Adipokines and Inflammation: Focus on Cardiovascular Diseases
Abstract
1. Introduction
2. Adipose Tissue Dysfunction and CVDs
3. Role of Some Adipokines in Inflammatory Processes Associated with CVDs
3.1. Leptin
3.2. Chemerin
3.3. Resistin
3.4. Oncostatin M
3.5. Adiponectin
3.6. Nesfatin-1
3.7. Relaxin
3.8. Omentin
3.9. Meteorin-Like Hormone
3.10. Fibroblast Growth Factor 21
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | AKT serine/threonine kinase 1 |
| AMI | Acute myocardial infarction |
| ApoE−/− | Apolipoprotein E double knockout |
| ARE | Antioxidant response elements |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CAP1 | Adenylyl cyclase-associated protein 1 |
| CaV1.2 | L-type Ca2+ channel |
| CCRL2 | C-C chemokine receptor-like 2 |
| cGMP | Cyclic guanosine monophosphate |
| CMDs | Cardiometabolic diseases |
| CMKLR1 | Chemokine-like receptor 1 |
| COX2 | Cyclooxygenase-2 |
| CRP | C-reactive protein |
| CVDs | Cardiovascular diseases |
| DCN | Decorin |
| EAT | Epicardial adipose tissue |
| ERK | Extracellular signal-regulated kinase |
| FGF-21 | Fibroblast growth factor-21 |
| eNOS | Endothelial nitric oxide synthase |
| FAS | Factor-associated suicide |
| GPR1 | G protein-coupled receptor 1 |
| GR | Glucocorticoid receptor |
| HbA1c | Glycated haemoglobin |
| HDL | High-density lipoprotein |
| HUVECs | Human umbilical vein endothelial cells |
| I/R | Ischemia/reperfusion |
| ICAM-1 | Intracellular molecular adhesion 1 |
| IFNγ | Interferon γ |
| IGF-1R | Insulin growth factor-1 receptor |
| IL | Interleukin |
| JAK | Janus kinase |
| JNK | C-Jun N-terminal kinases |
| KD | Kawasaki disease |
| LepR | Leptin receptor |
| LIF | Leukaemia inhibitory factor |
| LIFR | Leukaemia inhibitory factor receptor |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| Metrnl | Meteorin-like |
| MI | Myocardial infarction |
| MyD88 | Myeloid differentiation factor 88 |
| NFAT | Nuclear factor of activated T-cells |
| NF-κβ | Nuclear factor |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NO | Nitric oxide |
| Nox | Nicotinamide adenine dinucleotide phosphate oxidase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NUCB2 | Nucleobindin 2 |
| OSM | Oncostatin M |
| OSMR | Oncostatin M receptor |
| oxLDL | Oxidised low-density lipoprotein |
| PBMCs | Peripheral blood mononuclear cells |
| PECAM | Platelet endothelial cell adhesion molecule |
| PI3K | Phosphoinositide 3-kinase |
| PGE2 | Prostaglandin E2 |
| PPARδ | Peroxisome proliferator-activated receptor δ |
| PVAT | Perivascular adipose tissue |
| RARRES2 | Retinoic acid receptor responder 2 |
| ROR-1 | Tyrosine kinase-like orphan receptor 1 |
| ROS | Reactive oxygen species |
| RXFP1 | Relaxin family peptide receptor 1 |
| SAT | Subcutaneous adipose tissue |
| STAT | Signal transducer and activator of transcription protein |
| T2DM | Type 2 diabetes mellitus |
| TGF-β | Transforming growth factor β |
| TIG2 | Tazarotene-induced gene 2 |
| TLR-4 | Toll-like receptor 4 |
| TNFα | Tumour necrosis factor α |
| TXNIP | Thioredoxin-interacting protein |
| VAT | Visceral adipose tissue |
| VCAM-1 | Vascular cell molecular adhesion 1 |
| VEGF | Vascular endothelial growth factor |
| VSMCs | Vascular smooth muscle cells |
| WAT | White adipose tissue |
References
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 22 July 2020).
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.V.; Du, M.; Wang, S.; Bergen, W.G.; Fernyhough-Culver, M.; Basu, U.; Poulos, S.P.; Hausman, G.J. Adipose depots differ in cellularity, adipokines produced, gene expression, and cell systems. Adipocyte 2014, 3, 236–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, W.J.; Song, K.-H. Metabolically healthy obesity: A friend or foe? Kor. J. Intern. Med. 2017, 32, 611–621. [Google Scholar] [CrossRef]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Invest. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Bremer, A.A.; Jialal, I. Adipose tissue dysfunction in nascent metabolic syndrome. J. Obes. 2013, 2013, 393192. [Google Scholar] [CrossRef]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell. Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Hernández-Pardos, A.W.; Frühbeck, G. Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. Curr. Opin. Pharmacol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.-P. Cardiovascular and Metabolic Heterogeneity of Obesity. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential Fat Deposition in Subcutaneous Versus Visceral Depots Is Associated with Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Moreno-Villegas, Z.; González-Bris, A.; Egido, J.; Lorenzo, Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 44. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Chen, J.; Zhao, L. Epicardial adipose tissue and atrial fibrillation: Possible mechanisms, potential therapies, and future directions. Pacing Clin. Electrophysiol. 2020, 43, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Le Jemtel, T.H.; Samson, R.; Ayinapudi, K.; Singh, T.; Oparil, S. Epicardial Adipose Tissue and Cardiovascular Disease. Curr. Hypertens. Rep. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, L.; Liang, H.; Zhang, C.; Guan, L.; Li, M. A new measurement site for echocardiographic epicardial adipose tissue thickness and its value in predicting metabolic syndrome. Adv. Clin. Exp. Med. 2019, 28, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Keresztesi, A.A.; Asofie, G.; Simion, M.A.; Jung, H. Correlation between epicardial adipose tissue thickness and the degree of coronary artery atherosclerosis. Turk. J. Med. Sci. 2018, 48, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef]
- Erkan, A.F.; Tanindi, A.; Kocaman, S.A.; Ugurlu, M.; Tore, H.F. Epicardial Adipose Tissue Thickness Is an Independent Predictor of Critical and Complex Coronary Artery Disease by Gensini and Syntax Scores. Texas Hear. Inst. J. 2016, 43, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lima-Martínez, M.M.; Colmenares, L.; Campanelli, Y.; Paoli, M.; Rodney, M.; Santos, R.D.; Iacobellis, G. Epicardial adipose tissue thickness and type 2 diabetes risk according to the FINDRISC modified for Latin America. Clín. Investig. Arterioscler. 2019, 31, 15–22. [Google Scholar] [CrossRef]
- Hu, H.; Garcia-Barrio, M.; Jiang, Z.; Chen, Y.E.; Chang, L. Roles of Perivascular Adipose Tissue in Hypertension and Atherosclerosis. Antioxid. Redox Signal. 2020, ars.2020.8103. [Google Scholar] [CrossRef] [PubMed]
- Quesada, I.; Cejas, J.; García, R.; Cannizzo, B.; Redondo, A.; Castro, C. Vascular dysfunction elicited by a cross talk between periaortic adipose tissue and the vascular wall is reversed by pioglitazone. Cardiovasc. Ther. 2018, 36, e12322. [Google Scholar] [CrossRef]
- Qi, X.-Y.; Qu, S.-L.; Xiong, W.-H.; Rom, O.; Chang, L.; Jiang, Z.-S. Perivascular adipose tissue (PVAT) in atherosclerosis: A double-edged sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar] [CrossRef]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef]
- Anthony, S.R.; Guarnieri, A.R.; Gozdiff, A.; Helsley, R.N.; Phillip Owens, A.; Tranter, M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin. Sci. 2019, 133, 2329–2344. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, S.; Kim, H.W.; Weintraub, N.L. Potential role of perivascular adipose tissue in modulating atherosclerosis. Clin. Sci. 2020, 134, 3–13. [Google Scholar] [CrossRef]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- DeVallance, E.; Branyan, K.W.; Lemaster, K.C.; Anderson, R.; Marshall, K.L.; Olfert, I.M.; Smith, D.M.; Kelley, E.E.; Bryner, R.W.; Frisbee, J.C.; et al. Exercise training prevents the perivascular adipose tissue-induced aortic dysfunction with metabolic syndrome. Redox Biol. 2019, 26, 101285. [Google Scholar] [CrossRef]
- Han, F.; Hou, N.; Liu, Y.; Huang, N.; Pan, R.; Zhang, X.; Mao, E.; Sun, X. Liraglutide improves vascular dysfunction by regulating a cAMP-independent PKA-AMPK pathway in perivascular adipose tissue in obese mice. Biomed. Pharmacother. 2019, 120, 109537. [Google Scholar] [CrossRef]
- Dong, M.; Ren, J. What fans the fire: Insights into mechanisms of leptin in metabolic syndrome-associated heart diseases. Curr. Pharm. Des. 2014, 20, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin Resistance. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef]
- Lee, M.-W.; Lee, M.; Oh, K.-J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef] [PubMed]
- Gainsford, T.; Willson, T.A.; Metcalf, D.; Handman, E.; McFarlane, C.; Ng, A.; Nicola, N.A.; Alexander, W.S.; Hilton, D.J. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 14564–14568. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Portolés, M.; Roselló-Lletí, E.; Rivera, M.; González-Juanatey, J.R.R.; Lago, F. 20 years of leptin: Role of leptin in cardiomyocyte physiology and physiopathology. Life Sci. 2015, 140, 10–18. [Google Scholar] [CrossRef] [PubMed]
- An, H.S.; Lee, J.Y.; Choi, E.B.; Jeong, E.A.; Shin, H.J.; Kim, K.E.; Park, K.-A.; Jin, Z.; Lee, J.E.; Koh, J.S.; et al. Caloric restriction reverses left ventricular hypertrophy through the regulation of cardiac iron homeostasis in impaired leptin signaling mice. Sci. Rep. 2020, 10, 7176. [Google Scholar] [CrossRef]
- Ren, J. Leptin and hyperleptinemia—From friend to foe for cardiovascular function. J. Endocrinol. 2004, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soderberg, S.; Ahren, B.; Jansson, J.-H.; Johnson, O.; Hallmans, G.; Asplund, K.; Olsson, T. Leptin is associated with increased risk of myocardial infarction. J. Intern. Med. 1999, 246, 409–418. [Google Scholar] [CrossRef]
- Kain, D.; Simon, A.J.; Greenberg, A.; Ben Zvi, D.; Gilburd, B.; Schneiderman, J. Cardiac leptin overexpression in the context of acute MI and reperfusion potentiates myocardial remodeling and left ventricular dysfunction. PLoS ONE 2018, 13, e0203902. [Google Scholar] [CrossRef]
- Demarchi, A.; Mazzucchelli, I.; Somaschini, A.; Cornara, S.; Dusi, V.; Mirizzi, A.M.; Ruffinazzi, M.; Crimi, G.; Ferlini, M.; Gnecchi, M.; et al. Leptin affects the inflammatory response after STEMI. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Ekmen, N.; Helvaci, A.; Gunaldi, M.; Sasani, H.; Yildirmak, S.T. Leptin as an important link between obesity and cardiovascular risk factors in men with acute myocardial infarction. Ind. Heart J. 2016, 68, 132–137. [Google Scholar] [CrossRef]
