Vimentin Association with Nuclear Grooves in Normal MEF 3T3 Cells
Abstract
1. Introduction
2. Results
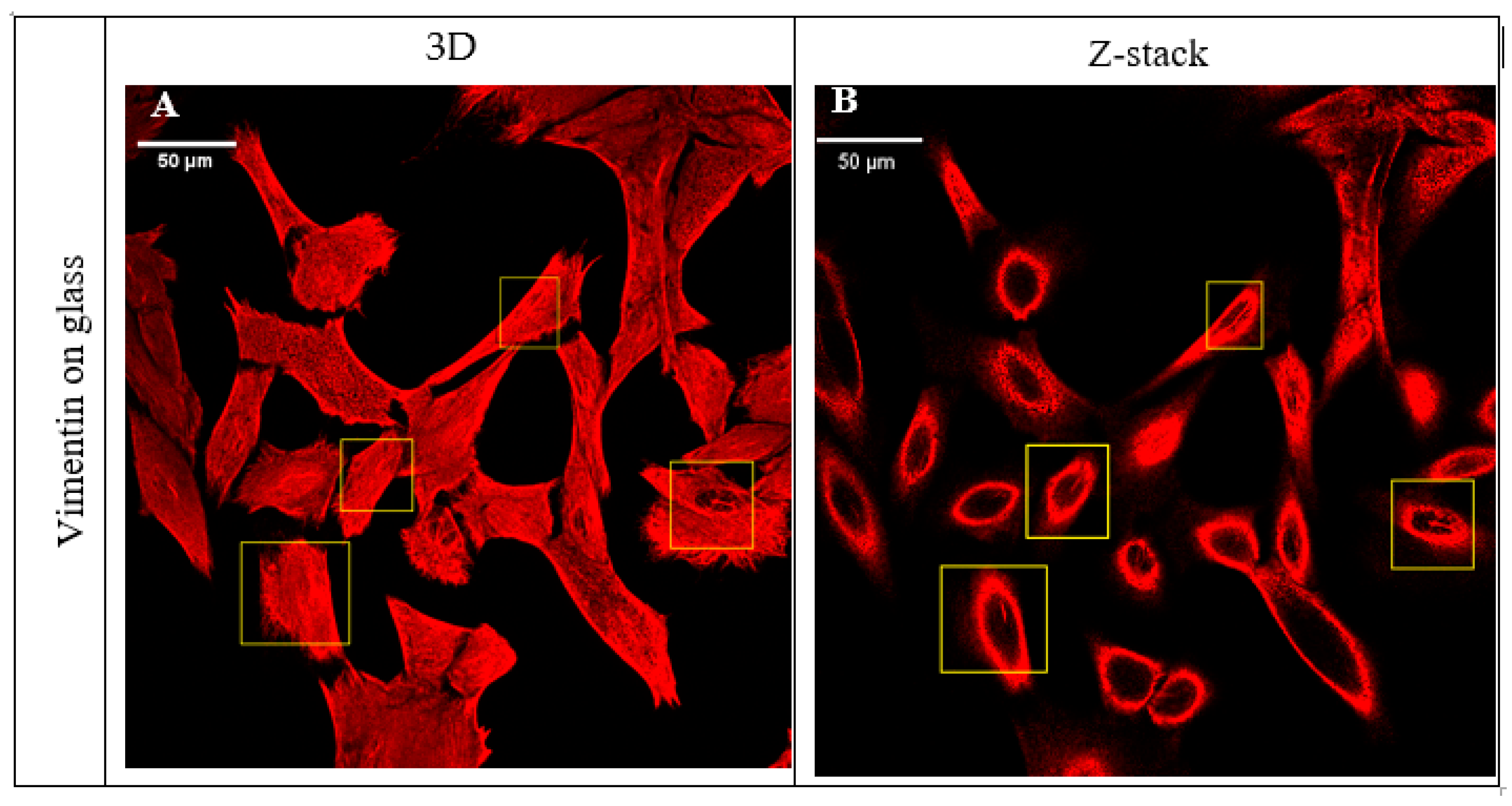
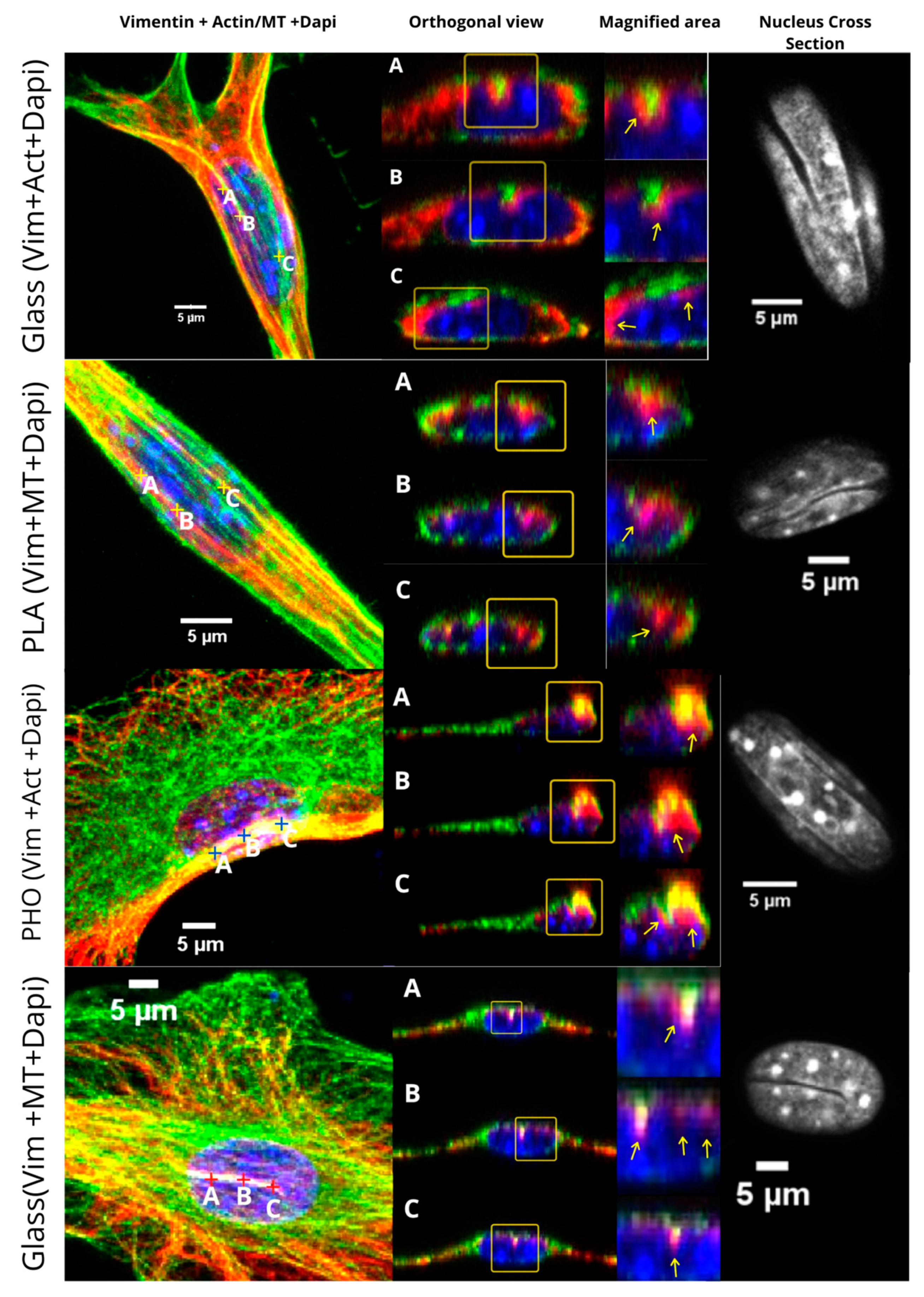
2.1. Vimentin Bundles Associated with Nuclear Grooves of the Cells
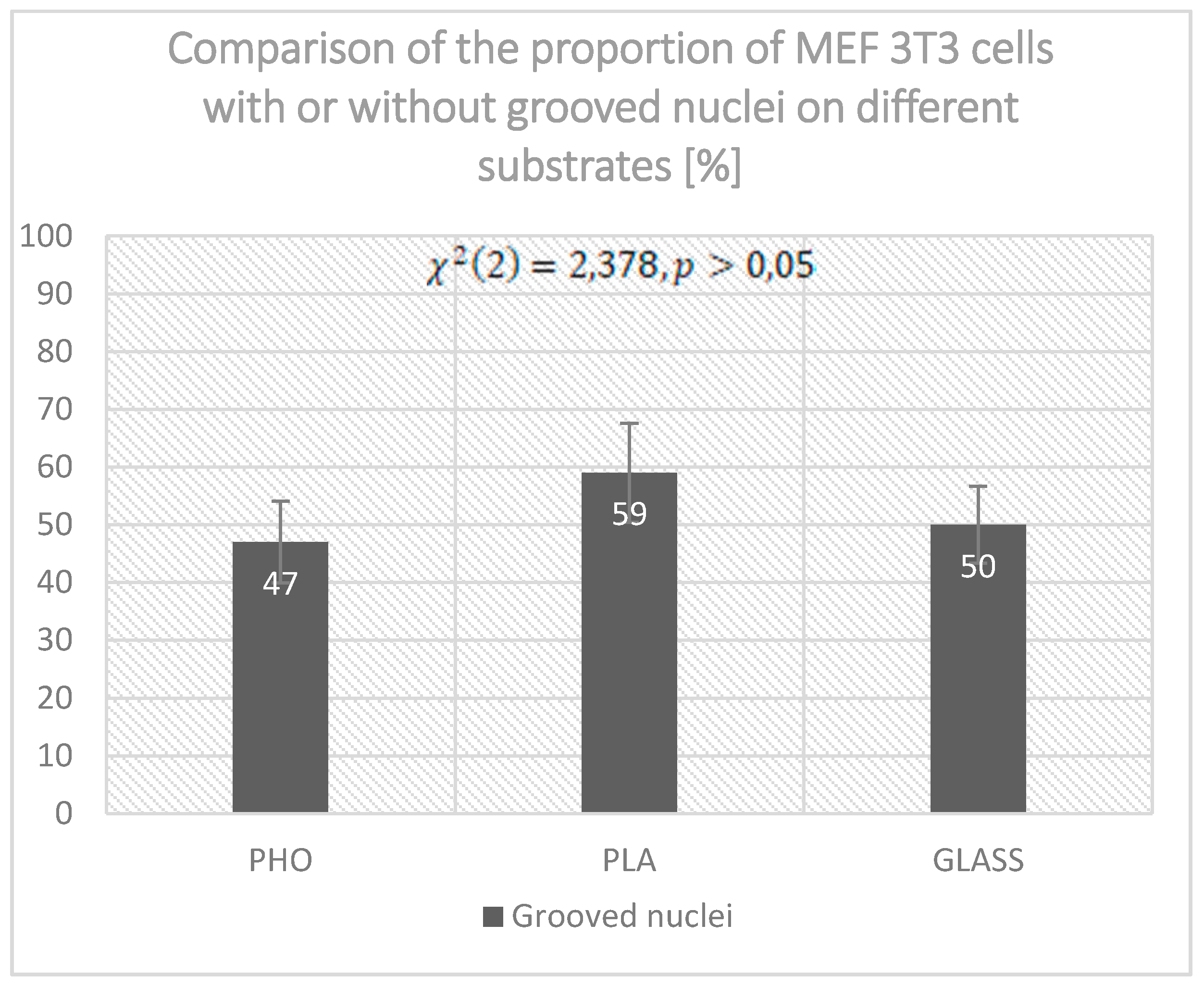
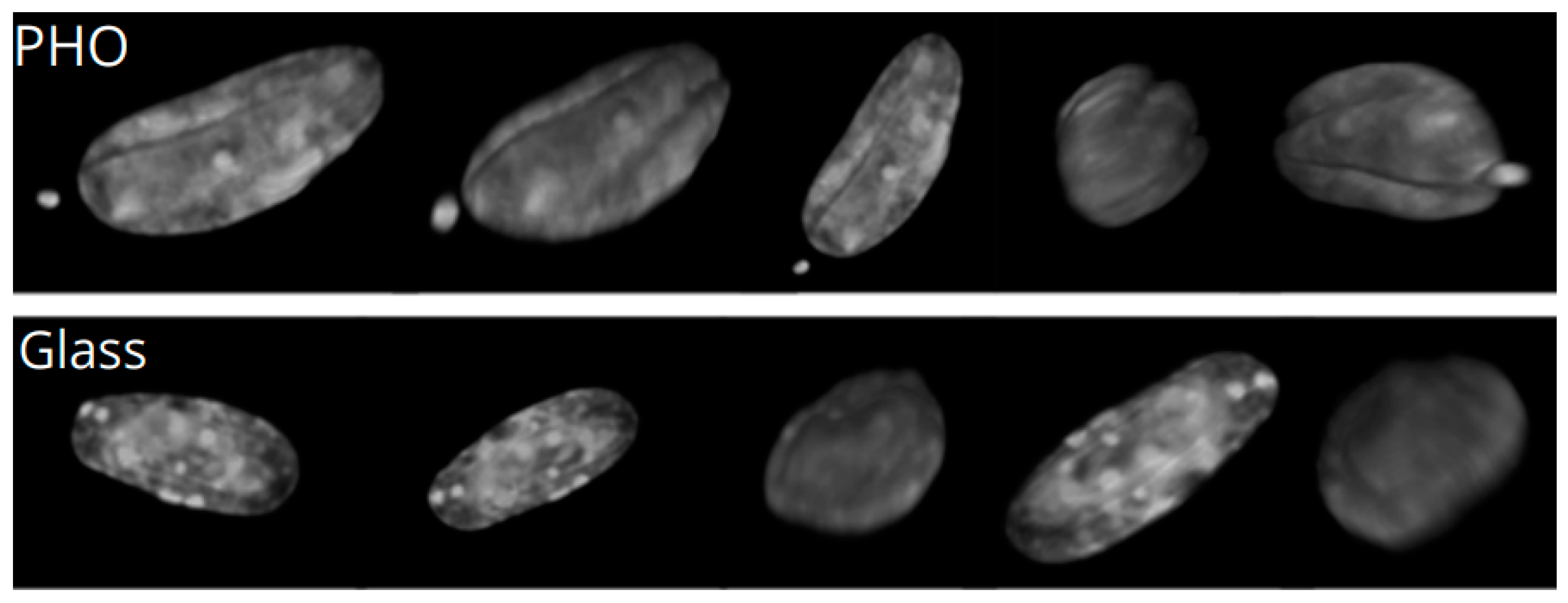
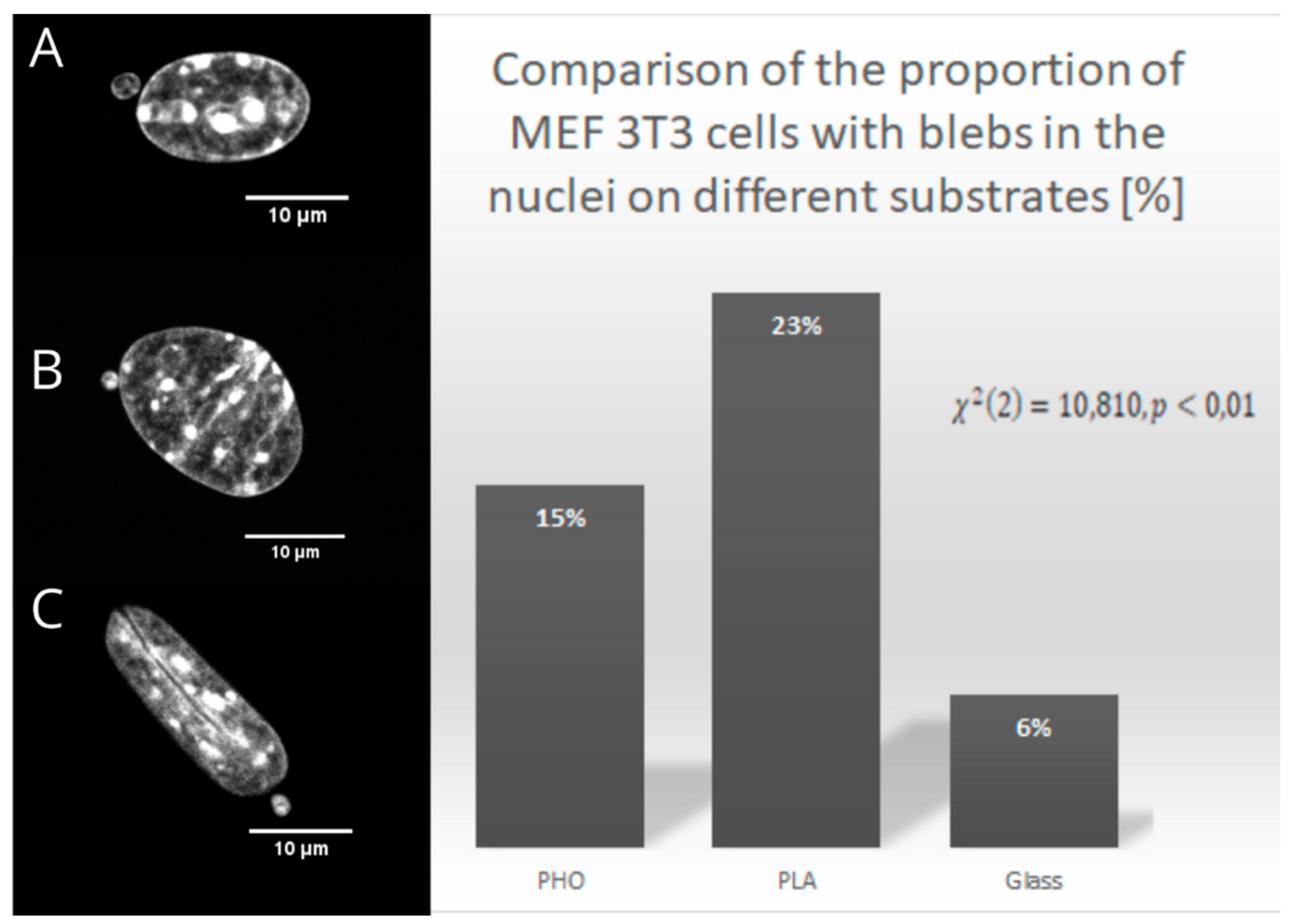
2.2. Nuclear Blebs Associated with the Type of the Substrate
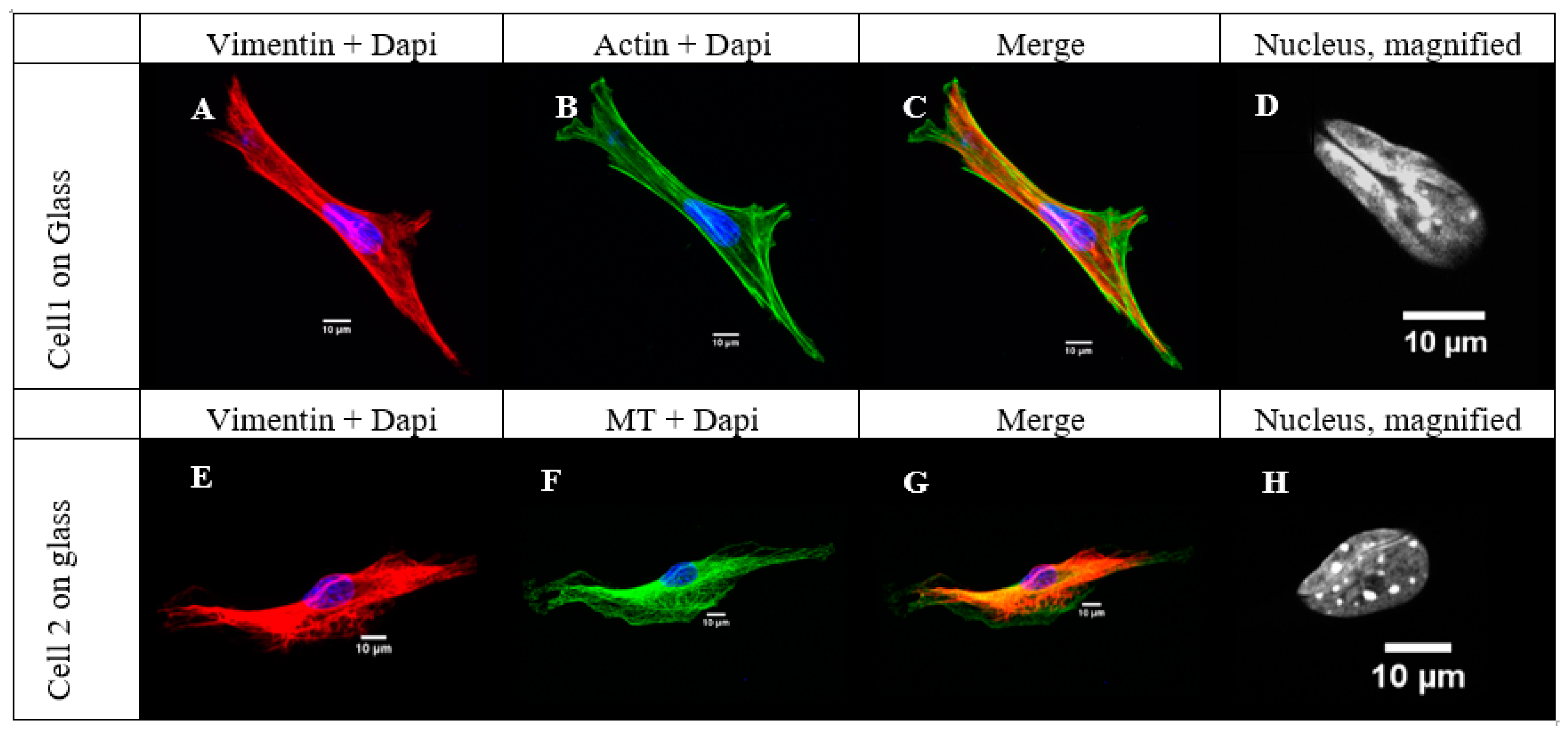
2.3. Vimentin Sinks into the Grooves Deeper than Microtubules or Microfilaments
3. Discussion
4. Materials and Methods
4.1. Cell Culturing on Glass, PHO, and PLA
4.2. Cells Preparation and Staining
4.3. Preparation of PHO and PLA Substrates
4.4. Microscopic Studies of Vimentin Cytoskeleton of MEF 3T3 Cells Grown on Different Substrates
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration [version 1; referees: 2 approved]. F1000Research 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Buehler, M.J. Rupture Mechanics of Vimentin Intermediate Filament Tetramers. J. Eng. Mech. 2009, 135, 422–433. [Google Scholar] [CrossRef][Green Version]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front. Cell Dev. Biol. 2017, 5, 1–19. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Zhang, F.; Lü, D.; Li, N.; Zheng, L.; Xu, Y.; Li, Z.; Sun, S.; Long, M. Mechanical remodeling of normally sized mammalian cells under a gravity vector. FASEB J. 2017, 31, 802–813. [Google Scholar] [CrossRef]
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A. Vimentin protects the structural integrity of the nucleus and suppresses nuclear damage caused by large deformations. bioRxiv 2019, 566174. [Google Scholar] [CrossRef]
- Hirokawa, M.; Miyake, Y.; Shimizu, M.; Kanahara, T.; Manabe, T.; Fujiwara, K.; Kohno, I. Nuclear Findings of Ovarian Surface Epithelial Tumors. Diagn. Cytopathol. 2000, 22, 27–29. [Google Scholar] [CrossRef]
- Reid, M.D.; Sigel, C.S. Pancreatoblastoma: Cytologic and Histologic Analysis of 12 Adult Cases Reveals Helpful Criteria in Their Diagnosis and Distinction From Common Mimics. Cancer Cytopathol. 2019, 127, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Cracolici, V.; Krausz, T.; Cipriani, N.A. Ubiquitin Immunostaining in Thyroid Neoplasms Marks True Intranuclear Cytoplasmic Pseudoinclusions and May Help Differentiate Papillary Carcinoma from NIFTP. Head Neck Pathol. 2018, 12, 522–528. [Google Scholar] [CrossRef]
