The Protective Effects of Pre- and Post-Administration of Micronized Palmitoylethanolamide Formulation on Postoperative Pain in Rats
Abstract
1. Introduction
2. Results
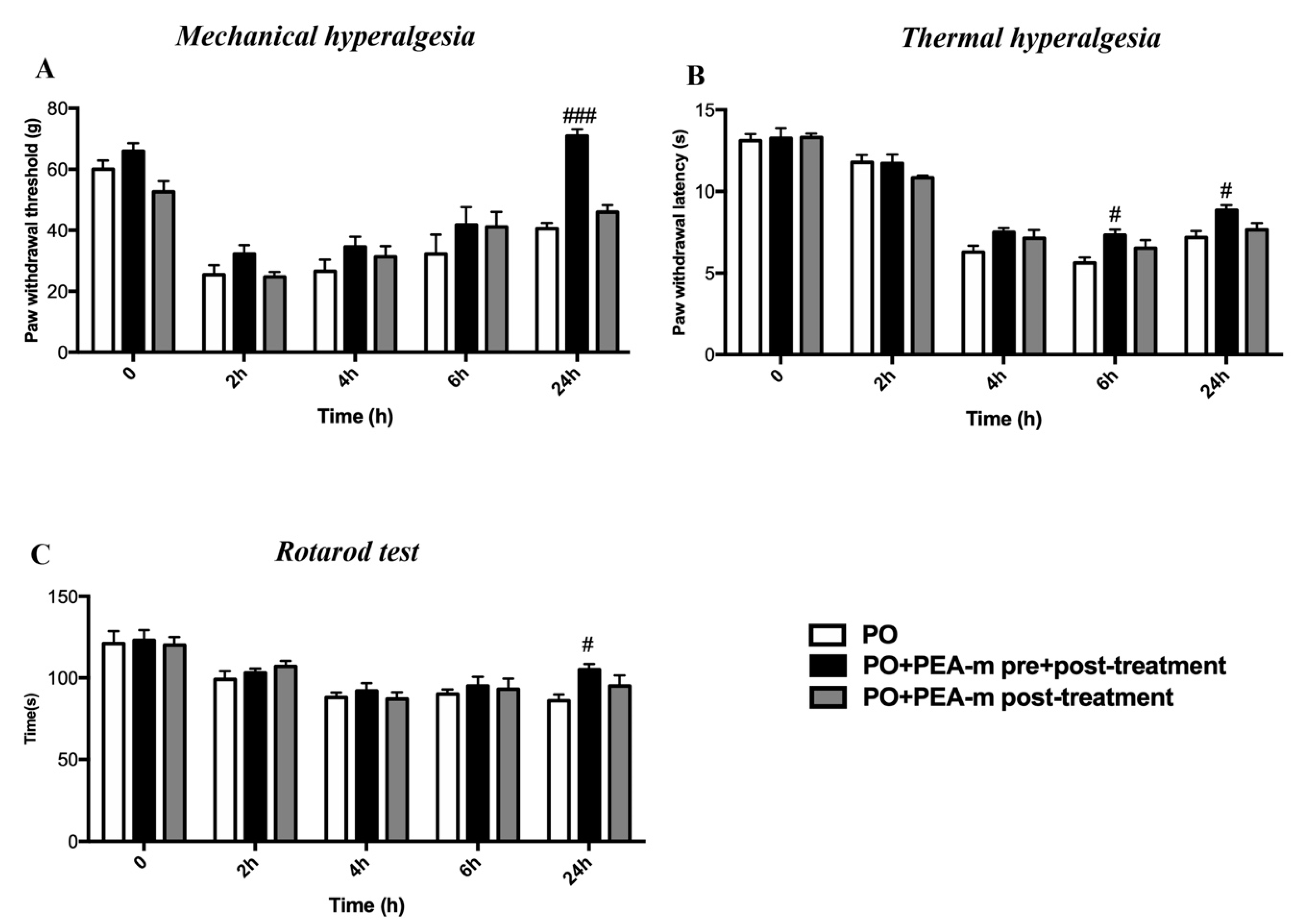
2.1. Treatment with PEA-m Relieved Mechanical Allodynia, Thermal Hyperalgesia, and Motor Coordination in Rat After Hind Paw Incision
