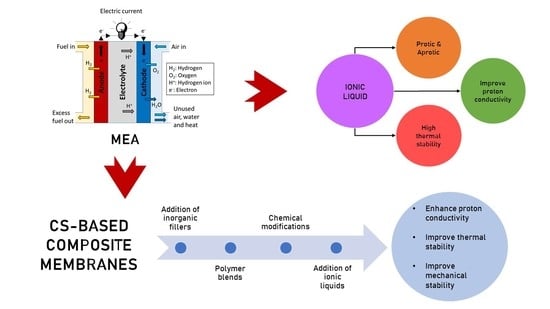
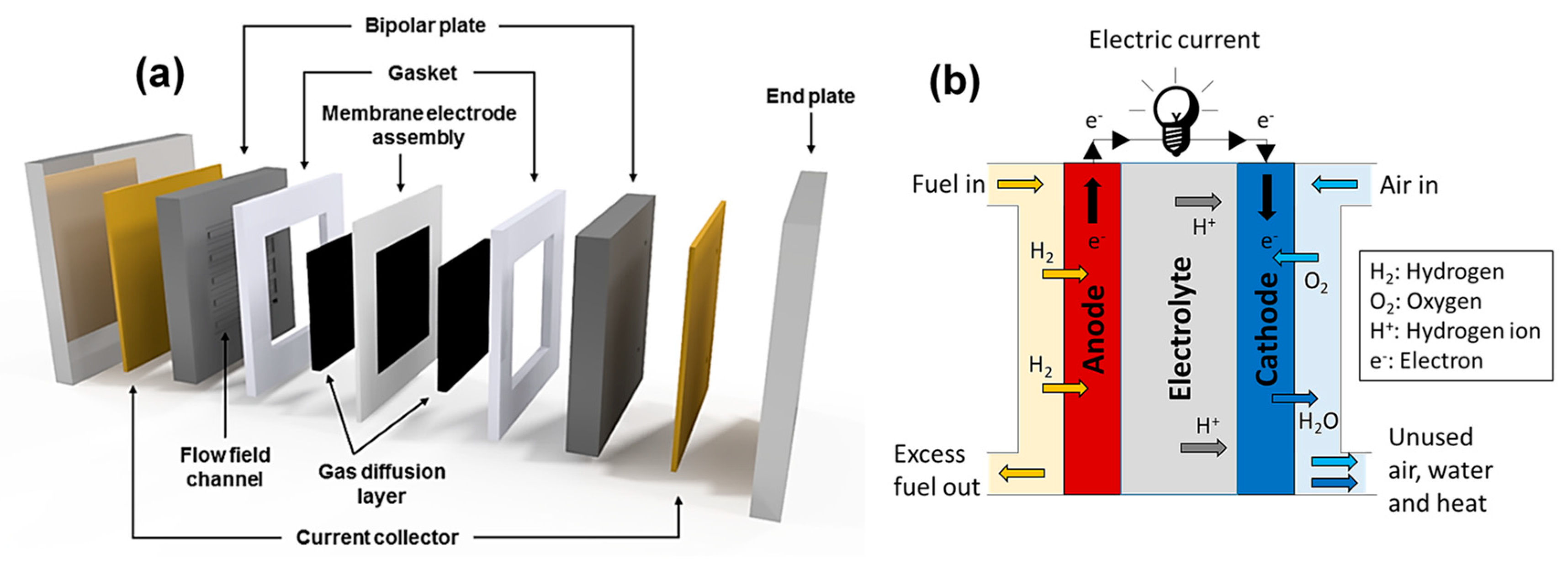
Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids
Abstract
1. Introduction
2. Biopolymer-Based Membranes
2.1. CS-Based Biopolymer Membranes
2.2. CS-Based Composite Membranes
2.2.1. CS/Inorganic Filler Composite Membranes
(1) Hygroscopic Oxides
(2) Heteropoly Acids
(3) Carbon Nanotubes
(4) Graphene Oxide
2.2.2. CS/Polymer Blend Membranes
2.3. Chemical Modifications of CS Biopolymer-Based Membrane