- Khafaji, H.A.R.H.; Bener, A.B.; Rizk, N.M.; Al Suwaidi, J. Elevated serum leptin levels in patients with acute myocardial infarction; correlation with coronary angiographic and echocardiographic findings. BMC Res. Notes 2012, 5, 262. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, P.; Li, T.; Gao, J.; Zhang, Y. Leptin Expression in Human Epicardial Adipose Tissue Is Associated with Local Coronary Atherosclerosis. Med. Sci. Monit. 2019, 25, 9913–9922. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Borodkina, D.; Akbasheva, O.; Antonova, L.; Matveeva, V.; Belik, E.; Ivanov, S.; Sotnikov, A.; et al. Adipocytes Directly Affect Coronary Artery Disease Pathogenesis via Induction of Adipokine and Cytokine Imbalances. Front. Immunol. 2019, 10, 2163. [Google Scholar] [CrossRef]
- Drosos, I.; Chalikias, G.; Pavlaki, M.; Kareli, D.; Epitropou, G.; Bougioukas, G.; Mikroulis, D.; Konstantinou, F.; Giatromanolaki, A.; Ritis, K.; et al. Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery: Possible role for the leptin-inflammation-fibrosis-hypoxia axis. Clin. Res. Cardiol. 2016, 105, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hang, T.; Cheng, X.-M.; Li, D.-M.; Zhang, Q.-G.; Wang, L.-J.; Peng, Y.-P.; Gong, J.-B. Associations of C1q/TNF-Related Protein-9 Levels in Serum and Epicardial Adipose Tissue with Coronary Atherosclerosis in Humans. Biomed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, V.-P.; Kiviniemi, A.; Lepojärvi, S.; Piira, O.-P.; Hedberg, P.; Junttila, J.; Ukkola, O.; Huikuri, H. Leptin predicts short-term major adverse cardiac events in patients with coronary artery disease. Ann. Med. 2017, 49, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, V.P.; Lepojärvi, E.S.; Piira, O.P.; Hedberg, P.; Junttila, M.J.; Ukkola, O.; Huikuri, H.V. High plasma leptin levels are associated with impaired diastolic function in patients with coronary artery disease. Peptides 2016, 84, 17–21. [Google Scholar] [CrossRef]
- Farcaş, A.D.; Rusu, A.; Stoia, M.A.; Vida-Simiti, L.A. Plasma leptin, but not resistin, TNF-α and adiponectin, is associated with echocardiographic parameters of cardiac remodeling in patients with coronary artery disease. Cytokine 2018, 103, 46–49. [Google Scholar] [CrossRef]
- Chen, M.-C.; Wang, J.-H.; Lee, C.-J.; Hsu, B.-G. Association between hyperleptinemia and cardiovascular outcomes in patients with coronary artery disease. Ther. Clin. Risk Manag. 2018, 14, 1855–1862. [Google Scholar] [CrossRef]
- Du, Y.; Yang, S.-H.; Li, S.; Zhao, X.; Zhang, Y.; Sun, D.; Zhu, C.-G.; Wu, N.-Q.; Guo, Y.-L.; Xu, R.-X.; et al. Increased Serum Leptin Levels in New-Onset, Untreated Female Patients with Coronary Artery Disease and Positively Associated with Inflammatory Markers. Ann. Nutr. Metab. 2018, 72, 142–148. [Google Scholar] [CrossRef]
- Bickel, C.; Schnabel, R.B.; Zeller, T.; Lackner, K.J.; Rupprecht, H.J.; Blankenberg, S.; Sinning, C.; Westermann, D. Predictors of leptin concentration and association with cardiovascular risk in patients with coronary artery disease: Results from the Athero Gene study. Biomarkers 2017, 22, 210–218. [Google Scholar] [CrossRef]
- Knudson, J.D.; Dincer, Ü.D.; Zhang, C.; Swafford, A.N.; Koshida, R.; Picchi, A.; Focardi, M.; Dick, G.M.; Tune, J.D. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am. J. Physiol. Circ. Physiol. 2005, 289, H48–H56. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qiao, J.; Hu, J.; Fan, M.; Zhao, Y.; Su, H.; Wang, Z.; Yu, Q.; Ma, Q.; Li, Y.; et al. Leptin promotes endothelial dysfunction in chronic kidney disease by modulating the MTA1-mediated WNT/β-catenin pathway. Mol. Cell. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Korda, M.; Kubant, R.; Patton, S.; Malinski, T. Leptin-induced endothelial dysfunction in obesity. Am. J. Physiol. Circ. Physiol. 2008, 295, H1514–H1521. [Google Scholar] [CrossRef]
- De Rosa, S.; Cirillo, P.; Pacileo, M.; Di Palma, V.; Paglia, A.; Chiariello, M. Leptin stimulated C-reactive protein production by human coronary artery endothelial cells. J. Vasc. Res. 2009, 46, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hoffmann, M.; Wolk, R.; Shamsuzzaman, A.S.M.; Somers, V.K. Leptin induces C-reactive protein expression in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, e302–e307. [Google Scholar] [CrossRef]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar] [CrossRef]
- Cirillo, P.; Angri, V.; De Rosa, S.; Calì, G.; Petrillo, G.; Maresca, F.; D’Ascoli, G.-L.; Maietta, P.; Brevetti, L.; Chiariello, M. Pro-atherothrombotic effects of leptin in human coronary endothelial cells. Thromb. Haemost. 2010, 103, 1065–1075. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-Y.; Leung, L.L.K. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim. Biophys. Sin. 2009, 41, 973–979. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.-D.; Vulcano, M.; Mirjolet, J.-F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.C.; Detheux, M.; et al. Specific Recruitment of Antigen-presenting Cells by Chemerin, a Novel Processed Ligand from Human Inflammatory Fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Nakae, S.; Zúñiga, L.; Kim, J.-Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J.; et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 2008, 205, 2207–2220. [Google Scholar] [CrossRef]
- Monnier, J.; Lewén, S.; O’Hara, E.; Huang, K.; Tu, H.; Butcher, E.C.; Zabel, B.A. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J. Immunol. 2012, 189. [Google Scholar] [CrossRef] [PubMed]
- Monnier, J.; Zabel, B.; Butcher, E. Regulation of the Atypical Chemerin Receptor, CCRL2, on Activated Brain Endothelial Cells. Clin. Immunol. 2010, 135, S63. [Google Scholar] [CrossRef]
- De Henau, O.; Degroot, G.-N.; Imbault, V.; Robert, V.; De Poorter, C.; Mcheik, S.; Galés, C.; Parmentier, M.; Springael, J.-Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Davenport, A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin 1) and GPR1 (Chemerin 2) Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2018, 70, 174–196. [Google Scholar] [CrossRef]
- Nagpal, S.; Patel, S.; Jacobe, H.; DiSepio, D.; Ghosn, C.; Malhotra, M.; Teng, M.; Duvic, M.; Chandraratna, R.A. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Invest. Dermatol. 1997, 109, 91–95. [Google Scholar] [CrossRef]
- Özcan, E.; Saygun, N.I.; Ilıkçı, R.; Karslıoğlu, Y.; Muşabak, U.; Yeşillik, S. Evaluation of chemerin and its receptors, ChemR23 and CCRL2, in gingival tissues with healthy and periodontitis. Odontology 2018, 106. [Google Scholar] [CrossRef]
- Chamberland, J.P.; Berman, R.L.; Aronis, K.N.; Mantzoros, C.S. Chemerin is expressed mainly in pancreas and liver, is regulated by energy deprivation, and lacks day/night variation in humans. Eur. J. Endocrinol. 2013, 169, 453–462. [Google Scholar] [CrossRef]
- Kostopoulos, C.G.; Spiroglou, S.G.; Varakis, J.N.; Apostolakis, E.; Papadaki, H.H. Chemerin and CMKLR1 expression in human arteries and periadventitial fat: A possible role for local chemerin in atherosclerosis? BMC Cardiovasc. Disord. 2014, 14, 56. [Google Scholar] [CrossRef]
- Berg, V.; Sveinbjörnsson, B.; Bendiksen, S.; Brox, J.; Meknas, K.; Figenschau, Y. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21-157). Arthritis Res. Ther. 2010, 12, R228. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Rame, C.; Cornuau, M.; Guérif, F.; Froment, P.; Dupont, J. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int. J. Mol. Sci. 2019, 20, 3778. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yoshizawa, H.; Seki, T.; Shirai, R.; Yamashita, T.; Okano, T.; Shibata, K.; Wakamatsu, M.J.; Mori, Y.; Morita, T.; et al. Chemerin-9, a potent agonist of chemerin receptor (ChemR23), prevents atherogenesis. Clin. Sci. 2019, 133, 1779–1796. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M.; Chishti, M.A.; Moustafa, A.S.; Fatma, S.; Alomaim, W.S.; Al-Naami, M.Y.; Bassas, A.F.; Chrousos, G.P.; Jo, H. Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: Adipose depot specificity and gender dimorphism. Mol. Cells 2012, 33, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Montes-Nieto, R.; Fernández-Durán, E.; Insenser, M.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Evidence for Masculinization of Adipokine Gene Expression in Visceral and Subcutaneous Adipose Tissue of Obese Women With Polycystic Ovary Syndrome (PCOS). J. Clin. Endocrinol. Metab. 2013, 98, E388–E396. [Google Scholar] [CrossRef] [PubMed]
- Zylla, S.; Pietzner, M.; Kühn, J.-P.; Völzke, H.; Dörr, M.; Nauck, M.; Friedrich, N. Serum chemerin is associated with inflammatory and metabolic parameters-results of a population-based study. Obesity 2017, 25, 468–475. [Google Scholar] [CrossRef]
- Gao, X.; Mi, S.; Zhang, F.; Gong, F.; Lai, Y.; Gao, F.; Zhang, X.; Wang, L.; Tao, H. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc. Diabetol. 2011, 10, 87. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Chen, S.; Wu, X.; Nong, W. Correlation between adiponectin, chemerin, vascular endothelial growth factor and epicardial fat volume in patients with coronary artery disease. Exp. Ther. Med. 2019, 19, 1095–1102. [Google Scholar] [CrossRef]
- Goralski, K.B.; Sinal, C.J. Elucidation of chemerin and chemokine-like receptor-1 function in adipocytes by adenoviral-mediated shRNA knockdown of gene expression. Methods Enzymol. 2009, 460. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, Y.; Takahashi, K.; Zolotaryov, F.N.; Hong, K.S.; Kitazawa, R.; Iida, K.; Okimura, Y.; Kaji, H.; Kitazawa, S.; et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008, 582, 573–578. [Google Scholar] [CrossRef]
