The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke
Abstract
1. Introduction
2. The Risk Factors for Developing Stroke
3. Inflammation in Stroke
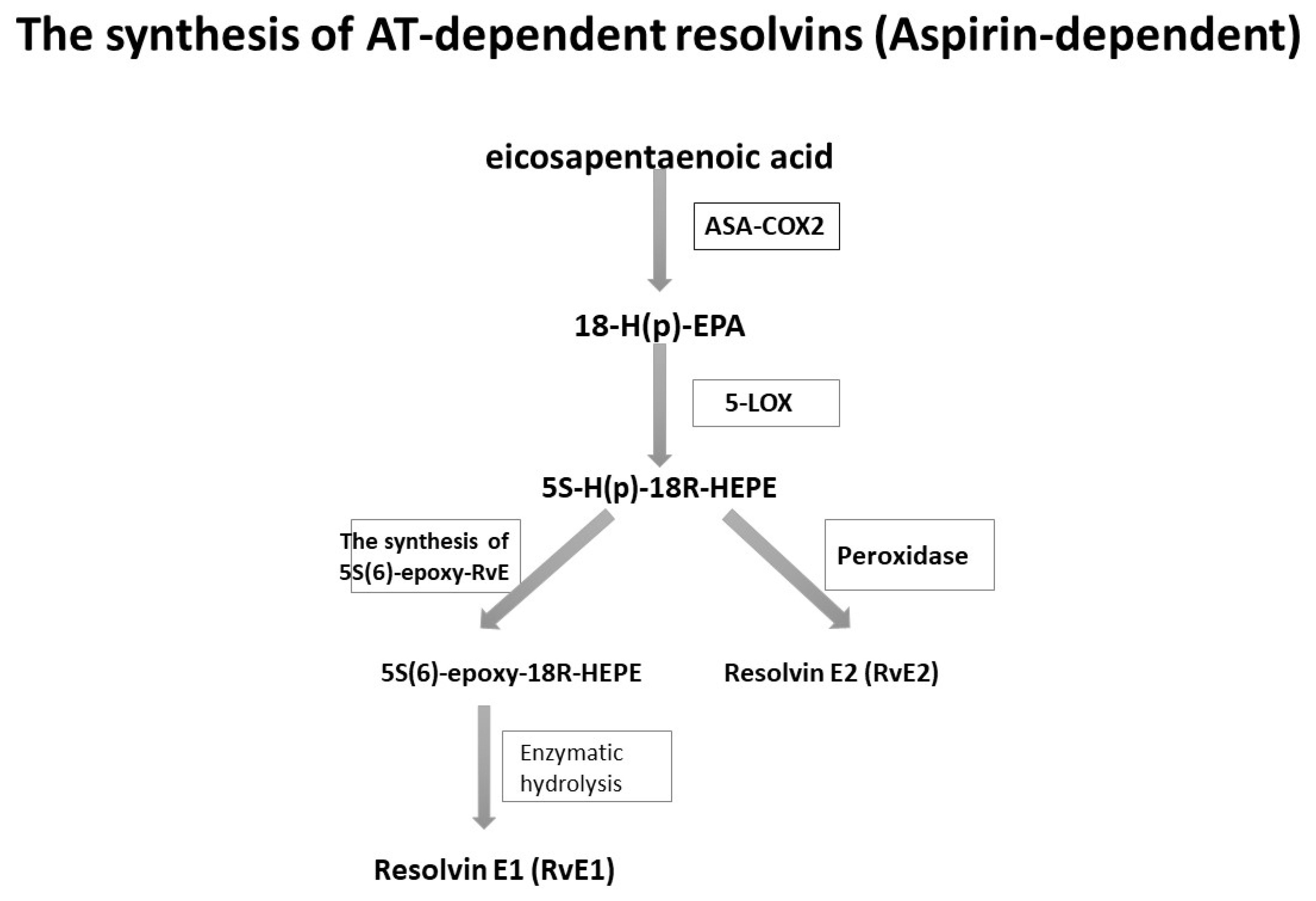
4. Resolvins: Synthesis
5. Receptors for Resolvins
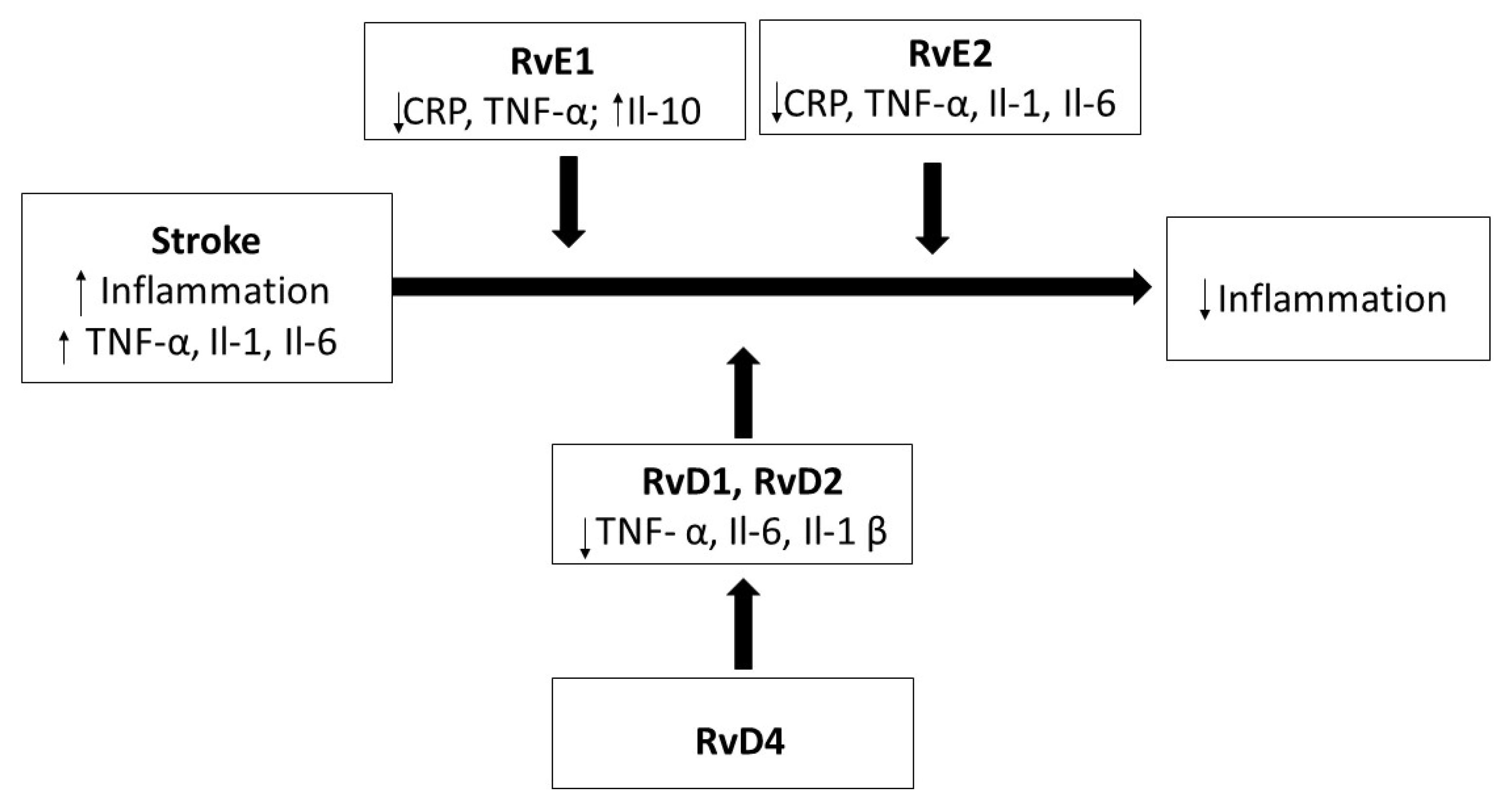
6. The Role of Resolvins in Stroke
7. Conclusions
8. Method of Article Search
Author Contributions
Funding
Conflicts of Interest
References
- Guzik, A.; Bushnell, C. Stroke epidemiology and risk factor management. Continuum (Minneap. Min.) 2017, 23, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Bejot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. La Presse Med. 2016, 45, e391–e398. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, J.M.; Muñoz-Vega, P.; Alberca, S.B.; Peral-Pacheco, D. Health-related quality of life and fatigue after transient ischemic attack and minor stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska-Bender, M.; Milewska, M.; Gołąbek, A.; Duda-Zalewska, A.; Staniszewska, A.J. The impact of ischemic cerebral stroke on the quality of life of patients based on clinical, social, and psychoemotional factors. Stroke Cerebrovasc. Dis. 2017, 26, 101–107. [Google Scholar] [CrossRef]
- Skoglund, E.; Westerlind, E.; Persson, H.C.; Sunnerhagen, K.S. Self-perceived impact of stroke: A longitudinal comparison between one and five years post-stroke. J. Rehabil. Med. 2019, 51, 660–664. [Google Scholar] [CrossRef]
- Davis, C.M.; Fairbanks, S.L.; Alkayed, N.J. Mechanism of the sex difference in endothelial dysfunction after stroke. Transl. Stroke Res. 2013, 4, 381–389. [Google Scholar] [CrossRef]
- Shu, S.; Pei, L.; Lu, Y. Promising targets of cell death signaling of NR2B receptor subunit in stroke pathogenesis. Regen. Med. Res. 2014, 2, 8. [Google Scholar] [CrossRef]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef]
- Kotlega, D.; Zembron-Lacny, A.; Golab-Janowska, M.; Nowacki, P.; Szczuko, M. The association of free fatty acids and eicosanoids with the severity of depressive symptoms in stroke patients. Int. J. Mol. Sci. 2020, 21, 5220. [Google Scholar] [CrossRef]
- Sakakibara, B.; Kim, A.; Eng, J. A systematic review and meta-analysis on self-management for improving risk factor control in stroke patients. Int. J. Behav. Med. 2017, 24, 42–53. [Google Scholar] [CrossRef]
- Kotlęga, D.; Gołąb-Janowska, M.; Meller, A.; Pawlukowska, W.; Nowacki, P. Detection of stroke risk factors over the decade in the polish population of ischemic stroke patients. Adv. Psychiatry Neurol. Postępy Psychiatrii i Neurologii 2019, 28, 83–87. [Google Scholar] [CrossRef]
