Effects of Plasma Treatment on the Bioactivity of Alkali-Treated Ceria-Stabilised Zirconia/Alumina Nanocomposite (NANOZR)
Abstract
1. Introduction
2. Results
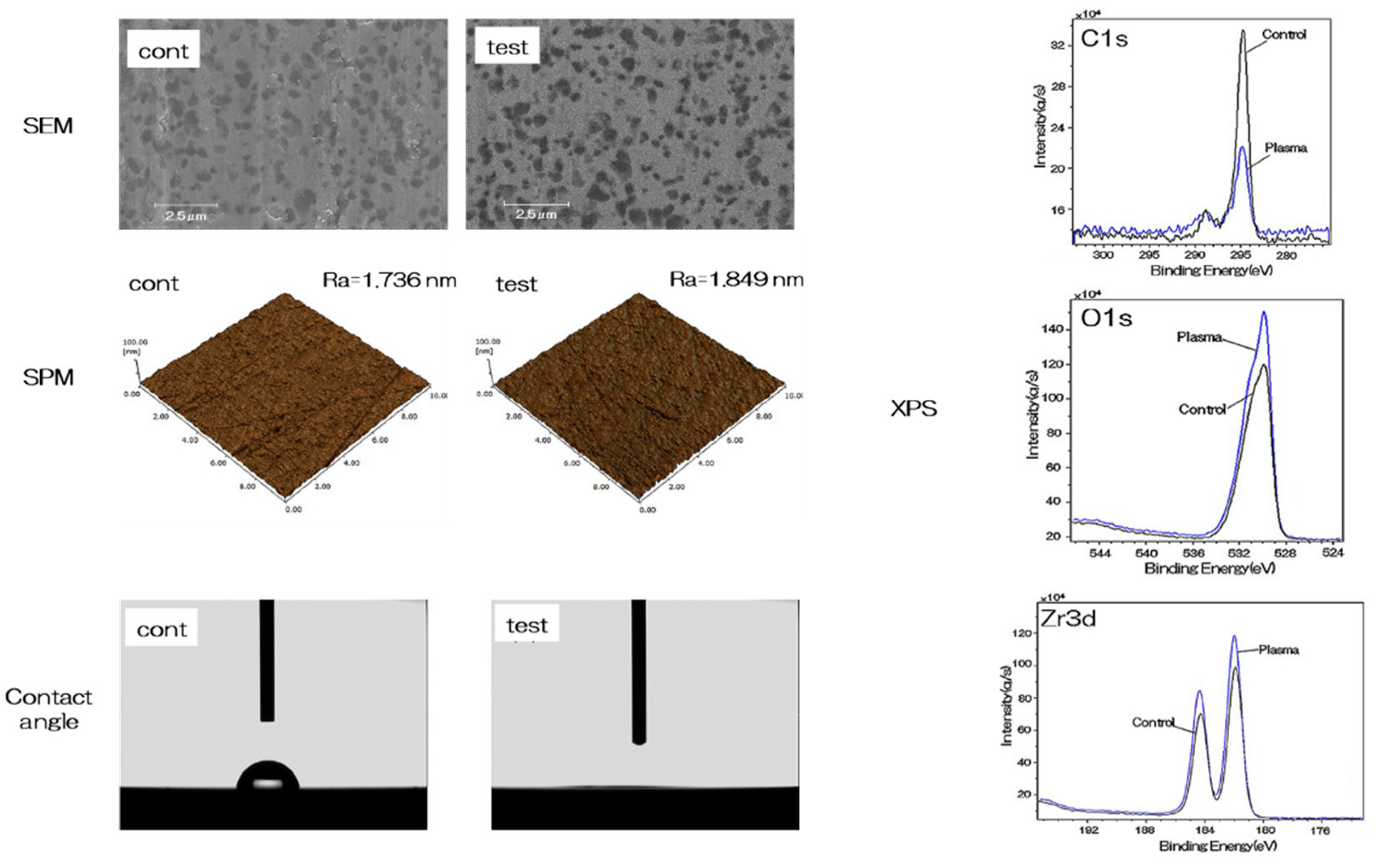
2.1. Evaluation of Nano-ZR Samples
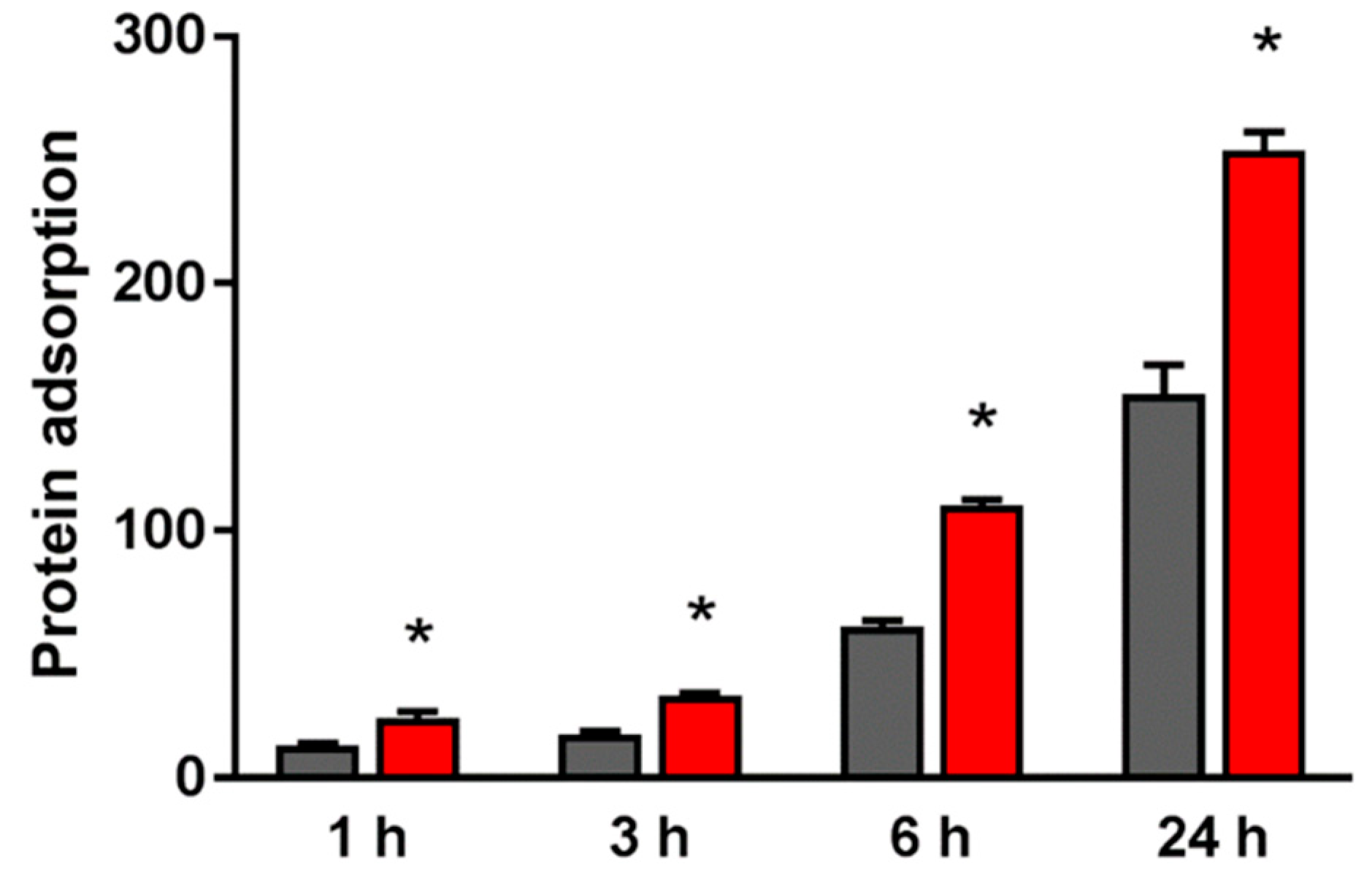
2.2. Evaluation of Protein Adsorption on the NANO-ZR Surface
2.3. Effects of the Nano-ZR Surface on Cell Adhesion and Morphology in rBMMSCs and HUVECs
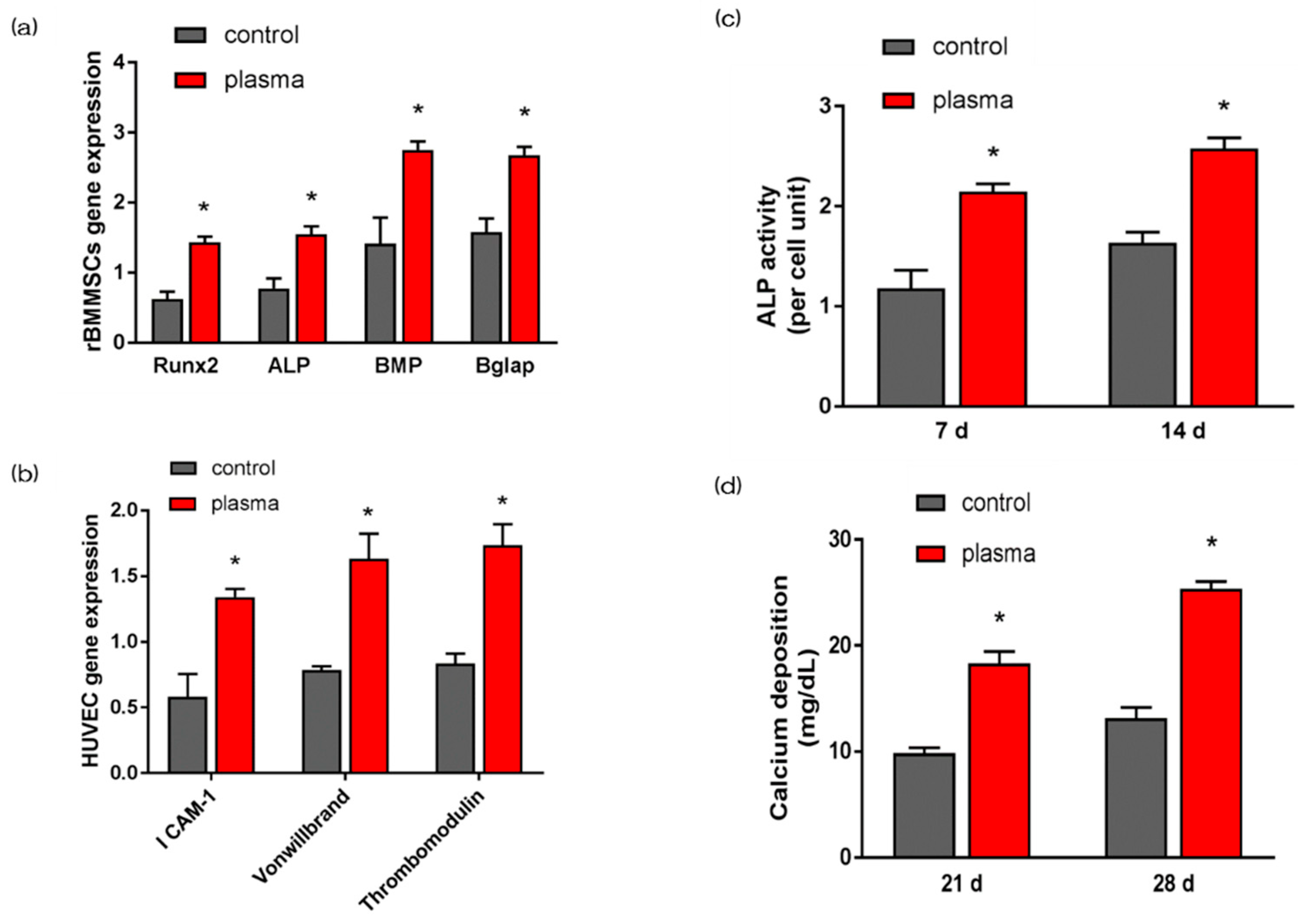
2.4. Evaluation of Hard Tissue Differentiation and Angiogenesis of rBMMSCs and HUVECs on Nano-ZR In Vitro
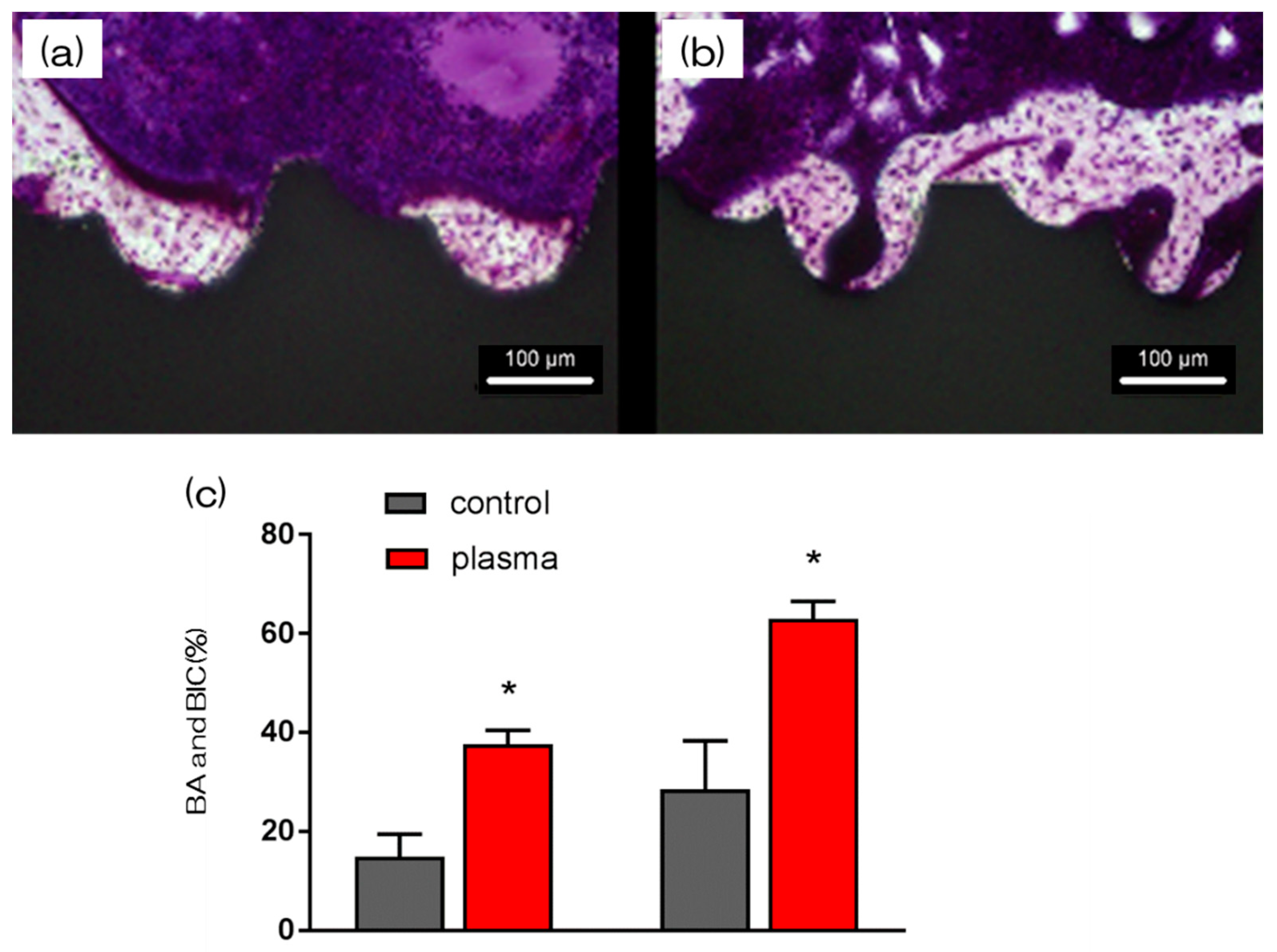
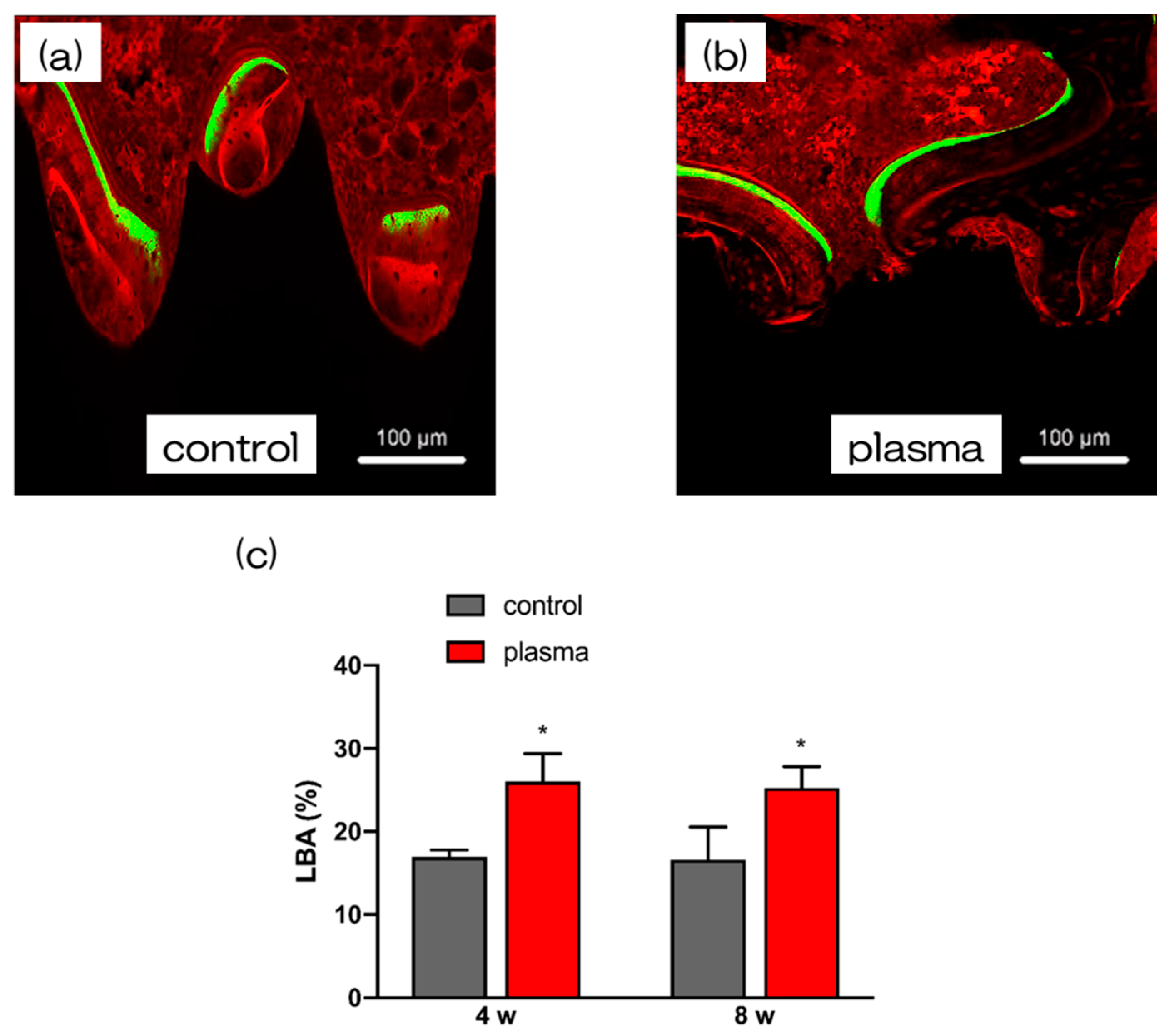
2.5. Evaluation of the Amount of New Bone Formation in the Tissue Surrounding the Nano-ZR Implant Placement In Vivo
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Characterization of NANO-ZR Samples
4.3. Protein Adsorption
4.4. Cell Culture
4.5. Cell Adhesion
4.6. QRT-PCR, Alkaline Phosphatase Activity, DNA Content, and Calcium Deposition
4.7. Animal Model and Surgical Procedures
4.8. Sequential Fluorescent Labeling
4.9. Morphological Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NANO-ZR | Ce-stabilised zirconia/alumina nanocomposite |
| rBMMSCs | Rat bone marrow mesenchymal stem cells |
| HUVECs | Human umbilical vein endothelial cells |
| SEM | Scanning electron microscopy |
| SPM | Scanning probe microscopy |
| XPS | X-ray photoelectron spectroscopy |
| BV/TV | Bone mass to total mass |
| Tb.N | Average trabecular number |
| Tb.Th | Average trabecular thickness |
| Tb.Sp | Mean trabecular separation |
| BA | Bone area ratio |
| BIC | Bone-to-implant contact |
| BSA | Bovine serum albumin |
| ALP | Alkaline phosphatase |
References
- Özkurt, Z.; Kazazoğlu, E. Zirconia dental implants: A literature review. J. Oral Implantol. 2011, 37, 367–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel zirconia materials in dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef]