- Kralisch, S.; Weise, S.; Sommer, G.; Lipfert, J.; Lossner, U.; Bluher, M.; Stumvoll, M.; Fasshauer, M. Interleukin-1ß induces the novel adipokine chemerin in adipocytes in vitro. Regul. Pept. 2009, 154, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Laurencikiene, J.; Taube, A.; Eckardt, K.; Cramer, A.; Horrighs, A.; Arner, P.; Eckel, J. Chemerin is a Novel Adipocyte-Derived Factor Inducing Insulin Resistance in Primary Human Skeletal Muscle Cells. Diabetes 2009, 58, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, S.; Guo, B.; Chang, L.; Li, Y. Chemerin Induces Insulin Resistance in Rat Cardiomyocytes in Part through the ERK1/2 Signaling Pathway. Pharmacology 2014, 94, 259–264. [Google Scholar] [CrossRef]
- Ernst, M.C.; Issa, M.; Goralski, K.B.; Sinal, C.J. Chemerin Exacerbates Glucose Intolerance in Mouse Models of Obesity and Diabetes. Endocrinology 2010, 151, 1998–2007. [Google Scholar] [CrossRef]
- Takahashi, M.; Okimura, Y.; Iguchi, G.; Nishizawa, H.; Yamamoto, M.; Suda, K.; Kitazawa, R.; Fujimoto, W.; Takahashi, K.; Zolotaryov, F.N.; et al. Chemerin regulates β-cell function in mice. Sci. Rep. 2011, 1, 123. [Google Scholar] [CrossRef]
- Ernst, M.C.; Haidl, I.D.; Zúñiga, L.A.; Dranse, H.J.; Rourke, J.L.; Zabel, B.A.; Butcher, E.C.; Sinal, C.J. Disruption of the Chemokine-Like Receptor-1 (CMKLR1) Gene Is Associated with Reduced Adiposity and Glucose Intolerance. Endocrinology 2012, 153, 672–682. [Google Scholar] [CrossRef]
- El-Deeb, T.S.; Bakkar, S.M.; Eltoony, L.; Zakhary, M.M.; Kamel, A.A.; Nafee, A.M.; Hetta, H.F. The adipokine Chemerin and Fetuin-A Serum Levels in Type 2 Diabetes Mellitus: Relation to Obesity and Inflammatory Markers. Egypt. J. Immunol. 2018, 25, 191–202. [Google Scholar]
- Ba, H.-J.; Xu, L.-L.; Qin, Y.-Z.; Chen, H.-S. Serum Chemerin Levels Correlate with Determinants of Metabolic Syndrome in Obese Children and Adolescents. Clin. Med. Insights. Pediatr. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, X.; Xu, J.; Yang, B.; Yu, J.; Gong, Q.; Zhang, X.; Sun, X.; Zhang, Q.; Xia, J.; et al. Association of serum chemerin and inflammatory factors with type 2 diabetes macroangiopathy and waist-to-stature ratio. Bosn. J. Basic Med. Sci. 2019, 19, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Skuratovskaia, D.; Zatolokin, P.; Vulf, M.; Mazunin, I.; Litvinova, L. Interrelation of chemerin and TNF-α with mtDNA copy number in adipose tissues and blood cells in obese patients with and without type 2 diabetes. BMC Med. Genomics 2019, 12, 40. [Google Scholar] [CrossRef]
- Liu, M.; Lin, X.; Wang, X. Decrease in serum chemerin through aerobic exercise plus dieting and its association with mitigation of cardio-metabolic risk in obese female adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 127–135. [Google Scholar] [CrossRef]
- Chakaroun, R.; Raschpichler, M.; Klöting, N.; Oberbach, A.; Flehmig, G.; Kern, M.; Schön, M.R.; Shang, E.; Lohmann, T.; Dreßler, M.; et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 2012, 61, 706–714. [Google Scholar] [CrossRef]
- Sell, H.; Divoux, A.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Bedossa, P.; Tordjman, J.; Eckel, J.; Clément, K. Chemerin Correlates with Markers for Fatty Liver in Morbidly Obese Patients and Strongly Decreases after Weight Loss Induced by Bariatric Surgery. J. Clin. Endocrinol. Metab. 2010, 95, 2892–2896. [Google Scholar] [CrossRef]
- Kolahdouzi, S.; Baghadam, M.; Kani-Golzar, F.A.; Saeidi, A.; Jabbour, G.; Ayadi, A.; De Sousa, M.; Zouita, A.; Abderrahmane, A.B.; Zouhal, H. Progressive circuit resistance training improves inflammatory biomarkers and insulin resistance in obese men. Physiol. Behav. 2019, 205, 15–21. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: Tumor necrosis factor-α stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg. Obes. Relat. Dis. 2013, 9, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.A.; Parlee, S.D.; Sinal, C.J. Chemerin: A potential endocrine link between obesity and type 2 diabetes. Endocrine 2012, 42, 243–251. [Google Scholar] [CrossRef]
- Ghosh, A.R.; Bhattacharya, R.; Bhattacharya, S.; Nargis, T.; Rahaman, O.; Duttagupta, P.; Raychaudhuri, D.; Liu, C.S.C.; Roy, S.; Ghosh, P.; et al. Adipose Recruitment and Activation of Plasmacytoid Dendritic Cells Fuel Metaflammation. Diabetes 2016, 65, 3440–3452. [Google Scholar] [CrossRef]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.C.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.L.; Greaves, D.R. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 2012, 423, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira, S.; Regan-Komito, D.; Iqbal, A.J.; Greaves, D.R.; Payne, S.J.; Orlowski, P. A model for the optimization of anti-inflammatory treatment with chemerin. Interface Focus 2018, 8, 20170007. [Google Scholar] [CrossRef]
- Valcamonica, E.; Chighizola, C.B.; Comi, D.; De Lucia, O.; Pisoni, L.; Murgo, A.; Salvi, V.; Sozzani, S.; Meroni, P.L. Levels of chemerin and interleukin 8 in the synovial fluid of patients with inflammatory arthritides and osteoarthritis. Clin. Exp. Rheumatol. 2014, 32, 243–250. [Google Scholar] [PubMed]
- Patnaik, K.; Pradeep, A.R.; Nagpal, K.; Karvekar, S.; Singh, P.; Raju, A. Human chemerin correlation in gingival crevicular fluid and tear fluid as markers of inflammation in chronic periodontitis and type-2 diabetes mellitus. J. Investig. Clin. Dent. 2017, 8, e12181. [Google Scholar] [CrossRef]
- Calvet, J.; Orellana, C.; Gratacós, J.; Berenguer-Llergo, A.; Caixàs, A.; Chillarón, J.J.; Pedro-Botet, J.; García-Manrique, M.; Navarro, N.; Larrosa, M. Synovial fluid adipokines are associated with clinical severity in knee osteoarthritis: A cross-sectional study in female patients with joint effusion. Arthritis Res. Ther. 2016, 18, 207. [Google Scholar] [CrossRef]
- Huang, K.; Du, G.; Li, L.; Liang, H.; Zhang, B. Association of chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers 2012, 17, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Ohyama, T.; Zuniga, L.; Kim, J.-Y.; Johnston, B.; Allen, S.J.; Guido, D.G.; Handel, T.M.; Butcher, E.C. Chemokine-like receptor 1 expression by macrophages in vivo: Regulation by TGF-β and TLR ligands. Exp. Hematol. 2006, 34, 1106–1114. [Google Scholar] [CrossRef]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007, 109, 3625–3632. [Google Scholar] [CrossRef]
- Zabel, B.A.; Silverio, A.M.; Butcher, E.C. Chemokine-Like Receptor 1 Expression and Chemerin-Directed Chemotaxis Distinguish Plasmacytoid from Myeloid Dendritic Cells in Human Blood. J. Immunol. 2005, 174, 244–251. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Greaves, D.R. Chemerin Contributes to Inflammation by Promoting Macrophage Adhesion to VCAM-1 and Fibronectin through Clustering of VLA-4 and VLA-5. J. Immunol. 2010, 185, 3728–3739. [Google Scholar] [CrossRef]
- Lehrke, M.; Becker, A.; Greif, M.; Stark, R.; Laubender, R.P.; von Ziegler, F.; Lebherz, C.; Tittus, J.; Reiser, M.; Becker, C.; et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur. J. Endocrinol. 2009, 161, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Jiang, W.; Lu, B.; Shi, Z. Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin. Exp. Hypertens. 2014, 36, 326–332. [Google Scholar] [CrossRef]
- Sawicka, K.; Michalska-Jakubus, M.; Potembska, E.; Kowal, M.; Pietrzak, A.; Krasowska, D. Visfatin and chemerin levels correspond with inflammation and might reflect the bridge between metabolism, inflammation and fibrosis in patients with systemic sclerosis. Adv. Dermatol. Allergol. 2019, 36, 551–565. [Google Scholar] [CrossRef]
- Kammerer, A.; Staab, H.; Herberg, M.; Kerner, C.; Klöting, N.; Aust, G. Increased circulating chemerin in patients with advanced carotid stenosis. BMC Cardiovasc. Disord. 2018, 18, 65. [Google Scholar] [CrossRef]
- Weigert, J.; Obermeier, F.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Aslanidis, C.; Rogler, G.; Ott, C.; Schäffler, A.; et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Xiaotao, L.; Xiaoxia, Z.; Yue, X.; Liye, W. Serum chemerin levels are associated with the presence and extent of coronary artery disease. Coron. Artery Dis. 2012, 23, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Ji, W.; Zhang, Y. Elevated Serum Chemerin Levels are Associated with the Presence of Coronary Artery Disease in Patients with Metabolic Syndrome. Intern. Med. 2011, 50, 1093–1097. [Google Scholar] [CrossRef]
- Aksan, G.; İnci, S.; Nar, G.; Soylu, K.; Gedikli, Ö.; Yüksel, S.; Özdemir, M.; Nar, R.; Meriç, M.; Şahin, M. Association of serum chemerin levels with the severity of coronary artery disease in patients with metabolic syndrome. Int. J. Clin. Exp. Med. 2014, 7, 5461–5468. [Google Scholar] [PubMed]
- Motawi, T.M.K.; Mahdy, S.G.; El-Sawalhi, M.M.; Ali, E.N.; El-Telbany, R.F.A. Serum levels of chemerin, apelin, vaspin, and omentin-1 in obese type 2 diabetic Egyptian patients with coronary artery stenosis. Can. J. Physiol. Pharmacol. 2018, 96, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Kojok, K.; El-Kadiry, A.E.-H.; Merhi, Y. Role of NF-κB in Platelet Function. Int. J. Mol. Sci. 2019, 20, 4185. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.K.; Kaur, J.; Adya, R.; Miras, A.D.; Mattu, H.S.; Hattersley, J.G.; Kaltsas, G.; Tan, B.K.; Randeva, H.S. Chemerin induces endothelial cell inflammation: Activation of nuclear factor-kappa beta and monocyte-endothelial adhesion. Oncotarget 2018, 9, 16678–16690. [Google Scholar] [CrossRef]
- Neves, K.B.; Nguyen Dinh Cat, A.; Lopes, R.A.M.; Rios, F.J.; Anagnostopoulou, A.; Lobato, N.S.; de Oliveira, A.M.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Chemerin Regulates Crosstalk Between Adipocytes and Vascular Cells Through Nox. Hypertension 2015, 66, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wang, L.; Zhang, Y.; Zhang, S.; Ning, L.; Zhao, J.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. Chemerin/ChemR23 axis promotes inflammation of glomerular endothelial cells in diabetic nephropathy. J. Cell. Mol. Med. 2019, 23, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, W.; Luo, Y.; Chen, H.; He, Y.; Cao, Y.; Dong, S. Adipokine Chemerin Stimulates Progression of Atherosclerosis in ApoE −/− Mice. Biomed Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Er, L.; Hsu, L.-A.; Juang, J.-M.; Chiang, F.-T.; Teng, M.-S.; Tzeng, I.-S.; Wu, S.; Lin, J.-F.; Ko, Y.-L. Circulating Chemerin Levels, but not the RARRES2 Polymorphisms, Predict the Long-Term Outcome of Angiographically Confirmed Coronary Artery Disease. Int. J. Mol. Sci. 2019, 20, 1174. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, M.; Zhang, L.; Zhao, Y.; Yang, Q. Association of serum chemerin concentrations with the presence of atrial fibrillation. Ann. Clin. Biochem. 2017, 54, 342–347. [Google Scholar] [CrossRef]
