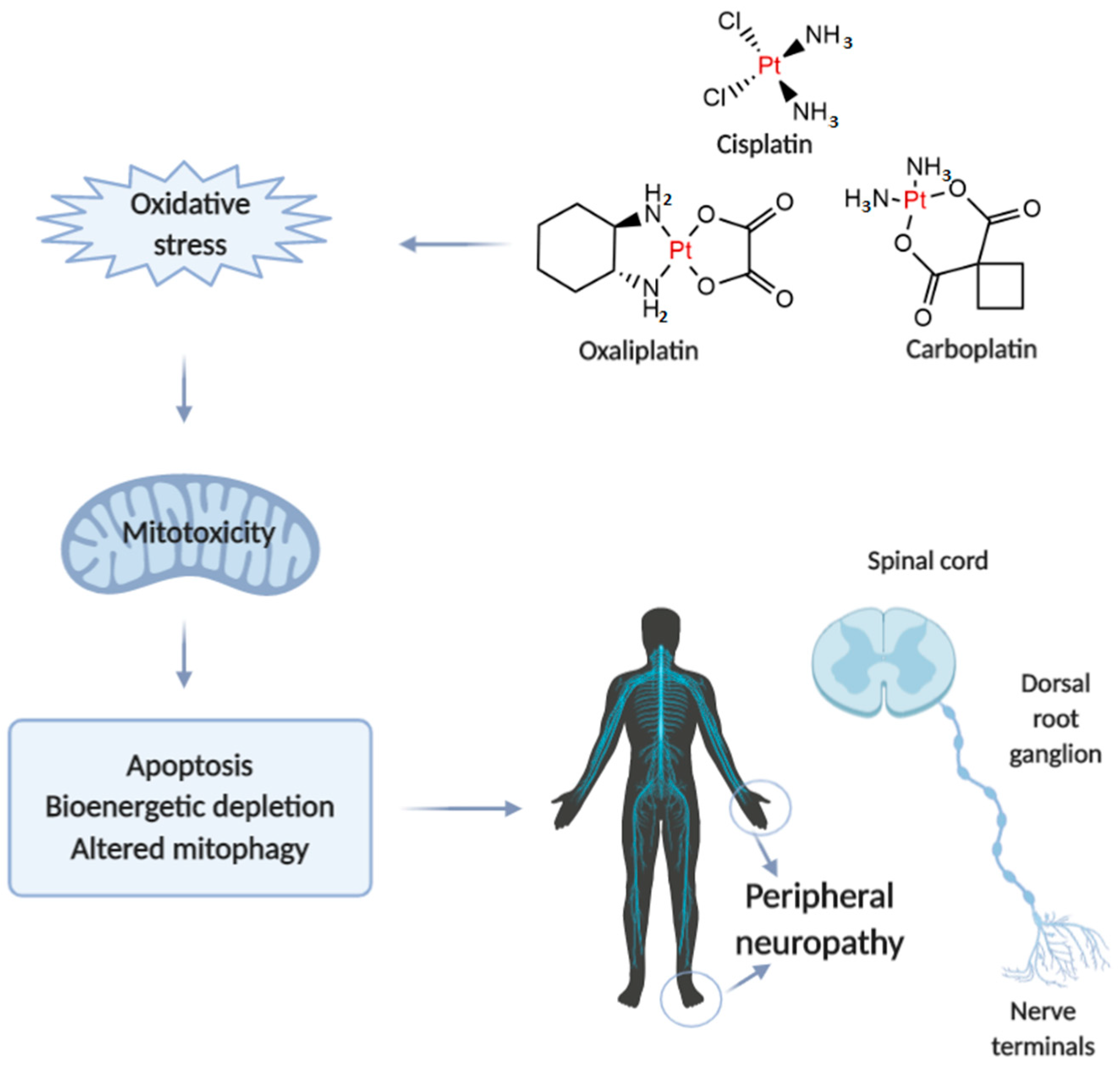
Neurotoxicity in response to platinum-based therapy is the leading clinical entity, aside from nephro- and hepatotoxicity that usually hampers platinum-based chemotherapy (Figure 1). The most frequently reported manifestations of neurotoxicity are due to the clinical appearance of peripheral neurotoxicity (numbness, tingling, or paresthesia in fingers and/or toes). With prolonged treatment, they gradually lead to disturbance of proprioception, which may result in ataxic gait [23]. The clinical manifestations of encephalopathy accompanying platinum-based therapy usually appear with an increase in cumulative dose [24]. Sensory manifestations of platinum compounds-induced neuropathy are often accompanied by ototoxicity [25].
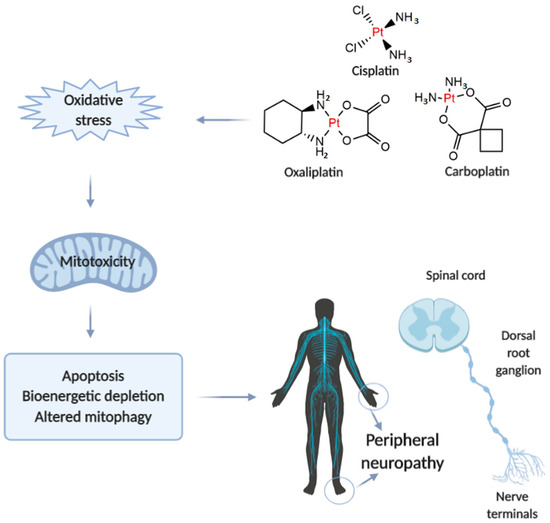
Figure 1.
Illustrative presentation of the development of platinum-based drug-induced peripheral neuropathy. Created in BioRender.com.
When comparing to the individual extension of neurotoxic manifestations for platinum-based drugs, it has been shown that carboplatin neurotoxicity is negligible compared to cisplatin and oxaliplatin. However, carboplatin, particularly when applied in high doses, can lead to the development of neurotoxic manifestations that may become irreversible in 30-50% of patients.
The evaluation of mechanisms underlying platinum-induced peripheral neurotoxicity (PIPN), in both in vivo and in vitro studies, confirmed that those compounds after passing the neuronal membrane initiate several proapoptotic phenomena including the activation of p53, Bax translocation, cytochrome c release, as well as the activation of caspase-3 and 9 [26]. Similar to the beneficial action of platinum compounds on tumor’s nuclear DNA, the undesirable impact has been observed on naïve cells mitochondrial DNA which results in inhibition of replication and translation, with consequent respiratory chain disturbance and energy deficiency [27]. Finally, the platinum compounds-induced mitochondrial dysfunction resulted in increased reactive oxygen species (ROS) production (with oxidative damage) and intracellular calcium accumulation [27]. Furthermore, the observed intracellular calcium accumulation was potentiated via up-regulation of calcium N channels induced by platinum compounds, that additionally potentiated apoptotic mechanisms [28]. An additional transmembrane mechanism is involved in the neurotoxic effect of platinum compounds on peripheral nerves. Namely, it has been reported that cisplatin-induced neurotoxicity increased expression of TRPA1 receptors in dorsal root ganglia that resulted in hyperalgesic response to thermal stimuli [29].
Interestingly, although it is still very questionable how cisplatin passes through an intact blood-brain barrier, there are certain literature data considering the central manifestations of neurotoxicity induced by platinum compounds. It has been shown that cisplatin administration strongly affects CNS progenitor cells and oligodendrocytes inducing the apoptotic events in hippocampal dentate gyrus and corpus callosum [30]. Only a few studies implicate that several mechanisms of platinum compounds action in CNS, including oxidative damage, inflammation and apoptosis, may be the cause of behavioral alterations manifested as increased anxiety [31], depression [32], as well as cognitive dysfunction [33].
Since there is a plethora of evidence that oxidative damage is crucially involved in the mechanisms of platinum-based compounds toxicities, it is not surprising that an enormous effort has been put in order to increase the safety of platinum-based therapy regimens by promoting the antioxidant supplementation as potentially protective interventions during the chemotherapy protocols-based platinum-containing antitumor agents.
4. Classification of Antioxidants
The root cause of many side effects and organ injury of platinum-based drugs is the generation of reactive oxygen species (ROS) [2]. The increase of ROS production associated with mitochondrial dysfunction in neurons was described as a potential mechanism of platinum drug neurotoxicity. The damage of mitochondrial DNA and interrupt RNA expression of Cytochrome B induced by platinum drugs applications give rise to disruption of neuronal mitochondrial function and overproduction of reactive oxygen species [34]. Therefore, antioxidant supplementation for the reduction of ROS, or alleviation of the effects of ROS, could also have significant influence on the neurotoxicity side effects of platinum drugs.
Oxidation reactions are very common in our body and they have a role to change a chemical substance, but also could produce highly reactive free radicals [35]. Xenobiotics, including drugs, represent pro-oxidant compounds and risk factors for the overproduction of free radicals. This process may lead to damage to biological molecules in their reaction with free radicals which tend to capture electrons from other molecules to stabilize themselves. All these reactions disrupt the structure and function of many tissues and organs, as well as the equilibrium between free radicals and antioxidants in the body [36]. The human body possesses a sophisticated and complex antioxidant protection system consisting of a variety of endogenous and exogenous originated substances which act interactively and synergistically, whether to actively inhibit oxidation reactions or to inhibit oxidation indirectly [37].
There are different definitions of antioxidants; the most comprehensive definition describes antioxidants as substances that directly scavenge free radicals, inhibit their production, or indirectly act to up-regulate antioxidant defenses. Furthermore, antioxidant compounds should have the ability to form a new stable radical that is inactive for further oxidation, after scavenging the free radical [38,39]. Antioxidant protection mechanisms in humans include three lines of defense: preventive antioxidants, radical-scavenging antioxidants, and repair and de novo antioxidants. There is also an adaptation mechanism of antioxidant defense that is referred to as ‘the fourth line of defense’ [40,41].
The first line of defense includes preventing mechanisms for biological molecules damage by suppressing or preventing the formation of free radicals and their derivatives. This defense line involves enzymatic antioxidants (Table 1) that catalyze the disproportionation reaction of reactive species such as superoxide anion (superoxide dismutase; SOD) or hydrogen peroxide (catalase; CAT, glutathione peroxidase; GPx, or glutathione reductase; GR). Also, the first line of antioxidant defense consists of proteins presented in blood plasma such as albumin, transferrin, ferritin, myoglobin, ceruloplasmin, and metallothioneins representing non-enzymatic antioxidants which bind polyvalent metal ion which may involve in redox reaction and produce free radicals. The second defense line consists of non-enzymatic molecules (Table 1) represented by vitamins (vitamin A, C, E, and K), glutathione (GSH), coenzyme Q10 (CoQ10), uric acid, melatonin, bilirubin, and alpha-lipoic acid (ALA) that scavenge ROS or reactive nitrogen species (RNS). The group of antioxidants that repair or eliminate structural damage of biomolecules caused by free radicals is classified as the third line of antioxidant defense (de novo antioxidants). This group mainly includes DNA repair enzyme systems, lipase, and proteolytic enzymes which can repair damaged molecules [40,42,43]. Furthermore, there is another important adaptation mechanism may be regarded as the fourth defense line that is activated by reaction and production of free radicals inducing the formation and transport of appropriate antioxidant enzymes to the right site. This mechanism includes, e.g., the formation of catalase and superoxide dismutase induced by ROS overproduction [40,41,44].