- Manzano, G.; Herrero, R.; Montero, J. Comparison of clinical performance of zirconia implants and titanium implants in animal models: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29. [Google Scholar] [CrossRef]
- Möller, B.; Terheyden, H.; Açil, Y.; Purcz, N.M.; Hertrampf, K. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: An in vivo and in vitro study. Int. J. Oral Maxillofac. Surg. 2012, 41, 638–645. [Google Scholar] [CrossRef]
- Al Qahtani, W.M.; Schille, C.; Spintzyk, S.; Al Qahtani, M.S.; Engel, E.; Geis-Gerstorfer, J.; Scheideler, L. Effect of surface modification of zirconia on cell adhesion, metabolic activity and proliferation of human osteoblasts. Biomed. Eng. Biomed. Tech. 2017, 62, 75–87. [Google Scholar] [CrossRef]
- Saulacic, N.; Erdösi, R.; Bosshardt, D.D.; Gruber, R.; Buser, D. Acid and alkaline etching of sandblasted zirconia implants: A histomorphometric study in miniature pigs. Clin. Implant Dent. Relat. Res. 2014, 16, 313–322. [Google Scholar] [CrossRef]
- Gahlert, M.; Roehling, S.; Sprecher, C.M.; Kniha, H.; Milz, S.; Bormann, K. In vivo performance of zirconia and titanium implants: A histmorphometric study in mini pig maxillae. Clin. Oral Implant. Res. 2012, 23, 281–286. [Google Scholar] [CrossRef]
- Bormann, K.; Gellrich, N.C.; Kniha, H.; Dard, M.; Wieland, M.; Gahlert, M. Biomechanical evaluation of a microstructured zirconia implant by a removal torque comparison with a standard Ti-SLA implant. Clin. Oral Implant. Res. 2012, 23, 1210–1216. [Google Scholar] [CrossRef]
- Kohal, R.J.; Baechle, M.; Han, J.S.; Hueren, D.; Huebner, U.; Butz, F. In vitro reaction of human osteoblasts on alumina-toughened zirconia. Clin. Oral Implant. Res. 2009, 20, 1265–1271. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef]
- Coelho, P.G.; Suzuki, M. Evaluation of an ibad thin-film process as an alternative method for surface incorporation of bioceramics on dental implants: A study in dogs. J. Appl. Oral Sci. 2005, 13, 87–92. [Google Scholar] [CrossRef]