- Zhao, D.; Bi, G.; Feng, J.; Huang, R.; Chen, X. Association of Serum Chemerin Levels with Acute Ischemic Stroke and Carotid Artery Atherosclerosis in a Chinese Population. Med. Sci. Monit. 2015, 21, 3121–3128. [Google Scholar] [CrossRef]
- Gu, P.; Cheng, M.; Hui, X.; Lu, B.; Jiang, W.; Shi, Z. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J. Hypertens. 2015, 33, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Canpolat, U.; Akin, S.; Dural, M.; Karakaya, J.; Aytemir, K.; Ozer, N.; Gurlek, A. Chemerin is not associated with subclinical atherosclerosis markers in prediabetes and diabetes. Anatol. J. Cardiol. 2015, 16, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Penas, D.; Feijóo-Bandín, S.; García-Rúa, V.; Mosquera-Leal, A.; Durán, D.; Varela, A.; Portolés, M.; Roselló-Lletí, E.; Rivera, M.; Diéguez, C.; et al. The adipokine chemerin induces apoptosis in cardiomyocytes. Cell. Physiol. Biochem. 2015, 37, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Kutlay, Ö.; Kaygısız, Z.; Kaygısız, B. The Effect of Chemerin on Cardiac Parameters and Gene Expressions in Isolated Perfused Rat Heart. Balkan Med. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hanthazi, A.; Jespers, P.; Vegh, G.; Degroot, G.-N.; Springael, J.-Y.; Lybaert, P.; Dewachter, L.; Mc Entee, K. Chemerin influences endothelin- and serotonin-induced pulmonary artery vasoconstriction in rats. Life Sci. 2019, 231, 116580. [Google Scholar] [CrossRef] [PubMed]
- Ferland, D.J.; Darios, E.S.; Neubig, R.R.; Sjögren, B.; Truong, N.; Torres, R.; Dexheimer, T.S.; Thompson, J.M.; Watts, S.W. Chemerin-induced arterial contraction is Gi- and calcium-dependent. Vascul. Pharmacol. 2017, 88, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Darios, E.S.; Winner, B.M.; Charvat, T.; Krasinksi, A.; Punna, S.; Watts, S.W. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am. J. Physiol. Circ. Physiol. 2016, 311, H498–H507. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Yang, P.; Read, C.; Kuc, R.E.; Yang, L.; Taylor, E.J.A.; Taylor, C.W.; Maguire, J.J.; Davenport, A.P. Chemerin Elicits Potent Constrictor Actions via Chemokine-Like Receptor 1 (CMKLR1), not G-Protein-Coupled Receptor 1 (GPR1), in Human and Rat Vasculature. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Lobato, N.S.; Neves, K.B.; Filgueira, F.P.; Fortes, Z.B.; Carvalho, M.H.C.; Webb, R.C.; Oliveira, A.M.; Tostes, R.C. The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway. Life Sci. 2012, 91, 600–606. [Google Scholar] [CrossRef]
- Kunimoto, H.; Kazama, K.; Takai, M.; Oda, M.; Okada, M.; Yamawaki, H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Circ. Physiol. 2015, 309, H1017–H1028. [Google Scholar] [CrossRef]
- Weng, C.; Shen, Z.; Li, X.; Jiang, W.; Peng, L.; Yuan, H.; Yang, K.; Wang, J. Effects of chemerin/CMKLR1 in obesity-induced hypertension and potential mechanism. Am. J. Transl. Res. 2017, 9, 3096–3104. [Google Scholar]
- Ferland, D.J.; Flood, E.D.; Garver, H.; Yeh, S.T.; Riney, S.; Mullick, A.E.; Fink, G.D.; Watts, S.W. Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high-salt conditions. Physiol. Genomics 2019, 51, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, G.; Dong, J.; Liu, Y.; Zong, H.; Liu, H.; Boden, G.; Li, L. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J. Investig. Med. 2010, 58, 883–886. [Google Scholar] [CrossRef]
- Meric, M.; Soylu, K.; Avci, B.; Yuksel, S.; Gulel, O.; Yenercag, M.; Coksevim, M.; Uzun, A. Evaluation of plasma chemerin levels in patients with non-dipper blood pressure patterns. Med. Sci. Monit. 2014, 20, 698–705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watts, S.W.; Dorrance, A.M.; Penfold, M.E.; Rourke, J.L.; Sinal, C.J.; Seitz, B.; Sullivan, T.J.; Charvat, T.T.; Thompson, J.M.; Burnett, R.; et al. Chemerin connects fat to arterial contraction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1320. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Brown, E.J.; Wright, C.M.; Bhat, S.; Banerjee, R.R.; Dai, C.Y.; Enders, G.H.; Silberg, D.G.; Wen, X.; Wu, G.D.; et al. A family of tissue-specific resistin-like molecules. Proc. Natl. Acad. Sci. USA 2001, 98, 502–506. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Moon, B.; Kwan, J.J.-M.; Duddy, N.; Sweeney, G.; Begum, N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am. J. Physiol. Metab. 2003, 285, E106–E115. [Google Scholar] [CrossRef]
- Rajala, M.W.; Obici, S.; Scherer, P.E.; Rossetti, L. Adipose-derived resistin and gut-derived resistin-like molecule–β selectively impair insulin action on glucose production. J. Clin. Invest. 2003, 111, 225–230. [Google Scholar] [CrossRef]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an Adipokine with Potent Proinflammatory Properties. J. Immunol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Reilly, M.P.; Millington, S.C.; Iqbal, N.; Rader, D.J.; Lazar, M.A. An Inflammatory Cascade Leading to Hyperresistinemia in Humans. PLoS Med. 2004, 1, e45. [Google Scholar] [CrossRef] [PubMed]
- Plutzky, J. Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am. J. Cardiol. 2001, 88, 10–15. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, J.; Lü, J.-M.; Chai, H.; Wang, X.; Lin, P.H.; Yao, Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am. J. Physiol. Circ. Physiol. 2010, 299, H193–H201. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Park, K.H.; Cho, Y.M.; Chung, S.S.; Cho, H.J.; Cho, S.Y.; Kim, S.J.; Kim, S.Y.; Lee, H.K.; Park, K.S. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc. Res. 2006, 69, 76–85. [Google Scholar] [CrossRef]
- Langheim, S.; Dreas, L.; Veschini, L.; Maisano, F.; Foglieni, C.; Ferrarello, S.; Sinagra, G.; Zingone, B.; Alfieri, O.; Ferrero, E.; et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am. J. Physiol. Circ. Physiol. 2010, 298, H746–H753. [Google Scholar] [CrossRef]
- Kawanami, D.; Maemura, K.; Takeda, N.; Harada, T.; Nojiri, T.; Imai, Y.; Manabe, I.; Utsunomiya, K.; Nagai, R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: A new insight into adipocytokine–endothelial cell interactions. Biochem. Biophys. Res. Commun. 2004, 314, 415–419. [Google Scholar] [CrossRef]
- Verma, S.; Li, S.-H.; Wang, C.-H.; Fedak, P.W.M.; Li, R.-K.; Weisel, R.D.; Mickle, D.A.G. Resistin Promotes Endothelial Cell Activation. Circulation 2003, 108, 736–740. [Google Scholar] [CrossRef]
- Luo, J.; Huang, L.; Wang, A.; Liu, Y.; Cai, R.; Li, W.; Zhou, M.-S. Resistin-Induced Endoplasmic Reticulum Stress Contributes to the Impairment of Insulin Signaling in Endothelium. Front. Pharmacol. 2018, 9, 1226. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391–405. [Google Scholar] [CrossRef]
- Liu, R.; He, B.; Gao, F.; Liu, Q.; Yi, Q. Relationship between adipokines and coronary artery aneurysm in children with Kawasaki disease. Transl. Res. 2012, 160, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Si, F.; Feng, S.; Yi, Q.; Liu, R. Resistin Enhances Inflammatory Cytokine Production in Coronary Artery Tissues by Activating the NF- κ B Signaling. Biomed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-Y.; Hsiao, Y.-W.; Guo, S.-M.; Chang, S.-L.; Lin, Y.-J.; Lo, L.-W.; Hu, Y.-F.; Chung, F.-P.; Chao, T.-F.; Liao, J.-N.; et al. Resistin as a Biomarker for the Prediction of Left Atrial Substrate and Recurrence in Patients with Drug-Refractory Atrial Fibrillation Undergoing Catheter Ablation. Int. Heart J. 2020, 61, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Zarling, J.M.; Shoyab, M.; Marquardt, H.; Hanson, M.B.; Lioubin, M.N.; Todaro, G.J. Oncostatin M: A growth regulator produced by differentiated histiocytic lymphoma cells. Proc. Natl. Acad. Sci. USA 1986, 83, 9739–9743. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, M.; Hurşitoğlu, M.; Toprak, Z.; Yoldemir, Ş.A.; Altun, Ö.; Toprak, I.D.; Özcan, M.; Yürüyen, G.; Uğurlukişi, B.; Erdem, M.G.; et al. Relationships among oncostatin M, insulin resistance, and chronic inflammation: A pilot study. Arch. Endocrinol. Metab. 2019, 64, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Demyanets, S.; Kaun, C.; Rychli, K.; Pfaffenberger, S.; Kastl, S.P.; Hohensinner, P.J.; Rega, G.; Katsaros, K.M.; Afonyushkin, T.; Bochkov, V.N.; et al. Oncostatin M-enhanced vascular endothelial growth factor expression in human vascular smooth muscle cells involves PI3K-, p38 MAPK-, Erk1/2- and STAT1/STAT3-dependent pathways and is attenuated by interferon-γ. Basic Res. Cardiol. 2011, 106, 217–231. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Prima, M.J.; Song, J.-A.; Kim, J.; Do, B.H.; Yoo, J.; Park, S.; Jang, J.; Lee, S.; Lee, E.; et al. Prokaryotic soluble overexpression and purification of oncostatin M using a fusion approach and genetically engineered E. coli strains. Sci. Rep. 2019, 9, 13706. [Google Scholar] [CrossRef]
- White, U.A.; Stewart, W.C.; Stephens, J.M. Gp130 cytokines exert differential patterns of crosstalk in adipocytes both in vitro and in vivo. Obesity 2011. [Google Scholar] [CrossRef]
- Lörchner, H.; Pöling, J.; Gajawada, P.; Hou, Y.; Polyakova, V.; Kostin, S.; Adrian-Segarra, J.M.; Boettger, T.; Wietelmann, A.; Warnecke, H.; et al. Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat. Med. 2015, 21, 353–362. [Google Scholar] [CrossRef]
- Luo, P.; Wang, P.-X.; Li, Z.-Z.; Zhang, X.-J.; Jiang, X.; Gong, J.; Qin, J.-J.; Guo, J.; Zhu, X.; Yang, S.; et al. Hepatic Oncostatin M Receptor β Regulates Obesity-Induced Steatosis and Insulin Resistance. Am. J. Pathol. 2016, 186, 1278–1292. [Google Scholar] [CrossRef]
- Richards, C.D. The Enigmatic Cytokine Oncostatin M and Roles in Disease. ISRN Inflamm. 2013, 2013, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Qin, J.-J.; Cheng, W.-L.; Zhu, X.; Gong, F.-H.; She, Z.; Huang, Z.; Xia, H.; Li, H. Oncostatin M receptor β deficiency attenuates atherogenesis by inhibiting JAK2/STAT3 signaling in macrophages. J. Lipid Res. 2017, 58, 895–906. [Google Scholar] [CrossRef]
