Age-Induced Differential Changes in the Central and Colonic Human Circadian Oscillators
Abstract
1. Introduction
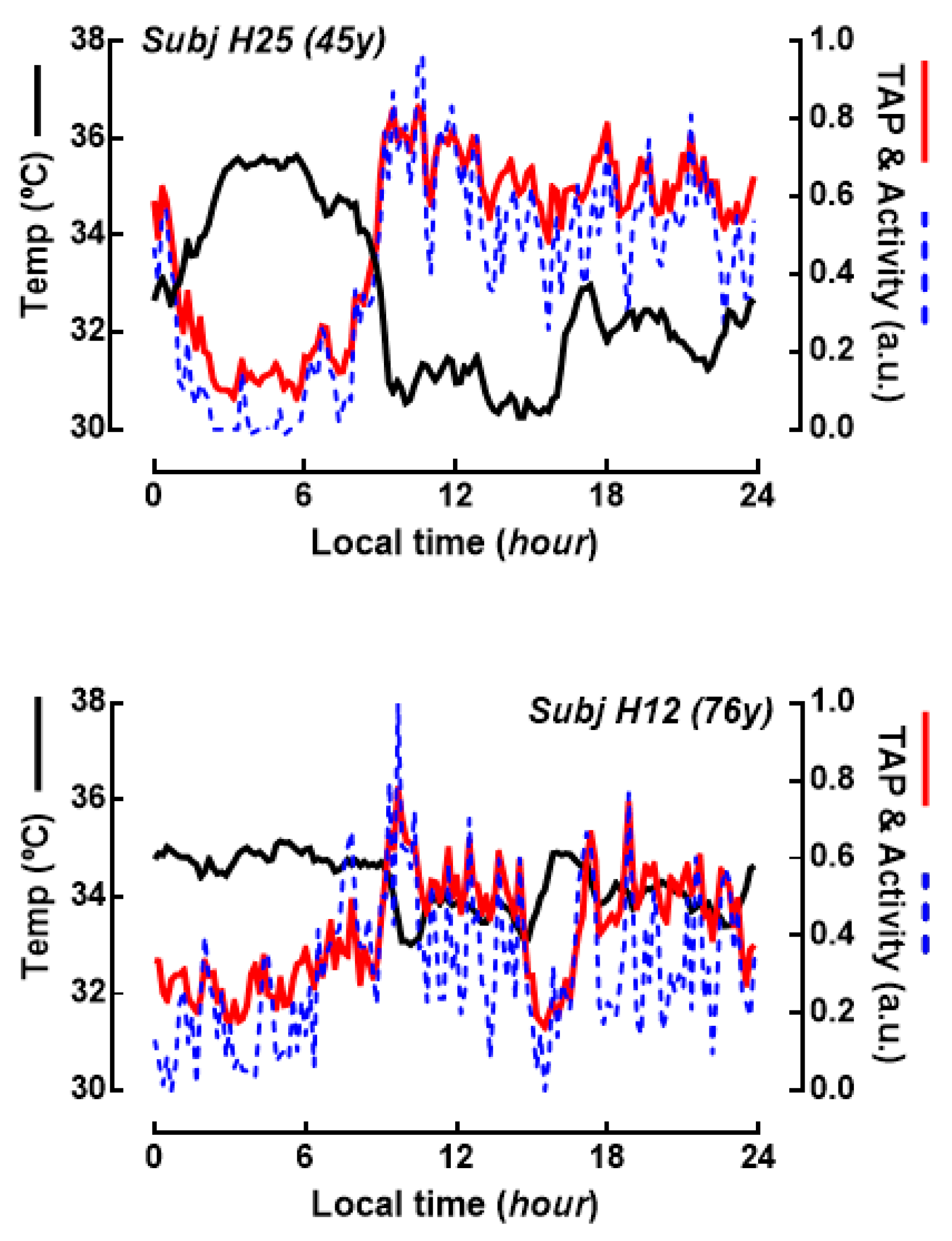
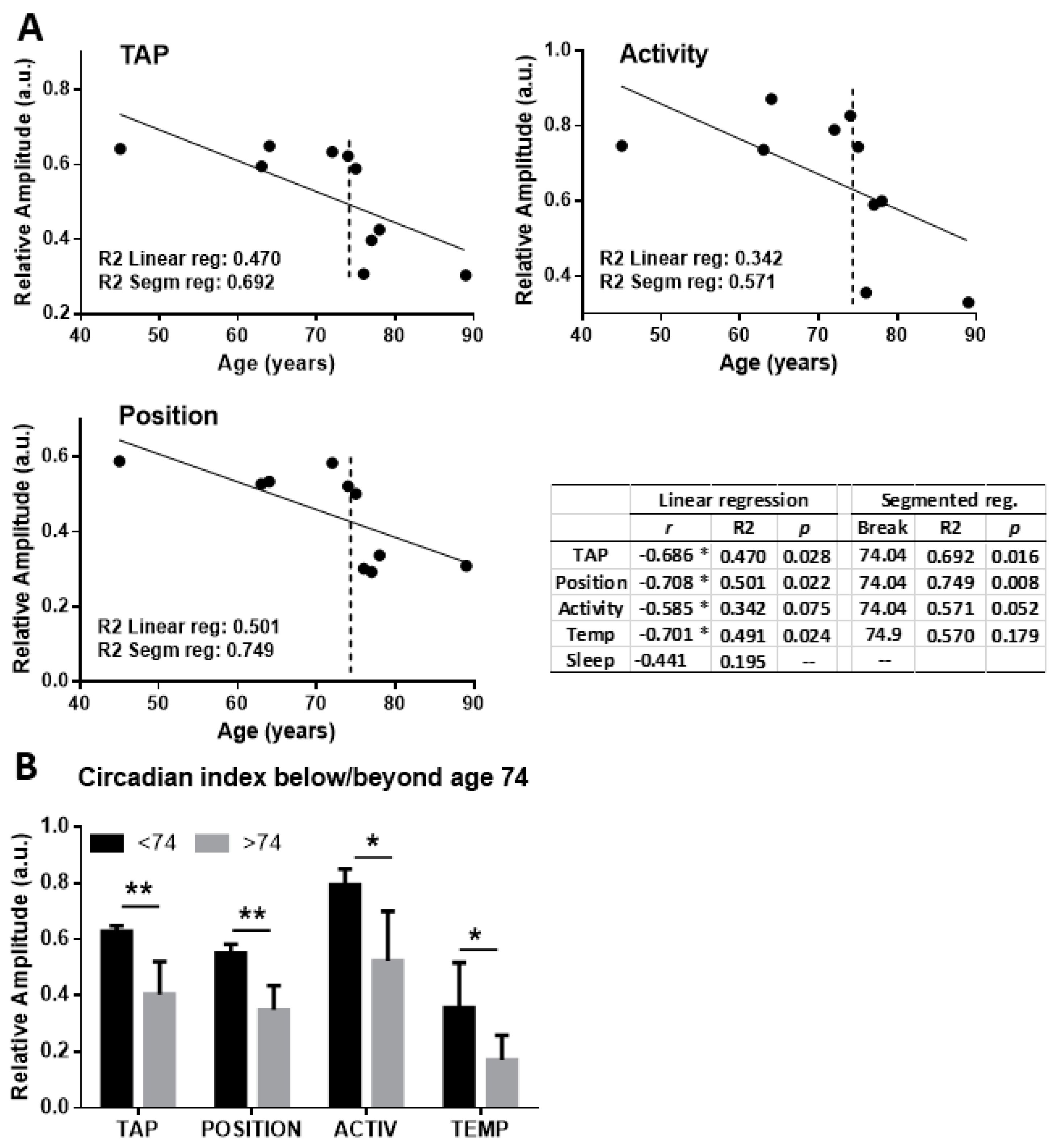
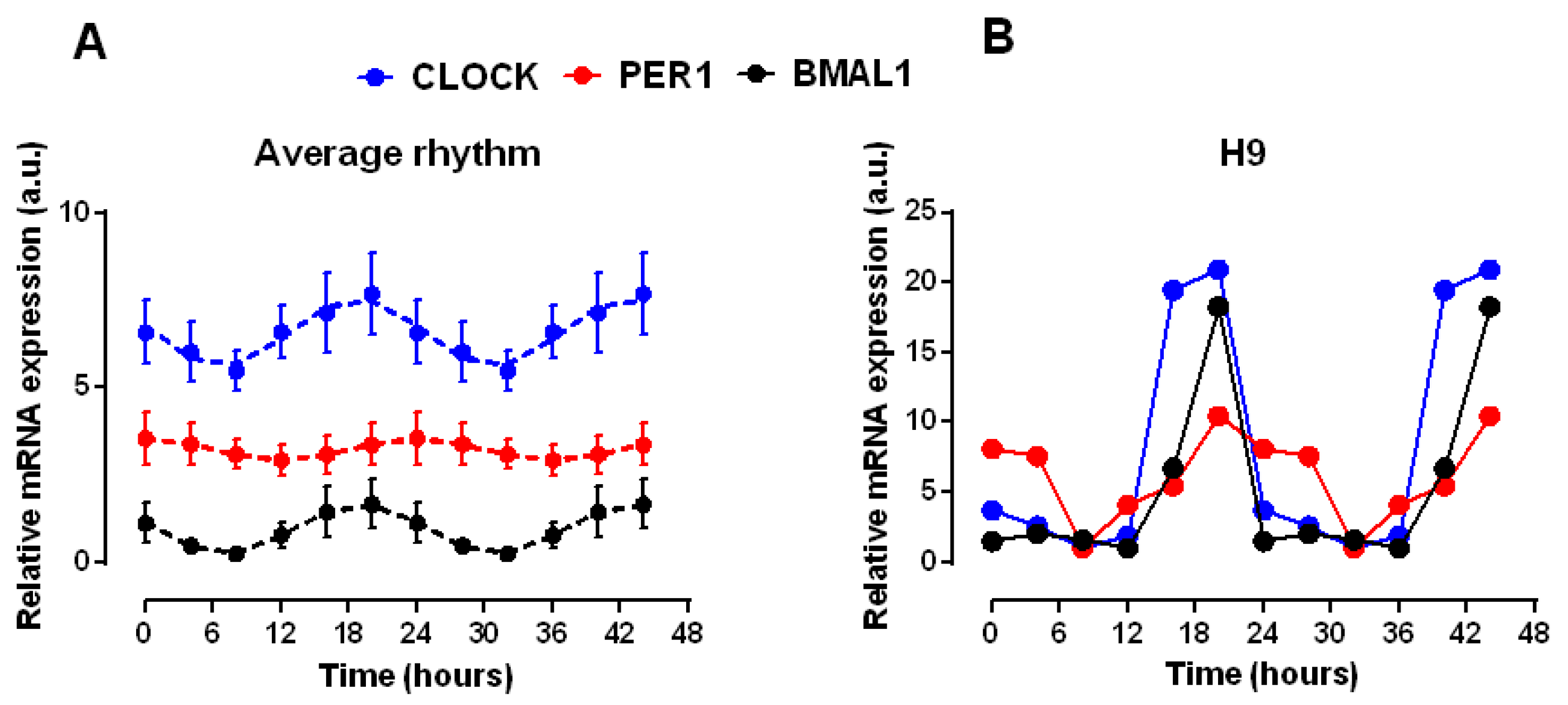
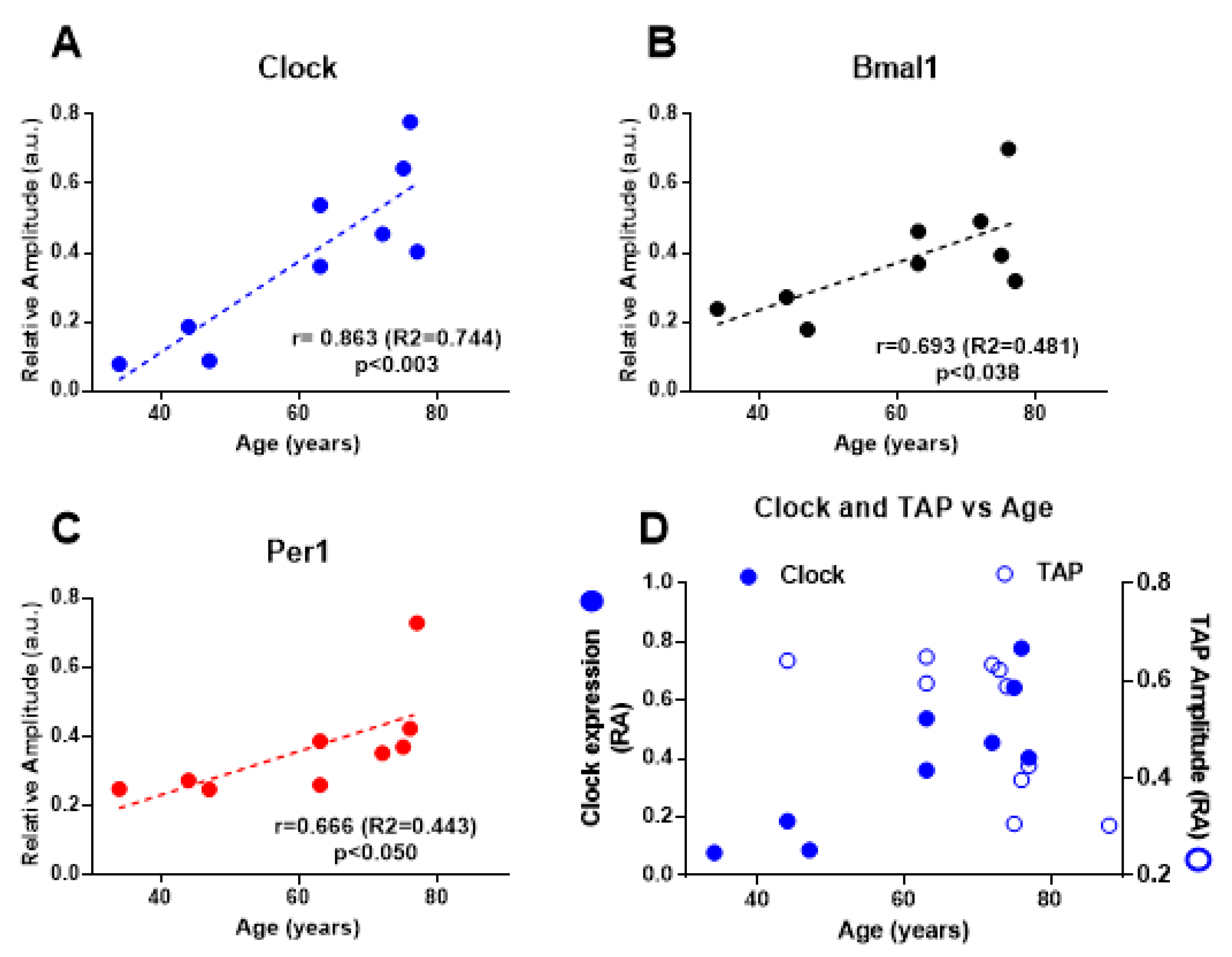
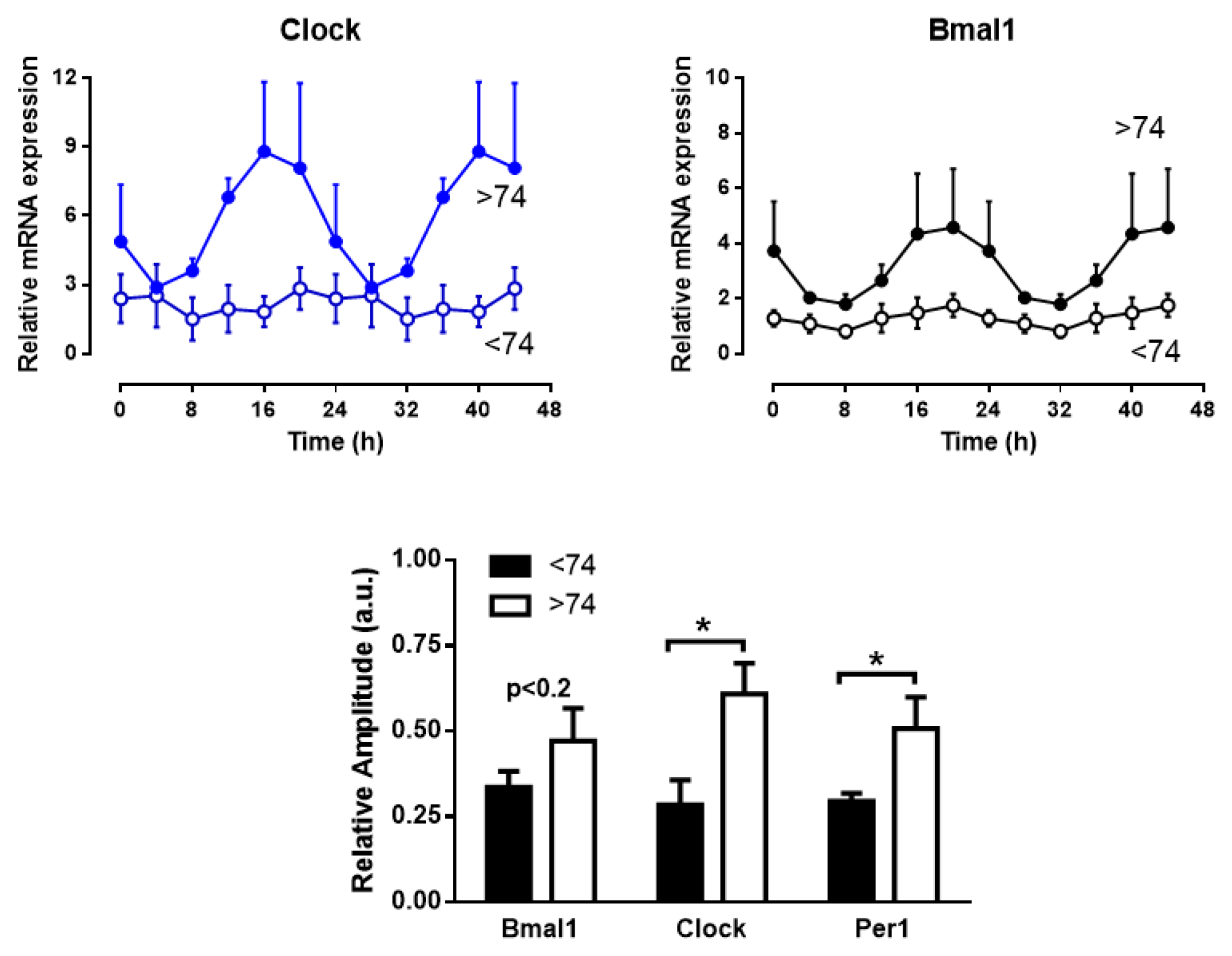
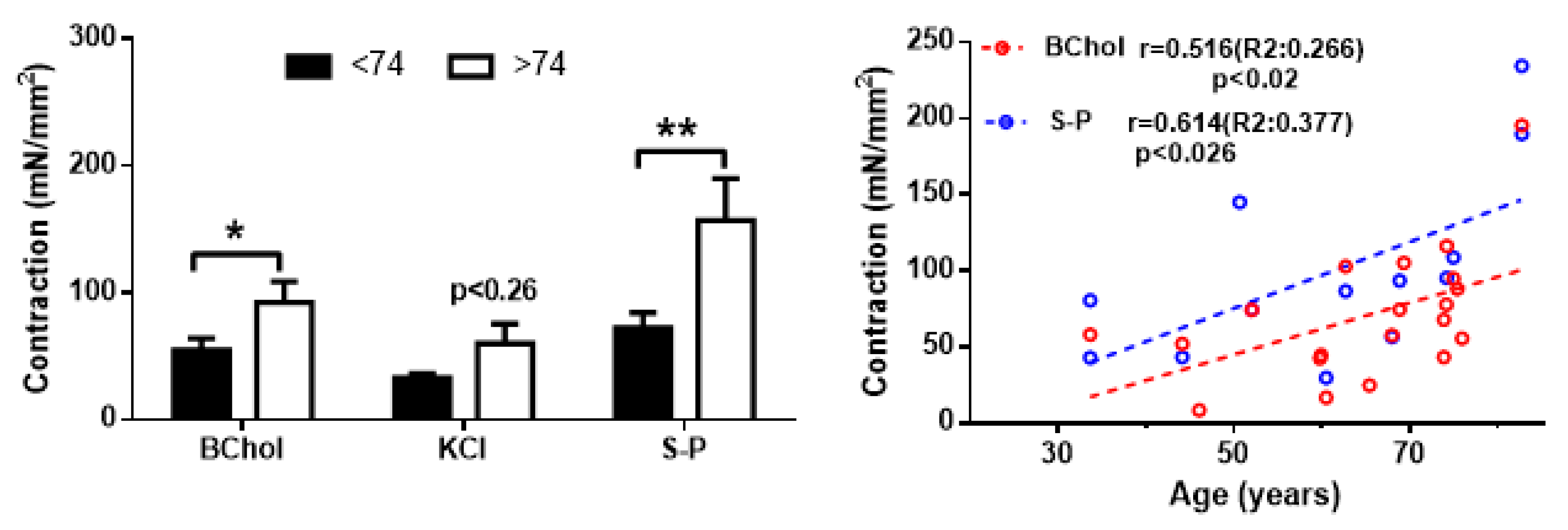
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Aacquisition and Processing of Circadian Marker Rhythms
4.3. Clock Genes Expression
4.4. Contraction Recording of Colonic Smooth Muscle Strips
4.5. Determination of Cytosolic Ca2+ Signals
4.6. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SCN | Suprachiasmatic nucleus |
| CFI | Circadian functional index |
| RA | Relative amplitude |
| Clock | Clock circadian regulator |
| Bmal1 | Aryl hydrocarbon receptor nuclear translocator like (ARNTL) |
| Per1 | Period circadian regulator 1 |
| K-HS | Krebs-Henseleit solution |
| [Ca2+]i | Cytosolic Ca2+ concentration |
References
- Camello, P.J.; Camello-Almaraz, C.; Pozo, M.J. Pharmacological Approaches to Improve Ageing. In Pharmacology; Gallelli, L., Ed.; InTech Open: London, UK, 2012; Available online: https://www.intechopen.com/books/pharmacology/pharmacological-approaches-to-improve-ageing (accessed on 15 November 2019). [CrossRef]
- Turek, F.W.; Penev, P.; Zhang, Y.; van Reeth, O.; Zee, P. Effects of age on the circadian system. Neurosci. Biobehav. Rev. 1995, 19, 53–58. [Google Scholar] [CrossRef]
