Global Analyses of Expressed Piwi-Interacting RNAs in Gastric Cancer
Abstract
1. Introduction
2. Results
2.1. Differential Expression Analysis
2.2. Identification of the Cancer-Related piRNAs Differentially Expressed Target Genes
3. Discussion
4. Materials and Methods
4.1. Biological Material and Clinical Data Collection
4.2. Total RNA Extraction
4.3. piRNA Library Preparation
4.4. NGS Reads Alignment and Quality Control
4.5. Differential Expression Analysis
4.6. Validation of DE piRNAs by qRT-PCR
4.7. Identification of DE piRNA Target RNAs
4.8. Dataset Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADJ | Samples of matched non-tumoral tumor-adjacent gastric tissue |
| AUC | Area Under the Curve (ROC analysis) |
| CAMs | Cell Adhesion Molecules |
| DE | Differentially Expressed |
| EMT | Epithelial-Mesenchymal Transition |
| GC | Gastric Cancer |
| KEGG | Kyotto Encyclopedia of Genes and Genomes |
| miRNA | MicroRNA |
| NC | Non-Cancer tissue |
| NGS | Next Generation Sequencing |
| piRNA | Piwi-Interacting RNA |
| pi-RISC | piRNA-mediated RNA silencing complex |
| qRT-PCR | Quantitative Real-Time PCR |
| sncRNA | Small non-coding RNA |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.; Le, L.C.; Pham, Y.T.-H.; Borron, C.; Park, J.Y.; Tran, C.T.D.; Tran, T.V.; Tran, H.T.-T.; Vu, K.T.; Do, C.D.; et al. Gastric cancer: epidemiology, biology, and prevention. Eur. J. of Cancer Prev. 2019, 28, 397–412. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; El-Omar, E.M. Genetics of gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 664–674. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Z.; Guo, W.; Li, J. Gene mutations in gastric cancer: a review of recent next-generation sequencing studies. Tumor Biol. 2015, 36, 7385–7394. [Google Scholar] [CrossRef]
- Pan, X.; Ji, X.; Zhang, R.; Zhou, Z.; Zhong, Y.; Peng, W.; Sun, N.; Xu, X.; Xia, L.; Li, P.; et al. Landscape of somatic mutations in gastric cancer assessed using next-generation sequencing analysis. Oncol. Lett. 2018. [Google Scholar] [CrossRef]
- Strand, M.S.; Lockhart, A.C.; Fields, R.C. Genetics of Gastric Cancer. Surg. Clin. of N. Am. 2017, 97, 345–370. [Google Scholar] [CrossRef]
- Astudillo, P. Wnt5a Signaling in Gastric Cancer. Front Cell. Dev. Biol. 2020, 8, 110. [Google Scholar] [CrossRef]