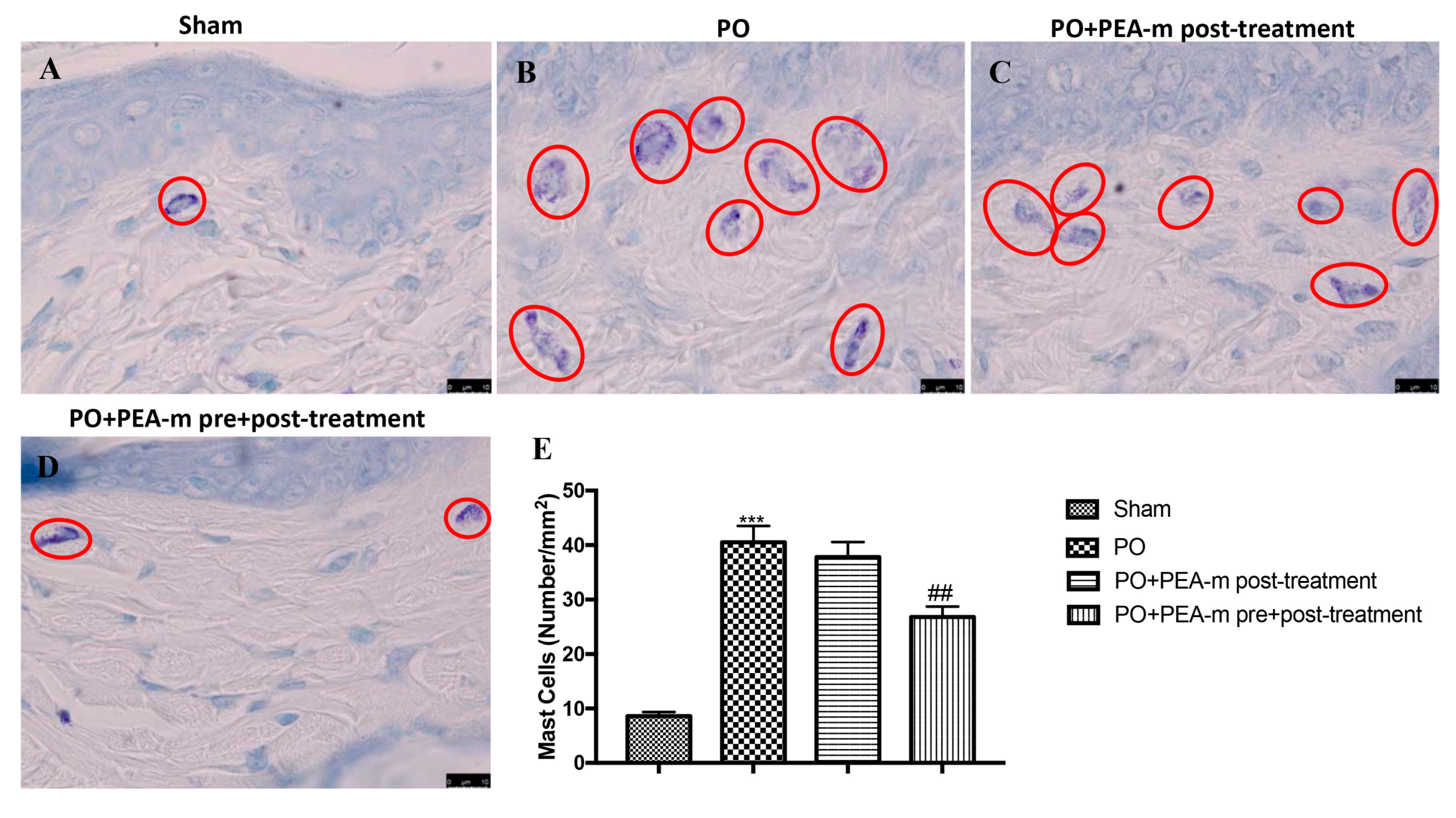
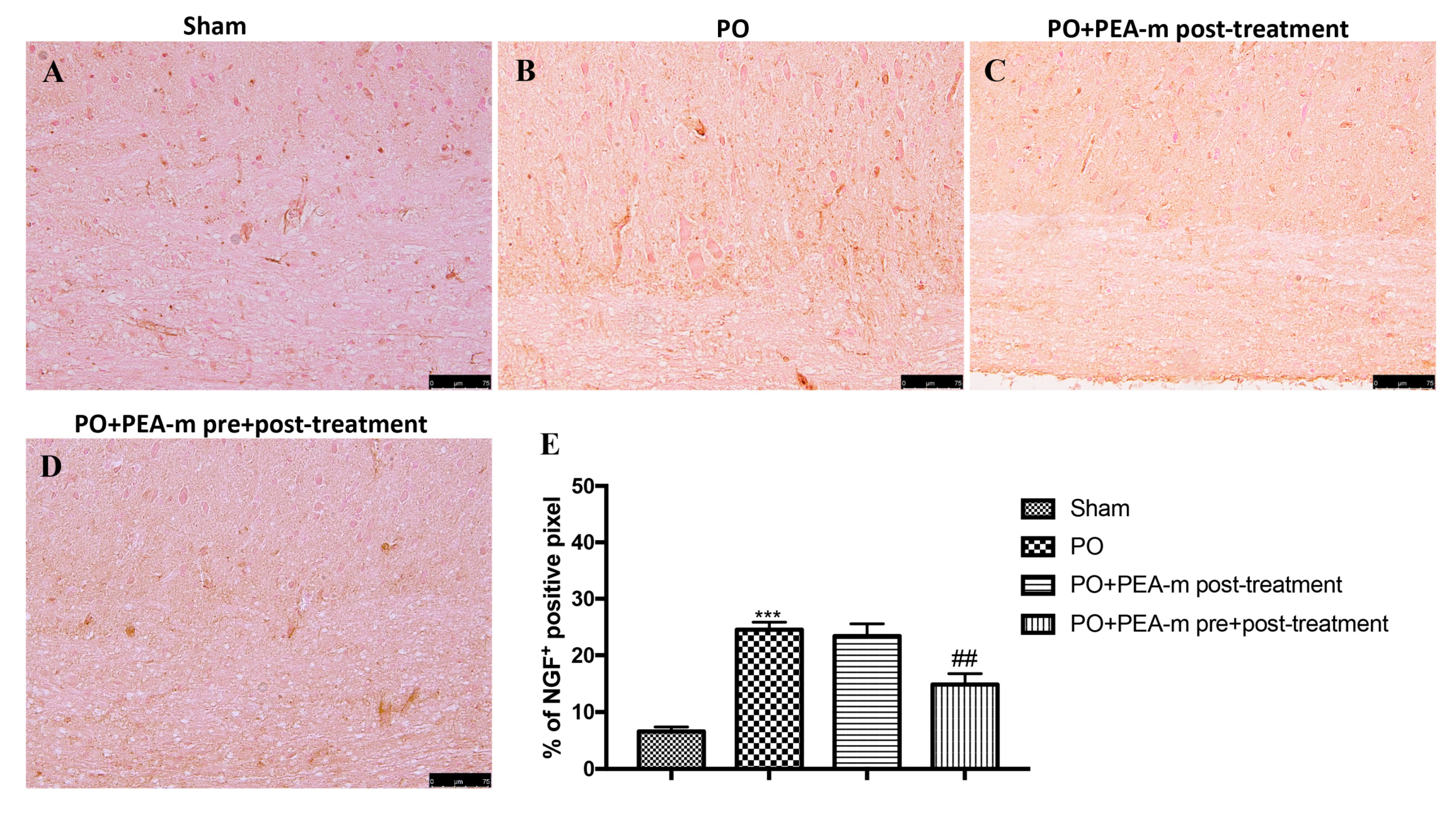
2.2. Effect of PEA-m on Mast Cells (MC) infiltration and NGF Levels
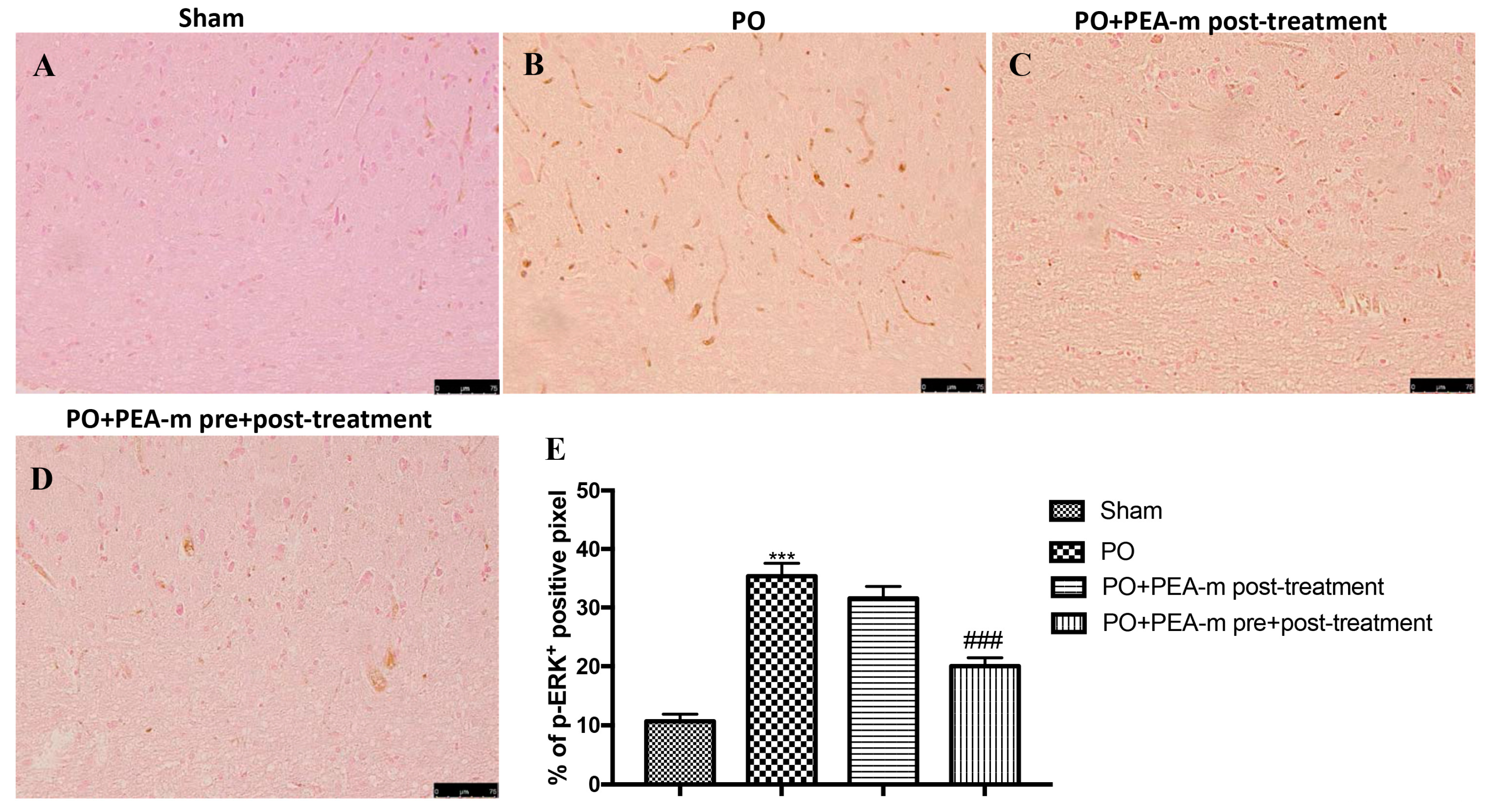
2.3. Effect of PEA-m on p-ERK Levels
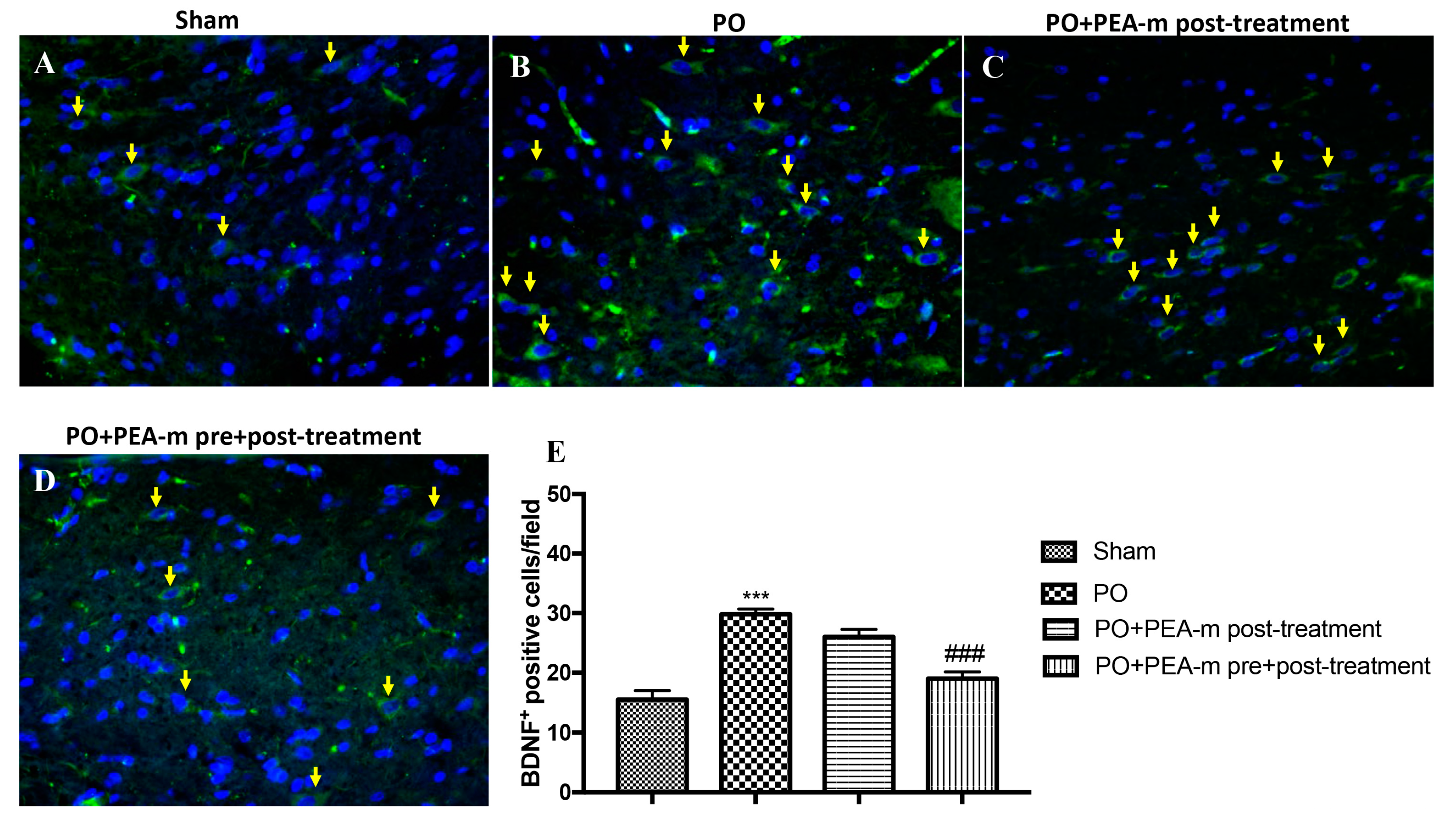
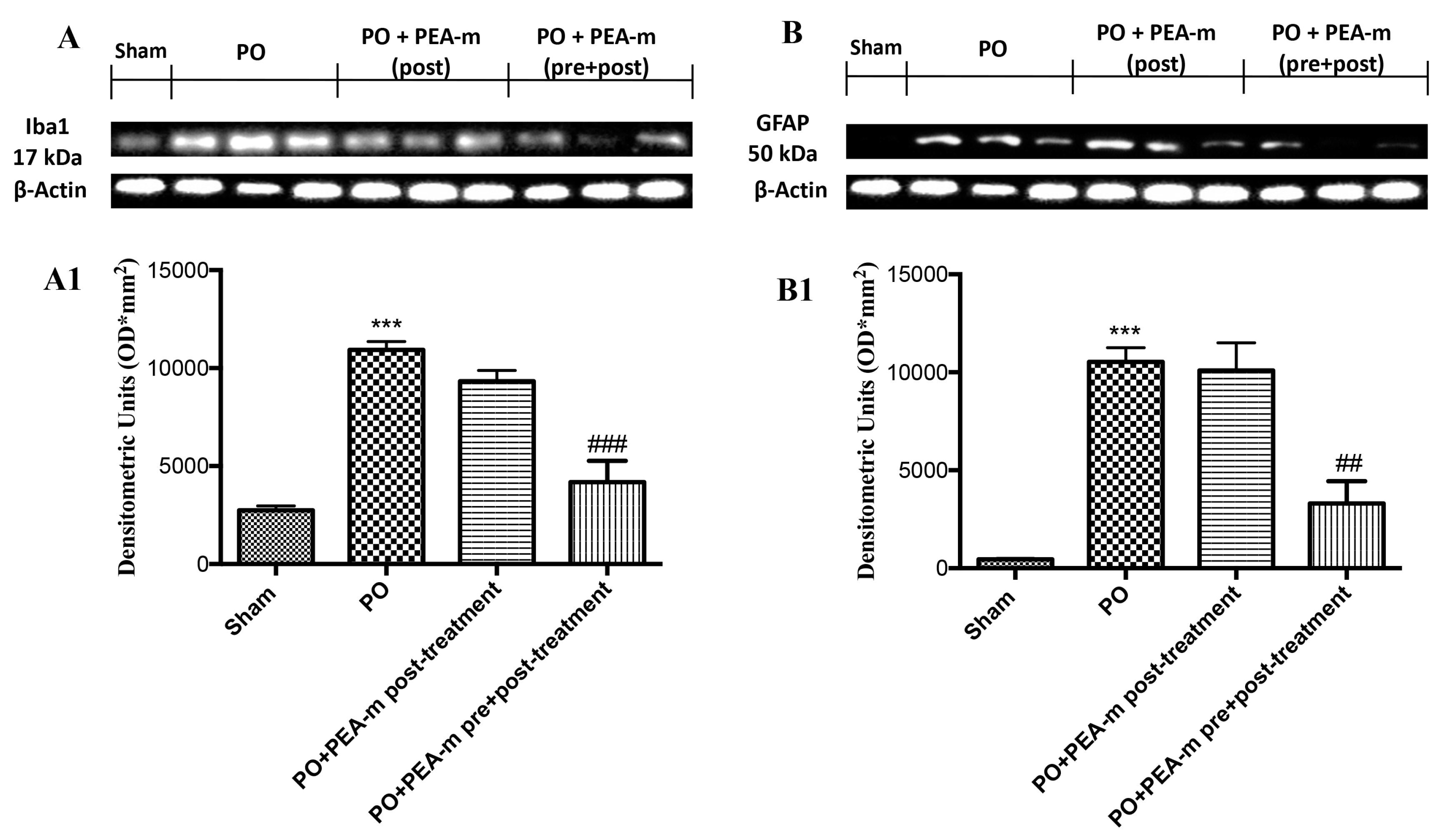
2.4. Effect of PEA-m on Expression Levels of BDNF, Iba1, and GFAP