Table 1.
Classification of antioxidants.
The human antioxidant defense mechanisms include both non-enzymatic and enzymatic antioxidants groups classified according to the molecular structure which forms endogenous antioxidant systems. But the human endogenous antioxidant system does not suffice, and the production of non-enzymatic antioxidants can be hardly producing in the human body. Therefore, there are exogenous non-enzymatic antioxidants (Table 1) such as mineral elements, nutritional antioxidants, and antioxidants obtained from natural resources (phytochemicals/phytonutrients) [36,39]. Exogenous non-enzymatic antioxidants extensively used in medicine and industry are divided into natural and synthetic. In view of the latest suspicions that synthetic antioxidants exert a noxious effect on human health, more attention is paid to natural antioxidants found in fruits, vegetables, nuts, and cereals, as well as medicinal and dietary plats for the use in food, cosmetic, and as therapeutics. The research studies carried in the field of natural antioxidants showed that phenolic compounds are the most studied phytochemicals. Among them, phenolic acids and flavonoids represent the most popular group of phenolic compounds because they may have excellent antioxidant activities but also good antimicrobial, anticancer, anti-inflammatory, and hormonal activities [44,45]. In this review, the authors aimed to summarize the most prominent research articles in terms of platinum-induced neurotoxicity and its treatment with different antioxidant supplementation. Scopus and PubMed Central databases were searched for published studies relevant to this matter, from 2010 until 2020. Since some papers referred to valuable older references, several were included in this review.
5. Antioxidants in the Treatment of Platinum-Based Chemotherapeutics-Induced Neurotoxicity
5.1. Vitamins, Minerals, and Dietetic Supplements
According to the World Health Organization recommendations nutrition is one of the major modifiable determinants of chronic disease [46]. Some essential nutrients, micronutrients, minerals, and trace elements, as well as nutritional supplements, beside their primary role for adequate functioning of an organism, possess anti-inflammatory, antihyperalgesic, and antioxidant effects through which they can influence on a chronic disease. One of the preventive and therapeutic strategies for alleviating neurotoxicity side effects in patients receiving platinum-based chemotherapeutics is the use of supplementation including vitamins, minerals and trace elements, as well as dietary supplements with antioxidant activity (Table 2) [47,48,49].

Table 2.
Vitamins, dietetic supplements, and medication with neuroprotective activity against neurotoxicity induced by platinum-based chemotherapeutics.
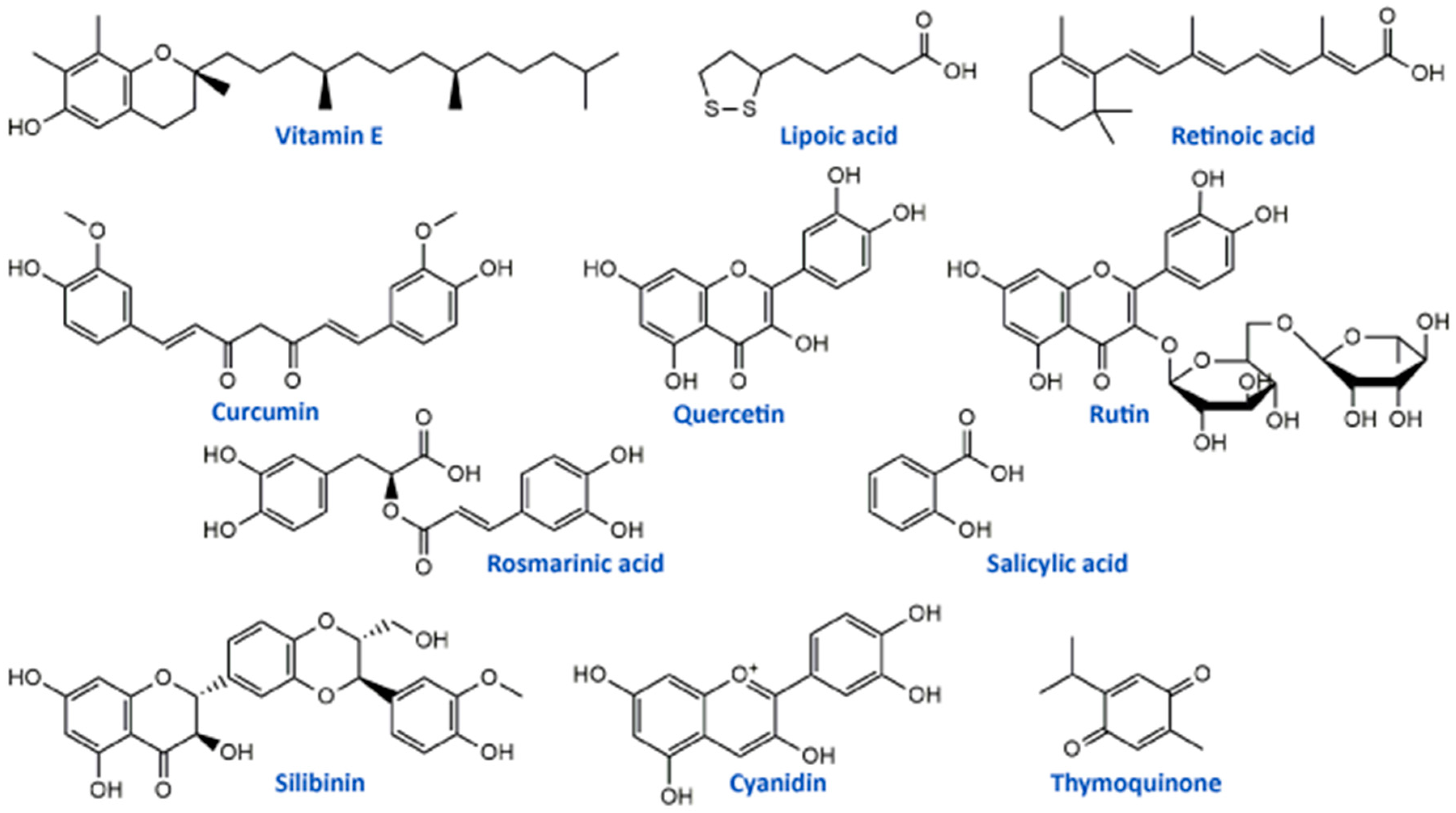
Vitamin E (Figure 2) is a potent antioxidant with high ability to neutralize ROS and protect cells and subcellular structures from lipid peroxidation [93]. Several studies have reported that cisplatin-induced neuropathy and vitamin E deficiency neuropathy manifest similar clinical and neuropathologic features [54,94]. Vitamin E is one of the most studied natural antioxidants in the prevention and therapy of the toxic effects of platinum drugs including fundamental and clinical studies. In vivo study on cisplatin-treated mice showed that vitamin E protected the peripheral nerve, decreased systemic toxicity, and increased antioxidant defense without interfering with its antitumor activity of cisplatin [52]. A randomized, placebo-controlled trial including 108 patients treated with cisplatin chemotherapy and vitamin E supplementation (400 mg/day) showed a significantly lower incidence of neurotoxicity in patients receiving vitamin E than in those receiving placebo. This phase III study provided evidence that vitamin E should be adopted in patients receiving cisplatin-based chemotherapy for reduction of risk of developing signs or symptoms of neurotoxicity [54]. Similar results were obtained in the study conducted by Argyriou et al. [53], suggesting the significantly lower incidence of neurotoxicity in patients with vitamin E supplementation (600 mg/day) during cisplatin chemotherapy. However, phase III trial study conducted in patients with neurotoxic chemotherapy (cisplatin, carboplatin, oxaliplatin, or a combination) supplemented with vitamin E (400 mg)/placebo showed no statistically significant difference between the incidence of sensory neuropathy in the studied groups (vitamin E and placebo) [55]. Also, studies designed to assess the influence of vitamin E supplementation (400 mg/day) for the prevention of oxaliplatin-induced peripheral neuropathy did not demonstrate any significant decrease in the incidence of peripheral neuropathy. Similar results were obtained in another study conducted by Salehi et al. [57]. The differences in numbers of patients included in clinical trials may be a possible explanation for the contrasting results.
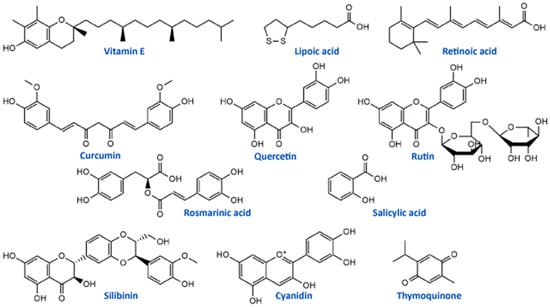
Retinoic acid (Figure 2, a metabolite of vitamin A) has also been studied as an antioxidant supplement in cisplatin-induced neuropathy in an animal model and patients. Tredici et al. [50] in the study investigating the prevention of retinoic acid on cisplatin-induced neurotoxicity in rats reported that retinoic acid-induced only a mild generalized protective effect without significant influence on morphometric, electrophysiological, functional and analytical disturbance on dorsal root ganglion neurons caused by cisplatin application. On the other hand, in the study of Arrieta et al. [51] retinoic acid reduced cisplatin-induced neuropathy in rats, reduced the electrophysiologic alterations and nerve growth factor in serum in patients with non-small-cell lung cancer with standard treatment based on cisplatin and paclitaxel.
Thiamine pyrophosphate and water-soluble formulation of provitamin coenzyme Q10 (WS-CoQ10) has also been studied for neurotoxicity prevention. The neuroprotective effect of WS-CoQ10 was tested on PC12 cells exposed to cisplatin. These cells possess neuronal cell features and respond positively to nerve growth factor. WS-CoQ10 showed neuroprotective effects on the PC12 cells, protection from cisplatin-induced DNA damage, and an increase in the total intracellular GSH [58]. The application of thiamine pyrophosphate significantly improved oxidative parameters in the brain tissue of rats treated with cisplatin [59].