- Kariasa, I.M.; Nurachmah, E.; Setyowati, S.; Koestoer, R.A. Analysis of participants’ characteristics and risk factors for stroke recurrence. Enferm. Clin. 2019, 29, 286–290. [Google Scholar] [CrossRef]
- Kernan, W.; Ovbiagele, B.; Black, H.; Bravata, D.; Chimowitz, M.; Ezekowitz, M.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef]
- Kotlęga, D.; Ciećwież, S.; Turowska-Kowalska, J.; Nowacki, P. Pathogenetic justification of statin use in ischaemic stroke prevention according to inflammatory theory in development of atherosclerosis. Advances in Psychiatry and Neurology/Postępy Psychiatrii i Neurologii. Neurol. Neurochir. Polska 2012, 46, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B.; Bushnell, C.D.; Adams, R.J.; Appel, L.J.; Braun, L.T.; Chaturvedi, S.; Creager, M.A.; Culebras, A.; Eckel, R.H.; Hart, R.G.; et al. Guidelines for the primary prevention of stroke. Stroke 2011, 42, 517–584. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, C.; Sandberg, O.; Gustafson, Y.; Bucht, G.; Carlberg, B.; Stenlund, H.; Franklin, G.A. Obstructiove sleep apnea is a risk factor for death in patients with stroke: A 10-year follow-up. Arch. Intern. Med. 2008, 168, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.; Inzucchi, S.; Sawan, C.; Macko, R.; Furie, K. Obesity: A stubbornly obvious target prevention. Stroke 2013, 44, 278–286. [Google Scholar] [CrossRef]
- Vemmos, K.; Ntaios, G.; Spengos, K.; Savvari, P.; Vemmou, A.; Pappa, T.; Manios, E.D.; Georgiopoulos, G.; Alevizaki, M. Association between obesity and mortality after acute first-ever stroke. Stroke 2011, 42, 30–36. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Bath, P.M.; Cotton, D.; Vinisko, R.; Diener, H.-C. Obesity and recurrent vascular risk after a recent ischemic stroke. Stroke 2011, 42, 3397–3402. [Google Scholar] [CrossRef]
- Haley, M.J.; Lawrence, C.B. Obesity and stroke: Can we translate from rodents to patients? Br. J. Pharmacol. 2016, 36, 2007–2021. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and stroke: An overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Jackman, K.; Iadecola, C. Neurovascular regulation in the ischemic brain. Antioxid. Redox Signal. 2015, 22, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Petrovic-Djergovic, D.; Goonewardena, S.N.; Pinsky, D.J. Inflammatory disequilibrium in stroke. Circ. Res. 2016, 119, 142–158. [Google Scholar] [CrossRef]
- Chapman, K.Z.; Dale, V.Q.; Dénes, Á.; Bennett, G.; Rothwell, N.J.; Allan, S.M.; McColl, B.W. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. Br. J. Pharmacol. 2009, 29, 1764–1768. [Google Scholar] [CrossRef]
- Kes, V.B.; Simundic, A.-M.; Nikolac, N.; Topic, E.; Demarin, V. Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin. Biochem. 2008, 41, 1330–1334. [Google Scholar] [CrossRef]