- Buijs, F.N.; Leon-Mercado, L.; Guzman-Ruiz, M.; Guerrero-Vargas, N.N.; Romo-Nava, F.; Buijs, R.M. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 2016, 31, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Cermakian, N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol. Int. 2007, 24, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011, 62, 139–150. [Google Scholar] [PubMed]
- Hoogerwerf, W.A. Role of clock genes in gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G549–G555. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A.; Hellmich, H.L.; Cornelissen, G.; Halberg, F.; Shahinian, V.B.; Bostwick, J.; Savidge, T.C.; Cassone, V.M. Clock gene expression in the murine gastrointestinal tract: Endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 2007, 133, 1250–1260. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A.; Shahinian, V.B.; Cornelissen, G.; Halberg, F.; Bostwick, J.; Timm, J.; Bartell, P.A.; Cassone, V.M. Rhythmic changes in colonic motility are regulated by period genes. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G143–G150. [Google Scholar] [CrossRef]
- Fung, C.H.; Vitiello, M.V.; Alessi, C.A.; Kuchel, G.A.; AGS/NIA Sleep Conference Planning Committee and Faculty; Arora, V.; Nisha Aurora, R.; Benveniste, H.; Bliwise, D.; Buysse, D.; et al. Report and Research Agenda of the American Geriatrics Society and National Institute on Aging Bedside-to-Bench Conference on Sleep, Circadian Rhythms, and Aging: New Avenues for Improving Brain Health, Physical Health, and Functioning. J. Am. Geriatr. Soc. 2016, 64, e238–e247. [Google Scholar] [CrossRef]
- Weinert, D. Circadian temperature variation and ageing. Ageing Res. Rev. 2010, 9, 51–60. [Google Scholar] [CrossRef]