- Wang, X.X.; Hayakawa, S.; Tsuru, K.; Osaka, A. A comparative study of in vitro apatite deposition on heat-, H(2)O(2)-, and NaOH-treated titanium surfaces. J. Biomater. Mater. Res. 2001, 54, 172–178. [Google Scholar] [CrossRef]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 2007, 28, 4748–4755. [Google Scholar] [CrossRef]
- Hart, A.; Gadegaard, N.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M.J. Osteoprogenitor response to low-adhesion nanotopographies originally fabricated by electron beam lithography. J. Mater. Sci. Mater. Med. 2007, 18, 1211–1218. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Factors influencing early dental implant failures. J. Dent. Res. 2016, 95, 995–1002. [Google Scholar] [CrossRef]
- Gupta, S. A Recent Updates on Zirconia Implants: A Literature Review. Dent. Implant. Dentures 2016, 1, 18–26. [Google Scholar]
- AlKahtani, R.N. The implications and applications of nanotechnology in dentistry: A review. Saudi Dent. J. 2018, 30, 107–116. [Google Scholar] [CrossRef]
- Naganuma, T. The relationship between cell adhesion force activation on nano/micro-topographical surfaces and temporal dependence of cell morphology. Nanoscale 2017, 9, 13171–13186. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 1, 25. [Google Scholar]
- Nawa, M.; Bamba, N.; Sekino, T.; Niihara, K. Tough and strong Ce-TZP/alumina nanocomposites doped with titania. Ceram. Int. 1998, 24, 497–506. [Google Scholar] [CrossRef]
- Nawa, M.; Bamba, N.; Sekino, T.; Niihara, K. The effect of TiO2 addition on strengthening and toughening in intragranular type of 12Ce-TZP/Al2O3 nanocomposites. J. Eur. Ceram. Soc. 1998, 18, 209–219. [Google Scholar] [CrossRef]
- Ban, S. Reliability and properties of core materials for all-ceramic dental restorations. Jpn. Dent. Sci. Rev. 2008, 44, 3–21. [Google Scholar] [CrossRef]