- Zhao, L.; Funk, C.D.; Zhao, L.; Funk, C.D. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc. Med. 2004, 14, 191–195. [Google Scholar] [CrossRef]
- Hampel, J.K.; Brownrigg, L.M.; Vignarajah, D.; Croft, K.D.; Dharmarajan, A.M.; Bentel, J.M.; Puddey, I.B.; Yeap, B.B. Differential modulation of cell cycle, apoptosis and PPARγ2 gene expression by PPARγ agonists ciglitazone and 9-hydroxyoctadecadienoic acid in monocytic cells. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 283–293. [Google Scholar] [CrossRef]
- Limor, R.; Sharon, O.; Knoll, E.; Many, A.; Weisinger, G.; Stern, N. Lipoxygenase-derived metabolites are regulators of peroxisome proliferator-activated receptor -2 expression in human vascular smooth muscle cells. Am. J. Hypertens. 2008, 21, 219–223. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A novel anti-inflammatory role of omega-3 PUFAs in prevention and treatment of atherosclerosis and vascular cognitive impairment and dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef]
- Viola, J.; Lemnitzer, P.; Jansen, Y.; Csaba, G.; Winter, C.; Neideck, C.; Silvestre-Roig, C.; Dittmar, G.; Döring, Y.; Drechsler, M.; et al. Resolving lipid mediatrs maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res. 2016, 119, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat Acids 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Burdge, G. Long-chain n-3 PUFA: Plant v. marine sources. Proc. Nutr. Soc. 2006, 65, 42–50. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Serhan, C.; Hong, S.; Gronert, K.; Colgan, S.; Devchand, P.; Mirick, G.; Moussignac, R.-L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Nowak, J.Z. Anti-inflammatory pro-resolving derivatives of omega-3 and omega-6 polyunsaturated fatty acids. Postępy Hig. Med. Dosw. 2010, 64, 115–132. [Google Scholar]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Biber, K.; Finsen, B. Inflammatory cytokines in experimental and human stroke. Br. J. Pharmacol. 2012, 32, 1677–1698. [Google Scholar] [CrossRef]
- Bannenberg, G.; Serhan, C. Pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2010, 1801, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Myint, P.K.; Zaman, M.J.S.; Li, Y.; Zhao, L.; Shi, P.; Ren, F.; Wu, Y. Relationship of serum interleukin-10 and its genetic variations with ischemic stroke in a chinese general population. PLoS ONE 2013, 8, e74126. [Google Scholar] [CrossRef] [PubMed]
- Arponen, O.; Muuronen, A.; Taina, M.; Sipola, P.; Hedman, M.; Jäkälä, P.; Vanninen, R.; Pulkki, K.; Mustonen, P. Acute phase IL-10 plasma concentration associates with the high risk sources of cardiogenic stroke. PLoS ONE 2015, 10, e0120910. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 2012, 188, 4527–4534. [Google Scholar] [CrossRef] [PubMed]