- Hofman, M.A.; Swaab, D.F. Living by the clock: The circadian pacemaker in older people. Ageing Res. Rev. 2006, 5, 33–51. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Warman, G.R.; Cheeseman, J.F. The functional changes of the circadian system organization in aging. Ageing Res. Rev. 2019, 52, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.A.; Madrid, J.A. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar] [CrossRef] [PubMed]
- Polidarova, L.; Sotak, M.; Sladek, M.; Pacha, J.; Sumova, A. Temporal gradient in the clock gene and cell-cycle checkpoint kinase Wee1 expression along the gut. Chronobiol. Int. 2009, 26, 607–620. [Google Scholar] [CrossRef]
- Martinez-Nicolas, A.; Madrid, J.A.; Garcia, F.J.; Campos, M.; Moreno-Casbas, M.T.; Almaida-Pagan, P.F.; Lucas-Sanchez, A.; Rol, M.A. Circadian monitoring as an aging predictor. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Fonnes, S.; Donatsky, A.M.; Gogenur, I. Expression of core clock genes in colorectal tumour cells compared with normal mucosa: A systematic review of clinical trials. Colorectal Dis. 2015, 17, 290–297. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Panza, A.; Valvano, M.R.; Palumbo, O.; Carella, M.; Pazienza, V.; Biscaglia, G.; Tavano, F.; Di Sebastiano, P.; Andriulli, A.; et al. Clock gene expression levels and relationship with clinical and pathological features in colorectal cancer patients. Chronobiol. Int. 2011, 28, 841–851. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Tatsumoto, M.; Matsumura, R.; Endo, T.; Hirata, K.; Tokuda, I.; Akashi, M. Normal peripheral circadian phase in the old-old with abnormal circadian behavior. Genes Cells 2018, 23, 849–859. [Google Scholar] [CrossRef]
