Bone Regeneration Potential of Human Dental Pulp Stem Cells Derived from Elderly Patients and Osteo-Induced by a Helioxanthin Derivative
Abstract
1. Introduction
2. Results
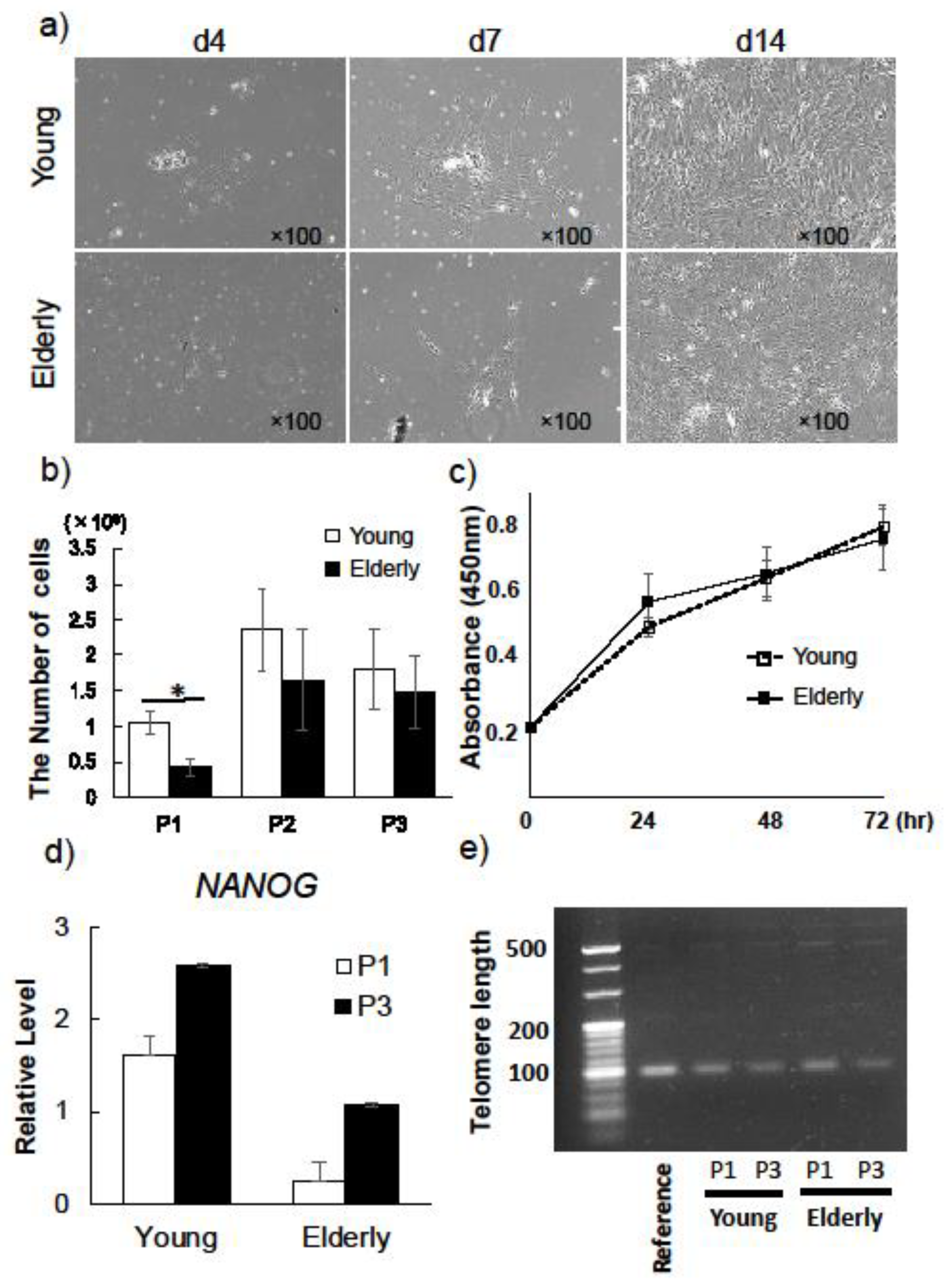
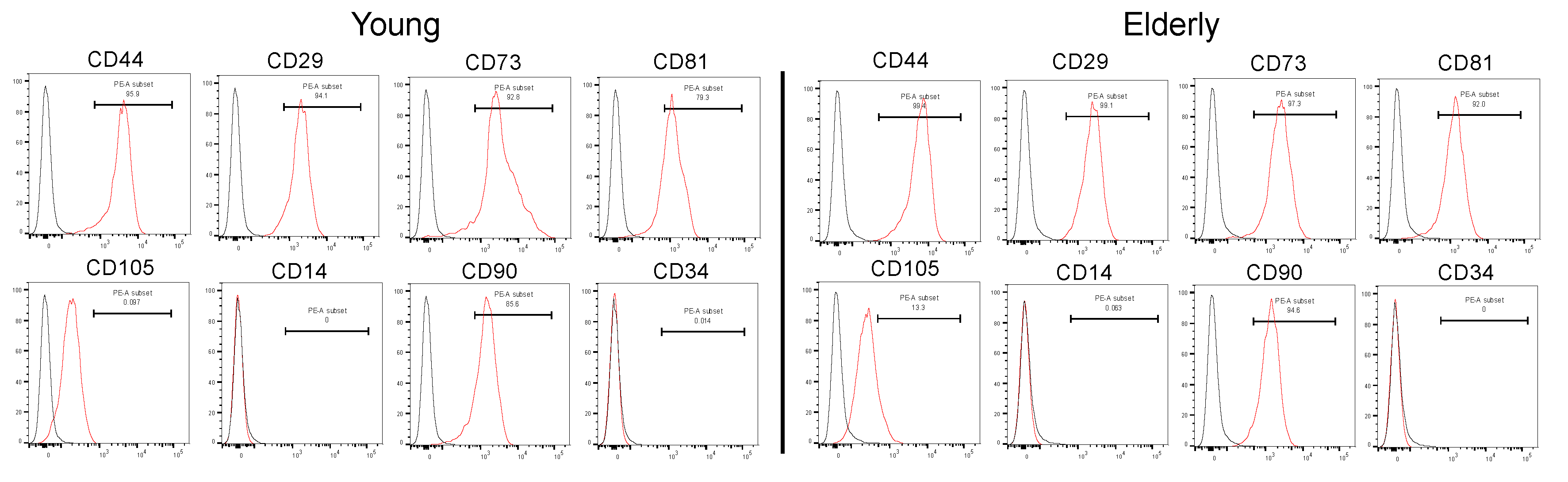
2.1. Characterization of Young and Elderly Dental Pulp Stem Cells
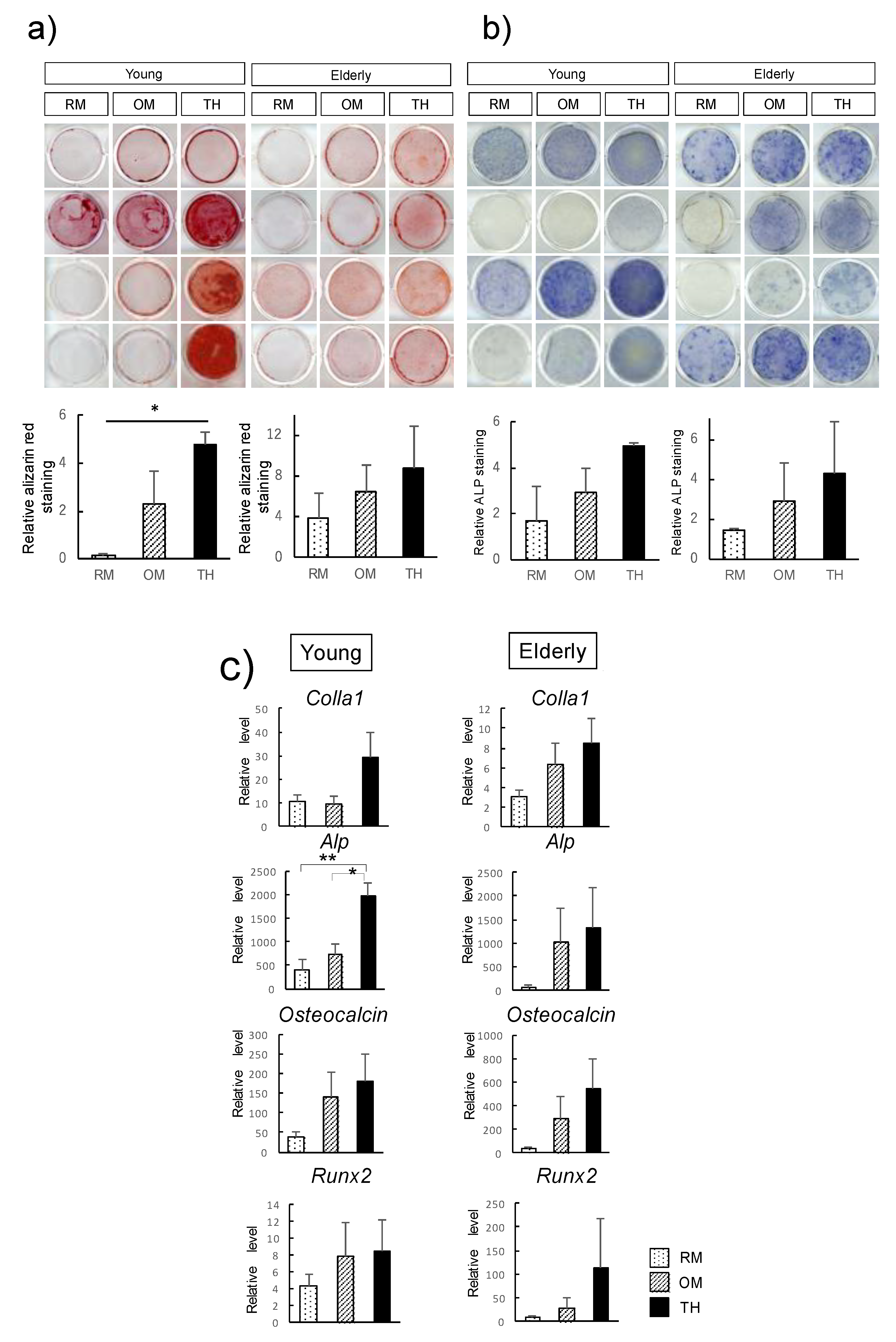
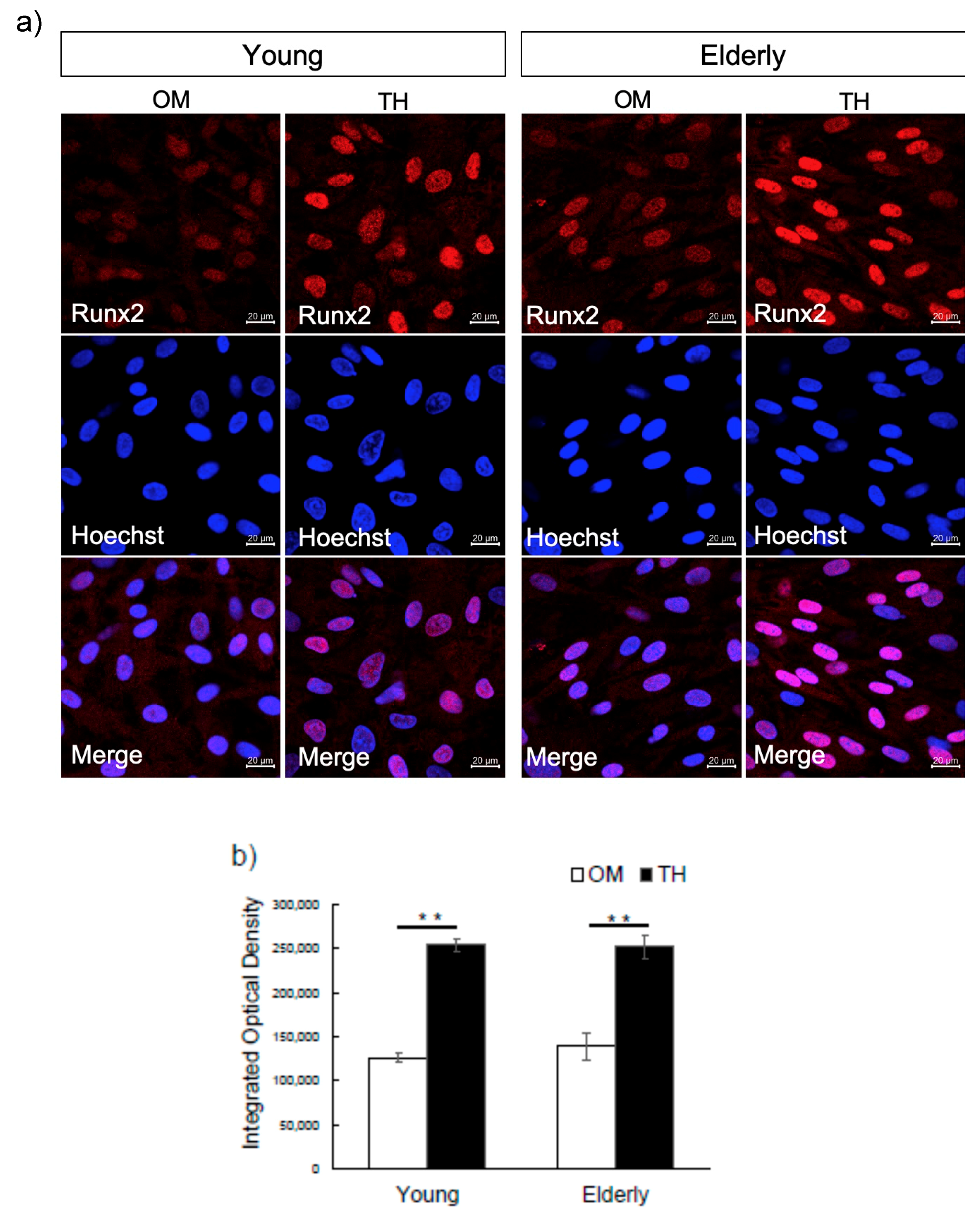
2.2. Effect of TH on Osteogenic Differentiation of Young and Elderly Dental Pulp Stem Cells
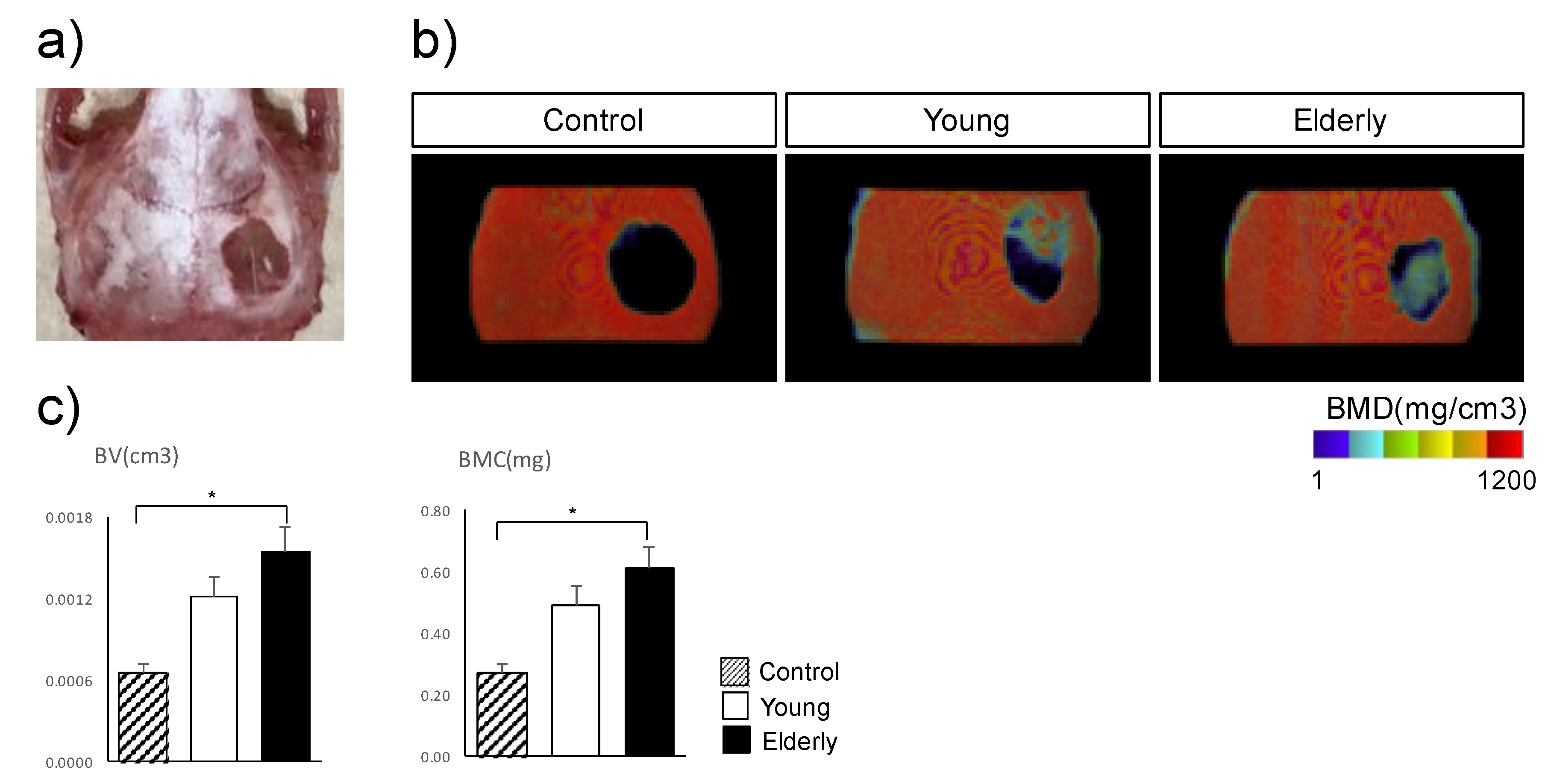
2.3. In Vivo Osteogenesis of TH-Induced Young and Elderly Dental Pulp Stem Cells
3. Discussion
4. Materials and Methods
4.1. Isolation of Dental Pulp Stem Cells
4.2. Cell Proliferation
4.3. Telomere Length
4.4. Flow Cytometry Analysis
4.5. Osteogenic Differentiation
4.6. Alizarin Red S Staining
4.7. Alkaline Phosphatase Staining
4.8. Immunocytochemistry
4.9. Reverse Transcription Polymerase Chain Reaction Analysis
4.10. Transplantation of Young and Elderly Dental Pulp Stem Cell Sheets into a Mouse Calvarial Defect Model
4.11. Radiological Evaluation
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | adipose tissue-derived stem cells |
| ALP | alkaline phosphatase |
| BMC | bone mineral content |
| BMD | bone mineral density |
| BMP | bone morphogenic protein |
| BMSCs | bone marrow stem cells |
| BV | bone volume |
| COLIA1 | type I collagen alpha 1 |