- Komori, T.; Morikawa, Y. Oncostatin M in the development of metabolic syndrome and its potential as a novel therapeutic target. Anat. Sci. Int. 2018, 93, 169–176. [Google Scholar] [CrossRef]
- Schnittker, D.; Kwofie, K.; Ashkar, A.; Trigatti, B.; Richards, C.D. Oncostatin M and TLR-4 Ligand Synergize to Induce MCP-1, IL-6, and VEGF in Human Aortic Adventitial Fibroblasts and Smooth Muscle Cells. Mediat. Inflamm. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Rychli, K.; Kaun, C.; Hohensinner, P.J.; Rega, G.; Pfaffenberger, S.; Vyskocil, E.; Breuss, J.M.; Furnkranz, A.; Uhrin, P.; Zaujec, J.; et al. The inflammatory mediator oncostatin M induces angiopoietin 2 expression in endothelial cells in vitro and in vivo. J. Thromb. Haemost. 2010, 8, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Kunderfranco, P.; Peano, C.; Carullo, P.; Cremonesi, M.; Schorn, T.; Carriero, R.; Termanini, A.; Colombo, F.S.; Jachetti, E.; et al. Single-Cell Sequencing of Mouse Heart Immune Infiltrate in Pressure Overload-Driven Heart Failure Reveals Extent of Immune Activation. Circulation 2019, 140, 2089–2107. [Google Scholar] [CrossRef] [PubMed]
- Pöling, J.; Gajawada, P.; Richter, M.; Lörchner, H.; Polyakova, V.; Kostin, S.; Shin, J.; Boettger, T.; Walther, T.; Rees, W.; et al. Therapeutic targeting of the oncostatin M receptor-β prevents inflammatory heart failure. Basic Res. Cardiol. 2014, 109, 396. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The reparative function of cardiomyocytes in the infarcted myocardium. Cell Metab. 2015, 21, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, H.; Yago, T.; Liu, Z.; McEver, R.P. Endothelial signaling by neutrophil-released oncostatin M enhances P-selectin–dependent inflammation and thrombosis. Blood Adv. 2019, 3, 168–183. [Google Scholar] [CrossRef]
- Kirchmer, M.N.; Franco, A.; Albasanz-Puig, A.; Murray, J.; Yagi, M.; Gao, L.; Dong, Z.M.; Wijelath, E.S. Modulation of vascular smooth muscle cell phenotype by STAT-1 and STAT-3. Atherosclerosis 2014, 234, 169–175. [Google Scholar] [CrossRef]
- Kakutani, Y.; Shioi, A.; Shoji, T.; Okazaki, H.; Koyama, H.; Emoto, M.; Inaba, M. Oncostatin M Promotes Osteoblastic Differentiation of Human Vascular Smooth Muscle Cells Through JAK3-STAT3 Pathway. J. Cell. Biochem. 2015, 116, 1325–1333. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque calcification during atherosclerosis progression and regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A.; Kuvalekar, A.A. Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018, 39, 151–158. [Google Scholar] [CrossRef]
- Oh, D.K.; Ciaraldi, T.; Henry, R.R. Adiponectin in health and disease. Diabetes Obes. Metab. 2007, 9, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Harwood, H.J.; Heal, D.J.; Smith, S.L.; Jones, R.B. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2012, 63, 57–75. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ahima, R.S. Neuroendocrine and metabolic effects of adipocyte-derived hormones. Clin. Sci. 2006, 110, 143–152. [Google Scholar] [CrossRef]
- Chou, I.-P.; Chiu, Y.-P.; Ding, S.-T.; Liu, B.-H.; Lin, Y.Y.; Chen, C.-Y. Adiponectin receptor 1 overexpression reduces lipid accumulation and hypertrophy in the heart of diet-induced obese mice—Possible involvement of oxidative stress and autophagy. Endocr. Res. 2014, 39, 173–179. [Google Scholar] [CrossRef]
- Koentges, C.; König, A.; Pfeil, K.; Hölscher, M.E.; Schnick, T.; Wende, A.R.; Schrepper, A.; Cimolai, M.C.; Kersting, S.; Hoffmann, M.M.; et al. Myocardial mitochondrial dysfunction in mice lacking adiponectin receptor 1. Basic Res. Cardiol. 2015, 110, 37. [Google Scholar] [CrossRef]
- Braun, M.; Hettinger, N.; Koentges, C.; Pfeil, K.; Cimolai, M.C.; Hoffmann, M.M.; Osterholt, M.; Doenst, T.; Bode, C.; Bugger, H. Myocardial mitochondrial and contractile function are preserved in mice lacking adiponectin. PLoS ONE 2015, 10, e0119416. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Combs, T.P.; Scherer, P.E. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002, 13, 84–89. [Google Scholar] [CrossRef]
- Hui, X.; Lam, K.S.L.; Vanhoutte, P.M.; Xu, A. Adiponectin and cardiovascular health: An update. Br. J. Pharmacol. 2012, 165, 574–590. [Google Scholar] [CrossRef]
- Ouchi, N.; Ohishi, M.; Kihara, S.; Funahashi, T.; Nakamura, T.; Nagaretani, H.; Kumada, M.; Ohashi, K.; Okamoto, Y.; Nishizawa, H.; et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 2003, 42, 231–234. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, L.; Zhang, F.; Zhu, D.; Xin, C.; Wang, H.; Zhang, F.; Guo, X.; Lee, Y.; Zhang, L.; et al. A novel mechanism of diabetic vascular endothelial dysfunction: Hypoadiponectinemia-induced NLRP3 inflammasome activation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, Y.; Katsuya, T.; Ishikawa, K.; Ouchi, N.; Ohishi, M.; Sugimoto, K.; Fu, Y.; Motone, M.; Yamamoto, K.; Matsuo, A.; et al. Hypoadiponectinemia Is an Independent Risk Factor for Hypertension. Hypertension 2004, 43, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.-S.; Cheung, B.M.Y.; Tso, A.W.K.; Xu, A.; Wat, N.M.S.; Fong, C.H.Y.; Ong, L.H.Y.; Tam, S.; Tan, K.C.B.; Janus, E.D.; et al. Hypoadiponectinemia as a Predictor for the Development of Hypertension. Hypertension 2007, 49, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Kanda, J.; Nakamura, T.; Horie, A.; Kurosawa, H.; Hashimoto, T.; Sato, K.; Kushida, S.; Suzuki, M.; Yano, S.; et al. Association of hypoadiponectinemia in men with early onset of coronary heart disease and multiple coronary artery stenoses. Metabolism 2006, 55, 1653–1657. [Google Scholar] [CrossRef]
- Kumada, M.; Kihara, S.; Sumitsuji, S.; Kawamoto, T.; Matsumoto, S.; Ouchi, N.; Arita, Y.; Okamoto, Y.; Shimomura, I.; Hiraoka, H.; et al. Association of Hypoadiponectinemia With Coronary Artery Disease in Men. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 85–89. [Google Scholar] [CrossRef]
- Mohty, D.; Pibarot, P.; Côté, N.; Cartier, A.; Audet, A.; Després, J.P.; Mathieu, P. Hypoadiponectinemia Is Associated with Valvular Inflammation and Faster Disease Progression in Patients with Aortic Stenosis. Cardiology 2011, 118, 140–146. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- Devaraj, S.; Torok, N.; Dasu, M.R.; Samols, D.; Jialal, I. Adiponectin Decreases C-Reactive Protein Synthesis and Secretion from Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1368–1374. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Wang, L.; Wang, X.; Du, X.; Sun, Z.; Dong, N.; Chen, X. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc. Diabetol. 2011, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nakano, Y.; Hattori, S.; Tomizawa, A.; Inukai, K.; Kasai, K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-κB activation in vascular endothelial cells. FEBS Lett. 2008, 582, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Mahadev, K.; Fuchsel, L.; Ouedraogo, R.; Xu, S.-Q.; Goldstein, B.J. Adiponectin suppresses IκB kinase activation induced by tumor necrosis factor-α or high glucose in endothelial cells: Role of cAMP and AMP kinase signaling. Am. J. Physiol. Metab. 2007, 293, E1836–E1844. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, R.; Gong, Y.; Berzins, B.; Wu, X.; Mahadev, K.; Hough, K.; Chan, L.; Goldstein, B.J.; Scalia, R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 2007, 117, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Park, Y.; Zhang, C. Coronary and Aortic Endothelial Function Affected by Feedback Between Adiponectin and Tumor Necrosis Factor α in Type 2 Diabetic Mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2156–2163. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Higa, N.; Asahi, T.; Oshiro, Y.; Takasu, N.; Tagawa, T.; Ueda, S.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y. Hypoadiponectinemia Is Closely Linked to Endothelial Dysfunction in Man. J. Clin. Endocrinol. Metab. 2003, 88, 3236–3240. [Google Scholar] [CrossRef]
- Cao, Y.; Tao, L.; Yuan, Y.; Jiao, X.; Lau, W.B.; Wang, Y.; Christopher, T.; Lopez, B.; Chan, L.; Goldstein, B.; et al. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J. Mol. Cell. Cardiol. 2009, 46, 413–419. [Google Scholar] [CrossRef]
- Deng, G.; Long, Y.; Yu, Y.-R.; Li, M.-R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int. J. Obes. 2010, 34, 165–171. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Van de Voorde, J.; Pauwels, B.; Boydens, C.; Decaluwé, K. Adipocytokines in relation to cardiovascular disease. Metabolism 2013, 62, 1513–1521. [Google Scholar] [CrossRef]
- Piñeiro, R.; Iglesias, M.J.; Gallego, R.; Raghay, K.; Eiras, S.; Rubio, J.; Diéguez, C.; Gualillo, O.; González-Juanatey, J.R.; Lago, F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005, 579, 5163–5169. [Google Scholar] [CrossRef]
- Boddu, N.J.; Theus, S.; Luo, S.; Wei, J.Y.; Ranganathan, G. Is the lack of adiponectin associated with increased ER/SR stress and inflammation in the heart? Adipocyte 2014, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, X.F.; Lang, Z.Q.; Jin, Y.C.; Fu, J.R.; Xv, X.M.; Sun, S.T.; Xin, X.; Zhang, L.S. Adiponectin is protective against endoplasmic reticulum stress-induced apoptosis of endothelial cells in sepsis. Braz. J. Med. Biol. Res. 2018, 51, e7747. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, X.; Huang, H.; Ding, N.; Zhang, S.; Hutchinson, S.Z.; Zhang, X. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS ONE 2014, 9, e94545. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bian, Y.; Bai, R.; Li, H.; Fu, M.; Xiao, C. Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca2+-ATPase activity and inhibiting endoplasmic reticulum stress. J. Cardiovasc. Pharmacol. 2013, 62, 143–153. [Google Scholar] [CrossRef]
- Bian, Y.-F.; Hao, X.-Y.; Gao, F.; Yang, H.-Y.; Zang, N.; Xiao, C.-S. Adiponectin attenuates hypoxia/reoxygenation-induced cardiomyocyte injury through inhibition of endoplasmic reticulum stress. J. Investig. Med. 2011, 59, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, Y.; Yu, Q.; Yang, Q.; Li, B.; Sun, L.; Yan, W.; Cai, X.; Gao, E.; Xiong, L.; et al. TNF-α antagonism ameliorates myocardial ischemia-reperfusion injury in mice by upregulating adiponectin. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1583–H1591. [Google Scholar] [CrossRef]