Selenium is a trace element that has an important role in cellular redox regulation and the protection of cellular components from oxidative damage. Selenium was shown to possess moderate neuroprotective effects on the cisplatin neurotoxic effect in rats. The study showed that selenium administration partly reverses nerve conduction velocity, the amplitude of compound action potential, and the number of axons in experiments with cisplatin application in rats [49].
Calcium and magnesium (Ca/Mg) infusions were suggested as supplementation in platinum-based drugs-induced peripheral neuropathy. Oxaliplatin is the most researched among them as chemotherapeutic agents because its metabolite oxalate has the potential to chelates both Ca and Mg ions disrupting the function of ion channels in nerve membranes [47]. The hypothesis that an increase of extracellular Ca and Mg may prevent or ameliorate oxaliplatin-induced neurotoxicity was approved in cell-based and animal studies [60,61]. There have been numerous clinical trials designed to evaluate the benefit of Ca/Mg infusions for decreasing oxaliplatin-induced neuropathy. Gamelin [95] concluded that Ca/Mg infusions reduced the incidence and intensity of acute oxaliplatin-induced symptoms in patients. Later studies did not confirm this observation, Loprinzi et al. [62] in the clinical study of intravenous Ca and Mg application for prevention of oxaliplatin-induced sensory neurotoxicity showed that Ca/Mg did not substantially decrease oxaliplatin-induced acute neuropathy.
Alpha lipoic acid (ALA) is one of the most studied dietary antioxidant supplements in the literature. This molecule (Figure 2) is essential for cell energy production in the Krebs cycle and represents endogenous antioxidant with free radical scavenging capability and the possibility to activate enzymes that reduce oxidative stress [65]. In an in vitro model using primary cultures of dorsal root ganglion, ALA protected these cells from cisplatin toxicity through its antioxidant and mitochondrial regulatory functions [63]. Also, another in vivo study on rats showed that ALA (100 mg/kg/day) restored conventional conduction velocity and conduction velocity distribution disturbed by cisplatin application [64]. Different clinical studies showed inconsistent results for use of ALA supplementation in cisplatin and oxaliplatin chemotherapy-induced peripheral neuropathy. Gedlicka et al. [66,67] in small clinical studies reported that ALA application in patients with established neuropathy secondary to the oxaliplatin/cisplatin treatment reduced the intensity of symptoms. On the other hand, Guo et al. [65] concluded in a randomized, double-blind, placebo-controlled trial with 243 patients that ALA was ineffective in preventing neurotoxicity caused by oxaliplatin or cisplatin. All these studies suggest that ALA may have benefits in platinum drug-induced neuropathy, but there is a need for deeper investigation, especially regarding clinical studies.
Melatonin is a pineal hormone, also known as a free radical scavenger, which manifested protection activity against oxaliplatin-induced alterations in motor activity and muscular strength in rats. Besides, this supplementation inactivated Bcl-2 and caspase 3 apoptotic protein, as well as decreased Cytochrome c release and modulated oxidative stress in rats’ brain caused by oxaliplatin application [71]. Tuncer et al. [64] reported a lower influence of melatonin on the modulation of cisplatin-induced neurotoxicity in rats compared to ALA.
Yehia et al. [68] reported that carnosine, an endogenous dipeptide occurring naturally in humans, reduced the symptoms when provided as a supplement to cancer patients with oxaliplatin-induced peripheral neuropathy by decreasing NF-κB and TNF-α, oxidative stress (reduced MDA and increased Nrf-2) and apoptosis (reduced caspase-3 activity).
D-Methionine is also one of the effective antioxidant supplements against cisplatin-induced neurotoxicity in experimental animals. It showed a positive influence on the cortical neurons damage in an in vitro model of cortical networks [72], prevented the decreased neurogenesis in the hippocampus of the adult rats [73], regulated electrophysiological recordings, and increased hippocampal neurogenesis in rodents after cisplatin application [74]. According to Gopal et al. [72], the optical isomer L-methionine was less protective on cisplatin-induced damage in cortical networks from mouse embryos compared to D-methionine. Also, some evidence showed that taurine (free intracellular amino acid also present in some food) has protective effects on cisplatin-induced toxicity modulating inflammation and oxidative damage. An in vivo study conducted on rats with cisplatin treatment confirmed significant amelioration of behavioral performance and antioxidant parameters in brain tissue, as well as a decrease in acetylcholinesterase activity in taurine-treated groups [75]. These amino acids with very promising results in fundamental studies have the potential for further investigations as nutritional supplements in clinical trials as co-adjuvant therapies in the reduction of neuropathy symptoms in patients with platinum-based drugs chemotherapy.
There are some studies about the use of different antioxidants which are usually not present or which are present in small amounts in the human diet, against cisplatin-induced neurotoxicity. Song et al. [69] reported that ergothioneine improved the learning and memory dysfunctions in mice treated with cisplatin, followed by the improvement of antioxidant status and the decrease of acetylcholinesterase activity in brain tissue. Ethoxyquin, a compound used as an antioxidant in animal feed, also showed protective effects against cisplatin-induced neurotoxicity in in vitro and in vivo studies [70]. Further safety and clinical studies are needed if this type of antioxidants is to be used as potential nutritional supplements adjuvant to neurotoxic chemotherapy.
5.2. Clinically Used Medications
The injurious effects of platinum-based drugs, especially a sensory peripheral neuropathy, are primarily dose-limiting, but they also can affect the quality of life of the patients. There are many possible therapies for the treatment of PIPN although none of them is completely efficient. That is the main reason for numerous investigations, presented in Table 2, aiming to develop promising adjuvant therapy for PIPN, without interfering with platinum cytostatic activity [96,97].
Amifostine is an organic thiophosphate that has cytoprotective and detoxicant activities. It is generally an inactive prodrug which activates after dephosphorilation in plasma membrane with alkaline phosphatase. When active metabolite enters the cell it act like free radical scavenger protecting DNA and cellular membranes [98]. There are many in vitro reports that showed good neuroprotective properties of amifostine against cisplatin and oxaliplatin, and some studies on patients claiming promising effects of amifostine against peripheral neurotoxicity induced by cisplatin, oxaliplatin, and carboplatin [99,100]. Moreover, a network meta-analysis of Fu and coworkers [76] confirmed that amifostine was the most active against both overall and severe platinum drugs-induced neurotoxicities compared to other most used therapies, such as vitamin E, GSH, and Ca/Mg infusion. Metformin, an anti-diabetic drug, showed neuroprotective effects against other chemotherapy-induced neuropathies, and therefore it has recently been tested in vivo for alleviating the oxaliplatin-induced neuropathy in rats. It was able to decrease levels of ROS and markers of oxidative stress and to ameliorate intraepidermal fibers degeneration, gliosis, and sensitivity [77]. The new drug in the treatment of multiple sclerosis - dimethyl fumarate (DMF) was tested because of its neuroprotective properties on cisplatin and oxaliplatin-induced neurodegeneration in the in vitro study [78]. DMF induced up-regulation of the nuclear factor-erythroid-2-related factor 2 (Nrf2)-dependent antioxidant response and prevented the inhibition of neurite outgrowth. The antioxidant and mitoprotective potential of carvedilol, an antihypertensive drug, was tested in vitro on neuronal cells (Neuro-2a) affected by oxaliplatin. Carvedilol is a non-selective beta-adrenergic receptor blocker (β1,β2) of the third generation, and alpha adrenergic receptor blocker (α1) exerting antioxidant potential. It showed significant antioxidant and free radical scavenging effects with the alleviation of functional and sensorimotor deficits, but without in vitro affecting the anti-tumor effects of oxaliplatin [79].
Cisplatin-induced neurotoxicity in rats was treated with oxytocin, a neurohypophyseal nonapeptide synthesized in the hypothalamus [80]. The results showed that oxytocin was able to reduce oxidative stress and inflammation in rats, but also to mitigate the electromyography (EMG) changes in rats treated with cisplatin. Another compound that can reduce the oxidative stress induced by oxaliplatin in rats is phosphatidylcholine [81], but also exhibited potential in the regulation of microglial activation and thus decreasing peripheral neuropathy in rats. In the study of Chiu et al. [34] chemotherapy (cisplatin)-induced cognitive impairment was treated with pifithrin-μ, an inhibitor of mitochondrial p53 accumulation. The use of this small molecule led to a significant improvement in preserving neuronal mitochondrial function. Patients with colorectal cancer treated with oxaliplatin-based chemotherapy were receiving co-treatment with monosialotetrahexosyl- ganglioside, known for its impacts on neuronal plasticity and repair mechanisms, and the release of neurotrophins in the brain [82]. This medication was able to significantly reduce the incidence of neuropathy induced by oxaliplatin, particularly severe neuropathy, with the absence of interfering with oxaliplatin activity.
N-Acetylcysteine (NAC) is a precursor of glutathione which can increase the concentration of glutathione in blood and thus increase the antioxidative defense in the organism [99]. The effects of NAC were studied in cisplatin-treated rats showing reduced levels of oxidative stress parameters in rodents as well as significant anxiolytic properties [83]. NAC was also potent in regulating cognitive performance and improving the histomorphological parameters that were disrupted by cisplatin administration [74]. Some PARP inhibitors may also be used for alleviating the chemotherapy-induced neurotoxicities. Since oxidative stress and mitochondrial dysfunction are closely connected with side effects of platinum chemotherapy-induced neuropathy, the inhibition of poly(ADP-ribose) polymerase (PARP), would be beneficial in terms of sensory and enteric neuroprotection [84]. Thus, several PARP inhibitors, such as GPI 21016 and ABT-888 analogue, showed alleviated sensory and enteric neuropathy against cisplatin and oxaliplatin therapy [84].