- Yeung, C.C.; Schjerling, P.; Heinemeier, K.M.; Boesen, A.P.; Dideriksen, K.; Kjaer, M. Investigating circadian clock gene expression in human tendon biopsies from acute exercise and immobilization studies. Eur. J. Appl. Physiol. 2019, 119, 1387–1394. [Google Scholar] [CrossRef]
- Chen, C.Y.; Logan, R.W.; Ma, T.; Lewis, D.A.; Tseng, G.C.; Sibille, E.; McClung, C.A. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2016, 113, 206–211. [Google Scholar] [CrossRef]
- Brzezinski, A.; Saada, A.; Miller, H.; Brzezinski-Sinai, N.A.; Ben-Meir, A. Is the aging human ovary still ticking? Expression of clock-genes in luteinized granulosa cells of young and older women. J. Ovarian Res. 2018, 11, 95. [Google Scholar] [CrossRef]
- Paulose, J.K.; Cassone, C.V.; Cassone, V.M. Aging, melatonin biosynthesis, and circadian clockworks in the gastrointestinal system of the laboratory mouse. Physiol. Genom. 2019, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Gorenz, A.; Shaikh, M.; Desai, V.; Kaminsky, T.; Van Den Berg, J.; Murphy, T.; Raeisi, S.; Fogg, L.; Vitaterna, M.H.; et al. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G192–G201. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Abellan, P.; Diez-Noguera, A.; Madrid, J.A.; Lujan, J.A.; Ordovas, J.M.; Garaulet, M. Glucocorticoids affect 24 h clock genes expression in human adipose tissue explant cultures. PLoS ONE 2012, 7, e50435. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, D.; Aoki, N.; Tanaka, M.; Aoyama, S.; Shibata, S. The circadian clock controls fluctuations of colonic cell proliferation during the light/dark cycle via feeding behavior in mice. Chronobiol. Int. 2015, 32, 1145–1155. [Google Scholar] [CrossRef]
- Wyse, C.A.; Coogan, A.N. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010, 1337, 21–31. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Bertrand, R.L.; Camello, P.J.; Pozo, M.J. Simultaneous measurement of serotonin and melatonin from the intestine of old mice: The effects of daily melatonin supplementation. J. Pineal Res. 2010, 49, 23–34. [Google Scholar] [CrossRef]
- Wiley, J.W.; Higgins, G.A.; Athey, B.D. Stress and glucocorticoid receptor transcriptional programming in time and space: Implications for the brain-gut axis. Neurogastroenterol. Motil. 2016, 28, 12–25. [Google Scholar] [CrossRef]
- Xu, L.; Wu, T.; Li, H.; Ni, Y.; Fu, Z. An individual 12-h shift of the light-dark cycle alters the pancreatic and duodenal circadian rhythm and digestive function. Acta Biochim. Biophys. Sin. 2017, 49, 954–961. [Google Scholar] [CrossRef]
- Polidarova, L.; Sladek, M.; Sotak, M.; Pacha, J.; Sumova, A. Hepatic, duodenal, and colonic circadian clocks differ in their persistence under conditions of constant light and in their entrainment by restricted feeding. Chronobiol. Int. 2011, 28, 204–215. [Google Scholar] [CrossRef]