- Oh-I, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Rodríguez-Penas, D.; García-Rúa, V.; Mosquera-Leal, A.; González-Juanatey, J.R.; Lago, F. Nesfatin-1: A new energy-regulating peptide with pleiotropic functions. Implications at cardiovascular level. Endocrine 2016, 52, 11–29. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Chen, J.; Brown, J.E.; Tripathi, G.; Hallschmid, M.; Patel, S.; Kern, W.; Hillhouse, E.W.; Lehnert, H.; Tan, B.K.; et al. Identification of nesfatin-1 in human and murine adipose tissue: A novel depot-specific adipokine with increased levels in obesity. Endocrinology 2010, 151, 3169–3180. [Google Scholar] [CrossRef]
- St-Pierre, D.H.; Martin, J.; Shimizu, H.; Tagaya, Y.; Tsuchiya, T.; Marceau, S.; Biertho, L.; Bastien, M.; Caron-Cantin, S.-M.; Simard, S.; et al. Association between nesfatin-1 levels and metabolic improvements in severely obese patients who underwent biliopancreatic derivation with duodenal switch. Peptides 2016, 86, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Reingold, B.K.; Gao, X.; Gaidhu, M.P.; Tsushima, R.G.; Unniappan, S. Nesfatin-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet β- and MIN6 cells. J. Endocrinol. 2011, 208, R9–R16. [Google Scholar] [CrossRef]
- Bonnet, M.S.; Pecchi, E.; Trouslard, J.; Jean, A.; Dallaporta, M.; Troadec, J.-D. Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J. Neuroinflamm. 2009, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, K.; Li, J.; Zhou, X.; Xu, L.; Wu, Z.; Ma, C.; Ran, J.; Hu, P.; Bao, J.; et al. Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging 2020, 12, 1760–1777. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Chen, S.-C.; Zou, X.-B.; Tian, L.-L.; Sui, S.-H.; Liu, N.-Z. Nesfatin-1 alleviates acute lung injury through reducing inflammation and oxidative stress via the regulation of HMGB1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5071–5081. [Google Scholar] [CrossRef]
- Tang, C.-H.; Fu, X.-J.; Xu, X.-L.; Wei, X.-J.; Pan, H.-S. The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in the traumatic rat brain. Peptides 2012, 36, 39–45. [Google Scholar] [CrossRef]
- Özsavcí, D.; Erşahin, M.; Şener, A.; Özakpinar, Ö.B.; Toklu, H.Z.; Akakín, D.; Şener, G.; Yeğen, B.Ç. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage-induced oxidative brain damage in rats. Neurosurgery 2011, 68, 1699–1708. [Google Scholar] [CrossRef]
- Acik, V.; Matyar, S.; Arslan, A.; İstemen, İ.; Olguner, S.K.; Arslan, B.; Gezercan, Y.; Ökten, A.İ. Relationshıp of spontaneous subarachnoid haemorrhage and cerebral aneurysm to serum Visfatin and Nesfatin-1 levels. Clin. Neurol. Neurosurg. 2020, 194, 105837. [Google Scholar] [CrossRef] [PubMed]
- Feijóo-Bandín, S.; Rodríguez-Penas, D.; García-Rúa, V.; Mosquera-Leal, A.; Otero, M.F.; Pereira, E.; Rubio, J.; Martínez, I.; Seoane, L.M.; Gualillo, O.; et al. Nesfatin-1 in human and murine cardiomyocytes: Synthesis, secretion, and mobilization of GLUT-4. Endocrinology 2013, 154, 4757–4767. [Google Scholar] [CrossRef]
- Ibe, S.; Kishimoto, Y.; Niki, H.; Saita, E.; Umei, T.; Miura, K.; Ikegami, Y.; Ohmori, R.; Kondo, K.; Momiyama, Y. Associations between plasma nesfatin-1 levels and the presence and severity of coronary artery disease. Heart Vessels 2019, 34, 965–970. [Google Scholar] [CrossRef]
- Serdar Kuyumcu, M.; Kuyumcu, A.; Yayla, Ç.; Bilal Özbay, M.; Ünal, S.; Açar, B.; Nural, C.; Şenat, A.; Samur, G. The Relationship between Nesfatin-1 Levels and SYNTAX Score in Patients with Non-ST Segment Elevation Myocardial Infarction. Acta Cardiol. Sin. 2018, 34, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, A.; Kuyumcu, M.S.; Ozbay, M.B.; Ertem, A.G.; Samur, G. Nesfatin-1: A novel regulatory peptide associated with acute myocardial infarction and Mediterranean diet. Peptides 2019, 114, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Li, X.; He, T.; Wang, Y.; Wang, Z.; Wang, S.; Xing, M.; Sun, W.; Ding, H. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides 2013, 46, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Sivri, S.; Sökmen, E.; Çelik, M.; Güçlü, K. Nesfatin-1 Levels Predict Angiographic No-Reflow in Patients with ST-Segment Elevation Myocardial Infarction. Acta Cardiol. Sin. 2020, 36, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kirisci, M.; Yardimci, M.M.; Kocarslan, A.; Sokmen, A.; Doganer, A.; Gunes, H. Nesfatin 1: A promising biomarker predicting successful reperfusion after coronary artery bypass surgery. Bratisl. Med. J. 2020, 121, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Tasatargil, A.; Kuscu, N.; Dalaklioglu, S.; Adiguzel, D.; Celik-Ozenci, C.; Ozdem, S.; Barutcigil, A.; Ozdem, S. Cardioprotective effect of nesfatin-1 against isoproterenol-induced myocardial infarction in rats: Role of the Akt/GSK-3β pathway. Peptides 2017, 95, 1–9. [Google Scholar] [CrossRef]
- Naseroleslami, M.; Sharifi, M.; Rakhshan, K.; Mokhtari, B.; Aboutaleb, N. Nesfatin-1 attenuates injury in a rat model of myocardial infarction by targeting autophagy, inflammation, and apoptosis. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Rodríguez-Penas, D.; Portolés, M.; Roselló-Lletí, E.; Rivera, M.; González-Juanatey, J.R.; Lago, F. Relaxin-2 in Cardiometabolic Diseases: Mechanisms of Action and Future Perspectives. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Saekow, M.; Kroc, R.L. Potentiation of insulin binding and insulin action by purified porcine relaxin. Ann. N. Y. Acad. Sci. 1982, 380, 200–216. [Google Scholar] [CrossRef]
- Jarrett, J.C.; Ballejo, G.; Saleem, T.H.; Tsibris, J.C.M.; Spellacy, W.N. The effect of prolactin and relaxin on insulin binding by adipocytes from pregnant women. Am. J. Obstet. Gynecol. 1984, 149, 250–255. [Google Scholar] [CrossRef]
- Bani, G.; Bianchi, S.; Formigli, L.; Bigazzi, M. Responsiveness of Mouse Parametrial Fat to Relaxin. Cells Tissues Organs 1989, 134, 128–132. [Google Scholar] [CrossRef]
- Martin, B.; Romero, G.; Salama, G. Cardioprotective actions of relaxin. Mol. Cell. Endocrinol. 2019, 487, 45–53. [Google Scholar] [CrossRef]
- Masini, E.; Bani, D.; Bigazzi, M.; Mannaioni, P.F.; Bani-Sacchi, T. Effects of relaxin on mast cells. In vitro and in vivo studies in rats and guinea pigs. J. Clin. Invest. 1994, 94, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Bani, D.; Bigazzi, M.; Masini, E.; Bani, G.; Sacchi, T.B. Relaxin depresses platelet aggregation: In vitro studies on isolated human and rabbit platelets. Lab. Invest. 1995, 73, 709–716. [Google Scholar]
- Bani, D.; Masini, E.; Bello, M.G.; Bigazzi, M.; Bani Sacchi, T.; Sacchi, T.B. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am. J. Pathol. 1998, 152, 1367–1376. [Google Scholar]
- Masini, E.; Bani, D.; Bello, M.G.; Bigazzi, M.; Mannaioni, P.F.; Sacchi, T.B. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: Evidence for an involvement of nitric oxide. Endocrinology 1997, 138, 4713–4720. [Google Scholar] [CrossRef]
- Nistri, S.; Cinci, L.; Perna, A.M.; Masini, E.; Mastroianni, R.; Bani, D. Relaxin induces mast cell inhibition and reduces ventricular arrhythmias in a swine model of acute myocardial infarction. Pharmacol. Res. 2008, 57, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Nistri, S.; Chiappini, L.; Sassoli, C.; Bani, D. Relaxin inhibits lipopolysaccharide-induced adhesion of neutrophils to coronary endothelial cells by a nitric oxide-mediated mechanism. FASEB J. 2003, 17, 2109–2111. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Nistri, S.; Vannacci, A.; Bani Sacchi, T.; Novelli, A.; Bani, D. Relaxin Inhibits the Activation of Human Neutrophils: Involvement of the Nitric Oxide Pathway. Endocrinology 2004, 145, 1106–1112. [Google Scholar] [CrossRef]
- Valle Raleigh, J.; Mauro, A.G.; Devarakonda, T.; Marchetti, C.; He, J.; Kim, E.; Filippone, S.; Das, A.; Toldo, S.; Abbate, A.; et al. Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism. Cardiovasc. Res. 2017, 2, 609–619. [Google Scholar] [CrossRef]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef] [PubMed]
- Beiert, T.; Tiyerili, V.; Knappe, V.; Effelsberg, V.; Linhart, M.; Stöckigt, F.; Klein, S.; Schierwagen, R.; Trebicka, J.; Nickenig, G.; et al. Relaxin reduces susceptibility to post-infarct atrial fibrillation in mice due to anti-fibrotic and anti-inflammatory properties. Biochem. Biophys. Res. Commun. 2017, 490, 643–649. [Google Scholar] [CrossRef]
- Beiert, T.; Knappe, V.; Tiyerili, V.; Stöckigt, F.; Effelsberg, V.; Linhart, M.; Steinmetz, M.; Klein, S.; Schierwagen, R.; Trebicka, J.; et al. Chronic lower-dose relaxin administration protects from arrhythmia in experimental myocardial infarction due to anti-inflammatory and anti-fibrotic properties. Int. J. Cardiol. 2018, 250, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mas, J.; Lax, A.; Asensio-Lopez, M.C.; Lencina, M.; Fernandez-del Palacio, M.J.; Soriano-Filiu, A.; de Boer, R.A.; Pascual-Figal, D.A. Early Anti-inflammatory and Pro-angiogenic Myocardial Effects of Intravenous Serelaxin Infusion for 72 H in an Experimental Rat Model of Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 460–469. [Google Scholar] [CrossRef]
- Gao, X.-M.; Su, Y.; Moore, S.; Han, L.-P.; Kiriazis, H.; Lu, Q.; Zhao, W.-B.; Ruze, A.; Fang, B.-B.; Duan, M.-J.; et al. Relaxin mitigates microvascular damage and inflammation following cardiac ischemia—Reperfusion. Basic Res. Cardiol. 2019, 114, 30. [Google Scholar] [CrossRef]
- Van den Berg, N.W.E.; de Groot, J.R. Myocardial infarction, atrial fibrillation and mortality: Timing is everything. Neth. Heart. J. 2015, 23, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.S.; Royce, S.G.; Hewitson, T.D.; Denton, K.M.; Cooney, T.E.; Bennett, R.G. Anti-fibrotic actions of relaxin. Br. J. Pharmacol. 2017, 174, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, H.; Yang, Q.; Sun, Y. Effects of relaxin on cardiac fibrosis, apoptosis, and tachyarrhythmia in rats with myocardial infarction. Biomed. Pharmacother. 2016, 84, 348–355. [Google Scholar] [CrossRef]