- Lee, J.; Han, E.M.; Lin, Z.; Wu, Z.; Lee, E.; Kim, Y. Clinicopathologic Significance of Nuclear Grooves and Inclusions in Renal Cell Carcinoma. Arch. Pathol. Lab. Med. 2008, 132, 940–946. [Google Scholar]
- Batistatou, A.; Scopa, C.D. Pathogenesis and Diagnostic Significance of Nuclear Grooves in Thyroid and Other Sites. Int. J. Surg. Pathol. 2009, 17, 107–110. [Google Scholar] [CrossRef]
- Collings, D.A.; Carter, C.N.; Rink, J.C.; Scott, A.C.; Wyatt, S.E.; Allen, N.S. Plant Nuclei Can Contain Extensive Grooves and Invaginations Plant Nuclei Can Contain Extensive Grooves and Invaginations. Plant Cell 2000, 12, 2425–2439. [Google Scholar] [CrossRef]
- Hampoelz, B.; Azou-gros, Y.; Fabre, R.; Markova, O.; Puech, P.; Lecuit, T. Microtubule-induced nuclear envelope fluctuations control chromatin dynamics in Drosophila embryos. Development 2011, 3386, 3377–3386. [Google Scholar] [CrossRef]
- Lowery, J.; Kuczmarski, E.R.; Herrmann, H.; Goldma, R.D. Intermediate filaments play a pivotal role in regulating cell architecture and function. J. Biol. Chem. 2015, 290, 17145–17153. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Etienne-Manneville, S. Intermediate filaments in cell migration and invasion: The unusual suspects. Curr. Opin. Cell Biol. 2015, 32, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.C.; Flores, L.R.; Dodhy, A.H.; Murray, E.R.; Gavara, N. Actomyosin and vimentin cytoskeletal networks regulate nuclear shape, mechanics and chromatin organization. Sci. Rep. 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Imbalzano, K.M.; Cohet, N.; Wu, Q.; Underwood, J.M.; Imbalzano, A.N.; Nickerson, J.A. Nuclear Shape Changes Are Induced by Knockdown of the SWI/SNF ATPase BRG1 and Are Independent of Cytoskeletal Connections. PLoS ONE 2013, 8, e055628. [Google Scholar] [CrossRef]
- Duarte, S.; Viedma-Poyatos, Á.; Navarro-Carrasco, E.; Martínez, A.E.; Pajares, M.A.; Pérez-Sala, D. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat. Commun. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Clubb, B.H.; Locke, M. 3T3 cells have nuclear invaginations containing F-actin. Tissue Cell. 1998, 30, 684–691. [Google Scholar] [CrossRef]
- Kamei, H. Relationship of Nuclear Invaginations to Perinuclear Rings Composed of Intermediate Filaments in MIA PaCa-2 and Some Other Cells. Cell Struct. Funct. 1994, 19, 123–132. [Google Scholar] [CrossRef][Green Version]