- Baba, Y.; Ishimoto, T.; Kurashige, J.; Iwatsuki, M.; Sakamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Epigenetic field cancerization in gastrointestinal cancers. Cancer Lett. 2016, 375, 360–366. [Google Scholar] [CrossRef]
- Pereira, A.L.; Magalhães, L.; Moreira, F.C.; Reis-das-Mercês, L.; Vidal, A.F.; Ribeiro-dos-Santos, A.M.; Demachki, S.; Anaissi, A.K.M.; Burbano, R.M.R.; Albuquerque, P.; et al. Epigenetic Field Cancerization in Gastric Cancer: microRNAs as Promising Biomarkers. J. Cancer 2019, 10, 1560–1569. [Google Scholar] [CrossRef]
- Figueiredo, C.; Camargo, M.C.; Leite, M.; Fuentes-Pananá, E.M.; Rabkin, C.S.; Machado, J.C. Pathogenesis of Gastric Cancer: Genetics and Molecular Classification. In Molecular Pathogenesis and Signal Transduction by Helicobacter Pylori; Tegtmeyer, N., Backert, S., Eds.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2017; Volume 400, pp. 277–304. ISBN 978-3-319-50519-0. [Google Scholar]
- Patel, T.N.; Roy, S.; Ravi, R. Gastric cancer and related epigenetic alterations. Ecancermedicalscience 2017, 11, 714. [Google Scholar] [CrossRef]
- Pereira, A.; Moreira, F.; Vinasco-Sandoval, T.; Cunha, A.; Vidal, A.; Ribeiro-dos-Santos, A.M.; Pinto, P.; Magalhães, L.; Assumpção, M.; Demachki, S.; et al. miRNome Reveals New Insights Into the Molecular Biology of Field Cancerization in Gastric Cancer. Front. Genet. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.; Quintana, L.G.; Lopes, D.C.F.; Vidal, A.F.; Pereira, A.L.; D’Araujo Pinto, L.C.; de Jesus Viana Pinheiro, J.; Khayat, A.S.; Goulart, L.R.; Burbano, R.; et al. APC gene is modulated by hsa-miR-135b-5p in both diffuse and intestinal gastric cancer subtypes. BMC Cancer 2018, 18, 1055. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.-B.; He, Y.-F.; Li, X.-Q.; Wang, K.; Wang, R.-L. The role of miRNA and lncRNA in gastric cancer. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.F.; Cruz, A.M.P.; Magalhães, L.; Pereira, A.L.; Anaissi, A.K.M.; Alves, N.C.F.; Albuquerque, P.J.B.S.; Burbano, R.M.R.; Demachki, S.; Ribeiro-dos-Santos, Â. hsa-miR-29c and hsa-miR-135b differential expression as potential biomarker of gastric carcinogenesis. World J. Gastroenterol. 2016, 22, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Assumpção, M.B.; Hamoy, I.G.; Darnet, S.; Burbano, R.; Khayat, A.; Gonçalves, A.N.; Alencar, D.O.; Cruz, A.; Magalhães, L.; et al. MiRNA Expression Profile for the Human Gastric Antrum Region Using Ultra-Deep Sequencing. PLoS ONE 2014, 9, e92300. [Google Scholar] [CrossRef]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006. [Google Scholar] [CrossRef]