| DPSCs | human dental pulp stem cells |
| FITC | fluorescein isothiocyanate |
| MSCs | mesenchymal stem cells |
| OM | osteogenic medium |
| P/S | penicillin/streptomycin |
| PBS | phosphate-buffered saline |
| PE | phycoerythrin |
| RM | regular medium |
| SD | standard deviation |
| TH | 4-(4-methoxyphenyl) pyrido[4 0,30:4,5]thieno[2,3-b]pyridine-2-carboxamide |
| αMEM | alpha-modified Eagle’s medium |
References
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Nakahara, T.; Ishikawa, H.; Sato, S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology 2013, 101, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Tvedesøe, C.; Rölfing, J.H.D.; Foldager, C.B.; Lysdahl, H.; Kraft, D.C.E.; Chen, M.; Baas, J.; Le, D.Q.S.; Bünger, C.E. Dental pulp-derived stromal cells exhibit a higher osteogenic potency than bone marrow-derived stromal cells in vitro and in a porcine critical-size bone defect model. Sicot J. 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; d’Aquino, R.; Laino, G.; Papaccio, G. Dental pulp stem cells: A promising tool for bone regeneration. Stem. Cell Rev. 2008, 4, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rando, T.A. Manifestations and mechanisms of stem cell aging. J. Cell Biol. 2011, 193, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Alt, E.U.; Senst, C.; Murthy, S.N.; Slakey, D.P.; Dupin, C.L.; Chaffin, A.E.; Kadowitz, P.J.; Izadpanaha, R. Aging alters tissue resident mesenchymal stem cell properties. Stem. Cell Res. 2012, 8, 215–225. [Google Scholar] [CrossRef]