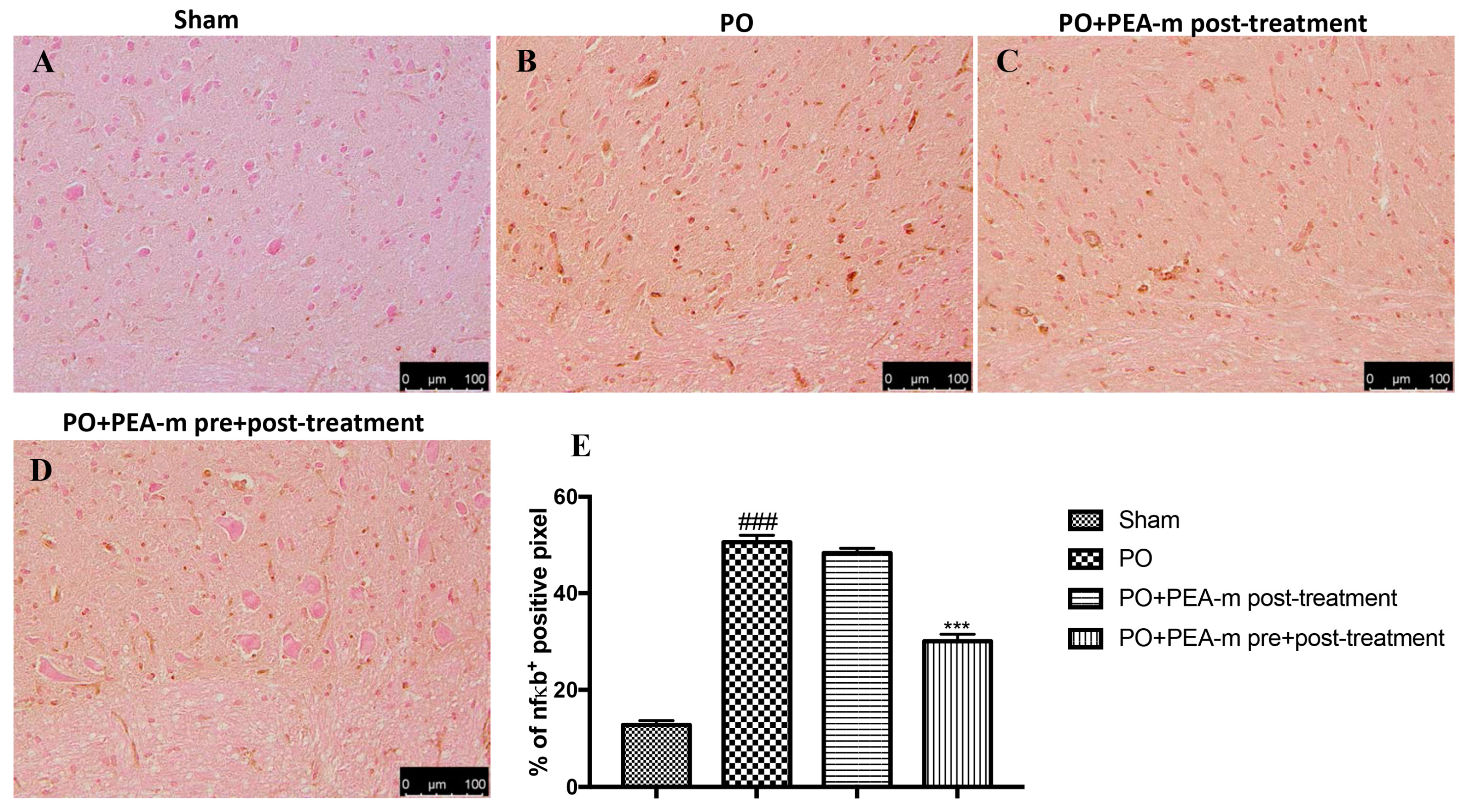
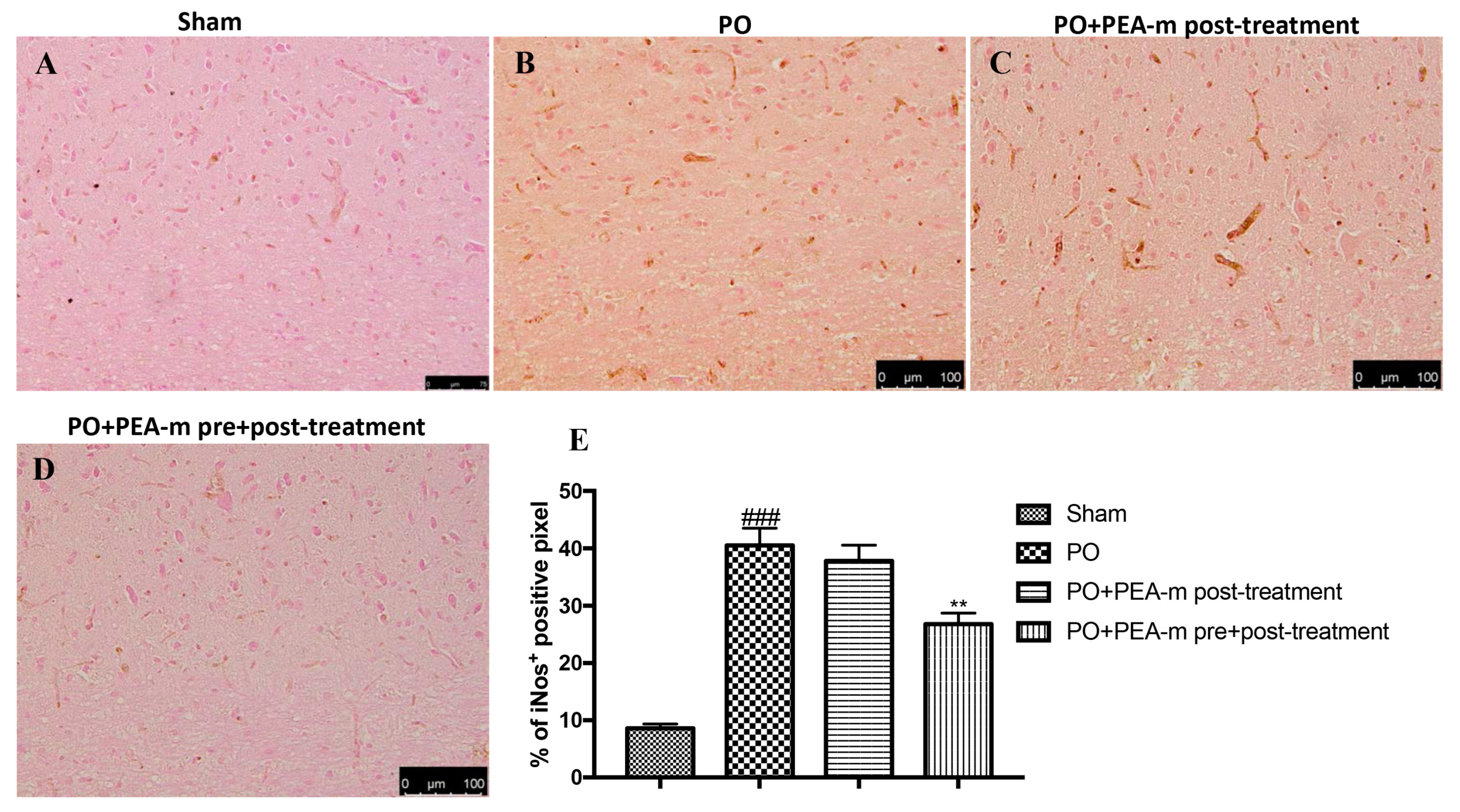
2.5. Effect of PEA-m on NF-κB and iNOS
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug
4.3. Postoperative Pain Model (PO)
4.4. Experimental Groups
- Sham + vehicle: rats in the control group received anesthesia but did not receive an incision. Vehicle solution (1% carboxymethylcellulose and saline) was administrated orally. (N = 10).
- Sham + PEA-m: rats in the control group received anesthesia but did not receive an incision. PEA-m was administrated orally 3 days before hind paw incision and 1, 6, and 8 h after surgery. (N = 10) (data not shown).
- PO + vehicle: after anesthesia, a longitudinal incision was performed on the right hind paw, and the vehicle (1% carboxymethylcellulose and saline) was then administered orally. (N = 10).
- PO + PEA-m post-treatment: the same as the PO + vehicle, but rats were treated with PEA-m (10 mg/kg oral somministration) 1, 6, and 8 h after hind paw incision. (N = 10).