- Grivna, S.T.; Beyret, E.; Wang, Z.; Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006. [Google Scholar] [CrossRef]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006. [Google Scholar] [CrossRef]
- Kim, V.N. Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev. 2006, 20, 1993–1997. [Google Scholar] [CrossRef]
- Ku, H.-Y.; Lin, H. PIWI proteins and their interactors in piRNA biogenesis, germline development and gene expression. Nat. Sci. Rev. 2014, 1, 205–218. [Google Scholar] [CrossRef]
- Chalbatani, G.M.; Dana, H.; Memari, F.; Gharagozlou, E.; Ashjaei, S.; Kheirandish, P.; Marmari, V.; Mahmoudzadeh, H.; Mozayani, F.; Maleki, A.R.; et al. Biological function and molecular mechanism of piRNA in cancer. Pract. Lab. Med. 2019, 13, e00113. [Google Scholar] [CrossRef] [PubMed]
- Fonseca Cabral, G.; Azevedo Dos Santos Pinheiro, J.; Vidal, A.F.; Santos, S.; Ribeiro-Dos-Santos, Â. piRNAs in Gastric Cancer: A New Approach Towards Translational Research. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. The epigenetic regulation of transposable elements by PIWI-interacting RNAs in Drosophila. Genes Genet. Syst. 2013, 88, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Siomi, M.C.; Sato, K.; Pezic, D.; Aravin, A.A. PIWI-interacting small RNAs: the vanguard of genome defence. Nat. Rev. Mol. 2011, 12, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.A.; Yin, H.; Sweeney, S.; Raha, D.; Snyder, M.; Lin, H. A Major Epigenetic Programming Mechanism Guided by piRNAs. Dev. Cell 2013, 24, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Robine, N.; Lau, N.C.; Balla, S.; Jin, Z.; Okamura, K.; Kuramochi-Miyagawa, S.; Blower, M.D.; Lai, E.C. A Broadly Conserved Pathway Generates 3′UTR-Directed Primary piRNAs. Curr. Bio. 2009, 19, 2066–2076. [Google Scholar] [CrossRef]
- Watanabe, T.; Lin, H. Posttranscriptional Regulation of Gene Expression by Piwi Proteins and piRNAs. Mol. Cell 2014, 56, 18–27. [Google Scholar] [CrossRef]
- Rojas-Ríos, P.; Simonelig, M. piRNAs and PIWI proteins: regulators of gene expression in development and stem cells. Development 2018, 145, dev161786. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Asemi, Z. Epigenetic roles of PIWI proteins and piRNAs in lung cancer. Cell Biosci. 2019, 9, 102. [Google Scholar] [CrossRef]