- Wu, W.; Niklason, L.; Steinbacher, D.M. The effect of age on human adipose-derived stem cells. Plast. Reconstr. Surg. 2013, 131, 27–37. [Google Scholar] [CrossRef]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003, 33, 919–926. [Google Scholar] [CrossRef]
- Smith, J.A.; Daniel, R. Stem cells and aging: A chicken-or-the-egg issue? Aging Dis. 2012, 3, 260–268. [Google Scholar]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Ito, M.; Tomokiyo, A.; Matsushita, K.; Nakashima, M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 2006, 24, 2493–2503. [Google Scholar] [CrossRef]
- Murray, P.E.; Stanley, H.R.; Matthews, J.B.; Sloan, A.J.; Smith, A.J. Age-related odontometric changes of human teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 474–482. [Google Scholar] [CrossRef]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Ito, E.; Sougawa, N.; Kawamura, T.; Daimon, T.; Shimizu, T.; et al. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation 2013, 128, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Iwaki, F.; Oki, M.; Aoki, K.; Ohba, S. An osteogenic helioxanthin derivative suppresses the formation of bone-resorbing osteoclasts. Regen. Ther. 2019, 11, 291–296. [Google Scholar] [CrossRef]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.I.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef]
- Wei, F.; Qu, C.; Song, T.; Ding, G.; Fan, Z.; Liu, D.; Zhang, C.; Shi, S.; Wang, S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J. Cell Physiol. 2012, 227, 3216–3224. [Google Scholar] [CrossRef]
- Stanko, P.; Kaiserova, K.; Altanerova, V.; Altaner, C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2014, 158, 373–377. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Alraies, A.; Alaidaroos, N.Y.; Waddington, R.J.; Moseley, R.; Sloan, A.J. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol. 2017, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Takeda-Kawaguchi, T.; Tezuka, Y.; Kunisada, T.; Shibata, T.; Tezuka, K. Hypoxia enhances colony formation and proliferation but inhibits differentiation of human dental pulp cells. Arch. Oral Biol. 2010, 55, 648–654. [Google Scholar] [CrossRef]
- Amir, L.R.; Suniarti, D.F.; Utami, S.; Abbas, B. Chitosan as a potential osteogenic factor compared with dexamethasone in cultured macaque dental pulp stromal cells. Cell Tissue Res. 2014, 358, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, N.; Fan, Y.; Wang, Y.; Gu, Y.; Li, Z.; Pan, Y.; Romila, G.; Zhou, Z.; Yu, J. The conditioned medium of calcined tooth powder promotes the osteogenic and odontogenic differentiation of human dental pulp stem cells via MAPK signaling pathways. Stem Cells Int. 2019, 2019, 4793518. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Pinton, P.; Stellini, E.; Botticelli, D.; Sivolella, S.; Zavan, B. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS ONE 2012, 7, e49146. [Google Scholar] [CrossRef]
- Ohba, S.; Nakajima, K.; Komiyama, Y.; Kugimiya, F.; Igawa, K.; Itaka, K.; Moro, T.; Nakamura, K.; Kawaguchi, H.; Takato, T.; et al. A novel osteogenic helioxanthin-derivative acts in a BMP-dependent manner. Biochem. Biophys. Res. Commun. 2007, 357, 854–860. [Google Scholar] [CrossRef]
- Karsenty, G.; Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S.; Stein, J.L.; Van Wijnen, A.J. Transcriptional control of osteoblast differentiation. Biochem. Soc. Trans. 1998, 26, 14–21. [Google Scholar] [CrossRef]
- Aubin, J.E. Bone stem cells. J. Cell Biochem. Suppl. 1998, 30–31, 73–82. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ito, K.; Nakamura, S.; Ueda, M.; Nagasaka, T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transpl. 2011, 20, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, C.; Geng, T.; Liu, Y.; Zhu, S.; Zhang, C.; Liu, Z.; Wang, P. EphrinB2 overexpression enhances osteogenic differentiation of dental pulp stem cells partially through ephrinB2-mediated reverse signaling. Stem Cell Res. Ther. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Bai, Y.; Zhang, D. Osteogenic stimulation of human dental pulp stem cells with a novel gelatin-hydroxyapatite-tricalcium phosphate scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 1851–1861. [Google Scholar] [CrossRef]
- Juhász, T.; Matta, C.; Katona, É.; Somogyi, C.; Takács, R.; Hajdú, T.; Helgadottir, S.L.; Fodor, J.; Csernoch, L.; Tóth, G. Pituitary adenylate cyclase-activating polypeptide (PACAP) signalling enhances osteogenesis in UMR-106 cell line. J. Mol. Neurosci. 2014, 54, 555–573. [Google Scholar] [CrossRef]
| Number of Collected Cells (×104) | Number of DPSCs at 70 % Confluence (×104) | Percentage of DPSCs (%) | Average Percentage of DPSCs (%) | |
|---|---|---|---|---|
| Young | ||||
| Y1 (18 year) | 1000 | 800 | 80 | 56 |
| Y2 (16 year) | 1000 | 500 | 50 | |
| Y3 (18 year) | 2600 | 600 | 23 | |
| Y4 (17 year) | 670 | 480 | 71 | |
| Elderly | ||||
| E1 (41 year) | 198 | 107 | 54 | 31 |
| E2 (54 year) | 60 | 10 | 16 | |
| E3 (50 year) | 760 | 60 | 7 | |
| E4 (42 year) | 63 | 28 | 44 |
| Gene | Primer Sequences (Forward and Reverse, 5′-3′) | Accession |
|---|---|---|
| GAPDH | GAAGGTGAAGGTCGGAGTCA GAAGATGGTGATGGGATTTC | BC023632 |
| NANOG | AACTGGCCGAAGAATAGCAA TGCACCAGGTCTGAGTGTTC | NM_024865 |
| RUNX2 | CAGACCAGCAGCACTCCATA CAGCGTCAACACCATCATTC | NM_004348 |
| ALP | ATGAAGGAAAAGCCAAGCAG ATGGAGACATTCTCTCGTTC | NM_000478 |
| COLIA1 | GTGCTAAAGGTGCCAATGGT CTCCTCGCTTTCCTTCCTCT | NM_000088 |