- PO + PEA-m pre + post-treatment: the same as the PO + vehicle group, but rats were treated with PEA-m (10 mg/kg oral somministration) 3 days before hind paw incision and 1, 6, and 8 h after surgery. (N = 10).
4.5. Behavioral Analysis
4.5.1. Mechanical Hyperalgesia
4.5.2. Thermal Hyperalgesia
4.5.3. Rotarod Test
4.6. Toluidine Blue Staining
4.7. Immunostaining of NGF, p-ERK, NF-kB, and iNOS
4.8. Immunofluorescence for BDNF
4.9. Western Blot Analysis for GFAP and Iba1
4.10. Statistical Evaluation
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PO | Postoperative pain |
| PEA-m | Micronized palmitoylethanolamide |
| NGF | Nerve growth factor |
| p-ERK | Phospho-extracellular signal-regulated kinases |
| Iba1 | Ionized calcium binding adaptor molecule 1 |
| GFAP | Glial fibrillary acidic protein |
| BDNF | Brain-derived neurotrophic factor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| CNS | Central nervous system |
References
- Grichnik, K.P.; Ferrante, F.M. The difference between acute and chronic pain. Mt. Sinai J. Med. 1991, 58, 217–220. [Google Scholar] [PubMed]
- Brennan, T.J.; Vandermeulen, E.P.; Gebhart, G.F. Characterization of a rat model of incisional pain. Pain 1996, 64, 493–501. [Google Scholar] [CrossRef]
- Zahn, P.K.; Pogatzki-Zahn, E.M.; Brennan, T.J. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain 2005, 114, 499–510. [Google Scholar] [CrossRef]
- Weiser, T.G.; Regenbogen, S.E.; Thompson, K.D.; Haynes, A.B.; Lipsitz, S.R.; Berry, W.R.; Gawande, A.A. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet 2008, 372, 139–144. [Google Scholar] [CrossRef]
- Itoh, H.; Kitamura, F.; Yokoyama, K. Estimates of annual medical costs of work-related low back pain in Japan. Ind. Health 2013, 51, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Gandhi, K.; Heitz, J.W.; Viscusi, E.R. Challenges in acute pain management. Anesthesiol. Clin. 2011, 29, 291–309. [Google Scholar] [CrossRef]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Wu, C.L.; Raja, S.N. Treatment of acute postoperative pain. Lancet 2011, 377, 2215–2225. [Google Scholar] [CrossRef]
- Kissin, I. The development of new analgesics over the past 50 years: A lack of real breakthrough drugs. Anesth. Analg. 2010, 110, 780–789. [Google Scholar] [CrossRef]
- Dahl, J.B.; Kehlet, H. [Treatment of postoperative pain—A status report]. Ugeskr. Laeger 2006, 168, 1986–1988. [Google Scholar]
- Alkaitis, M.S.; Solorzano, C.; Landry, R.P.; Piomelli, D.; DeLeo, J.A.; Romero-Sandoval, E.A. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PLoS ONE 2010, 5, e10891. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef]
- Burston, J.J.; Woodhams, S.G. Conference on ‘PUFA mediators: Implications for human health’ Symposium 3: Cannabinoids in human health Endocannabinoid system and pain: An introduction. Proc. Nutr. Soc. 2014, 73, 106–117. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Jonsson, K.O.; Vandevoorde, S.; Lambert, D.M.; Tiger, G.; Fowler, C.J. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br. J. Pharmacol. 2001, 133, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Iuvone, T.; Di Marzo, V. N-palmitoyl-ethanolamine: Biochemistry and new therapeutic opportunities. Biochimie 2010, 92, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Di Marzo, V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell Introduction. Philos. Trans. R. Soc. B 2012, 367, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Mazzari, S.; Canella, R.; Petrelli, L.; Marcolongo, G.; Leon, A. N-(2-Hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996, 300, 227–236. [Google Scholar] [CrossRef]
- Capasso, R.; Izzo, A.A.; Fezza, F.; Pinto, A.; Capasso, F.; Mascolo, N.; Di Marzo, V. Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br. J. Pharmacol. 2001, 134, 945–950. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; D’Alessandro, A.; Iuvone, T.; Capoccia, E.; Gigli, S.; Pesce, M.; Seguella, L.; Nobile, N.; Aprea, G.; Maione, F.; et al. Palmitoylethanolamide Modulates Inflammation-Associated Vascular Endothelial Growth Factor (VEGF) Signaling via the Akt/mTOR Pathway in a Selective Peroxisome Proliferator-Activated Receptor Alpha (PPAR-alpha)-Dependent Manner. PLoS ONE 2016, 11, e0156198. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tuting, T.; Bisogno, T.; De Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef]
- Karsak, M.; Gaffal, E.; Date, R.; Wang-Eckhardt, L.; Rehnelt, J.; Petrosino, S.; Starowicz, K.; Steuder, R.; Schlicker, E.; Cravatt, B.; et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 2007, 316, 1494–1497. [Google Scholar] [CrossRef]
- Abramo, F.; Campora, L.; Albanese, F.; della Valle, M.F.; Cristino, L.; Petrosino, S.; Di Marzo, V.; Miragliotta, V. Increased levels of palmitoylethanolamide and other bioactive lipid mediators and enhanced local mast cell proliferation in canine atopic dermatitis. BMC Vet. Res. 2014, 10, 21. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Ahmad, A.; Bruschetta, G.; Di Paola, R.; Crupi, R.; Paterniti, I.; Esposito, E.; Cuzzocrea, S. The anti-inflammatory effects of palmitoylethanolamide (PEA) on endotoxin-induced uveitis in rats. Eur. J. Pharmacol. 2015, 761, 28–35. [Google Scholar] [CrossRef]
- Paterniti, I.; Di Paola, R.; Campolo, M.; Siracusa, R.; Cordaro, M.; Bruschetta, G.; Tremolada, G.; Maestroni, A.; Bandello, F.; Esposito, E.; et al. Palmitoylethanolamide treatment reduces retinal inflammation in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2015, 769, 313–323. [Google Scholar] [CrossRef]
- Pessina, F.; Capasso, R.; Borrelli, F.; Aveta, T.; Buono, L.; Valacchi, G.; Fiorenzani, P.; Di Marzo, V.; Orlando, P.; Izzo, A.A. Protective effect of palmitoylethanolamide in a rat model of cystitis. J. Urol. 2015, 193, 1401–1408. [Google Scholar] [CrossRef]
- Jaggar, S.I.; Hasnie, F.S.; Sellaturay, S.; Rice, A.S.C. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain 1998, 76, 189–199. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Jaggar, S.I.; Rice, A.S.C. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB1 and CB2-like receptors. Pain 2002, 97, 11–21. [Google Scholar] [CrossRef]