- Guo, B.; Li, D.; Du, L.; Zhu, X. piRNAs: biogenesis and their potential roles in cancer. Cancer Metastasis Rev. 2020. [Google Scholar] [CrossRef]
- Hyun, S. Small RNA Pathways That Protect the Somatic Genome. Int. J. Mol. Sci. 2017, 18, 912. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.J.; Weiner, M.M.; Lin, H. PIWI proteins and PIWI-interacting RNAs in the soma. Nature 2014, 505, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Song, L.; Liu, C.; Lv, X.; Li, X.; Jie, J.; Zhao, D.; Li, D. piR-55490 inhibits the growth of lung carcinoma by suppressing mTOR signaling. Tumor Biol. 2016, 37, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Guo, J.-M.; Xiao, B.-X.; Miao, Y.; Jiang, Z.; Zhou, H.; Li, Q. piRNA, the new non-coding RNA, is aberrantly expressed in human cancer cells. Clin. Chim. Acta 2011, 412, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.; Rizzo, F.; Marchese, G.; Ravo, M.; Tarallo, R.; Nassa, G.; Giurato, G.; Santamaria, G.; Cordella, A.; Cantarella, C.; et al. RNA sequencing identifies specific PIWI-interacting small non-coding RNA expression patterns in breast cancer. Oncotarget 2014, 5, 9901–9910. [Google Scholar] [CrossRef]
- Huang, G.; Hu, H.; Xue, X.; Shen, S.; Gao, E.; Guo, G.; Shen, X.; Zhang, X. Altered expression of piRNAs and their relation with clinicopathologic features of breast cancer. Clin. Transl. Oncol. 2013, 15, 563–568. [Google Scholar] [CrossRef]
- Martinez, V.D.; Enfield, K.S.S.; Rowbotham, D.A.; Lam, W.L. An atlas of gastric PIWI-interacting RNA transcriptomes and their utility for identifying signatures of gastric cancer recurrence. Gastric. Cancer 2016, 19, 660–665. [Google Scholar] [CrossRef]
- Cui, L.; Lou, Y.; Zhang, X.; Zhou, H.; Deng, H.; Song, H.; Yu, X.; Xiao, B.; Wang, W.; Guo, J. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clin. Biochem. 2011, 44, 1050–1057. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Assumpção, C.B.; Calcagno, D.Q.; Araújo, T.M.T.; dos Santos, S.E.B.; dos Santos, Â.K.C.R.; Riggins, G.J.; Burbano, R.R.; Assumpção, P.P. The role of piRNA and its potential clinical implications in cancer. Epigenomics 2015, 7, 975–984. [Google Scholar] [CrossRef]