- Nojkov, B.; Rubenstein, J.H.; Chey, W.D.; Hoogerwerf, W.A. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am. J. Gastroenterol. 2010, 105, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, P.J.; Gomez, M.F.; Hedlund, P.; Sward, K.; Hellstrand, P.; Camello, P.J.; Pozo, M.J.; Andersson, K.E. Effect of melatonin on age associated changes in Guinea pig bladder function. J. Urol. 2007, 177, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, P.J.; Camello-Almaraz, C.; Moreno, R.; Camello, P.J.; Pozo, M.J. Melatonin treatment reverts age-related changes in Guinea pig gallbladder neuromuscular transmission and contractility. J. Pharmacol. Exp. Ther. 2006, 319, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Bitar, K.; Greenwood-Van Meerveld, B.; Saad, R.; Wiley, J.W. Aging and gastrointestinal neuromuscular function: Insights from within and outside the gut. Neurogastroenterol. Motil. 2011, 23, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal Tract Disorders in Older Age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6757524. [Google Scholar] [CrossRef] [PubMed]
- Pascua, P.; Camello-Almaraz, C.; Camello, P.J.; Martin-Cano, F.E.; Vara, E.; Fernandez-Tresguerres, J.A.; Pozo, M.J. Melatonin, and to a lesser extent growth hormone, restores colonic smooth muscle physiology in old rats. J. Pineal Res. 2011, 51, 405–415. [Google Scholar] [CrossRef]
- Dinning, P.G. Recording In Vivo Human Colonic Motility: What Have We Learnt Over the Past 100 Years? Adv. Exp. Med. Biol. 2016, 891, 213–222. [Google Scholar] [CrossRef]
- Schmutz, I.; Chavan, R.; Ripperger, J.A.; Maywood, E.S.; Langwieser, N.; Jurik, A.; Stauffer, A.; Delorme, J.E.; Moosmang, S.; Hastings, M.H.; et al. A specific role for the REV-ERBalpha-controlled L-Type Voltage-Gated Calcium Channel CaV1.2 in resetting the circadian clock in the late night. J. Biol. Rhythm. 2014, 29, 288–298. [Google Scholar] [CrossRef]