- Martin, B.; Gabris-Weber, B.A.; Reddy, R.; Romero, G.; Chattopadhyay, A.; Salama, G. Relaxin reverses inflammatory and immune signals in aged hearts. PLoS ONE 2018, 13, e0190935. [Google Scholar] [CrossRef]
- Brecht, A.; Bartsch, C.; Baumann, G.; Stangl, K.; Dschietzig, T. Relaxin inhibits early steps in vascular inflammation. Regul. Pept. 2011, 166, 76–82. [Google Scholar] [CrossRef]
- Dschietzig, T.; Bartsch, C.; Baumann, G.; Stangl, K. RXFP1-inactive relaxin activates human glucocorticoid receptor: Further investigations into the relaxin-GR pathway. Regul. Pept. 2009, 154, 77–84. [Google Scholar] [CrossRef]
- Bathgate, R.A.D.; Halls, M.L.; van der Westhuizen, E.T.; Callander, G.E.; Kocan, M.; Summers, R.J. Relaxin family peptides and their receptors. Physiol. Rev. 2013, 93, 405–480. [Google Scholar] [CrossRef] [PubMed]
- Collino, M.; Rogazzo, M.; Pini, A.; Benetti, E.; Rosa, A.C.; Chiazza, F.; Fantozzi, R.; Bani, D.; Masini, E. Acute treatment with relaxin protects the kidney against ischaemia/reperfusion injury. J. Cell. Mol. Med. 2013, 17, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Boehnert, M.U.; Armbruster, F.P.; Hilbig, H. Relaxin as a protective substance in the preserving solution for liver transplantation: Spectrophotometric in vivo imaging of local oxygen supply in an isolated perfused rat liver model. Ann. N. Y. Acad. Sci. 2009, 1160, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Tanigawa, Y.; Hirohashi, S. Cloning of the Novel Gene Intelectin, Which Is Expressed in Intestinal Paneth Cells in Mice. Biochem. Biophys. Res. Commun. 1998, 251, 759–762. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Shin, K.; Lönnerdal, B. Molecular Cloning and Functional Expression of a Human Intestinal Lactoferrin Receptor ‡. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef]
- Lee, J.-K.; Schnee, J.; Pang, M.; Wolfert, M.; Baum, L.G.; Moremen, K.W.; Pierce, M. Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology 2001, 11, 65–73. [Google Scholar] [CrossRef]
- Schäffler, A.; Neumeier, M.; Herfarth, H.; Fürst, A.; Schölmerich, J.; Büchler, C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim. Biophys. Acta Gene Struct. Expr. 2005, 1732, 96–102. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Fain, J.N.; Sacks, H.S.; Buehrer, B.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Carter, R.A.; Tichansky, D.S.; Madan, A.K. Identification of omentin mRNA in human epicardial adipose tissue: Comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int. J. Obes. 2008, 32, 810–815. [Google Scholar] [CrossRef]
- Svensson, H.; Odén, B.; Edén, S.; Lönn, M. Adiponectin, chemerin, cytokines, and dipeptidyl peptidase 4 are released from human adipose tissue in a depot-dependent manner: An in vitro system including human serum albumin. BMC Endocr. Disord. 2014, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ji, Q.; Cai, L.; Huang, F.; Lai, Y.; Liu, Y.; Yu, J.; Han, B.; Zhu, E.; Zhang, J.; et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc. Diabetol. 2016, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Matloch, Z.; Kratochvílová, H.; Cinkajzlová, A.; Lipš, M.; Kopecký, P.; Pořízka, M.; Haluzíková, D.; Lindner, J.; Mráz, M.; Kloučková, J.; et al. Changes in Omentin Levels and Its mRNA Expression in Epicardial Adipose Tissue in Patients Undergoing Elective Cardiac Surgery: The Influence of Type 2 Diabetes and Coronary Heart Disease. Physiol. Res. 2018, 67, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Shibata, R.; Ouchi, N.; Tokuda, Y.; Funakubo, H.; Suzuki, M.; Kataoka, T.; Nagao, T.; Okumura, S.; Shinoda, N.; et al. Increased expression of the adipocytokine omentin in the epicardial adipose tissue of coronary artery disease patients. Atherosclerosis 2016, 251, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Berti, L.; Hartwig, S.; Irmler, M.; Rädle, B.; Siegel-Axel, D.; Beckers, J.; Lehr, S.; Al-Hasani, H.; Häring, H.-U.; Hrabě de Angelis, M.; et al. Impact of fibroblast growth factor 21 on the secretome of human perivascular preadipocytes and adipocytes: A targeted proteomics approach. Arch. Physiol. Biochem. 2016, 122, 281–288. [Google Scholar] [CrossRef]
- De Souza Batista, C.M.; Yang, R.-Z.; Lee, M.-J.; Glynn, N.M.; Yu, D.-Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef]
- Fernández-Trasancos, Á.; Agra, R.M.; García-Acuña, J.M.; Fernández, Á.L.; González-Juanatey, J.R.; Eiras, S. Omentin treatment of epicardial fat improves its anti-inflammatory activity and paracrine benefit on smooth muscle cells. Obesity 2017, 25, 1042–1049. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Qian, G.; Zhou, J. Omentin-1 attenuates adipose tissue inflammation via restoration of TXNIP/NLRP3 signaling in high-fat diet-induced obese mice. Fundam. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Lin, F.; Han, K.; Wang, X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch. Biochem. Biophys. 2020, 679, 108187. [Google Scholar] [CrossRef]
- Yıldız, S.S.; Sahin, I.; Cetinkal, G.; Aksan, G.; Kucuk, S.H.; Keskin, K.; Cetin, S.; Sigirci, S.; Avcı, İ.İ.; Kilci, H.; et al. Usefulness of Serum Omentin-1 Levels for the Prediction of Adverse Cardiac Events in Patients with Hypertrophic Cardiomyopathy. Med. Princ. Pract. 2018, 27, 107–114. [Google Scholar] [CrossRef]
- Wang, X.-H.; Dou, L.-Z.; Gu, C.; Wang, X.-Q. Plasma levels of omentin-1 and visfatin in senile patients with coronary heart disease and heart failure. Asian Pac. J. Trop. Med. 2014, 7, 55–62. [Google Scholar] [CrossRef]
- Narumi, T.; Watanabe, T.; Kadowaki, S.; Kinoshita, D.; Yokoyama, M.; Honda, Y.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; et al. Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovasc. Diabetol. 2014, 13, 84. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Y.; Zhang, S.; Wang, Z.; Zhang, J.; Chang, C.; Liu, L.; Ji, Q.; Liu, X. Circulating Omentin-1 Levels Are Decreased in Dilated Cardiomyopathy Patients with Overt Heart Failure. Dis. Markers 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Bonadia, N.; Angelini, F.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Association between plasma omentin-1 levels in type 2 diabetic patients and peripheral artery disease. Cardiovasc. Diabetol. 2019, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Onur, I.; Oz, F.; Yildiz, S.; Kuplay, H.; Yucel, C.; Sigirci, S.; Elitok, A.; Pilten, S.; Kasali, K.; Yasar Cizgici, A.; et al. A decreased serum omentin-1 level may be an independent risk factor for peripheral arterial disease. Int. Angiol. 2014, 33, 455–460. [Google Scholar]
- Baig, M.; Alghalayini, K.W.; Gazzaz, Z.J.; Atta, H. Association of Serum Omentin-1, Chemerin, and Leptin with Acute Myocardial Infarction and its Risk Factors. Pakistan J. Med. Sci. 2020, 36, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Tahmatzidis, D.K.; Giannakoulas, C.; Kapelouzou, A.; Gkontopoulos, A.; Parissis, J.; Lampropoulos, S.; Kottas, G. Serum levels of novel adipokines, omentin-1 and chemerin, in patients with acute myocardial infarction: KOZANI STUDY. J. Cardiovasc. Med. 2015, 16, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, F.; Han, F.; Lv, L.; Tang, C.-E.; Luo, F. Omentin-1 Ameliorated Free Fatty Acid-Induced Impairment in Proliferation, Migration, and Inflammatory States of HUVECs. Cardiol. Res. Pract. 2020, 20, 3054379. [Google Scholar] [CrossRef]
- Çimen, A.R.; Cerit, E.T.; Iyidir, O.T.; Karakus, R.; Uyar, B.B.; Toruner, F.B.; Cakir, N.; Arslan, M. Serum omentin-1 levels and endothelial dysfunction in obesity. Acta Endocrinol. 2017, 13, 138–143. [Google Scholar] [CrossRef]
- Hayashi, M.; Morioka, T.; Hatamori, M.; Kakutani, Y.; Yamazaki, Y.; Kurajoh, M.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shioi, A.; et al. Plasma omentin levels are associated with vascular endothelial function in patients with type 2 diabetes at elevated cardiovascular risk. Diabetes Res. Clin. Pract. 2019, 148, 160–168. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Castro, A.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Circulating Omentin as a Novel Biomarker of Endothelial Dysfunction. Obesity 2011, 19, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fang, S.; Liu, X.; Li, J.; Wang, X.; Cui, J.; Chen, T.; Li, Z.; Yang, F.; Tian, J.; et al. Omentin-1 protects against high glucose-induced endothelial dysfunction via the AMPK/PPARδ signaling pathway. Biochem. Pharmacol. 2020, 174, 113830. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, X.; Liu, F.; Tan, H.; Shang, D. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem. Biophys. Res. Commun. 2012, 425, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Binti Kamaruddin, N.A.; Fong, L.Y.; Tan, J.J.; Abdullah, M.N.H.; Singh Cheema, M.; Bin Yakop, F.; Yong, Y.K. Cytoprotective Role of Omentin Against Oxidative Stress-Induced Vascular Endothelial Cells Injury. Molecules 2020, 25, 2534. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Ushach, I.; Arrevillaga-Boni, G.; Heller, G.N.; Pone, E.; Hernandez-Ruiz, M.; Catalan-Dibene, J.; Hevezi, P.; Zlotnik, A. Meteorin-like/Meteorin-β Is a Novel Immunoregulatory Cytokine Associated with Inflammation. J. Immunol. 2018, 201, 3669–3676. [Google Scholar] [CrossRef]
- Lee, J.O.; Byun, W.S.; Kang, M.J.; Han, J.A.; Moon, J.; Shin, M.-J.; Lee, H.J.; Chung, J.H.; Lee, J.-S.; Son, C.-G.; et al. The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKα2. FEBS J. 2020, 287, 2087–2104. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, Y.E.; Kim, J.M.; Choung, S.; Joung, K.H.; Kim, H.J.; Ku, B.J. Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 135, 7–10. [Google Scholar] [CrossRef]
- Dadmanesh, M.; Aghajani, H.; Fadaei, R.; Ghorban, K. Lower serum levels of Meteorin-like/Subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PLoS ONE 2018, 13, e0204180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-X.; Ji, H.-H.; Yao, M.-P.; Wang, L.; Wang, Y.; Zhou, P.; Liu, Y.; Zheng, X.-F.; He, H.-W.; Wang, L.-S.; et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J. Cell. Mol. Med. 2019, 23, 271–280. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, H.M.; Selim, F.O.; Hosny, T.A.M.; Almassry, H.N. Association of low serum Meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res. Clin. Pract. 2019, 150, 57–63. [Google Scholar] [CrossRef]