- Fu, A.; Jacobs, D.I.; Zhu, Y. Epigenome-wide analysis of piRNAs in gene-specific DNA methylation. RNA Biol. 2014, 11, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.D.; Vucic, E.A.; Thu, K.L.; Hubaux, R.; Enfield, K.S.S.; Pikor, L.A.; Becker-Santos, D.D.; Brown, C.J.; Lam, S.; Lam, W.L. Unique somatic and malignant expression patterns implicate PIWI-interacting RNAs in cancer-type specific biology. Sci. Rep. 2015, 5, 10423. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Liu, N.; Toiyama, Y.; Kusunoki, M.; Nagasaka, T.; Fujiwara, T.; Wei, Q.; Qin, H.; Lin, H.; Ma, Y.; et al. Novel evidence for a PIWI-interacting RNA (piRNA) as an oncogenic mediator of disease progression, and a potential prognostic biomarker in colorectal cancer. Mol. Cancer 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.; Odom, D.T.; Kutter, C. The emergence of piRNAs against transposon invasion to preserve mammalian genome integrity. Nat. Commun. 2017, 8, 1411. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Hui, G.; Yuan, L.; Shi, D.; Wang, Y.; Du, M.; Zhong, D.; Ma, L.; Tong, N.; Qin, C.; et al. Identification of novel piRNAs in bladder cancer. Cancer Lett. 2015, 356, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Siomi, M.C. The piRNA pathway in Drosophila ovarian germ and somatic cells. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2020, 96, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Fagegaltier, D.; Falciatori, I.; Czech, B.; Castel, S.; Perrimon, N.; Simcox, A.; Hannon, G.J. Oncogenic transformation of Drosophila somatic cells induces a functional piRNA pathway. Genes Dev. 2016, 30, 1623–1635. [Google Scholar] [CrossRef]
- Ishizu, H.; Iwasaki, Y.W.; Hirakata, S.; Ozaki, H.; Iwasaki, W.; Siomi, H.; Siomi, M.C. Somatic Primary piRNA Biogenesis Driven by cis-Acting RNA Elements and trans-Acting Yb. Cell Rep. 2015, 12, 429–440. [Google Scholar] [CrossRef]
- Cheng, J.; Deng, H.; Xiao, B.; Zhou, H.; Zhou, F.; Shen, Z.; Guo, J. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Lett. 2012, 315, 12–17. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Hu, D.; Mao, Q.; Yu, Z.; Zhang, H.; Li, C.; Chen, G.; Liu, F.; Zhu, W.; et al. Transcriptome-wide piRNA profiling in human gastric cancer. Oncol. Rep. 2019. [Google Scholar] [CrossRef]