- Jenny, N.; Callas, P.; Judd, S.; McClure, L.; Kissela, B.; Zakai, N.; Cuchman, M. Inflammatory cytokines and schemic stroke risk: The REGARDS cohort. Neurology 2019, 92, e2375–e2384. [Google Scholar] [CrossRef]
- Cherpokova, D.; Jouvene, C.C.; Libreros, S.; DeRoo, E.P.; Chu, L.; De La Rosa, X.; Norris, P.C.; Wagner, D.D.; Serhan, C.N. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood 2019, 134, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Pirault, J.; Bäck, M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Elajami, T.K.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N.; Welty, F.K. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016, 30, 2792–2801. [Google Scholar] [CrossRef]
- Fredman, G.; Spite, M. Specialized pro-resolving mediators in cardiovascular diseases. Mol. Asp. Med. 2017, 58, 65–71. [Google Scholar] [CrossRef]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arter. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef]
- Karagiannis, G.; Weile, J.; Bader, G.; Minta, J. Integrative pathway dissection of molecular mechanisms od moxLDL-induced vascular muscle phenotype transformation. BMC Cardiovasc. Disord. 2013, 13, 4. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.-P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and chemr23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Laguna-Fernandez, A.; Checa, A.; Carracedo, M.; Artiach, G.; Petri, M.; Baumgartner, R.; Forteza, M.J.; Jiang, X.; Andonova, T.; Waler, M.E.; et al. ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low-density lipoprotein uptake and phagocytosis in macrophages. Circulation 2018, 138, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Zhang, D.; Mu, R.; Shen, H.; Li, X.; Wang, Z.; Li, H.; Chen, G. Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats. Mol. Brain 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Bäck, M. Omega-3 fatty acids in atherosclerosis and coronary artery disease. Futur. Sci. OA 2017, 3. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gartung, A.; Yang, J.; Yang, H.; Gilligan, M.M.; Sulciner, M.L.; Bhasin, S.S.; Bielenberg, D.R.; Chang, J.; Schmidt, B.A.; et al. Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. J. Clin. Investig. 2019, 129, 2964–2979. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Gao, J.; Zhang, C.Y.; Hayworth, C.; Frank, M.; Wang, Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano 2019, 13, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef]