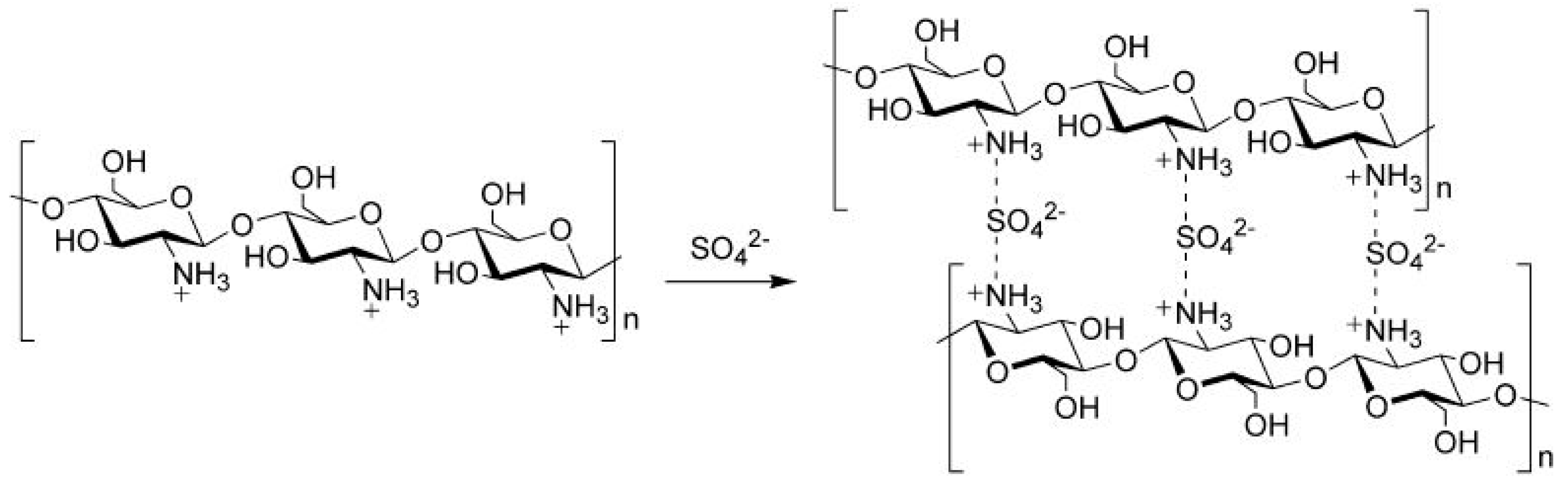
2.3.1. Sulphonation
2.3.2. Phosphorylation
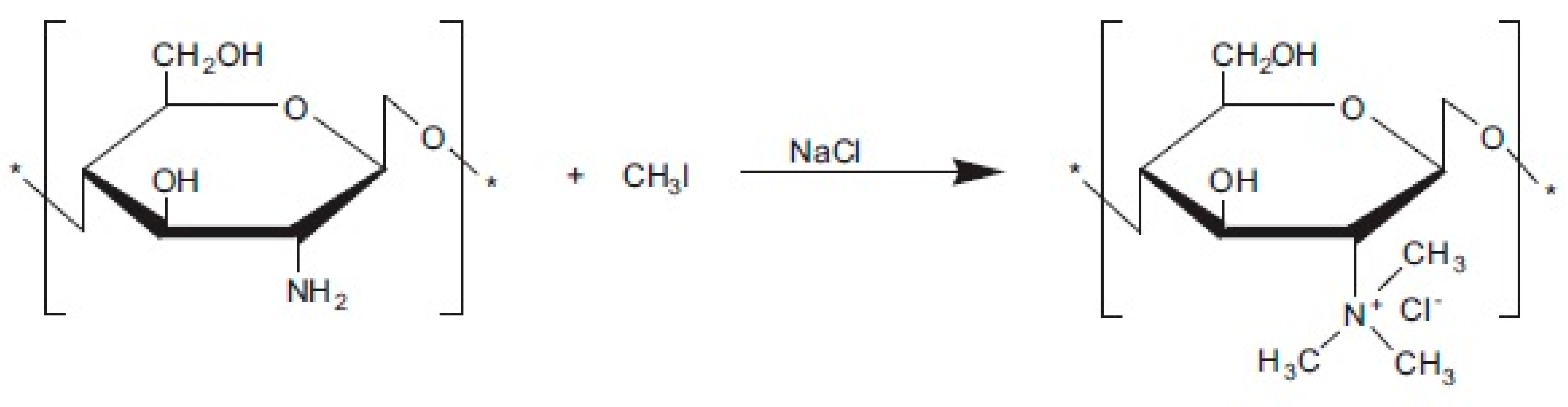
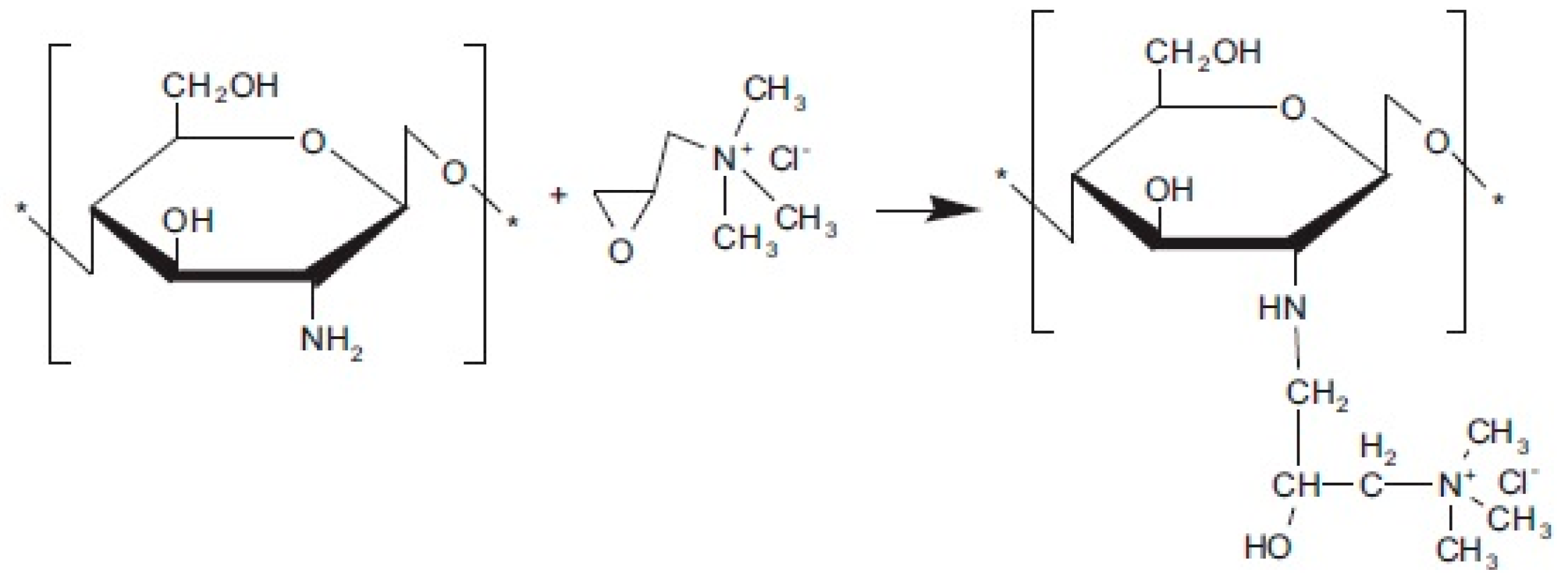
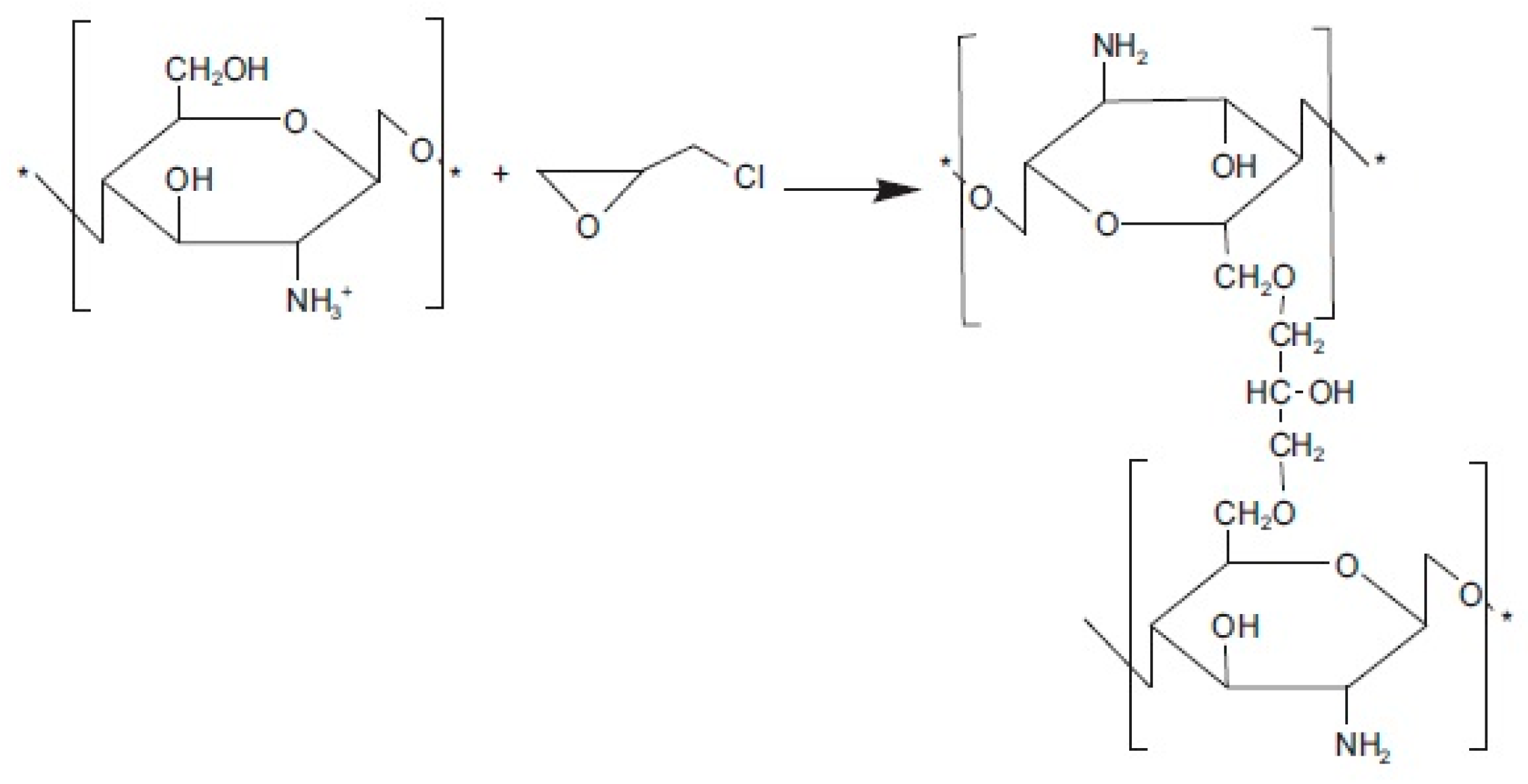
2.3.3. Quaternization
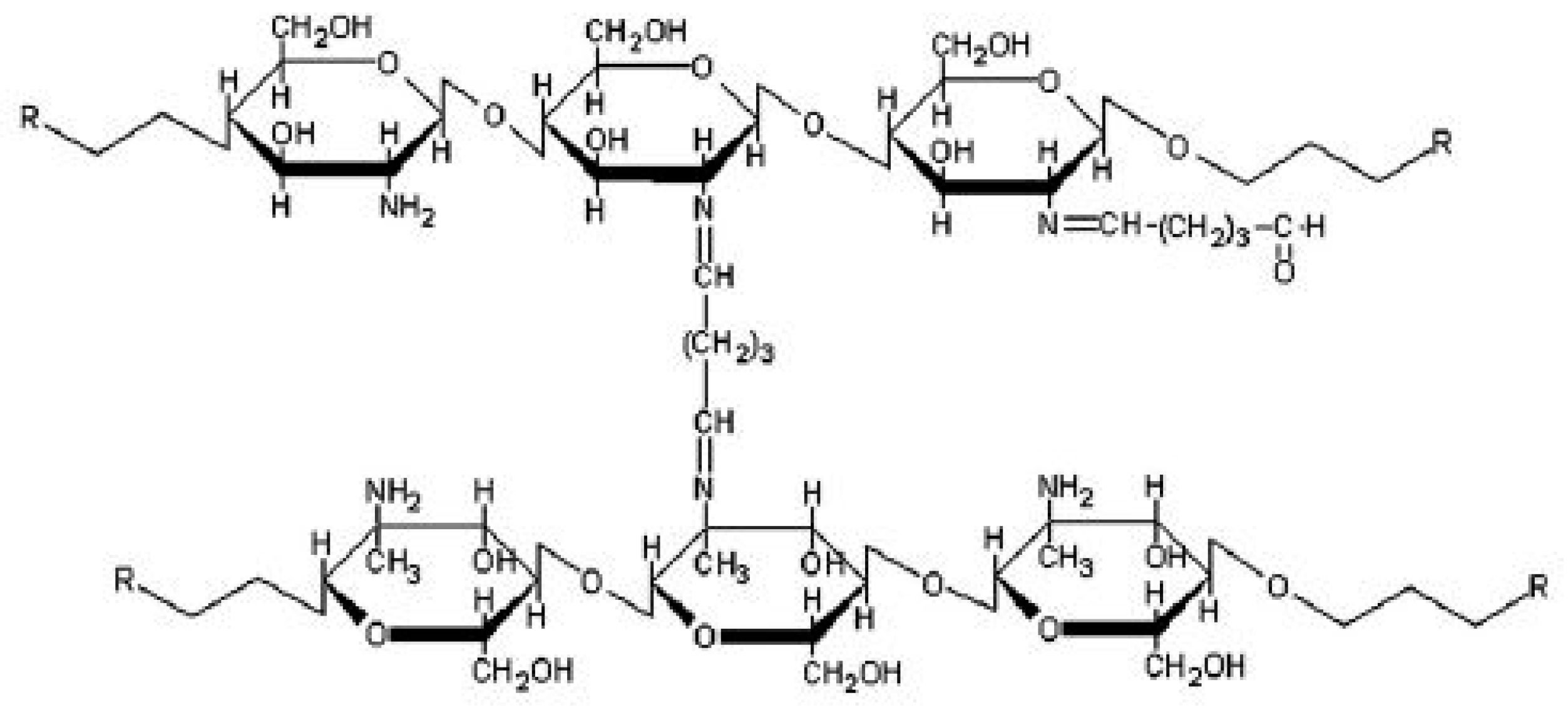
2.3.4. Chemical Cross-Linking
- CS cross-linked with itself,
- Hybrid polymer networks, in which cross-linking reaction occurs between a structural unit of a CS chain and a structural unit of a polymeric chain of another type,