Many studies have dealt with the neuroprotective activity of different medications against platinum drugs-induced neuropathy. Several compounds were considered to have a number of favorable properties. For example, an chemoprotectant BNP7787 showed good neuroprotective effects in phase II randomized study [99]. A therapy based on chelation properties can be used in the treatment of metal overexposures, including platinum, supporting their excretion from the body. Generally, based on the Lewis theory of hard and soft acids and bases, Pt2+ as a soft acid (soft metal) has a high affinity for chelation with compounds possessing soft bases (soft ligands) [101]. For example, the organosulfur complex diethyldithiocarbamate (DDTC) was thought to be able to act as an antioxidant by chelation of Pt2+ ions, increasing renal excretion of platinum thereafter influencing on neurophysiological aspects of patients treated with cisplatin. But in the randomized placebo-controlled multicenter evaluation of DDTC in cisplatin treatment, it was shown that DDTC was not able to alleviate cisplatin-induced toxicities [87]. On the other hand, a chelation complex fodipir (DPDP), a derivative of vitamin B6, acts by lowering oxidative and nitrosative stress in PIPN patients. The probable mechanism of PIPN development is via retention of Pt2+ ions in the dorsal root ganglion and where they can bind to proteins, that lead to the development of oxidative and nitrosative stress [102]. In the recent study of Stehr et al. [88] was shown that Pt2+ has an affinity for DPDP, and the newly formed complex of metal with DPDP has fast excretion and low toxicity which are positive aspects for use of DPDP as chelation therapy of PIPN. Another chelation complex of manganese and the ligand fodipir, mangafodipir, displayed significant results in the study conducted by Coriat et al. [89] on oxaliplatin-induced neurotoxicity in a mouse model as well as in patients with oxaliplatin neuropathy (grade ≥2). This complex is generally inactive, yet while it is indicated that it has to be subjected to metabolism into N,N’-dipyridoxyl ethylenedi amine-N,N’-diacetic acid (MnPLED) in organism so it can show its cytoprotective effects. The results of the above-mentioned research showed that intravenous administration of mangafodipir along with oxaliplatin led to the alleviation of oxaliplatin-induced peripheral neuropathy in patients with grade 2 neuropathy and thus can be a successful choice for adjuvant therapy. A mixed metal complex calmangafodipir (Ca2+/Mn2+) was patented in 2015 by Karlsson et al. [90] as a new stable complex, whose toxic effects are reduced compared to mangafodipir, acting as a reducer of oxidative stress and levels of reactive oxygen species. Thus it became the object of different investigations, one of which dealt with its activity on oxaliplatin-induced peripheral neuropathy in a placebo-controlled randomized phase II study [103]. The authors concluded that calmangafodipir was able to prevent the development of oxaliplatin-induced peripheral neuropathy during and after treatment, at a dose of 5 mmol/kg. However, Karlsson & Jynge [104] criticized the implementation and interpretation of the results by Glimelius et al., proposing a revision of methodology and design of the experiment (phase II study) so that it can be accurate and authoritative for the further development of a phase III study. There are still no approved chelation-based treatments of PIPN, but research work is encouraged to find appropriate therapy without affecting the platinum-drug activity and without side-effects [101].
Xaliproden, a 5HT1A agonist that possesses neuroprotective activity, showed a reduction in grade 3–4 neurotoxicity by activation of MAPK pathways in oxaliplatin-induced neuropathy without affecting FOLFOX4 antitumor activity in first-line treatment of patients with metastatic colorectal cancer (MCRC) [91]. Some new perspectives of drug preparation and application are being investigated. Therefore, basalin-coated silver nanoparticles were developed and applied as a potent antioxidant for the amelioration of oxaliplatin-induced peripheral neuropathy in mice [92]. They were able to significantly perform the chelation of aluminum and consequently ameliorate neuropathic pain in mice.
5.3. Natural Products and Medicinal Plants
Compounds of the natural origin are in use in traditional and modern medicine regarding their numerous beneficial effects. They can be used in the prevention and/or therapy of various pathological conditions such as cardiovascular diseases, metabolic disorders, carcinogenesis, and neural impairments [105]. The most widely studied phytochemicals are definitely phenolic compounds or polyphenolics from medicinal plants. They exerted a wide range of biological activities, including antioxidant, anti-inflammatory, anti-apoptotic, and anti-cancer effects, which can be the main pillars of their actions as neuroprotectors, particularly towards platinum drugs-induced neurotoxicities (Table 3) [48,106,107,108,109]. One of the well-studied phenolic compounds with neuroprotective properties is curcumin (Figure 2), a yellow pigment isolated from Curcuma longa (Zingiberaceae) rhizomes. It showed protective activity in vitro, in terms of neurite outgrowth inhibition in PC12 cells treated with cisplatin, but on the other hand, curcumin had not affected the anticancer activity of cisplatin in HepG2 tumor cells [110]. The in vivo studies of Oz et al. [111] in rats placed curcumin as a compound that significantly alleviated cisplatin-induced learning and memory impairments. It was able to lower the oxidative stress level and improve the cognition and cholinergic functions affected by cisplatin. These results in different models suggested that curcumin may diminish cisplatin-induced neurotoxicity. Apart from its properties against cisplatin, curcumin can be used to prevent the neurotoxicity induced by oxaliplatin. It was able to significantly restore enzymatic and non-enzymatic antioxidant capacity and to alleviate the mitochondrial activity in the brain mitochondria of rats as well as to lower neurotensin plasma concentration, improve motor and behavioral parameters and reduce the concentration of oxaliplatin in the sciatic nerve of male Wistar rats [112,113].

Table 3.
Compounds from natural sources, natural products, and medicinal plants with promising protective activity against platinum-drugs induced neuropathy.
Flavonoids, the most common class of polyphenolic compounds in the plant kingdom, are well-known for their biological potential which mostly lies in the fact that they have exceptional antioxidant properties [140]. Quercetin, a bioactive flavonol, and its glucoside rutin (quercetin-3-O-rutinoside) (Figure 2) were tested for their ability to restore increased thermal and mechanical nociceptive response induced by oxaliplatin in mice [114]. Both compounds significantly reduced oxidative stress and prevented oxaliplatin-induced chronic painful peripheral neuropathy. Rutin confirmed its neuroprotective action against cisplatin in an in vivo study conducted by Almutairi and coworkers [115]. The high expressions of PON-1, PON-3, PPAR-δ, and GPX in rat brain tissues caused by cisplatin were restored by rutin application while PON-2 expression levels were increased. It was clear that rutin manifests its activity via the antioxidant pathway. Another flavonoid compound, 6-methoxyflavon, was tested against cisplatin-induced neuropathic allodynia and hypoalgesia in rats [116]. This compound exerted both peripheral and central antinociceptive activities, reducing the chemotherapy-induced peripheral neuropathy, but with the absence of motor side-effects characteristic to gabapentin as a control.
Phenolic acids are known for their significant antioxidant, antitumor, and antimicrobial activity and thanks to that they display many benefits on human health [141,142]. Many members of this group of phenolics showed powerful effects against different neurological disorders acting as neuroprotectors [143]. These compounds also showed significant alleviation of disrupted parameters during the cisplatin treatment, particularly rosmarinic acid [144], ellagic acid [145], protocatechuic acid [146], and caffeic acid phenethyl ester [147]. Therefore, it is not a surprising fact that rosmarinic acid (Figure 2) was able to mitigate mitochondrial dysfunction and spinal glial activation in vitro and in vivo in oxaliplatin-induced peripheral neuropathy [119]. Salicylic acid (Figure 2) showed a reduction in cisplatin neurotoxicity by antioxidant effects in rat primary neuron cell cultures in vitro [117]. Moreover, in cisplatin-induced neurotoxicity in rats, caffeic acid phenethyl ester, a derivative of caffeic acid, was capable to restore to normal activities of brain metabolic enzymes (hexokinase, glucose-6-phosphate dehydrogenase, lactate dehydrogenase, and malate dehydrogenase) showing vital activity against the development of neuropathy [118].
In the study of Li et al. [120] cyanidin, a natural phenolic compound belonging to the group of anthocyanidins present in many fruits and vegetables, especially colored berries, cherries and grapes (Figure 2), was able to suppress oxidative stress in cisplatin-induced neurotoxicity on PC12 cells based on its notable antioxidant potential against ROS overproduction. The main bioactive compound of Nigela sativa (black cumin) seed oil is thymoquinone (Figure 2). Due to its antioxidant, anti-inflammatory, anti-neoplastic, and neuroprotective properties, it exhibited protective activity against cisplatin neurotoxicity in cultured DRG neurons [121]. Namely, thymoquinone promoted the neuronal cell viability and neurite outgrowth in a dose-dependent manner. In another in vivo study, thymoquinone reduced oxidative stress downregulated the apoptotic markers (p38 mitogen-activated protein kinase (MAPK), STAT-1, p53, p21, and MMP9) in rats and protected brain tissue against cisplatin action [122]. In the same study [122], geraniol, monoterpenoid alcohol, showed similar effects as thymoquinone during cisplatin-induced neurotoxicity in rats. Chen et al. [123] studied the effects of ginsenoside Rb1, a ginsenoside found in Panax ginseng and Panax japonicus var. major roots, on cisplatin-induced memory impairments in rats. In assays such as novel objects recognition task and Morris water maze task it was shown that ginsenoside Rb1 significantly ameliorated memory impairments caused by cisplatin, as well as reduced the neuronal loss induced by cisplatin in different regions (CA1, CA3, and dentate gyrus) of the hippocampus. Moreover, this compound exhibited the ability to rescue the cholinergic neuron function in rat brain and also lowered oxidative stress and neuroinflammation.
Isothiocyanates, a group of natural bioactive compounds mostly present in cruciferous vegetables, are characterized by their antioxidant properties [148]. That is what enabled them to be used in the alleviation of platinum drug-induced neurotoxicity. Allyl-isothiocyanate alleviated neuropathic pain induced by oxaliplatin in rats and reduced the hypersensitivity to cold non-noxious stimuli by releasing H2S [124]. Similarly, glucosinolate glucoraphanin and derived isothiocyanate sulforaphane exerted reducing effects on oxaliplatin induced-neuropathic pain in mice, in a dose-dependent manner by releasing H2S and modulating Kv7 channels [125]. The same research group, Di Cesare Mannelli et al. [126] conducted in vivo experiments on oxaliplatin-induced neuropathy using silibinin as an antioxidant compound. Silibinin (Figure 2) is a flavonolignan isolated from Silybum marianum that possesses antioxidant and antineoplastic activities. In this study, silibinin reduced oxidative damage of oxaliplatin in rats at a concentration of 100 mg/kg. It also recovered motor coordination and showed a reduction in oxaliplatin-dependent pain induced by mechanical and thermal stimuli. Silibinin is one of the components in silymarin complex mixture which also contains three more flavonolignans (silychristin, silydianin, and taxifolin) and its use is mainly focused on liver disorders treatment [149]. Silymarin in rats, at a concentration of 100 mg/kg, decreased the anxiogenic effect of cisplatin treatment and exhibited significant antioxidant activity in brain tissues by lowering lipid peroxidation and elevating GSH levels and CAT and SOD activities [127].