- Kaneko, K.; Yamada, T.; Tsukita, S.; Takahashi, K.; Ishigaki, Y.; Oka, Y.; Katagiri, H. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 2009, 1263, 58–68. [Google Scholar] [CrossRef]
- Sladek, M.; Rybova, M.; Jindrakova, Z.; Zemanova, Z.; Polidarova, L.; Mrnka, L.; O’Neill, J.; Pacha, J.; Sumova, A. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology 2007, 133, 1240–1249. [Google Scholar] [CrossRef]
- Sotak, M.; Polidarova, L.; Musilkova, J.; Hock, M.; Sumova, A.; Pacha, J. Circadian regulation of electrolyte absorption in the rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1066–G1074. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cano, F.E.; Camello-Almaraz, C.; Acuna-Castroviejo, D.; Pozo, M.J.; Camello, P.J. Age-related changes in mitochondrial function of mouse colonic smooth muscle: Beneficial effects of melatonin. J. Pineal Res. 2014, 56, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cano, F.E.; Camello-Almaraz, C.; Macias, J.G.; Pozo, M.J.; Camello, P.J. Propagation of Intracellular Ca2+ Signals in Aged Exocrine Cells. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.; Diez, A.; Puyet, A.; Camello, P.J.; Camello-Almaraz, C.; Bautista, J.M.; Pozo, M.J. Calcium controls smooth muscle TRPC gene transcription via the CaMK/calcineurin-dependent pathways. Am. J. Physiol. Cell Physiol. 2007, 292, C553–C563. [Google Scholar] [CrossRef] [PubMed]