- Abe, T.; Takano, K.; Suzuki, A.; Shimada, Y.; Inagaki, M.; Sato, N.; Obinata, T.; Endo, T. Myocyte differentiation generates nuclear invaginations traversed by myofibrils associating with sarcomeric protein mRNAs. J. Cell Sci. 2004, 117, 6523–6534. [Google Scholar] [CrossRef]
- Johnson, N.; Krebs, M.; Boudreau, R.; Giorgi, G.; LeGros, M.; Larabell, C. Actin-filled nuclear invaginations indicate degree of cell de-differentiation. Differentiation 2003, 71, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A.; Mandal, K.; Kittisopikul, M.; Sivagurunathan, S.; Goldman, A.; Goldman, R.D.; Janmey, P.A. Vimentin protects cells against nuclear rupture and DNA damage during migration. J. Cell Biol. 2019, 218, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Sarria, A.J.; Lieber, J.G.; Nordeen, S.K.; Evans, R.M. The presence or absence of a vimentin-type intermediate filament network affects the shape of the nucleus in human SW-13 cells. J. Cell Sci. 1994, 107, 1593–1607. [Google Scholar] [PubMed]
- Terriac, E.; Schütz, S.; Lautenschläger, F. Vimentin Intermediate Filament Rings Deform the Nucleus During the First Steps of Adhesion. Front. Cell Dev. Biol. 2019, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Witko, T.; Solarz, D.; Feliksiak, K.; Haraźna, K.; Rajfur, Z.; Guzik, M. Insights into In Vitro Wound Closure on Two Biopolyesters—Polylactide and Polyhydroxyoctanoate. Materials 2020, 13, 2793. [Google Scholar] [CrossRef]