- Ban, S.; Sato, H.; Suehiro, Y.; Nakanishi, H.; Nawa, M. Biaxial flexure strength and low temperature degradation of Ce-TZP/Al2O3 nanocomposite and Y-TZP as dental restoratives. J. Biomed. Mater. Res. B 2008, 87, 492–498. [Google Scholar] [CrossRef]
- Takano, T.; Tasaka, A.; Yoshinari, M.; Sakurai, K. Fatigue strength of Ce-TZP/Al2O3 nanocomposites with different surfaces. J. Dent. Res. 2012, 91, 800–804. [Google Scholar] [CrossRef]
- Camargo, W.A.; Takemoto, S.; Hoekstra, J.W.; Leeuwenburgh, S.C.; Jansen, J.A.; van den Beucken, J.J.; Alghamdi, H.S. Effect of surface alkali-based treatment of titanium implants on ability to promote in vitro mineralization and in vivo bone formation. Acta Biomater. 2017, 57, 511–523. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Jang, J.H.; Kwon, T.G.; Bae, Y.C.; Suh, J.Y. Effects of phosphoric acid treatment of titanium surfaces on surface properties, osteoblast response and removal of torque forces. Acta Biomater. 2010, 6, 1661–1670. [Google Scholar] [CrossRef]
- Ko, H.C.; Han, J.S.; Bachle, M.; Jang, J.H.; Shin, S.W.; Kim, D.J. Initial osteoblast-like cell response to pure titanium and zirconia/alumina ceramics. Dent. Mater. 2007, 23, 1349–1355. [Google Scholar] [CrossRef]
- Depprich, R.; Ommerborn, M.; Zipprich, H.; Naujoks, C.; Handschel, J.; Wiesmann, H.P.; Meyer, U. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008, 4, 29. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Komasa, S.; Taguchi, Y.; Nishida, H.; Tanaka, M.; Kawazoe, T. Bioactivity of nanostructure on titanium surface modified by chemical processing at room temperature. J. Periodontics. 2012, 56, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surface with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Taguchi, Y.; Komasa, S.; Sekino, T.; Tanaka, M. Cell differentiation on nanoscale feature of a titanium surface: Effects of deposition time in NaOH solution. J. Hard Tissue Biol. 2014, 23, 63–70. [Google Scholar] [CrossRef]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef]