- Zhang, M.; Du, X. Noncoding RNAs in gastric cancer: Research progress and prospects. World J. Gastroenterol. 2016, 22, 6610–6618. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.F.; Ribeiro-dos-Santos, A.M.; Vinasco-Sandoval, T.; Magalhães, L.; Pinto, P.; Anaissi, A.K.M.; Demachki, S.; Assumpção, P.P.; Santos, S.E.B.; Ribeiro-dos-Santos, Â. The comprehensive expression analysis of circular RNAs in gastric cancer and its association with field cancerization. Sci. Rep. 2017, 7, 14551. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Anderson, C.; Marshall, E.A.; Minatel, B.C.; Enfield, K.S.S.; Saprunoff, H.L.; Lam, W.L.; Martinez, V.D. Piwi-interacting RNAs in cancer: emerging functions and clinical utility. Mol. Cancer 2016, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, B.J.M.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Brown, R.E. Field effect in cancer-an update. Ann. Clin. Lab. Sci. 2009, 39, 331–337. [Google Scholar]
- Graham, T.a.; McDonald, S.A.; Wright, N. A Field cancerization in the GI tract. Future Oncol. 2011, 7, 981–993. [Google Scholar] [CrossRef]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef]
- Labelle, M.; Hynes, R.O. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012, 2, 1091–1099. [Google Scholar] [CrossRef]
- Poli, A.; Zaurito, A.E.; Abdul-Hamid, S.; Fiume, R.; Faenza, I.; Divecha, N. Phosphatidylinositol 5 Phosphate (PI5P): From Behind the Scenes to the Front (Nuclear) Stage. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Yin, J.; Jiang, X.-Y.; Qi, W.; Ji, C.-G.; Xie, X.-L.; Zhang, D.-X.; Cui, Z.-J.; Wang, C.-K.; Bai, Y.; Wang, J.; et al. piR-823 contributes to colorectal tumorigenesis by enhancing the transcriptional activity of HSF1. Cancer Sci. 2017, 108, 1746–1756. [Google Scholar] [CrossRef]
- Krishnan, P.; Ghosh, S.; Graham, K.; Mackey, J.R.; Kovalchuk, O.; Damaraju, S. Piwi-interacting RNAs and PIWI genes as novel prognostic markers for breast cancer. Oncotarget 2016, 7, 37944–37956. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.E.; Firek, M.; Jliedi, A.; Amaar, Y.G. Identification and characterization of RASSF1C piRNA target genes in lung cancer cells. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sökeland, G.; Schumacher, U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol. Cancer 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Hu, Z.; Wen, J.; Wang, K.; Liu, Y. TGF- promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta Bioch. Bioph. Sin. 2009, 41, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, R.