- Haller, V.L.; Cichewicz, D.L.; Welch, S.P. Non-cannabinoid CB1, non-cannabinoid CB2 antinociceptive effects of several novel compounds in the PPQ stretch test in mice. Eur. J. Pharmacol. 2006, 546, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Comelli, F.; Bettoni, I.; Colleoni, M.; Giagnoni, G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: Involvement of CB1, TRPV1 and PPAR gamma receptors and neurotrophic factors. Pain 2008, 139, 541–550. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; D’Amico, A.; Cipriano, M.; Petrosino, S.; Orlando, P.; Di Marzo, V.; Iuvone, T.; Grp, E.R. Levels of endocannabinoids and palmitoylethanolamide and their pharmacological manipulation in chronic granulomatous inflammation in rats. Pharmacol. Res. 2010, 61, 321–328. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Luongo, L.; Cipriano, M.; Palazzo, E.; Cinelli, M.P.; de Novellis, V.; Maione, S.; Iuvone, T. Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol. Pain 2011, 7. [Google Scholar] [CrossRef]
- Luongo, L.; Guida, F.; Boccella, S.; Bellini, G.; Gatta, L.; Rossi, F.; de Novellis, V.; Maione, S. Palmitoylethanolamide Reduces Formalin-Induced Neuropathic-Like Behaviour Through Spinal Glial/Microglial Phenotypical Changes in Mice. CNS Neurol. Disord. Drug Targets 2013, 12, 45–54. [Google Scholar] [CrossRef]
- Mannelli, L.D.; D’Agostino, G.; Pacini, A.; Russo, R.; Zanardelli, M.; Ghelardini, C.; Calignano, A. Palmitoylethanolamide Is a Disease-Modifying Agent in Peripheral Neuropathy: Pain Relief and Neuroprotection Share a PPAR-Alpha-Mediated Mechanism. Mediat. Inflamm. 2013. [Google Scholar] [CrossRef]
- Seol, T.K.; Lee, W.; Park, S.; Kim, K.N.; Kim, T.Y.; Oh, Y.N.; Jun, J.H. Effect of palmitoylethanolamide on inflammatory and neuropathic pain in rats. Korean J. Anesthesiol. 2017, 70, 561–566. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S.; et al. The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J. 2019, 33, 11364–11380. [Google Scholar] [CrossRef]
- Nestmann, E.R. Safety of micronized palmitoylethanolamide (microPEA): Lack of toxicity and genotoxic potential. Food Sci. Nutr. 2017, 5, 292–309. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Zhdanov, A.V.; Mallel, G.; Dinan, T.G.; Cryan, J.F. The mouse cyclophosphamide model of bladder pain syndrome: Tissue characterization, immune profiling, and relationship to metabotropic glutamate receptors. Physiol. Rep. 2014, 2, e00260. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Overcoming obstacles to developing new analgesics. Nat. Med. 2010, 16, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J. Pathophysiology of postoperative pain. Pain 2011, 152, S33–S40. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F.; Goehler, L.E. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 1995, 63, 289–302. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflamm. 2014, 11, 136. [Google Scholar] [CrossRef]
- Bartolucci, M.L.; Marini, I.; Bortolotti, F.; Impellizzeri, D.; Di Paola, R.; Bruschetta, G.; Crupi, R.; Portelli, M.; Militi, A.; Oteri, G.; et al. Micronized palmitoylethanolamide reduces joint pain and glial cell activation. Inflamm. Res. 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, T.; Abazov, V.M.; Abbott, B.; Acharya, B.S.; Adams, M.; Adams, T.; Agnew, J.P.; Alexeev, G.D.; Alkhazov, G.; Alton, A.; et al. Tevatron Combination of Single-Top-Quark Cross Sections and Determination of the Magnitude of the Cabibbo-Kobayashi-Maskawa Matrix Element V_{tb}. Phys. Rev. Lett. 2015, 115, 152003. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Casili, G.; Evangelista, M.; Cuzzocrea, S. N-palmitoylethanolamide Prevents Parkinsonian Phenotypes in Aged Mice. Mol. Neurobiol. 2018, 55, 8455–8472. [Google Scholar] [CrossRef]
- Passavanti, M.B.; Alfieri, A.; Pace, M.C.; Pota, V.; Sansone, P.; Piccinno, G.; Barbarisi, M.; Aurilio, C.; Fiore, M. Clinical applications of palmitoylethanolamide in pain management: Protocol for a scoping review. Syst. Rev. 2019, 8, 9. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Micheli, L.; Lucarini, E.; Ghelardini, C. Ultramicronized N-Palmitoylethanolamine Supplementation for Long-Lasting, Low-Dosed Morphine Antinociception. Front. Pharmacol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Petrosino, S.; Cordaro, M.; Verde, R.; Moriello, A.S.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Stubhaug, A.; Breivik, H.; Eide, P.K.; Kreunen, M.; Foss, A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol. Scand. 1997, 41, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Fletcher, D.; Bouhassira, D.; Sessler, D.I.; Chauvin, M. The evolution of primary hyperalgesia in orthopedic surgery: Quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth. Analg. 2007, 105, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Brennan, T.J. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain 2009, 144, 329–339. [Google Scholar] [CrossRef]
- Xu, J.; Brennan, T.J. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 2010, 112, 153–164. [Google Scholar] [CrossRef]
- Spofford, C.M.; Brennan, T.J. Gene expression in skin, muscle, and dorsal root ganglion after plantar incision in the rat. Anesthesiology 2012, 117, 161–172. [Google Scholar] [CrossRef]
- Xu, J.; Richebe, P.; Brennan, T.J. Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. Eur. J. Pain 2009, 13, 820–828. [Google Scholar] [CrossRef]
- Pogatzki, E.M.; Gebhart, G.F.; Brennan, T.J. Characterization of Adelta—and C-fibers innervating the plantar rat hindpaw one day after an incision. J. Neurophysiol. 2002, 87, 721–731. [Google Scholar] [CrossRef]
- Mills, C.D.; Nguyen, T.; Tanga, F.Y.; Zhong, C.; Gauvin, D.M.; Mikusa, J.; Gomez, E.J.; Salyers, A.K.; Bannon, A.W. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur. J. Pain 2013, 17, 469–479. [Google Scholar] [CrossRef]
- Osikowicz, M.; Longo, G.; Allard, S.; Cuello, A.C.; Ribeiro-da-Silva, A. Inhibition of endogenous NGF degradation induces mechanical allodynia and thermal hyperalgesia in rats. Mol. Pain 2013, 9, 37. [Google Scholar] [CrossRef]