Many herbal mixtures are in use for relieving chemotherapy-induced neuropathic pain. One that showed significant improvement regarding the negative effects of platinum-based drugs is goshajinkigan, a traditional Japanese Kampo medicine consisted of ten medicinal plants that is used clinically to treat pain in Japan. Ushio et al. [128] showed that goshajinkigan was able to prevent cold hyperalgesia and mechanical allodynia during the oxaliplatin-induced neuropathy in rats. The treatment with goshajinkigan also exerted significant improvements of oxaliplatin neuropathy in non-resectable or recurrent colorectal cancer patients [129]. Moreover, in phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan was shown its benefits towards preventing oxaliplatin-induced neuropathy without affecting the activity of the chemotherapeutic [130]. Wei and coworkers [131] monitored the neuroprotective effects of some mixtures of Traditional Chinese Medicine against oxaliplatin in cancer patients. The highest neuroprotective potential had Huangqi Injection which consisted of an extract of Astragalus membranaceus radix and Huangqi Guizhi Wuwu Decoction that contained a mixture of Astragalus membranaceus radix, Cinnamomum cassia, Paeonia lactiflora, Ziziphus jujuba, and Zingiberis recens rhizoma. The common feature for both mixtures was Astragali radix (Huang Qi). The extracts of Astragalus roots, aqueous and two hydroalcoholic extracts, were tested in vitro against oxaliplatin-induced neurotoxicity in the neuronal-derived cell line SH-SY5Y and in primary cultures of rat cortical astrocytes [132]. The Astragali radix extracts exhibited strong antioxidant activity, ameliorating the lipid peroxidation, proteins, and DNA oxidation. The 50% hydroalcoholic extract was dominant in the prevention of caspase-3 activation and it stimulates astrocyte viability.
Another immensely important medicinal plant in traditional Chinese medicine is Danshen or Salvia miltiorrhiza, mainly used in the treatment of cardiovascular diseases and neurasthenic insomnia [150]. Nevertheless, Danshen and its active constituents tanshinones (tanshinone IIA and cryptotanshinone) exhibited promising activity against oxaliplatin-induced neuropathic pain where they reduced chemotherapy-induced nociceptive hypersensitivity in mice and attenuated glioblastoma cells malignancy. Danshen and tanshinones had the long-lasting pain-relieving effects so they could serve as adjuvant therapy of choice in the oxaliplatin treatment. Another plant tested, because of its significant antioxidant potential, in the model of cisplatin-induced neuropathy in mice, was chamomile (Matricaria chamomilla). The ethanol extract of chamomile flowers showed anti-inflammatory effects and reduction of pain responses in the formalin test in mice with intensive analgesic effect [134]. Hypericum perforatum L. (St. John’s wort) is well-known for its antioxidant, anti-inflammatory, analgesic, and neuroprotective effects [151,152,153]. Its extract showed significant protective activity against oxaliplatin-induced neurotoxicity in vitro and reduced caspase-3 activity in rat astrocytes without the reduction of oxaliplatin cytotoxicity on HT-29 cells [135]. Satureja hortensis aerial part extract, applied in rats at three different concentrations (50, 100 and 200 mg/kg) along with cisplatin injection, exhibited strong anxiolytic activity and lowered apoptotic parameters in rat hippocampal tissues [127]. Moreover, it was able to significantly reduce the oxidative stress in brain tissues by alleviating CAT and SOD activities and GSH levels and decreasing the levels of lipid peroxidation indicators.
Vitis vinifera L., the common grapevine, is known for many benefits on human health, particularly for the antioxidant potential of its main bioactive compounds proanthocyanidins but also many phenolic acids and flavonoids [106,141]. The V. vinifera hydroalcoholic extract in the model of oxaliplatin-induced neurotoxicity showed extensive activity towards the reduction of superoxide anion concentration and lipid peroxidation in rat astrocytes [136]. It also suppressed mechanical and thermal hypersensitivity in rats while a decline of astrocytes activation in the spinal cord was monitored. The grape seed proanthocyanidin extract also showed neuroprotective power against carboplatin in a study reported by Yousef et al. [137]. The main way of acting was through decreasing the oxidative stress in brain tissue and reducing cytokines, p53, neurotransmitters and biochemical parameters. The extract also inhibited brain cell apoptosis and alleviated carboplatin effects on the histological parameters. Green tea (Camellia sinensis) can attenuate toxicities linked to treatment with platinum-based drugs (cisplatin, oxaliplatin) [154]. Its extract had notable potential on oxaliplatin-induced peripheral neuropathy in rats where caused alleviation of sensory symptoms after oxaliplatin treatment, such as allodynia, but it was inefficient in the prevention of morphometric or electrophysiological alterations [138]. Lithospermum erythrorhizon is a plant used in traditional Chinese medicine for eczema and other skin diseases as well as for wound healing. However, it was shown that Lithospermi radix extract can be an excellent neuroprotective agent against oxaliplatin-induced peripheral neuropathy in both in vitro and in vivo models [139].
6. Conclusions
In summary, we intentionally presented the variety of previously reported beneficial effects of antioxidant supplementation in the treatment of neurotoxicity, as one of the most frequent dose- and time-limiting factors in chemotherapeutic protocols that include platinum-based compounds. It seems reasonable that future investigations that should analyze the protective role of specific antioxidants more precisely may result in achieving even more subtle coverage of chemotherapeutic protocols with adverse effects preventing strategy.
Author Contributions
Conceptualization, J.S.K.S., D.S., V.M. and G.R.; methodology, J.S.K.S., D.S., V.M. and G.R.; formal analysis, J.S.K.S., D.S., V.M. and G.R.; investigation, J.S.K.S., D.S., V.M. and G.R.; resources, J.S.K.S., D.S., V.M. and G.R.; data curation, J.S.K.S., D.S., V.M. and G.R.; writing—original draft preparation, J.S.K.S., D.S., V.M. and G.R.; writing—review and editing, J.S.K.S., D.S., V.M. and G.R.; visualization, J.S.K.S., D.S., V.M. and G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the Faculty of Medical Sciences (JP 01/19), University of Kragujevac, Serbia. J.S.K.S. and V.M. thank the Ministry of Education, Science, and Technological Development of the Republic of Serbia for the support in the realization of their scientific research in 2020 (contracts No: 451-03-68/2020-14/200378 and 451-03-68/2020-14/200122). The authors are grateful to Igor Stankovic for providing his competent graphical design skills for the preparation of manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galanski, M.; Jakupec, M.; Keppler, B. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalt. Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Sapalidis, K.; Zarogoulidis, P.; Sardeli, C.; Koulouris, C.; Giannakidis, D.; Pavlidis, E.; Katsaounis, A.; Michalopoulos, N.; Mantalobas, S.; et al. Inhaled cisplatin for NSCLC: Facts and results. Int. J. Mol. Sci. 2019, 20, 2005. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, A.; Pisano, C.; Di Napoli, M.; Arenare, L.; Della Pepa, C.; Tambaro, R.; Facchini, G.; Gargiulo, P.; Rossetti, S.; Mangili, G.; et al. Cisplatin can be safely administered to ovarian cancer patients with hypersensitivity to carboplatin. Gynecol. Oncol. 2017, 144, 72–76. [Google Scholar] [CrossRef]
- Bucher-Johannessen, C.; Page, C.M.; Haugen, T.B.; Wojewodzic, M.W.; Fosså, S.D.; Grotmol, T.; Haugnes, H.S.; Rounge, T.B. Cisplatin treatment of testicular cancer patients introduces long-term changes in the epigenome. Clin. Epigenetics 2019, 11, 179. [Google Scholar] [CrossRef]
- Baek, D.W.; Park, J.-Y.; Lee, S.J.; Chae, Y.S. Impressive effect of cisplatin monotherapy on a patient with heavily pretreated triple-negative breast cancer with poor performance. Yeungnam Univ. J. Med. 2020, 37, 230–235. [Google Scholar] [CrossRef]
- Angeli, E.; Nguyen, T.T.; Janin, A.; Bousquet, G. How to make anticancer drugs cross the blood-brain barrier to treat brain metastases. Int. J. Mol. Sci. 2020, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, S.; Ma, Y.; Li, L.; Li, X.; Wang, X.; Fu, X.; Ma, W.; Qin, Y.; Li, W.; et al. Efficacy and safety of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) regimen in newly diagnosed, advanced-stage extranodal natural killer/T-cell lymphoma: Interim analysis of a phase 4 study NCT01501149. Oncotarget 2016, 7, 55721–55731. [Google Scholar] [CrossRef]
- Nguyen, J.; Solimando, D.A.; Waddell, J.A. Carboplatin and Liposomal Doxorubicin for Ovarian Cancer. Hosp. Pharm. 2016, 51, 442–449. [Google Scholar] [CrossRef]
- Xiang, M.; Colevas, A.D.; Holsinger, F.C.; Le, Q.T.X.; Beadle, B.M. Survival after definitive chemoradiotherapy with concurrent cisplatin or carboplatin for head and neck cancer. JNCCN J. Natl. Compr. Cancer Netw. 2019, 17, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, H.; Ninomiya, K.; Kenmotsu, H.; Morise, M.; Daga, H.; Goto, Y.; Kozuki, T.; Miura, S.; Sasaki, T.; Tamiya, A.; et al. The Japanese Lung Cancer Society Guideline for Non-Small Cell Lung Cancer, Stage IV; Springer: Singapore, 2019; Volume 24, ISBN 0123456789. [Google Scholar]