-L.; Xu, A.-M. Epithelial-mesenchymal transition in gastric cancer. Am. J. Transl. Res. 2015, 7, 2141–2158. [Google Scholar]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Griffiths-Jones, S. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Lu, Y.; Li, Y.; Zheng, Y.; Kan, Y.; Chen, R.; He, S. piRBase: a comprehensive database of piRNA sequences. Nucleic Acids Res. 2019, 47, D175–D180. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
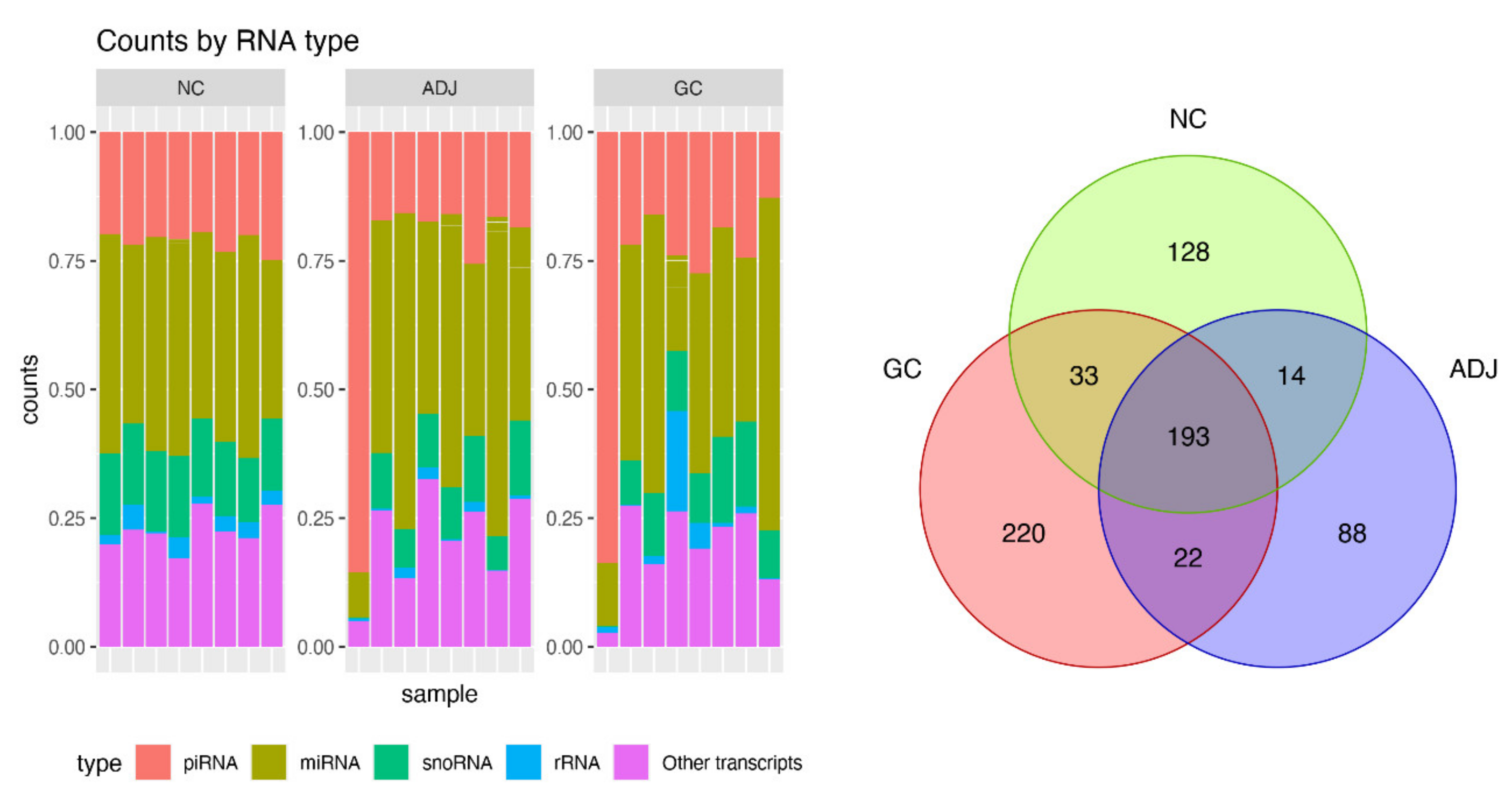
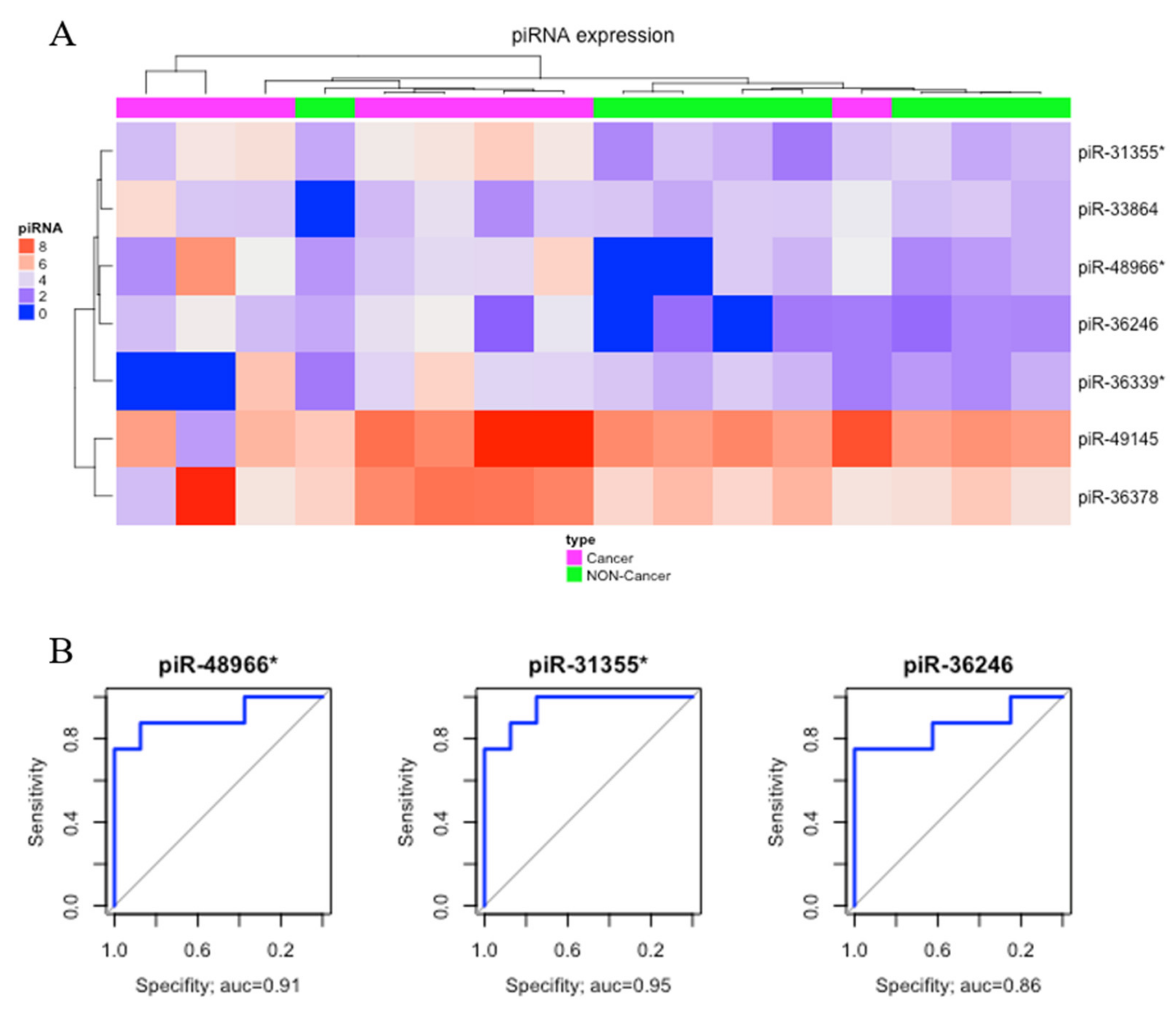
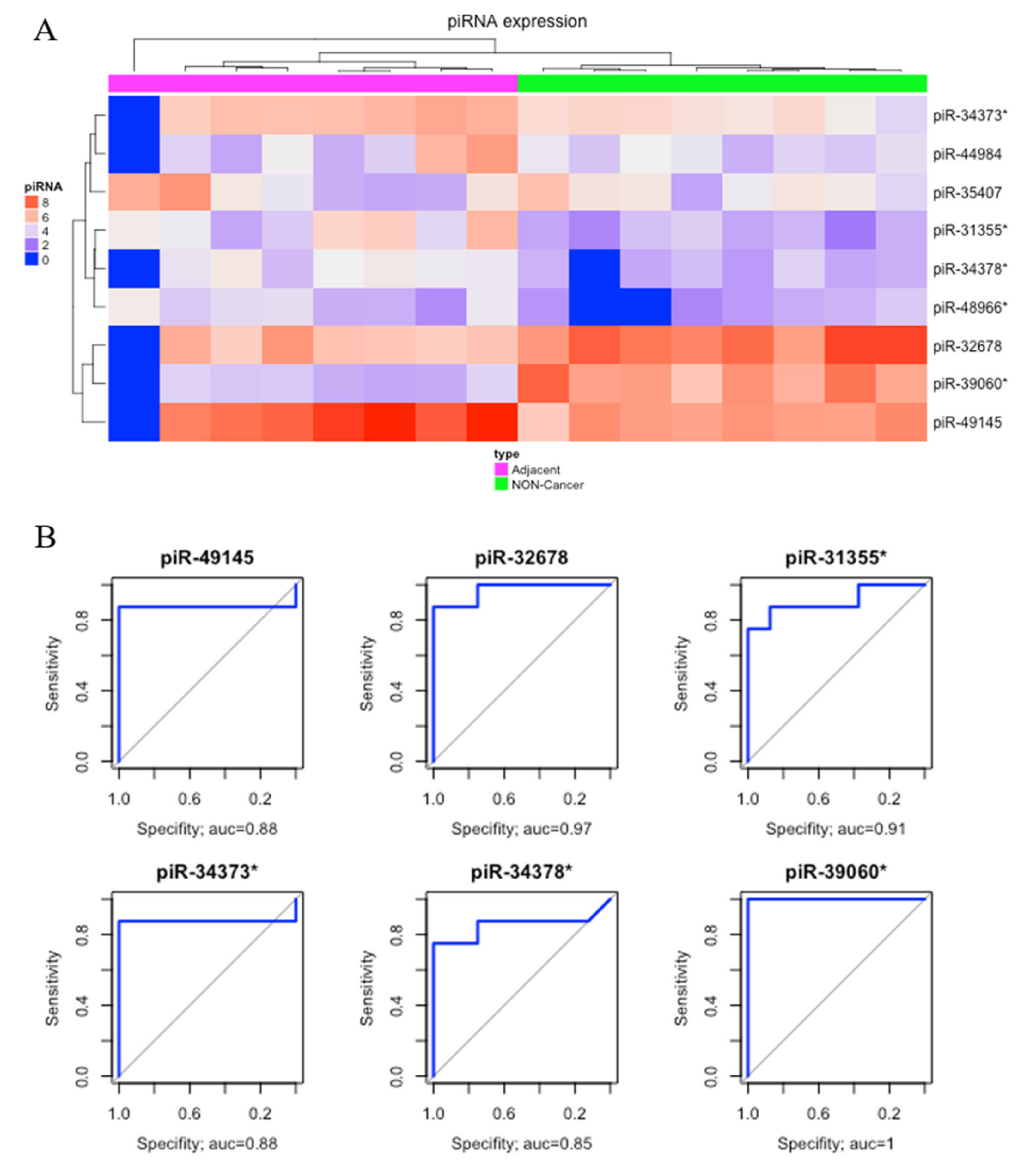
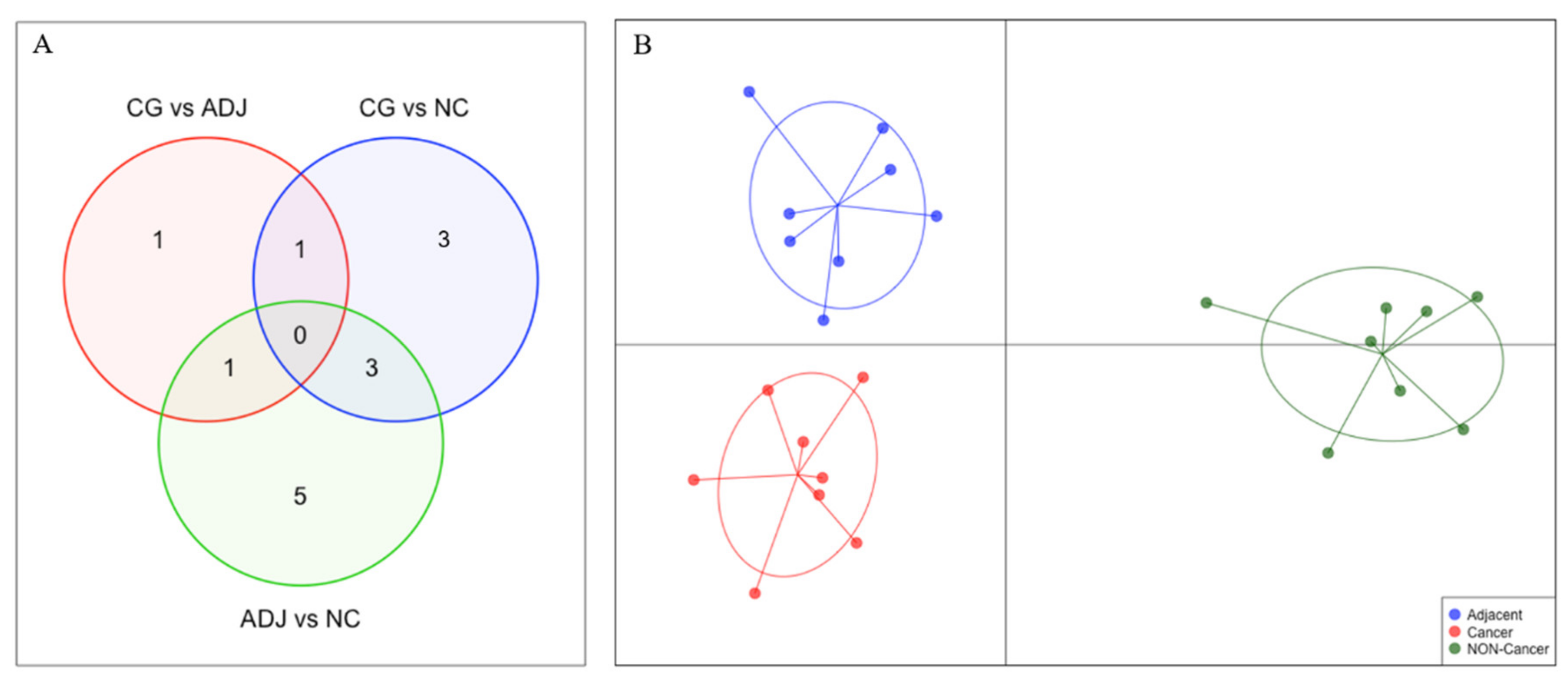
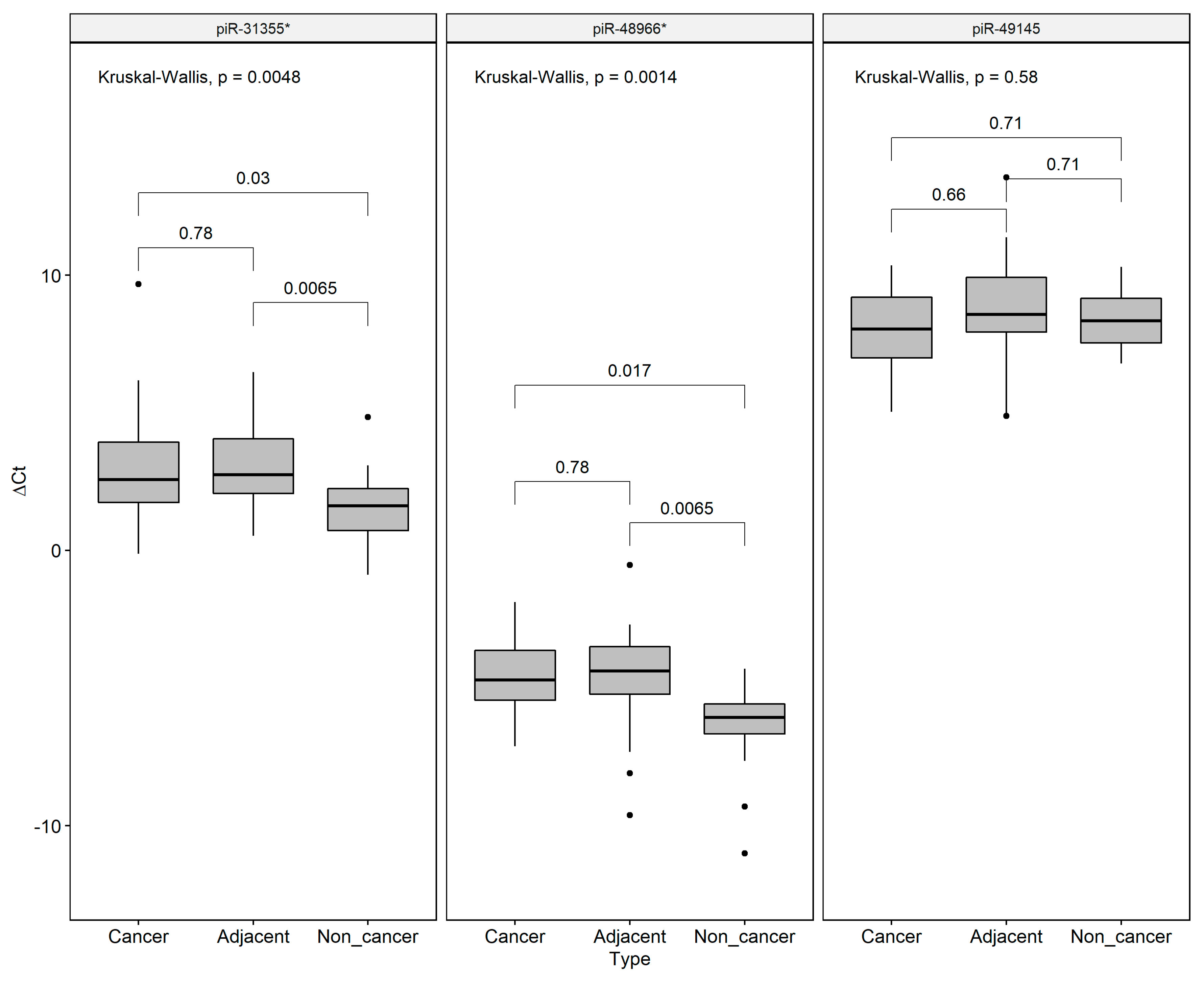
| Differentially Expressed piRNAs | GC vs NC | AD vs NC | GC vs AD | |||
|---|---|---|---|---|---|---|
| log2Fold Change | pAdj | log2Fold Change | pAdj | log2Fold Change | pAdj | |
| piR-48966 * | 2.26 | 3.49 × 10−3 | l.78 | 0.015 | ||
| piR-49145 | 2.18 | 7 × 10−3 | 3.01 | 2 × 10−8 | ||
| piR-31355 * | 2.24 | 2.42 × 10−5 | 2.72 | 7.09 × 10−6 | ||
| piR-33864 | 2.59 | 0.01 | ||||
| piR-36246 | 2.49 | 9.70 × 10−3 | ||||
| piR-36339 * | 2.04 | 0.02 | ||||
| piR-36378 | 2.02 | 0.01 | 2.34 | 0.03 | ||
| piR-33534 * | 3.11 | 3.14 × 10−4 | ||||
| piR-39060 * | −4.27 | 1.92 × 10−17 | 3.21 | 3.62 × 10−4 | ||
| piR-32678 | −2.23 | 5.64 × 10−6 | ||||
| piR-34373 * | 1.75 | 2.28 × 10−6 | ||||
| piR-34378 * | 1.99 | 2.26 × 10−5 | ||||
| piR-35407 | 2.25 | 0.03 | ||||
| piR-44984 | 1.83 | 0.02 | ||||
| piRNA | Target Gene | # of Complementary Sites | Energy to Most Probably Complementary Site | Score to Most Probably Complementary Site |
|---|---|---|---|---|
| piR-48966 * | LYN† | 48 | −24.43 | 219 |
| piR-31355 * | PIKFYVE | 7 | −22.92 | 147 |
| piR-33864 | BIN1 | 16 | −25.37 | 162 |
| FGFR2 † | 33 | −23.60 | 173 | |
| piR-33534 | FBXO31 | 1 | −30.08 | 147 |
| piR-39060 | PRUNE1 | 25 | −20.67 | 166 |
| PEAK1 † | 123 | −35.75 | 175 | |
| CRLF3 | 34 | −26.07 | 161 |
| piRNA | No. of Complementary Sites | No. of Genes | Maximum Score | Minimum Energy | Maximum Complementarity |
|---|---|---|---|---|---|
| piR-31355 * | 169 | 63 | 230 | −49.6 | 100 |
| piR-32678 | 4 | 3 | 185 | −36.9 | 85.7 |
| piR-33534 * | 57 | 23 | 190 | −34.45 | 85.2 |
| piR-33864 | 11 | 8 | 178 | −33.27 | 84.0 |
| piR-34373 * | 10 | 2 | 192 | −31.08 | 92.0 |
| piR-34378 * | 460 | 119 | 204 | −38.75 | 86.2 |