- Pawar, R.P.; Tekale, S.U.; Shisodia, S.U.; Totre, J.T.; Domb, A.J. Biomedical applications of poly(lactic acid). Rec. Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Sofińska, K.; Barbasz, J.; Witko, T.; Dryzek, J.; Haraźna, K.; Witko, M.; Kryściak-Czerwenka, J.; Guzik, M. Structural, topographical, and mechanical characteristics of purified polyhydroxyoctanoate polymer. J. Appl. Polym. Sci. 2019, 136, 47192. [Google Scholar] [CrossRef]
- Witko, T.; Solarz, D.; Feliksiak, K.; Rajfur, Z.; Guzik, M. Cellular architecture and migration behavior of fibroblast cells on polyhydroxyoctanoate (PHO): A natural polymer of bacterial origin. Biopolymers 2019. [Google Scholar] [CrossRef]
- Vtyurin, B.V.; Chekmaryova, I.A.; Dubova, E.A.; Podgornova, M.N.; Shchegolev, A.I. Ultrastructural Characteristics of Solid Pseudopapillary Tumors of the Pancreas. Bull. Exp. Biol. Med. 2011, 151, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zheng, Y. Sculpting the Nucleus with Dynamic Microtubules. Dev. Cell. 2013, 27, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hartig, R.; Shoeman, R.L.; Janetzko, A.; Tolstonog, G.; Traub, P. DNA-mediated transport of the intermediate filament protein vimentin into the nucleus of cultured cells. J. Cell Sci. 1998, 3584, 3573–3584. [Google Scholar]