- Nakano, Y.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Rat endothelial cell attachment, behavior and gene expression on NaOH-treated titanium surfaces. J. Oral Tissue Eng. 2013, 11, 189–200. [Google Scholar]
- Hara, Y.; Komasa, S.; Yoshimine, S.; Nishizaki, H.; Okazaki, J. effect of nano modified titanium surface on adsorption of rat periodontal ligament cells. J. Osaka Dent. Univ. 2018, 52, 37–44. [Google Scholar]
- Nishizaki, M.; Komasa, S.; Taguchi, Y.; Nishizaki, H.; Okazaki, J. Bioactivity of NANOZR induced by Alkali Treatment. Int. J. Mol. Sci. 2017, 18, 780. [Google Scholar] [CrossRef]
- Komasa, S.; Nishizaki, M.; Zhang, H.; Takao, S.; Yin, D.; Terada, C.; Okazaki, J. Osseointegration of alkali-modified NANOZR implants: An in vivo study. Int. J. Mol. Sci. 2019, 20, 842. [Google Scholar] [CrossRef]
- Fridman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Ohl, A.; Lüthen, F.; Bergemann, C.; Nebe, B.; Neumann, H.G. Capability of differently charged plasma polymer coatings for control of tissue interactions with titanium surfaces. J. Adhes. Sci. Techol. 2010, 24, 1191–1205. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Jesswein, H.; Lüthen, F.; Diener, A.; Ihrke, R.; Nebe, J.B. Similarities between plasma amino functionalized PEEK and titanium surfaces concerning enhancement of osteoblast cell adhesion. J. Adhes. Sci. Techol. 2010, 24, 905–923. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Polak, M.; Lüthen, F.; Nebe, B.; Rychly, J.; Ohl, A. Gas-discharge plasma-assisted functionalization of titanium implant surfaces. Mater. Sci. Forum. 2019, 638–642, 700–705. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of argon affects the earliest biological response of different implant surfaces: An in vitro comparative study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.W.K.; Chan, R.Y.L.; Lam, K.O.; Wu, S.L.; Liu, X.M.; Chung, C.Y.; Cheung, K.M.C. In vitro and in vivo characterization of novel plasma treated nickel titanium shape memory alloy for orthopedic implantation. Surf. Coat. Technol. 