- Martín-Aragón, T.; Serrano, J.; Benedí, J.; Meiriño, R.M.; García-Alonso, P.; Calvo, F.A. The value of oxaliplatin in the systemic treatment of locally advanced rectal cancer. J. Gastrointest. Oncol. 2018, 9, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Jia, Z.; Wu, H.; Gu, K. Oxaliplatin-Based regimen is superior to cisplatin-Based regimen in tumour remission as first-line chemotherapy for advanced gastric cancer: A meta-analysis. J. Cancer 2019, 10, 1923–1929. [Google Scholar] [CrossRef]
- Bogliolo, S.; Cassani, C.; Gardella, B.; Musacchi, V.; Babilonti, L.; Venturini, P.-L.; Ferrero, S.; Spinillo, A. Oxaliplatin for the treatment of ovarian cancer. Expert Opin. Investig. Drugs 2015, 24, 1275–1286. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Ma, H.; Yin, R.; Zhu, M.; Shen, W.; Dai, J.; Shu, Y.; Xu, L.; Hu, Z.; et al. Genome-wide association study on platinum-induced hepatotoxicity in non-small cell lung cancer patients. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Guan, Y.; Liu, X.J.; Zhang, C.F.; Wang, P.; Liang, H.L.; Guo, Q. Sen Clinical comparative investigation of efficacy and toxicity of cisplatin plus gemcitabine or plus abraxane as first-line chemotherapy for stage III/IV non-small-cell lung cancer. Onco Targets Ther. 2016, 9, 5693–5698. [Google Scholar] [CrossRef]
- Tixier, F.; Ranchon, F.; Iltis, A.; Vantard, N.; Schwiertz, V.; Bachy, E.; Bouafia-Sauvy, F.; Sarkozy, C.; Tournamille, J.F.; Gyan, E.; et al. Comparative toxicities of 3 platinum-containing chemotherapy regimens in relapsed/refractory lymphoma patients. Hematol. Oncol. 2017, 35, 584–590. [Google Scholar] [CrossRef]
- Kart, A.; Cigremis, Y.; Karaman, M.; Ozen, H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 2010, 62, 45–52. [Google Scholar] [CrossRef]
- Khan, M.W.; Zhao, P.; Khan, A.; Raza, F.; Raza, S.M.; Sarfraz, M.; Chen, Y.; Li, M.; Yang, T.; Ma, X.; et al. Synergism of cisplatin-oleanolic acid co-loaded calcium carbonate nanoparticles on hepatocellular carcinoma cells for enhanced apoptosis and reduced hepatotoxicity. Int. J. Nanomed. 2019, 14, 3753–3771. [Google Scholar] [CrossRef]
- Eoh, K.J.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, Y.T.; Kim, S.W. Long-term survival analysis of intraperitoneal versus intravenous chemotherapy for primary ovarian cancer and comparison between carboplatin- and cisplatin-based intraperitoneal chemotherapy. J. Korean Med. Sci. 2017, 32, 2021–2028. [Google Scholar] [CrossRef]
- Grigorian, A.; Brien, C.B.O. Hepatotoxicity Secondary to Chemotherapy. J. Clin. Trans. Hepatol. 2014, 2, 95–102. [Google Scholar]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Karavelioglu, E.; Boyaci, M.G.; Simsek, N.; Sonmez, M.A.; Koc, R.; Karademir, M.; Guven, M.; Eser, O. Selenium protects cerebral cells by cisplatin induced neurotoxicity. Acta Cir. Bras. 2015, 30, 394–400. [Google Scholar] [CrossRef]
- Salman, M.; Naseem, I.; Hassan, I.; Khan, A.A.; Alhazza, I.M. Riboflavin arrests cisplatin-induced neurotoxicity by ameliorating cellular damage in dorsal root ganglion cells. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Randon, K.R.; Knight, A.; Windebank, A.J. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: A potential mechanism for neurotoxicity. Neurobiol. Dis. 2005, 18, 305–313. [Google Scholar] [CrossRef]
- Podratz, J.L.; Knight, A.M.; Ta, L.E.; Staff, N.P.; Gass, J.M.; Genelin, K.; Schlattau, A.; Lathroum, L.; Windebank, A.J. Cisplatin induced Mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011, 41, 661–668. [Google Scholar] [CrossRef]
- Leo, M.; Schmitt, L.I.; Erkel, M.; Melnikova, M.; Thomale, J.; Hagenacker, T. Cisplatin-induced neuropathic pain is mediated by upregulation of N-type voltage-gated calcium channels in dorsal root ganglion neurons. Exp. Neurol. 2017, 288, 62–74. [Google Scholar] [CrossRef]
- Zhao, M.; Isami, K.; Nakamura, S.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol. Pain 2012, 8, 1–11. [Google Scholar] [CrossRef]
- Dietrich, J.; Han, R.; Yang, Y.; Mayer-Pröschel, M.; Noble, M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 2006, 5. [Google Scholar] [CrossRef]
- Pantic, M.; Minic, M. The Evaluation of the Effects of N-Acetylcysteine on Cisplatin-Induced Alterations in Exploratory Activity in Elevated Plus Maze Test in Rats. Serbian J. Exp. Clin. Res. 2019, 20, 65–72. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; Saad, M.A.; Abdelsalam, R.M. Neuroprotective effect of nebivolol against cisplatin-associated depressive-like behavior in rats. J. Neurochem. 2017, 141, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Lomeli, N.; Di, K.; Czerniawski, J.; Guzowski, J.F.; Bota, D.A. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic. Biol. Med. 2017, 102, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Chiu, G.S.; Maj, M.A.; Rizvi, S.; Dantzer, R.; Vichaya, E.G.; Laumet, G.; Kavelaars, A.; Heijnen, C.J. Pifithrin-m prevents cisplatin-induced chemobrain by preserving neuronal mitochondrial function. Cancer Res. 2017, 77, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Amr, M. Antioxidant Enzyme; El-Missiry, M.A., Ed.; InTechOpen Limited: London, UK, 2012; ISBN 978-953-51-0789-7. [Google Scholar]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Warraich, U.E.A.; Hussain, F.; Kayani, H.U.R. Aging - Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Niki, E. Antioxidant defenses in eukaryotic cells. In Advances in DNA Damage and Repair; Springer US: Boston, MA, USA, 1999; pp. 313–318. [Google Scholar]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2003; 149p.
- Watson, R.R.; Zibadi, S. Nutritional Modulators of Pain in the Aging Population. Nutr. Modul. Pain Aging Popul. 2017, 1–298. [Google Scholar] [CrossRef]
- Pellacani, C.; Eleftheriou, G. Neurotoxicity of antineoplastic drugs: Mechanisms, susceptibility, and neuroprotective strategies. Adv. Med. Sci. 2020, 65, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Erken, H.A.; Koç, E.R.; Yazici, H.; Yay, A.; Önder, G.Ö.; Sarici, S.F. Selenium partially prevents cisplatin-induced neurotoxicity: A preliminary study. Neurotoxicology 2014, 42, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tredici, G.; Tredici, S.; Fabbrica, D.; Minoia, C.; Cavaletti, G. Experimental cisplatin neuronopathy in rats and the effect of retinoic acid administration. J. Neurooncol. 1998, 36, 31–40. [Google Scholar] [CrossRef]
- Arrieta, O.; Hernández-Pedro, N.; Fernández-González-Aragón, M.C.; Saavedra-Pérez, D.; Campos-Parra, A.D.; Ríos-Trejo, M.A.; Cerón-Lizárraga, T.; Martínez-Barrera, L.; Pineda, B.; Ordóñez, G.; et al. Retinoic acid reduces chemotherapy-induced neuropathy in an animal model and patients with lung cancer. Neurology 2011, 77, 987–995. [Google Scholar] [CrossRef]
- Leonetti, C.; Biroccio, A.; Gabellini, C.; Scarsella, M.; Maresca, V.; Flori, E.; Bove, L.; Pace, A.; Stopacciaro, A.; Zupi, G.; et al. α-Tocopherol Protects Against Cisplatin-Induced Toxicity Without Interfering With Antitumor Efficacy. Int. J. Cancer 2003, 104, 243–250. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Chroni, E.; Koutras, A.; Iconomou, G.; Papapetropoulos, S.; Polychronopoulos, P.; Kalofonos, H.P. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: Final results. Support. Care Cancer 2006, 14, 1134–1140. [Google Scholar] [CrossRef]
- Pace, A.; Giannarelli, D.; Galiè, E.; Savarese, A.; Carpano, S.; Della Giulia, M.; Pozzi, A.; Silvani, A.; Gaviani, P.; Scaioli, V.; et al. Vitamin e neuroprotection for cisplatin neuropathy: A randomized, placebo-controlled trial. Neurology 2010, 74, 762–766. [Google Scholar] [CrossRef]
- Kottschade, L.A.; Sloan, J.A.; Mazurczak, M.A.; Johnson, D.B.; Murphy, B.P.; Rowland, K.M.; Smith, D.A.; Berg, A.R.; Stella, P.J.; Loprinzi, C.L. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support. Care Cancer 2011, 19, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Afonseca, S.O.D.; Cruz, F.M.; Cubero, D.D.I.G.; Lera, A.T.; Schindler, F.; Okawara, M.; Souza, L.F.D.; Rodrigues, N.P.; Giglio, A.D. Vitamin E for prevention of oxaliplatin-induced peripheral neuropathy: A pilot randomized clinical trial. Sao Paulo Med. J. 2013, 131, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Z.; Roayaei, M. Effect of Vitamin E on oxaliplatin-induced peripheral neuropathy prevention: A randomized controlled trial. Int. J. Prev. Med. 2015, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Machado, C.; Mendonça, L.M.; Venancio, V.D.P.; Bianchi, M.L.P.; Antunes, L.M.G. Coenzyme Q10 protects Pc12 cells from cisplatin-induced DNA damage and neurotoxicity. Neurotoxicology 2013, 36, 10–16. [Google Scholar] [CrossRef]
- Turan, M.I.; Cayir, A.; Cetin, N.; Suleyman, H.; Turan, I.S.; Tan, H. An investigation of the effect of thiamine pyrophosphate on cisplatin-induced oxidative stress and DNA damage in rat brain tissue compared with thiamine: Thiamine and thiamine pyrophosphate effects on cisplatin neurotoxicity. Hum. Exp. Toxicol. 2014, 33, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, M.; Banno, Y.; Nakamura, M.; Otsuka, M.; Teramachi, H.; Tsuchiya, T.; Itoh, Y. The Pivotal Role of Intracellular Calcium in Oxaliplatin-Induced Inhibition of Neurite Outgrowth but Not Cell Death in Differentiated PC12 Cells. Chem. Res. Toxicol. 2011, 24, 1845–1852. [Google Scholar] [CrossRef]
- Sakurai, M.; Egashira, N.; Kawashiri, T.; Yano, T.; Ikesue, H.; Oishi, R. Oxaliplatin-induced neuropathy in the rat: Involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 2009, 147, 165–174. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Qin, R.; Dakhil, S.R.; Fehrenbacher, L.; Flynn, K.A.; Atherton, P.; Seisler, D.; Qamar, R.; Lewis, G.C.; Grothey, A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J. Clin. Oncol. 2014, 32, 997–1005. [Google Scholar] [CrossRef]
- Melli, G.; Taiana, M.; Camozzi, F.; Triolo, D.; Podini, P.; Quattrini, A.; Taroni, F.; Lauria, G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp. Neurol. 2008, 214, 276–284. [Google Scholar] [CrossRef]