- Vadivelu, N.; Mitra, S.; Narayan, D. Recent advances in postoperative pain management. Yale J. Biol. Med. 2010, 83, 11–25. [Google Scholar]
- Wilder-Smith, O.H.; Arendt-Nielsen, L. Postoperative hyperalgesia: Its clinical importance and relevance. Anesthesiology 2006, 104, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.D.; Shi, X.; Li, X.; Qiao, Y.; Liang, D.; Angst, M.S.; Yeomans, D.C. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol. Pain 2007, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- De Winter, B.Y.; van den Wijngaard, R.M.; de Jonge, W.J. Intestinal mast cells in gut inflammation and motility disturbances. Biochim. Biophys. Acta 2012, 1822, 66–73. [Google Scholar] [CrossRef]
- Weller, K.; Foitzik, K.; Paus, R.; Syska, W.; Maurer, M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006, 20, 2366–2368. [Google Scholar] [CrossRef]
- Indraccolo, U.; Barbieri, F. Effect of palmitoylethanolamide-polydatin combination on chronic pelvic pain associated with endometriosis: Preliminary observations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 150, 76–79. [Google Scholar] [CrossRef]
- Murina, F.; Graziottin, A.; Felice, R.; Radici, G.; Tognocchi, C. Vestibulodynia: Synergy between palmitoylethanolamide + transpolydatin and transcutaneous electrical nerve stimulation. J. Low Genit. Tract. Dis. 2013, 17, 111–116. [Google Scholar] [CrossRef]
- Hunt, S.P.; Mantyh, P.W. The molecular dynamics of pain control. Nature reviews. Neuroscience 2001, 2, 83–91. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002, 82, 981–1011. [Google Scholar] [CrossRef]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Boustany, L.; Liang, H.; Brennan, T.J. Nerve growth factor expression after plantar incision in the rat. Anesthesiology 2007, 107, 128–135. [Google Scholar] [CrossRef]
- Campillo, A.; Gonzalez-Cuello, A.; Cabanero, D.; Garcia-Nogales, P.; Romero, A.; Milanes, M.V.; Laorden, M.L.; Puig, M.M. Increased spinal dynorphin levels and phospho-extracellular signal-regulated kinases 1 and 2 and c-Fos immunoreactivity after surgery under remifentanil anesthesia in mice. Mol. Pharmacol. 2010, 77, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, I.; Reichl, S.; Segelcke, D.; Zahn, P.K.; Pogatzki-Zahn, E.M. Selective prevention of mechanical hyperalgesia after incision by spinal ERK1/2 inhibition. Eur. J. Pain 2015, 19, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Masaki, E.; Mizuta, K.; Ohtani, N.; Kido, K. Early Postoperative Nociceptive Threshold and Production of Brain-Derived Neurotrophic Factor Induced by Plantar Incision Are Not Influenced with Minocycline in a Rat: Role of Spinal Microglia. Neurosignals 2016, 24, 15–24. [Google Scholar] [CrossRef]
- Lee, K.M.; Kang, B.S.; Lee, H.L.; Son, S.J.; Hwang, S.H.; Kim, D.S.; Park, J.S.; Cho, H.J. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci. 2004, 19, 3375–3381. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Impellizzeri, D.; Di Paola, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. A new co-ultramicronized composite including palmitoylethanolamide and luteolin to prevent neuroinflammation in spinal cord injury. J. Neuroinflamm. 2013, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Reddi, D. Preventing chronic postoperative pain. Anaesthesia 2016, 71, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Thorn, D.A.; Qiu, Y.; Peng, B.W.; Zhang, Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Br. J. Pharmacol. 2014, 171, 1580–1590. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Rozas, G.; Guerra, M.J.; Labandeira-Garcia, J.L. An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res. Brain Res. Protoc. 1997, 2, 75–84. [Google Scholar] [CrossRef]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Gugliandolo, E.; Peritore, A.F.; Di Paola, R.; Cuzzocrea, S. Topical Application of Adelmidrol + Trans-Traumatic Acid Enhances Skin Wound Healing in a Streptozotocin-Induced Diabetic Mouse Model. Front. Pharmacol. 2018, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Crupi, R.; Campolo, M.; Genovese, T.; Esposito, E.; Cuzzocrea, S. Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS ONE 2013, 8, e57208. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Ahmad, A.; Di Paola, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of Toll like receptor 4 signaling pathway in the secondary damage induced by experimental spinal cord injury. Immunobiology 2015, 220, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, R.; Fusco, R.; Cordaro, M.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Crupi, R.; Genovese, T.; Evangelista, M.; Di Paola, R.; et al. The Protective Effects of Pre- and Post-Administration of Micronized Palmitoylethanolamide Formulation on Postoperative Pain in Rats. Int. J. Mol. Sci. 2020, 21, 7700. https://doi.org/10.3390/ijms21207700
Siracusa R, Fusco R, Cordaro M, Peritore AF, D’Amico R, Gugliandolo E, Crupi R, Genovese T, Evangelista M, Di Paola R, et al. The Protective Effects of Pre- and Post-Administration of Micronized Palmitoylethanolamide Formulation on Postoperative Pain in Rats. International Journal of Molecular Sciences. 2020; 21(20):7700. https://doi.org/10.3390/ijms21207700
Chicago/Turabian StyleSiracusa, Rosalba, Roberta Fusco, Marika Cordaro, Alessio F. Peritore, Ramona D’Amico, Enrico Gugliandolo, Rosalia Crupi, Tiziana Genovese, Maurizio Evangelista, Rosanna Di Paola, and et al. 2020. "The Protective Effects of Pre- and Post-Administration of Micronized Palmitoylethanolamide Formulation on Postoperative Pain in Rats" International Journal of Molecular Sciences 21, no. 20: 7700. https://doi.org/10.3390/ijms21207700
APA StyleSiracusa, R., Fusco, R., Cordaro, M., Peritore, A. F., D’Amico, R., Gugliandolo, E., Crupi, R., Genovese, T., Evangelista, M., Di Paola, R., Cuzzocrea, S., & Impellizzeri, D. (2020). The Protective Effects of Pre- and Post-Administration of Micronized Palmitoylethanolamide Formulation on Postoperative Pain in Rats. International Journal of Molecular Sciences, 21(20), 7700. https://doi.org/10.3390/ijms21207700