| Osteocalcin | GGCAGCGAGGTAGTGAAGAG AGCAGAGCGACACCCTAGAC | NM_199173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, M.; Kawase-Koga, Y.; Yamakawa, D.; Fujii, Y.; Chikazu, D. Bone Regeneration Potential of Human Dental Pulp Stem Cells Derived from Elderly Patients and Osteo-Induced by a Helioxanthin Derivative. Int. J. Mol. Sci. 2020, 21, 7731. https://doi.org/10.3390/ijms21207731
Sato M, Kawase-Koga Y, Yamakawa D, Fujii Y, Chikazu D. Bone Regeneration Potential of Human Dental Pulp Stem Cells Derived from Elderly Patients and Osteo-Induced by a Helioxanthin Derivative. International Journal of Molecular Sciences. 2020; 21(20):7731. https://doi.org/10.3390/ijms21207731
Chicago/Turabian StyleSato, Marika, Yoko Kawase-Koga, Daiki Yamakawa, Yasuyuki Fujii, and Daichi Chikazu. 2020. "Bone Regeneration Potential of Human Dental Pulp Stem Cells Derived from Elderly Patients and Osteo-Induced by a Helioxanthin Derivative" International Journal of Molecular Sciences 21, no. 20: 7731. https://doi.org/10.3390/ijms21207731
APA StyleSato, M., Kawase-Koga, Y., Yamakawa, D., Fujii, Y., & Chikazu, D. (2020). Bone Regeneration Potential of Human Dental Pulp Stem Cells Derived from Elderly Patients and Osteo-Induced by a Helioxanthin Derivative. International Journal of Molecular Sciences, 21(20), 7731. https://doi.org/10.3390/ijms21207731