- Oosterbaan, R.J. Crop production and soil salinity: Evaluation of field data from India by segmented linear regression with breakpoint. In Proceedings of the Symposium on Land Drainage for Salinity Control in Arid and Semi-Arid Regions, Cairo, Egypt, 25 February–2 March 1990; Volume 3, pp. 373–383. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camello-Almaraz, C.; Martin-Cano, F.E.; Santos, F.J.; Espin, M.T.; Antonio Madrid, J.; Pozo, M.J.; Camello, P.J. Age-Induced Differential Changes in the Central and Colonic Human Circadian Oscillators. Int. J. Mol. Sci. 2020, 21, 674. https://doi.org/10.3390/ijms21020674
Camello-Almaraz C, Martin-Cano FE, Santos FJ, Espin MT, Antonio Madrid J, Pozo MJ, Camello PJ. Age-Induced Differential Changes in the Central and Colonic Human Circadian Oscillators. International Journal of Molecular Sciences. 2020; 21(2):674. https://doi.org/10.3390/ijms21020674
Chicago/Turabian StyleCamello-Almaraz, Cristina, Francisco E. Martin-Cano, Francisco J. Santos, Mª Teresa Espin, Juan Antonio Madrid, Maria J. Pozo, and Pedro J. Camello. 2020. "Age-Induced Differential Changes in the Central and Colonic Human Circadian Oscillators" International Journal of Molecular Sciences 21, no. 2: 674. https://doi.org/10.3390/ijms21020674
APA StyleCamello-Almaraz, C., Martin-Cano, F. E., Santos, F. J., Espin, M. T., Antonio Madrid, J., Pozo, M. J., & Camello, P. J. (2020). Age-Induced Differential Changes in the Central and Colonic Human Circadian Oscillators. International Journal of Molecular Sciences, 21(2), 674. https://doi.org/10.3390/ijms21020674