- Semi- or full-interpenetrating polymer networks, in which a polymer of another kind is entrapped in self-cross-linked CS network.
3. Ionic Liquids in Polymer Electrolyte Membrane Fuel Cell
3.1. Proton-Conducting Ionic Liquids (PCILs)
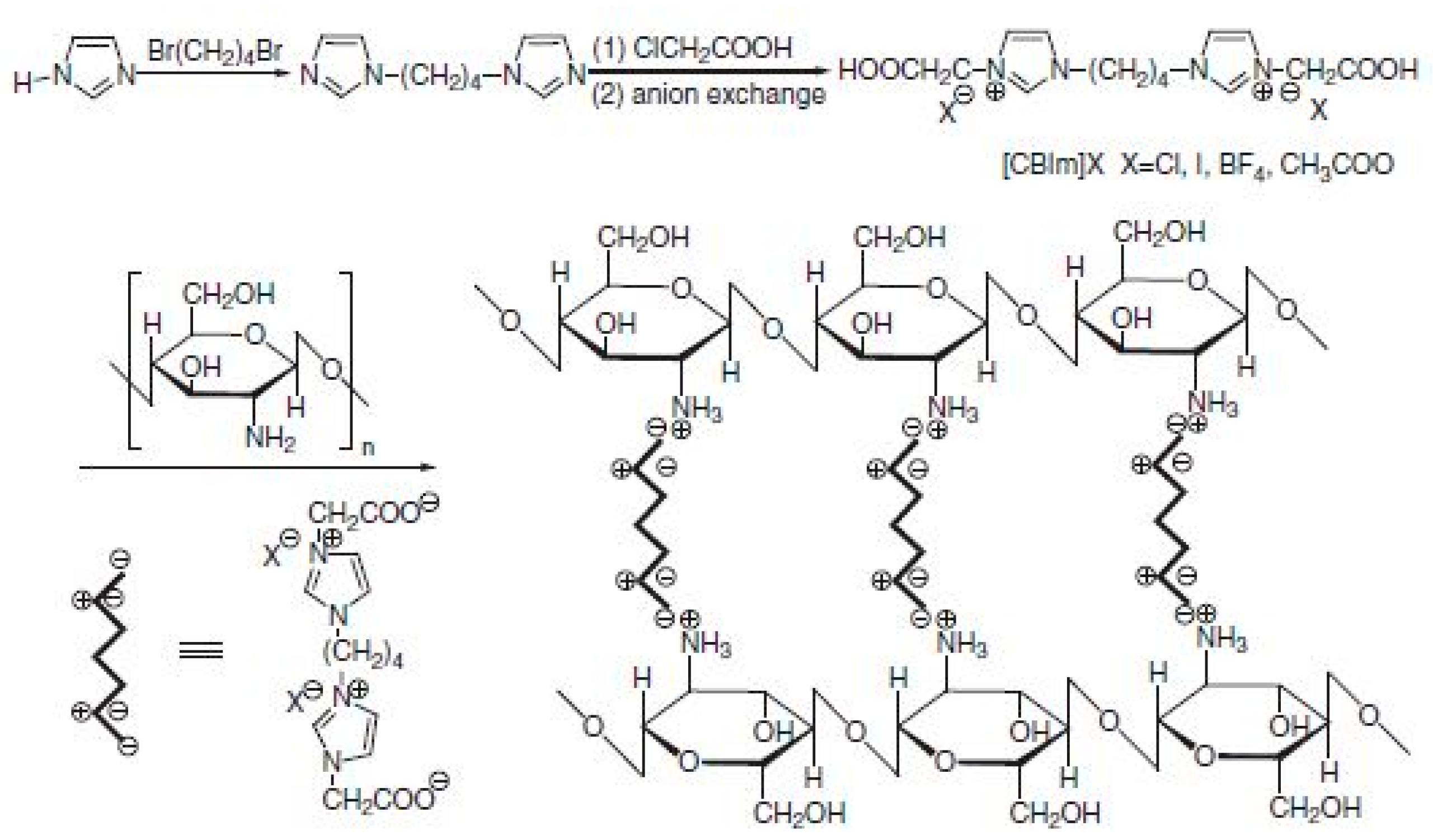
3.2. Modification of CS in Ionic Liquids
3.3. CS-Ionic Liquid Composite Membrane
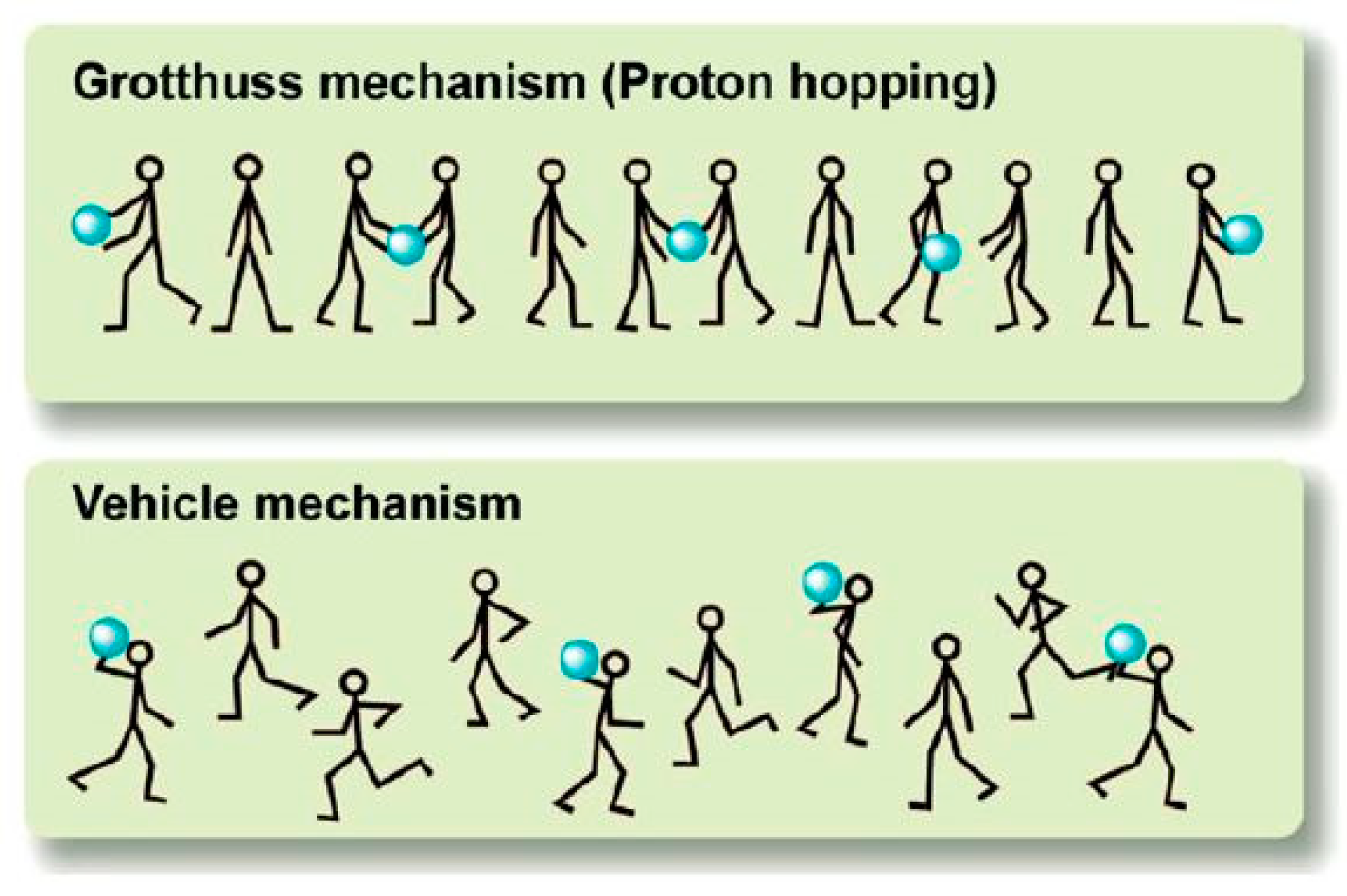