- Xu, L.; Cai, Y.; Wang, Y.; Xu, C. Meteorin-Like (METRNL) Attenuates Myocardial Ischemia/Reperfusion Injury-Induced Cardiomyocytes Apoptosis by Alleviating Endoplasmic Reticulum Stress via Activation of AMPK-PAK2 Signaling in H9C2 Cells. Med. Sci. Monit. 2020, 26, e924564. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Haberka, M.; Machnik, G.; Kowalówka, A.; Biedroń, M.; Skudrzyk, E.; Regulska-Ilow, B.; Gajos, G.; Manka, R.; Deja, M.; Okopień, B.; et al. Epicardial, paracardial and perivascular fat quantity, genes expression and serum cytokines in coronary artery disease and diabetes. Pol. Arch. Intern. Med. 2019, 129, 738–746. [Google Scholar] [CrossRef]
- Akyildiz, Z.I.; Polat, S.; Yurekli, B.S.; Kocabas, G.U.; Tuluce, K.; Tuluce, S.Y.; Kocabas, U.; Bozkaya, G.; Yuksel, A.; Nazli, C. Epicardial fat, body mass index, and triglyceride are independent contributors of serum fibroblast growth factor 21 level in obese premenopausal women. J. Endocrinol. Invest. 2015, 38, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kotulák, T.; Drápalová, J.; Kopecký, P.; Lacinová, Z.; Kramář, P.; Říha, H.; Netuka, I.; Malý, J.; Housa, D.; Bláha, J.; et al. Increased circulating and epicardial adipose tissue mRNA expression of fibroblast growth factor-21 after cardiac surgery: Possible role in postoperative inflammatory response and insulin resistance. Physiol. Res. 2011, 60, 757–767. [Google Scholar] [CrossRef]
- Wang, W.-F.; Li, S.-M.; Ren, G.-P.; Zheng, W.; Lu, Y.-J.; Yu, Y.-H.; Xu, W.-J.; Li, T.-H.; Zhou, L.-H.; Liu, Y.; et al. Recombinant murine fibroblast growth factor 21 ameliorates obesity-related inflammation in monosodium glutamate-induced obesity rats. Endocrine 2015, 49, 119–129. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, T.-T.; Li, S.; Sun, X.; Li, Z.; Li, Y.; Li, D.-S.; Wang, W.-F. Fibroblast Growth Factor 21 Exerts its Anti-inflammatory Effects on Multiple Cell Types of Adipose Tissue in Obesity. Obesity 2019, 27, 399–408. [Google Scholar] [CrossRef]
- Keinicke, H.; Sun, G.; Mentzel, C.M.J.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr. Connect. 2020, 9, 755–768. [Google Scholar] [CrossRef]
- Singhal, G.; Fisher, F.M.; Chee, M.J.; Tan, T.G.; El Ouaamari, A.; Adams, A.C.; Najarian, R.; Kulkarni, R.N.; Benoist, C.; Flier, J.S.; et al. Fibroblast Growth Factor 21 (FGF21) Protects against High Fat Diet Induced Inflammation and Islet Hyperplasia in Pancreas. PLoS ONE 2016, 11, e0148252. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Li, J.; Hu, C.; Zhao, W.; Ma, W.; Yao, M.; Xing, L. Fibroblast Growth Factor 21 dependent TLR4/MYD88/NF-κB signaling activation is involved in lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2020, 80, 106219. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.G.; Yi, S.-A.; Choi, S.-E.; Kang, Y.; Kim, T.H.; Jeon, J.Y.; Bae, M.A.; Ahn, J.H.; Jeong, H.; Hwang, E.S.; et al. TM-25659-Induced Activation of FGF21 Level Decreases Insulin Resistance and Inflammation in Skeletal Muscle via GCN2 Pathways. Mol. Cells 2015, 38, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, J.-Y.; Li, S.; Guo, X.-C.; Wu, T.; Wang, W.-F.; Li, D.-S. Fibroblast growth factor 21 regulates foam cells formation and inflammatory response in Ox-LDL-induced THP-1 macrophages. Biomed. Pharmacother. 2018, 108, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J.; Li, S.; Song, L.; Guo, X.; Yao, W.; Zou, D.; Gao, X.; Liu, Y.; Bai, F.; et al. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. Int. Immunopharmacol. 2016, 38, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zheng, Q.; Chen, J.; Tan, X.; Li, Q.; Ding, L.; Zhang, R.; Lin, X. FGF21 mitigates atherosclerosis via inhibition of NLRP3 inflammasome-mediated vascular endothelial cells pyroptosis. Exp. Cell Res. 2020, 393, 112108. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gou, Z.; Li, Y.; Wang, Y.; Zhu, J.; Xu, G.; Zhang, Q. Fibroblast growth factor 21 inhibits atherosclerosis in apoE-/- mice by ameliorating Fas-mediated apoptosis. Lipids Health Dis. 2018, 17, 203. [Google Scholar] [CrossRef]
- Xiaolong, L.; Dongmin, G.; Liu, M.; Zuo, W.; Huijun, H.; Qiufen, T.; XueMei, H.; Wensheng, L.; Yuping, P.; Jun, L.; et al. FGF21 induces autophagy-mediated cholesterol efflux to inhibit atherogenesis via RACK1 up-regulation. J. Cell. Mol. Med. 2020, 24, 4992–5006. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Qi, Y.-F.; Chang, J.-R.; Lu, W.-W.; Zhang, J.-S.; Wang, S.-P.; Cheng, S.-J.; Zhang, M.; Fan, Q.; Lv, Y.; et al. Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE(-/-) mice. Heart Vessels 2015, 30, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Pan, X.; Wu, F.; Ye, D.; Zhang, Y.; Wang, Y.; Jin, L.; Lian, Q.; Huang, Y.; Ding, H.; et al. Fibroblast Growth Factor 21 Prevents Atherosclerosis by Suppression of Hepatic Sterol Regulatory Element-Binding Protein-2 and Induction of Adiponectin in Mice. Circulation 2015, 131, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Cheng, J.; Zhou, Q.; Peng, J.; Pan, Y.; Han, H. Fibroblast growth factor 21 attenuates inflammation and oxidative stress in atherosclerotic rat via enhancing the Nrf1-ARE signaling pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 1308–1317. [Google Scholar]
- Zhang, Y.; Liu, Z.; Zhou, M.; Liu, C. Therapeutic effects of fibroblast growth factor-21 against atherosclerosis via the NF-κB pathway. Mol. Med. Rep. 2018, 17, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Li, N.; He, Z.; Zeng, X.; Nan, Y.; Chen, J.; Miao, P.; Ying, Y.; Lin, W.; Zhao, X.; et al. Fibroblast growth factor 21 Ameliorates diabetes-induced endothelial dysfunction in mouse aorta via activation of the CaMKK2/AMPKα signaling pathway. Cell Death Dis. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Basurto, L.; Gregory, M.A.; Hernández, S.B.; Sánchez-Huerta, L.; Martínez, A.D.; Manuel-Apolinar, L.; Avelar, F.J.; Alonso, L.A.M.; Sánchez-Arenas, R. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp. Gerontol. 2019, 124, 110624. [Google Scholar] [CrossRef] [PubMed]
- Yafei, S.; Elsewy, F.; Youssef, E.; Ayman, M.; El-Shafei, M. Fibroblast growth factor 21 association with subclinical atherosclerosis and arterial stiffness in type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qian, L.; Zhang, L.; Zhang, J.; Zhou, J.; Li, Y.; Hou, X.; Fang, Q.; Li, H.; Jia, W. Fibroblast Growth Factor 21 is Related to Atherosclerosis Independent of Nonalcoholic Fatty Liver Disease and Predicts Atherosclerotic Cardiovascular Events. J. Am. Heart Assoc. 2020, 9, e015226. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Hou, J. Relationship between Serum fibroblast growth factor 21 levels and morphological atherosclerotic plaque characteristics in patients with coronary heart disease. Eur. Heart J. Suppl. 2016, 18, F37. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, L.; Xu, A.; Zhou, P.; Long, Z.; Tu, Y.; Chen, X.; Tang, W.; Huang, G.; Zhou, Z. Serum fibroblast growth factor 21 levels are related to subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 72. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Saita, E.; Kishimoto, Y.; Ibe, S.; Seki, T.; Miura, K.; Suzuki-Sugihara, N.; Ikegami, Y.; Ohmori, R.; Kondo, K.; et al. Low Plasma Levels of Fibroblast Growth Factor-21 in Patients with Peripheral Artery Disease. J. Atheroscler. Thromb. 2018, 25, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, A.; Lin, S.; Yu, L.; Lin, X.; Yan, X.; Lu, X.; Zhang, C. Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart. Cell Death Dis. 2018, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Tanajak, P.; Sa-Nguanmoo, P.; Wang, X.; Liang, G.; Li, X.; Jiang, C.; Chattipakorn, S.C.; Chattipakorn, N. Fibroblast growth factor 21 (FGF21) therapy attenuates left ventricular dysfunction and metabolic disturbance by improving FGF21 sensitivity, cardiac mitochondrial redox homoeostasis and structural changes in pre-diabetic rats. Acta Physiol. 2016, 217, 287–299. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Z.; Xue, M.; Yi, X.; Liang, J.; Niu, C.; Chen, G.; Shen, Y.; Zhang, H.; Zheng, J.; et al. Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Planavila, A.; Redondo-Angulo, I.; Ribas, F.; Garrabou, G.; Casademont, J.; Giralt, M.; Villarroya, F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc. Res. 2015, 106, 19–31. [Google Scholar] [CrossRef]
- Planavila, A.; Redondo, I.; Hondares, E.; Vinciguerra, M.; Munts, C.; Iglesias, R.; Gabrielli, L.A.; Sitges, M.; Giralt, M.; van Bilsen, M.; et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 2013, 4, 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, C.; Liu, Y.; Li, Y.; Du, S.; Zhang, R.; Sun, Y.; Zhang, R.; Wang, Y.; Xue, H.; et al. Fibroblast growth factor 21 inhibited ischemic arrhythmias via targeting miR-143/EGR1 axis. Basic Res. Cardiol. 2020, 115, 9. [Google Scholar] [CrossRef] [PubMed]
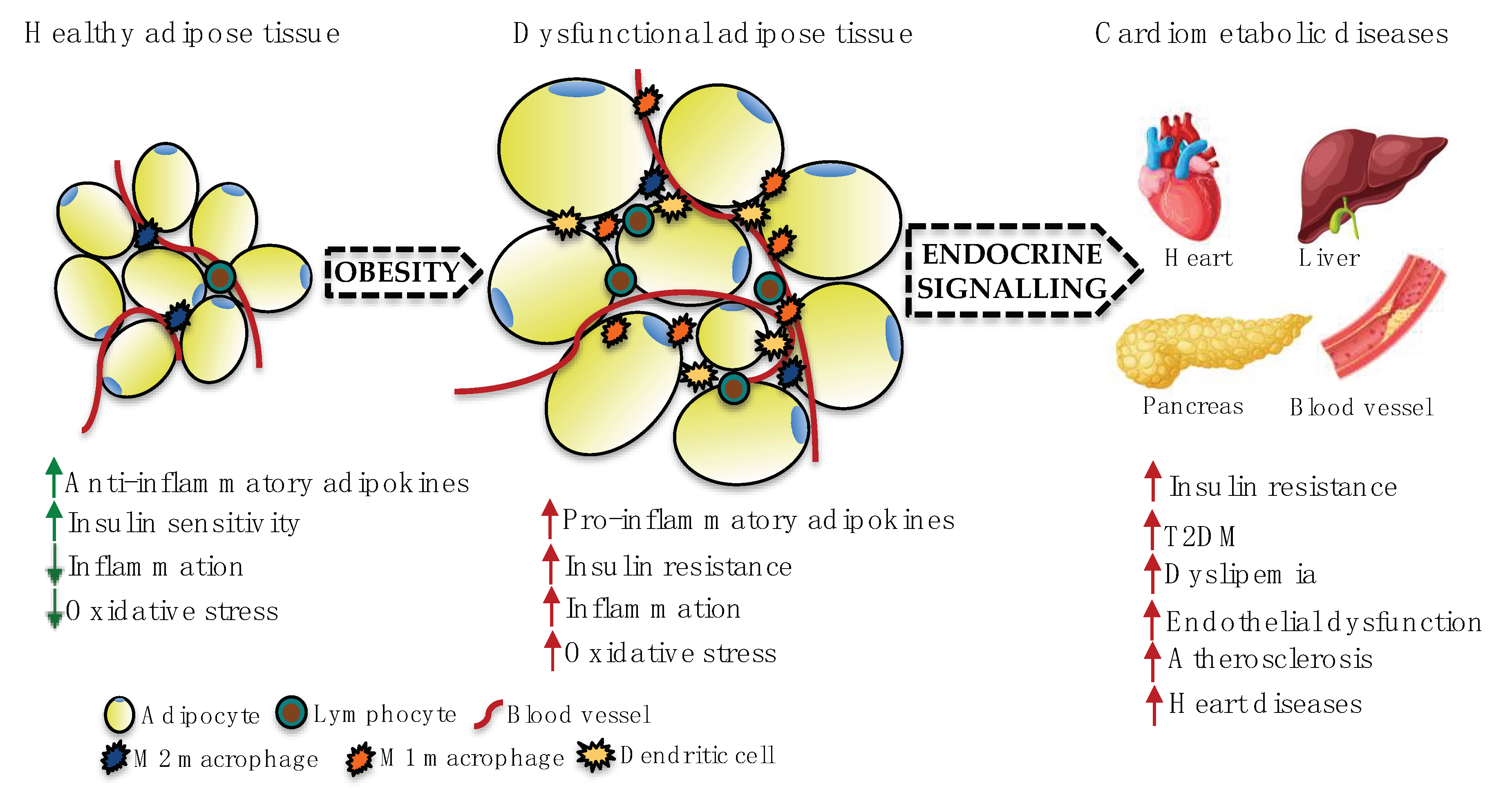
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7711. https://doi.org/10.3390/ijms21207711
Feijóo-Bandín S, Aragón-Herrera A, Moraña-Fernández S, Anido-Varela L, Tarazón E, Roselló-Lletí E, Portolés M, Moscoso I, Gualillo O, González-Juanatey JR, et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. International Journal of Molecular Sciences. 2020; 21(20):7711. https://doi.org/10.3390/ijms21207711
Chicago/Turabian StyleFeijóo-Bandín, Sandra, Alana Aragón-Herrera, Sandra Moraña-Fernández, Laura Anido-Varela, Estefanía Tarazón, Esther Roselló-Lletí, Manuel Portolés, Isabel Moscoso, Oreste Gualillo, José Ramón González-Juanatey, and et al. 2020. "Adipokines and Inflammation: Focus on Cardiovascular Diseases" International Journal of Molecular Sciences 21, no. 20: 7711. https://doi.org/10.3390/ijms21207711
APA StyleFeijóo-Bandín, S., Aragón-Herrera, A., Moraña-Fernández, S., Anido-Varela, L., Tarazón, E., Roselló-Lletí, E., Portolés, M., Moscoso, I., Gualillo, O., González-Juanatey, J. R., & Lago, F. (2020). Adipokines and Inflammation: Focus on Cardiovascular Diseases. International Journal of Molecular Sciences, 21(20), 7711. https://doi.org/10.3390/ijms21207711