- Traub, P.; Shoeman, R.L. Hypothesis: Intermediate filament and related proteins: Potential activators of nucleosomes during transcription initiation and elongation? BioEssays 1994, 16, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Nitkiewicz, T.; Wojnarowska, M.; Sołtysik, M.; Kaczmarski, A.; Witko, T.; Ingrao, C.; Guzik, M. How sustainable are biopolymers? Findings from a life cycle assessment of polyhydroxyalkanoate production from rapeseed-oil derivatives. Sci. Total Environ. 2020, 749, 141279. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.H.; Chou, I.N.; Tseng, S.H.; Wu, H.S. Application of biodegradable polyhydroxyalkanoates as surgical films for ventral hernia repair in mice. Int. J. Polym. Sci. 2014, 2014, 789681. [Google Scholar] [CrossRef]
- Mallardé, D.; Valière, M.; David, C.; Menet, M.; Guérin, P. Hydrolytic degradability of poly(3-hydroxyoctanoate) and of a poly(3-hydroxyoctanoate)/poly(R,S-lactic acid) blend. Polymer 1998, 39, 3387–3392. [Google Scholar] [CrossRef]
- Cichoń, E.; Haraźna, K.; Skibiński, S.; Witko, T.; Zima, A.; Ślósarczyk, A.; Zimowska, M.; Witko, M.; Leszczyński, B.; Wróbel, A.; et al. Novel bioresorbable tricalcium phosphate/polyhydroxyoctanoate (TCP/PHO) composites as scaffolds for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 98, 235–245. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliksiak, K.; Witko, T.; Solarz, D.; Guzik, M.; Rajfur, Z. Vimentin Association with Nuclear Grooves in Normal MEF 3T3 Cells. Int. J. Mol. Sci. 2020, 21, 7478. https://doi.org/10.3390/ijms21207478
Feliksiak K, Witko T, Solarz D, Guzik M, Rajfur Z. Vimentin Association with Nuclear Grooves in Normal MEF 3T3 Cells. International Journal of Molecular Sciences. 2020; 21(20):7478. https://doi.org/10.3390/ijms21207478
Chicago/Turabian StyleFeliksiak, Karolina, Tomasz Witko, Daria Solarz, Maciej Guzik, and Zenon Rajfur. 2020. "Vimentin Association with Nuclear Grooves in Normal MEF 3T3 Cells" International Journal of Molecular Sciences 21, no. 20: 7478. https://doi.org/10.3390/ijms21207478
APA StyleFeliksiak, K., Witko, T., Solarz, D., Guzik, M., & Rajfur, Z. (2020). Vimentin Association with Nuclear Grooves in Normal MEF 3T3 Cells. International Journal of Molecular Sciences, 21(20), 7478. https://doi.org/10.3390/ijms21207478