- Tuncer, S.; Dalkilic, N.; Akif Dunbar, M.; Keles, B. Comparative effects of alpha lipoic acid and melatonin on cisplatin-induced neurotoxicity. Int. J. Neurosci. 2010, 120, 655–663. [Google Scholar] [CrossRef]
- Guo, Y.; Jones, D.; Palmer, J.L.; Forman, A.; Dakhil, S.R.; Velasco, M.R.; Weiss, M.; Gilman, P.; Mills, G.M.; Noga, S.J.; et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: A randomized, double-blind, placebo-controlled trial. Support. Care Cancer 2014, 22, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Gedlicka, C.; Scheithauer, W.; Schull, B.; Kornek, G.V. Effective Treatment of Oxaliplatin-Induced Cumulative Polyneuropathy With Alpha-Lipoic Acid. J. Clin. Oncol. 2002, 20, 3356–3357. [Google Scholar] [CrossRef] [PubMed]
- Gedlicka, C.; Kornek, G.V.; Schmid, K.; Scheithauer, W. Amelioration of docetaxel/cisplatin induced polyneuropathy by α-lipoic acid. Ann. Oncol. 2003, 14, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.; Saleh, S.; El Abhar, H.; Saad, A.S.; Schaalan, M. L-Carnosine protects against Oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: A perspective on targeting Nrf-2 and NF-κB pathways. Toxicol. Appl. Pharmacol. 2019, 365, 41–50. [Google Scholar] [CrossRef]
- Song, T.Y.; Chen, C.L.; Liao, J.W.; Ou, H.C.; Tsai, M.S. Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem. Toxicol. 2010, 48, 3492–3499. [Google Scholar] [CrossRef]
- Zhu, J.; Carozzi, V.A.; Reed, N.; Mi, R.; Marmiroli, P.; Cavaletti, G.; Hoke, A. Ethoxyquin provides neuroprotection against cisplatin-induced neurotoxicity. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Waseem, M.; Tabassum, H.; Parvez, S. Neuroprotective effects of melatonin as evidenced by abrogation of oxaliplatin induced behavioral alterations, mitochondrial dysfunction and neurotoxicity in rat brain. Mitochondrion 2016, 30, 168–176. [Google Scholar] [CrossRef]
- Gopal, K.V.; Wu, C.; Shrestha, B.; Campbell, K.C.M.; Moore, E.J.; Gross, G.W. D-Methionine protects against cisplatin-induced neurotoxicity in cortical networks. Neurotoxicol. Teratol. 2012, 34, 495–504. [Google Scholar] [CrossRef]
- Hinduja, S.; Kraus, K.S.; Manohar, S.; Salvi, R.J. D-Methionine Protects Against Cisplatin-Induced Neurotoxicity in the Hippocampus of the Adult Rat. Neurotox. Res. 2015, 27, 199–204. [Google Scholar] [CrossRef]
- Rosic, G.; Joksimovic, J.; Selakovic, D.; Jakovljevic, V.; Zivkovic, V.; Srejovic, I.; Djuric, M.; Djuric, D. The Beneficial Effects of Sulfur-containing Amino Acids on Cisplatin-induced Cardiotoxicity and Neurotoxicity in Rodents. Curr. Med. Chem. 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Owoeye, O.; Adedara, I.A.; Farombi, E.O. Pretreatment with taurine prevented brain injury and exploratory behaviour associated with administration of anticancer drug cisplatin in rats. Biomed. Pharmacother. 2018, 102, 375–384. [Google Scholar] [CrossRef]
- Fu, X.; Wu, H.; Li, J.; Wang, C.; Li, M.; Ma, Q.; Yang, W. Efficacy of drug interventions for chemotherapy-induced chronic peripheral neurotoxicity: A network meta-analysis. Front. Neurol. 2017, 8, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.W.; Sánchez, A.; Diaz, P.; Broekhuizen, R.; Godoy, J.; Mondaca, S.; Catenaccio, A.; Macanas, P.; Nervi, B.; Calvo, M.; et al. Metformin protects from oxaliplatin induced peripheral neuropathy in rats. Neurobiol. Pain 2020, 8, 100048. [Google Scholar] [CrossRef]
- Kawashiri, T.; Miyagi, A.; Shimizu, S.; Shigematsu, N.; Kobayashi, D.; Shimazoe, T. Dimethyl fumarate ameliorates chemotherapy agent-induced neurotoxicity in vitro. J. Pharmacol. Sci. 2018, 137, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Komirishetty, P.; Kumar, A. Carvedilol prevents functional deficits in peripheral nerve mitochondria of rats with oxaliplatin-evoked painful peripheral neuropathy. Toxicol. Appl. Pharmacol. 2017, 322, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Akman, T.; Akman, L.; Erbas, O.; Terek, M.C.; Taskiran, D.; Ozsaran, A. The preventive effect of oxytocin to cisplatin-induced neurotoxicity: An experimental rat model. BioMed Res. Int. 2015, 2015, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Chung, Y.H.; Lee, H.S.; Chung, S.J.; Lee, J.H.; Sohn, U.D.; Shin, Y.K.; Park, E.S.; Kim, H.C.; Bang, J.S.; et al. Protective effects of phosphatidylcholine on oxaliplatin-induced neuropathy in rats. Life Sci. 2015, 130, 81–87. [Google Scholar] [CrossRef]
- Chen, X.F.; Wang, R.; Yin, Y.M.; Røe, O.D.; Li, J.; Zhu, L.J.; Guo, R.H.; Wu, T.; Shu, Y.Q. The effect of monosialotetrahexosylganglioside (GM1) in prevention of oxaliplatin induced neurotoxicity: A retrospective study. Biomed. Pharmacother. 2012, 66, 279–284. [Google Scholar] [CrossRef]
- Vukovic, R.; Kumburovic, I.; Joksimovic Jovic, J.; Jovicic, N.; Katanic Stankovic, J.S.; Mihailovic, V.; Djuric, M.; Velickovic, S.; Arnaut, A.; Selakovic, D.; et al. N-acetylcysteine protects against the anxiogenic response to cisplatin in rats. Biomolecules 2019, 9, 892. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. PARP inhibition in platinum-based chemotherapy: Chemopotentiation and neuroprotection. Pharmacol. Res. 2018, 137, 104–113. [Google Scholar] [CrossRef]
- Parker, A.R.; Petluru, P.N.; Nienaber, V.L.; Zhao, M.; Ayala, P.Y.; Badger, J.; Chie-Leon, B.; Sridhar, V.; Logan, C.; Kochat, H.; et al. Novel covalent modification of human anaplastic lymphoma kinase (ALK) and potentiation of crizotinib-mediated inhibition of ALK activity by BNP7787. Onco Targets Ther. 2015, 8, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hausheer, F.H.; Shanmugarajah, D.; Leverett, B.D.; Chen, X.; Huang, Q.; Kochat, H.; Petluru, P.N.; Parker, A.R. Mechanistic study of BNP7787-mediated cisplatin nephroprotection: Modulation of gamma-glutamyl transpeptidase. Cancer Chemother. Pharmacol. 2010, 65, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Gandara, D.R.; Nahhas, W.A.; Adelson, M.D.; Lichtman, S.M.; Podczaski, E.S.; Yanovich, S.; Homesley, H.D.; Braly, P.; Ritch, P.S.; Weisberg, S.R.; et al. Randomized placebo-controlled multicenter evaluation of diethyldithiocarbamate for chemoprotection against cisplatin-induced toxicities. J. Clin. Oncol. 1995, 13, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Stehr, J.E.; Lundström, I.; Karlsson, J.O.G. Evidence that fodipir (DPDP) binds neurotoxic Pt2+ with a high affinity: An electron paramagnetic resonance study. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Coriat, R.; Alexandre, J.; Nicco, C.; Quinquis, L.; Benoit, E.; Chéreau, C.; Lemaréchal, H.; Mir, O.; Borderie, D.; Tréluyer, J.M.; et al. Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir. J. Clin. Investig. 2014, 124, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.-O.; Reineke, K.; Kurz, T.; Andersson, R.; Hall, M.; McLaughlin, C.; Jacobsson, S.; Nasstrom, J. Calmangafodipir, A New Chemical Entity, and Other Mixed Metal Complexes, Methods of Preparation, Compositions, and Methods of Treatment. U.S. Patent Application No. 14/922,555, 26 October 2015. [Google Scholar]
- Cassidy, J.; Bjarnason, G.A.; Hickish, T.; Topham, C.; Provencio, M.; Bodoky, G.; Landherr, L.; Koralewski, P.; Lopez-Vivanco, G.; Said, G. Randomized double blind (DB) placebo (Plcb) controlled phase III study assessing the efficacy of xaliproden (X) in reducing the cumulative peripheral sensory neuropathy (PSN) induced by the oxaliplatin (Ox) and 5-FU/LV combination (FOLFOX4) in first-line. J. Clin. Oncol. 2006, 24, 3507. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, Y.; Zhao, C.; Teng, H. Investigation on effect of basalin coated silver nanoparticles as antioxidant for alleviating peripheral neuropathy in mice treated with oxaliplatin. J. Photochem. Photobiol. B Biol. 2017, 177, 56–61. [Google Scholar] [CrossRef]
- Prša, P.; Karademir, B.; Biçim, G.; Mahmoud, H.; Dahan, I.; Yalçın, A.S.; Mahajna, J.; Milisav, I. The potential use of natural products to negate hepatic, renal and neuronal toxicity induced by cancer therapeutics. Biochem. Pharmacol. 2020, 173, 113551. [Google Scholar] [CrossRef]
- Brami, C.; Bao, T.; Deng, G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit. Rev. Oncol. Hematol. 2016, 98, 325–334. [Google Scholar] [CrossRef]
- Gamelin, L. Prevention of Oxaliplatin-Related Neurotoxicity by Calcium and Magnesium Infusions: A Retrospective Study of 161 Patients Receiving Oxaliplatin Combined with 5-Fluorouracil and Leucovorin for Advanced Colorectal Cancer. Clin. Cancer Res. 2004, 10, 4055–4061. [Google Scholar] [CrossRef]
- Calls, A.; Carozzi, V.; Navarro, X.; Monza, L.; Bruna, J. Pathogenesis of platinum-induced peripheral neurotoxicity: Insights from preclinical studies. Exp. Neurol. 2020, 325, 113141. [Google Scholar] [CrossRef] [PubMed]
- Amptoulach, S.; Tsavaris, N. Neurotoxicity Caused by the Treatment with Platinum Analogues. Chemother. Res. Pract. 2011, 2011, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector. Oncologist 2007, 12, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-Induced Neurotoxicity and Preventive Strategies: Past, Present, and Future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O. Chelation Treatment during Acute and Chronic Metal Overexposures-Experimental and Clinical Studies; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128030721. [Google Scholar]