| piR-35407 | 286 | 146 | 200 | −48.77 | 81.5 |
| piR-36246 | 33 | 16 | 186 | −40.18 | 88.0 |
| piR-36339 * | 409 | 157 | 195 | −43.17 | 84.6 |
| piR-36378 | 82 | 46 | 231 | −55.10 | 100 |
| piR-39060 | 33 | 19 | 187 | −35.14 | 81.5 |
| piR-44984 | 59 | 42 | 235 | −55.40 | 100 |
| piR-48966 * | 191 | 101 | 198 | −35.30 | 88 |
| piR-49145 | 444 | 252 | 192 | −36.46 | 88.9 |
| Molecular Pathway | Observed Gene Count | FDR | Genes |
|---|---|---|---|
| Cell adhesion molecules (CAMs) | 9 | 9.77 × 106 | CD276, CD8A, CD99, CLDN19, HLA-G, MAG, NCAM1, NLGN4Y, SIGLEC1 |
| Renal cell carcinoma | 4 | 0.0248 | HIF1A, PAK6, PTPN11, TGFB3 |
| TGF-beta signaling pathway | 4 | 0.0318 | BMP5, BMPR1A, SMAD2, TGFB3 |
| Hippo signaling pathway | 6 | 0.0338 | BMP5, BMPR1A, SMAD2, TGFB3, WNT4, BMPR2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinasco-Sandoval, T.; Moreira, F.C.; F. Vidal, A.; Pinto, P.; Ribeiro-dos-Santos, A.M.; Cruz, R.L.S.; Fonseca Cabral, G.; Anaissi, A.K.M.; Lopes, K.d.P.; Ribeiro-dos-Santos, A.; et al. Global Analyses of Expressed Piwi-Interacting RNAs in Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 7656. https://doi.org/10.3390/ijms21207656
Vinasco-Sandoval T, Moreira FC, F. Vidal A, Pinto P, Ribeiro-dos-Santos AM, Cruz RLS, Fonseca Cabral G, Anaissi AKM, Lopes KdP, Ribeiro-dos-Santos A, et al. Global Analyses of Expressed Piwi-Interacting RNAs in Gastric Cancer. International Journal of Molecular Sciences. 2020; 21(20):7656. https://doi.org/10.3390/ijms21207656
Chicago/Turabian StyleVinasco-Sandoval, Tatiana, Fabiano Cordeiro Moreira, Amanda F. Vidal, Pablo Pinto, André M. Ribeiro-dos-Santos, Rebecca L. S. Cruz, Gleyce Fonseca Cabral, Ana K. M. Anaissi, Katia de Paiva Lopes, Arthur Ribeiro-dos-Santos, and et al. 2020. "Global Analyses of Expressed Piwi-Interacting RNAs in Gastric Cancer" International Journal of Molecular Sciences 21, no. 20: 7656. https://doi.org/10.3390/ijms21207656
APA StyleVinasco-Sandoval, T., Moreira, F. C., F. Vidal, A., Pinto, P., Ribeiro-dos-Santos, A. M., Cruz, R. L. S., Fonseca Cabral, G., Anaissi, A. K. M., Lopes, K. d. P., Ribeiro-dos-Santos, A., Demachki, S., de Assumpção, P. P., Ribeiro-dos-Santos, Â., & Santos, S. (2020). Global Analyses of Expressed Piwi-Interacting RNAs in Gastric Cancer. International Journal of Molecular Sciences, 21(20), 7656. https://doi.org/10.3390/ijms21207656