- Wang, C.-S.; Maruyama, C.L.; Easley, J.T.; Trump, B.G.; Baker, O.J. AT-RvD1 promotes resolution of inflammation in NOD/ShiLtJ mice. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Bacarin, C.C.; Mori, M.A.; Ferreira, E.D.F.; Romanini, C.V.; De Oliveira, R.M.W.; Milani, H. Fish oil provides robust and sustained memory recovery after cerebral ischemia: Influence of treatment regimen. Physiol. Behav. 2013, 119, 61–71. [Google Scholar] [CrossRef]
- Xu, M.; Tan, B.; Zhou, W.; Wei, T.; Lai, W.; Tan, J.-W.; Dong, J. Resolvin D1, an endogenous lipid mediator for inactivation of inflammation-related signaling pathways in microglial cells, prevents lipopolysaccharide-induced inflammatory responses. CNS Neurosci. Ther. 2013, 19, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011, 89, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, K.; Bryniarski, K. The biological activity of macrophages in health and disease. Postępy Hig. Med. Dosw. 2012, 66, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Akagi, D.; Chen, M.; Toy, R.; Chatterjee, A.; Conte, M.S. Systemic delivery of proresolving lipid mediators resolvin D 2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 2015, 29, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Eitos, E.; Rius, B.; Gonzalez-Periz, A.; Lopez-Vicario, C.; Moran-Salvador, E.; Martinez-Clemente, M.; Arroyo, V.; Claria, J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization towar dan M2-like phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar]
- Hsiao, H.-M.; Sapinoro, R.E.; Thatcher, T.H.; Croasdell, A.; Levy, E.P.; Fulton, R.A.; Olsen, K.C.; Pollock, S.J.; Serhan, C.N.; Phipps, R.P.; et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS ONE 2013, 8, e58258. [Google Scholar] [CrossRef]
- Salic, K.; Morrison, M.C.; Verschuren, L.; Wielinga, P.Y.; Wu, L.; Kleemann, R.; Gjorstrup, P.; Kooistra, T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 2016, 250, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Hasturk, H.; Abdallah, R.; Kantarci, A.; Nguyen, D.; Giordano, N.; Hamilton, J.; Van Dyke, T.E. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arter. Thromb. Vasc. Biol. 2015, 35, 1123–1133. [Google Scholar] [CrossRef]
- Capó, X.; Martorell, M.; Busquets-Cortés, C.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Resolvins as proresolving inflammatory mediators in cardiovascular disease. Eur. J. Med. Chem. 2018, 153, 123–130. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Risk and Prevention Study Collaborative Group; Roncaglioni, M.C.; Tombesi, M.; Avanzini, F.; Barlera, S.; Caimi, V.; Longoni, P.; Marzona, I.; Milani, V.; Silletta, M.G.; et al. n–3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]