- Karlsson, J.O.G.; Andersson, R.G.; Jynge, P. Mangafodipir a Selective Cytoprotectant—With Special Reference to Oxaliplatin and Its Association to Chemotherapy-Induced Peripheral Neuropathy (CIPN). Transl. Oncol. 2017, 10, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Manojlovic, N.; Pfeiffer, P.; Mosidze, B.; Kurteva, G.; Karlberg, M.; Mahalingam, D.; Buhl Jensen, P.; Kowalski, J.; Bengtson, M.; et al. Persistent prevention of oxaliplatin-induced peripheral neuropathy using calmangafodipir (PledOx®): A placebo-controlled randomised phase II study (PLIANT). Acta Oncol. (Madr.) 2018, 57, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.O.G.; Jynge, P. Is it possible to draw firm conclusions from the PLIANT trial? Acta Oncol. (Madr.) 2018, 57, 862–864. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Zhang, C.; Wu, R.; Yang, A.Y.; Gaspar, J.; Kong, A.-N.T. Dietary Phytochemicals and Cancer Chemoprevention: A Perspective on Oxidative Stress, Inflammation, and Epigenetics. Chem. Res. Toxicol. 2016, 29, 2071–2095. [Google Scholar] [CrossRef]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef]
- Mendonça, L.M.; da Silva Machado, C.; Correia Teixeira, C.C.; Pedro de Freitas, L.A.; Pires Bianchi, M.D.L.; Greggi Antunes, L.M. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013, 34, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Nurullahoglu Atalik, K.E.; Yerlikaya, F.H.; Demir, E.A. Curcumin alleviates cisplatin-induced learning and memory impairments. Neurobiol. Learn. Mem. 2015, 123, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Parvez, S. Neuroprotective activities of curcumin and quercetin with potential relevance to mitochondrial dysfunction induced by oxaliplatin. Protoplasma 2016, 253, 417–430. [Google Scholar] [CrossRef]
- Al Moundhri, M.S.; Al-Salam, S.; Al Mahrouqee, A.; Beegam, S.; Ali, B.H. The Effect of Curcumin on Oxaliplatin and Cisplatin Neurotoxicity in Rats: Some Behavioral, Biochemical, and Histopathological Studies. J. Med. Toxicol. 2013, 9, 25–33. [Google Scholar] [CrossRef]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.C.; Wong, D.V.T.; Lima-Júnior, R.C.P.; de Albuquerque Ribeiro, R.; Vale, M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.M.; Alanazi, W.A.; Alshammari, M.A.; Alotaibi, M.R.; Alhoshani, A.R.; Al-Rejaie, S.S.; Hafez, M.M.; Al-Shabanah, O.A. Neuro-protective effect of rutin against Cisplatin-induced neurotoxic rat model. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Subhan, F.; Ahmad, N.; Sewell, R.D.E. The flavonoid 6-methoxyflavone allays cisplatin-induced neuropathic allodynia and hypoalgesia. Biomed. Pharmacother. 2017, 95, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Cetin, D.; Hacımuftuoglu, A.; Tatar, A.; Turkez, H.; Togar, B. The in vitro protective effect of salicylic acid against paclitaxel and cisplatin-induced neurotoxicity. Cytotechnology 2016, 68, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, B.; Güleç, M.; Özyurt, H.; Ekici, F.; Atış, Ö.; Akbaş, A. The effect of antioxidant caffeic acid phenethyl ester (CAPE) on some enzyme activities in cisplatin-induced neurotoxicity in rats. Eur. J. Gen. Med. 2006, 3, 167–172. [Google Scholar] [CrossRef]
- Areti, A.; Komirishetty, P.; Kalvala, A.K.; Nellaiappan, K.; Kumar, A. Rosmarinic Acid Mitigates Mitochondrial Dysfunction and Spinal Glial Activation in Oxaliplatin-induced Peripheral Neuropathy. Mol. Neurobiol. 2018, 55, 7463–7475. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Sun, J.Y.; Wang, K.; Zhang, S.; Hou, Y.J.; Yang, M.F.; Fu, X.Y.; Zhang, Z.Y.; Mao, L.L.; Yuan, H.; et al. Attenuation of Cisplatin-Induced Neurotoxicity by Cyanidin, a Natural Inhibitor of ROS-Mediated Apoptosis in PC12 Cells. Cell. Mol. Neurobiol. 2015, 35, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Üstün, R.; Oğuz, E.K.; Şeker, A.; Korkaya, H. Thymoquinone prevents cisplatin neurotoxicity in primary DRG neurons. Neurotoxicology 2018, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, M.A.; Mahmoud, M.O.; Abdel-Razik, A.R.H.; Gomaa, S.B. Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci. 2019, 228, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, H.; Xu, H.; Zheng, Y.; Wu, T.; Lian, Y. Ginsenoside Rb1 ameliorates cisplatin-induced learning and memory impairments. J. Ginseng Res. 2019, 43, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Lucarini, E.; Micheli, L.; Mosca, I.; Ambrosino, P.; Soldovieri, M.V.; Martelli, A.; Testai, L.; Taglialatela, M.; Calderone, V.; et al. Effects of natural and synthetic isothiocyanate-based H2S-releasers against chemotherapy-induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology 2017, 121, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Micheli, L.; Trallori, E.; Citi, V.; Martelli, A.; Testai, L.; De Nicola, G.R.; Iori, R.; Calderone, V.; Ghelardini, C.; et al. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2S release in vivo. Phyther. Res. 2018, 32, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-induced neuropathy: Oxidative Stress as Pathological Mechanism. Protective Effect of Silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kumburovic, I.; Selakovic, D.; Juric, T.; Jovicic, N.; Mihailovic, V.; Stankovic, J.K.; Sreckovic, N.; Kumburovic, D.; Jakovljevic, V.; Rosic, G. Antioxidant Effects of Satureja hortensis L. Attenuate the Anxiogenic Effect of Cisplatin in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 8307196. [Google Scholar] [CrossRef] [PubMed]
- Ushio, S.; Egashira, N.; Sada, H.; Kawashiri, T.; Shirahama, M.; Masuguchi, K.; Oishi, R. Goshajinkigan reduces oxaliplatin-induced peripheral neuropathy without affecting anti-tumour efficacy in rodents. Eur. J. Cancer 2012, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Shimada, M.; Kurita, N.; Iwata, T.; Morimoto, S.; Yoshikawa, K.; Higashijima, J.; Miyatani, T.; Kono, T. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int. J. Clin. Oncol. 2011, 16, 322–327. [Google Scholar] [CrossRef]
- Kono, T.; Hata, T.; Morita, S.; Munemoto, Y.; Matsui, T.; Kojima, H.; Takemoto, H.; Fukunaga, M.; Nagata, N.; Shimada, M.; et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): A phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother. Pharmacol. 2013, 72, 1283–1290. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, L.; Wang, H.; Wang, C.; Deng, Q.; Li, X. Efficacy of Traditional Chinese Medicines in Preventing Oxaliplatin-induced Peripheral Neurotoxicity in Cancer Patients: A Network Meta-analysis. Chin. Herb. Med. 2017, 9, 161–168. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Zanardelli, M.; Bartolucci, G.; Karioti, A.; Bilia, A.R.; Vannacci, A.; Mugelli, A.; Ghelardini, C. In Vitro Evidence for the Use of Astragali Radix Extracts as Adjuvant against Oxaliplatin-Induced Neurotoxicity. Planta Med. 2015, 81, 1045–1055. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Piccolo, M.; Maione, F.; Ferraro, M.G.; Irace, C.; De Feo, V.; Ghelardini, C.; Mascolo, N. Tanshinones from Salvia miltiorrhiza Bunge revert chemotherapy-induced neuropathic pain and reduce glioblastoma cells malignancy. Biomed. Pharmacother. 2018, 105, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Abad, A.N.A.; Nouri, M.H.K.; Gharjanie, A.; Tavakoli, F. Effect of Matricaria chamomilla Hydroalcoholic Extract on Cisplatin-induced Neuropathy in Mice. Chin. J. Nat. Med. 2011, 9, 126–131. [Google Scholar] [CrossRef]
- Cinci, L.; Di Cesare Mannelli, L.; Maidecchi, A.; Mattoli, L.; Ghelardini, C. Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: In vitro evaluations. Z. Naturforsch. C 2017, 72, 219–226. [Google Scholar] [CrossRef]
- Micheli, L.; Mattoli, L.; Maidecchi, A.; Pacini, A.; Ghelardini, C.; Di Cesare Mannelli, L. Effect of Vitis vinifera hydroalcoholic extract against oxaliplatin neurotoxicity: In vitro and in vivo evidence. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Yousef, M.I.; Khalil, D.K.A.M.; Abdou, H.M. Neuro- and nephroprotective effect of grape seed proanthocyanidin extract against carboplatin and thalidomide through modulation of inflammation, tumor suppressor protein p53, neurotransmitters, oxidative stress and histology. Toxicol. Rep. 2018, 5, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.T.; Jeon, E.K.; Won, H.S.; Cho, Y.S.; Ko, Y.H. Effect of green tea extracts on oxaliplatin-induced peripheral neuropathy in rats. BMC Complement. Altern. Med. 2012, 12, 1. [Google Scholar] [CrossRef]
- Cho, E.S.; Yi, J.M.; Park, J.S.; Lee, Y.J.; Lim, C.J.; Bang, O.S.; Kim, N.S. Aqueous extract of Lithospermi radix attenuates oxaliplatin-induced neurotoxicity in both in vitro and in vivo models. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Domitrović, R.; Potočnjak, I.; Crnčević-Orlić, Ž.; Škoda, M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem. Toxicol. 2014, 66, 321–328. [Google Scholar] [CrossRef]
- Yüce, A.; Ateşşahin, A.; Ceribaşi, A.O.; Aksakal, M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 345–349. [Google Scholar] [CrossRef]
- Yamabe, N.; Park, J.Y.; Lee, S.S.; Cho, E.-J.J.; Lee, S.S.; Kang, K.S.; Hwang, G.S.; Kim, S.-N.N.; Kim, H.Y.; Shibamoto, T. Protective effects of protocatechuic acid against cisplatin-induced renal damage in rats. J. Funct. Foods 2015, 19, 20–27. [Google Scholar] [CrossRef]
- Chirino, Y.I.; Pedraza-Chaverri, J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009, 61, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, S.; Filho, S.; Nogueira-Machado, J.; Caligiorne, R. The Anti-Oxidant Properties of Isothiocyanates: A Review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Putnik, P.; Bursać Kovačević, D.; Petrović, M.; Munekata, P.E.; Gómez, B.; Marszałek, K.; Roohinejad, S.; Barba, F.J. Silymarin compounds: Chemistry, innovative extraction techniques and synthesis. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 111–130. [Google Scholar]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Rizshsky, L.; Hauck, C.; Nikolau, B.J.; Murphy, P.A.; Birt, D.F. Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry 2011, 72, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of green tea and its main constituents against natural and chemical toxins: A comprehensive review. Food Chem. Toxicol. 2017, 100, 115–137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).