2007, 202, 1247–1251. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Heiland, M.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieldan, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of micro structured titanium implant surfaces. J. Biomed. Mater. Res. 2006, 76, 323–334. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Jimbo, R.; Sawase, T.; Baba, K.; Kurogi, T.; Shibata, Y.; Atsuta, M. Enhanced initial cell responses to chemically modified anodized titanium. Clin. Implant. Dent. Res. 2008, 10, 55–61. [Google Scholar] [CrossRef]
- Foest, R.; Kindel, E.; Ohl, A.; Stieber, M.; Weltmann, K.M. Non-thermal atmospheric pressure discharges for surface modification. Plasma Phys. Contr. Fusion 2005, 47, 525–536. [Google Scholar] [CrossRef]
- Ujino, D.; Nishizaki, H.; Higuchi, S.; Komasa, S.; Okazaki, J. effect of plasma treatment of titanium surface on bioactivity. Appl. Sci. 2019, 9, 2257. [Google Scholar] [CrossRef]
- Cho, B.H.; Han, G.J.; Oh, K.H.; Chung, S.N.; Chun, B.H. The effect of plasma polymer coating using atmospheric-pressure glow discharge on the shear bond strength of composite resin to ceramic. J. Mater. Sci. 2011, 46, 2755–2763. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of Biomaterials. Mater. Sci. Eng. R Rep. 2012, 36, 143–206. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Shohet, J.L. Plasma-aided manufacturing. Science 1991, 19, 725–733. [Google Scholar]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Sawase, T.; Jimbo, R.; Wennerberg, A. A novel characteristic of porous titanium oxide implants. Clin. Oral Implant. Res. 2007, 18, 680–685. [Google Scholar] [CrossRef]
- Hasan, M.I.; Walsh, J.L. Influence of gas flow velocity on the transport of chemical species in an atmospheric pressure air plasma discharge. Appl. Phys. Lett. 2017, 110, 134102. [Google Scholar] [CrossRef]