| Omega-3 | Resolvin Subtypes | Corresponding Receptors | Localisation | Function |
|---|---|---|---|---|
| EPA | RvE1 | ChemR23 (ERV, CMKLR1) | Chemerin receptor 23 is expressed on NK cells, ILCs, macrophages, dendritic cells, and epithelial cells | stimulation of phagocytosis decrease in the level of proinflammatory cytokines |
| BLT1 | ||||
| RvE2 | BLT1 | Leukotriene LTB4 is expressed on human neutrophils, eosinophils, monocytes, macrophages, mast cells, dendritic cells, and T cells | reduction in neutrophil mobilization | |
| DHA- | RvD1 | ALX/FPR2 | Expression on neutrophils, macrophages, monocytes, macrophages, and T cells | increase of phagocytosis prevention of the differentiation of T-lymphocytes towards Th1 and Th12, promotion of regulatory cell (Tr) formation |
| DRV1/GPR32 | The G-23 protein coupled receptor is expressed on human neutrophils, lymphocytes, macrophages, and monocytes, as well as vascular tissues | |||
| RvD2 | DRV1/GPR32 | development of CD8a lymphocytes in the small intestine migration ability of immune cells recruitment of granulocytes decrease in blood pressure | ||
| DRV2/GPR18 | The G-18 protein coupled receptor is expressed on human and murine neutrophils, monocytes, and macrophages | |||
| RvD3 | DRV1/GPR32 | The G-23 protein coupled receptor is expressed on human neutrophils, lymphocytes, macrophages, and monocytes, as well as vascular tissues | promotion of macrophage phagocytosis | |
| RvD4 | G protein-coupled receptors: no data | inhibition of metastases and induced T cell responses | ||
| RvD5 | DRV1/GPR32 | The G-23 protein coupled receptor is expressed on human neutrophils, lymphocytes, macrophages, and monocytes, as well as vascular tissues | expression of macrophages increase of phagocytosis. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tułowiecka, N.; Kotlęga, D.; Prowans, P.; Szczuko, M. The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7628. https://doi.org/10.3390/ijms21207628
Tułowiecka N, Kotlęga D, Prowans P, Szczuko M. The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke. International Journal of Molecular Sciences. 2020; 21(20):7628. https://doi.org/10.3390/ijms21207628
Chicago/Turabian StyleTułowiecka, Nikola, Dariusz Kotlęga, Piotr Prowans, and Małgorzata Szczuko. 2020. "The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke" International Journal of Molecular Sciences 21, no. 20: 7628. https://doi.org/10.3390/ijms21207628
APA StyleTułowiecka, N., Kotlęga, D., Prowans, P., & Szczuko, M. (2020). The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke. International Journal of Molecular Sciences, 21(20), 7628. https://doi.org/10.3390/ijms21207628