- Almaguer-Flores, A.; Olivares-Navarrete, R.; Wieland, M.; Ximénez-Fyvie, L.A.; Schwartz, Z.; Boyan, B.D. Influence of topography and hydrophilicity on initial oral biofilm formation on microstructure titanium surfaces in vitro. Clin. Oral Implant. Res. 2012, 23, 301–307. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium to promote to human mesenchymal stem cell migration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Eichler, M.; Geis-Gerstorfer, J. Wetting behavior of dental implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 1256–1266. [Google Scholar]
- Sela, M.N.; Badihi, L.; Rosen, G.; Steinberg, D.; Kohavi, D. adsorption of human plasma proteins to modified titanium surfaces. Clin. Oral Implant. Res. 2007, 18, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Yu, H.S.; Choi, E.H.; Hwang, C.J. Corrigendum: Time-dependent effects of ultraviolet and nonthermal atmospheric pressure plasma on the biological activity of titanium. Sci. Rep. 2016, 6, 33421. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Pattanayek, S.K.; Pandey, L.M. Effect of functional groups of self-assembled monolayers on protein adsorption and initial cell adhesion. ACS Biomater. Sci. Eng. 2018, 4, 3224–3233. [Google Scholar] [CrossRef]
- Ngandu Mpoyi, E.; Cantini, M.; Reynolds, P.M.; Gadegaard, N.; Dalby, M.J.; Salmerón-Sánchez, M. Protein adsorption as a key mediator in the nanotopographical control of cell behavior. ACS Nano 2016, 10, 6638–6647. [Google Scholar] [CrossRef]
- D’Sa, R.A.; Burke, G.A.; Meenan, B.J. Protein adhesion and cell response on atmospheric pressure dielectric barrier discharge-modified polymer surfaces. Acta Biomater. 2010, 6, 2609–2620. [Google Scholar] [CrossRef]
- Kusumoto, T.; Yin, D.; Zhang, H.; Chen, L.; Nishizaki, H.; Komasa, Y.; Komasa, S. Evaluation of the osteointegration of a novel alkali-treated inplant system in vivo. J. Hard Tissue Biol. 2017, 26, 355–360. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Y.; Chen, L.; Yin, D.; Zhang, H.; Tashiro, Y.; Okazaki, J.; Komasa, S. Optimized surface characteristic and enhanced in vivo osseointegration of alkali-treated titanium with nanonetwork structure. Int. J. Mol. Sci. 2019, 20, 1127. [Google Scholar] [CrossRef]
- Terada, C.; Komasa, S.; Kusumoto, T.; Kawazoe, T.; Okazaki, J. Effect of amelogenin coating of a nano-modified titanium surface on bioactivity. Int. J. Mol. Sci. 2018, 19, 1274. [Google Scholar] [CrossRef]
- Su, Y.; Komasa, S.; Sekino, T.; Nishizaki, H.; Okazaki, J. Characterization and bone differentiation of nanoporous structure fabricated on Ti6Al4V Alloy. J. Nanomater. 2015. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takao, S.; Komasa, S.; Agariguchi, A.; Kusumoto, T.; Pezzotti, G.; Okazaki, J. Effects of Plasma Treatment on the Bioactivity of Alkali-Treated Ceria-Stabilised Zirconia/Alumina Nanocomposite (NANOZR). Int. J. Mol. Sci. 2020, 21, 7476. https://doi.org/10.3390/ijms21207476
Takao S, Komasa S, Agariguchi A, Kusumoto T, Pezzotti G, Okazaki J. Effects of Plasma Treatment on the Bioactivity of Alkali-Treated Ceria-Stabilised Zirconia/Alumina Nanocomposite (NANOZR). International Journal of Molecular Sciences. 2020; 21(20):7476. https://doi.org/10.3390/ijms21207476
Chicago/Turabian StyleTakao, Seiji, Satoshi Komasa, Akinori Agariguchi, Tetsuji Kusumoto, Giuseppe Pezzotti, and Joji Okazaki. 2020. "Effects of Plasma Treatment on the Bioactivity of Alkali-Treated Ceria-Stabilised Zirconia/Alumina Nanocomposite (NANOZR)" International Journal of Molecular Sciences 21, no. 20: 7476. https://doi.org/10.3390/ijms21207476
APA StyleTakao, S., Komasa, S., Agariguchi, A., Kusumoto, T., Pezzotti, G., & Okazaki, J. (2020). Effects of Plasma Treatment on the Bioactivity of Alkali-Treated Ceria-Stabilised Zirconia/Alumina Nanocomposite (NANOZR). International Journal of Molecular Sciences, 21(20), 7476. https://doi.org/10.3390/ijms21207476