3.4. Proton Transport Mechanism through Ionic Liquids
4. Conclusions and Future Perspectives
- Mechanical stability. A composite membrane with improved mechanical strength should be produced to ensure its sustainability in long-duration fuel cell operations.
- Water retention capacity. Composite membranes with good retention capacity must be developed to prevent the loss of bound water when tested at high temperatures.
- Proton conductivity. Biopolymer composite membranes with increased conductivity and that are comparable with Nafion membranes must be developed.
- Low cost. Inexpensive composite membranes that are environmentally friendly and have attractive properties must be developed.
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APTES | 3-aminopropyl-triethoxysilane |
| ATMP | Amino tris(methylene phosphonic acid) |
| CCSM | Chitosan sulphate blending membrane |
| CNTs | Carbon nanotubes |
| CS | Chitosan |
| CVD | Chemical vapor deposition |
| DMAFC | Direct methanol alkaline fuel cell |
| DMFC | Direct methanol fuel cell |
| DQ | Degree of quaternization |
| DWCNTs | Double-walled carbon nanotubes |
| GA | Glutaraldehyde |
| GO | Graphene oxide |
| GPTMS | γ-glycidoxypropyltrimethoxysilane |
| GTMAC | 2,3-epoxypropyltrimethylammonium chloride |
| HDT | Hexadecyltrimethylammonium bromide |
| HPAs | Heteropoly acids |
| HTCC | N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride |
| HTFSI | Solid bis-(trifluoromethanesulfonyl)amide |
| IHPA | Silica-supported silicotungstic acid |
| MEA | Membrane electrode assembly |
| MFC | Microbial fuel cell |
| MPTMS | 3-mercaptopropyl-trimethoxysilane |
| MSA | Methanesulphonic acid |
| MWCNTs | Multiwalled carbon nanotubes |
| NMPC | N-methylene phosphonic chitosan |
| NMPSGO | N,N-di-methylenephosphonic acid propylsilane graphene oxide |
| NSBC | N-o-sulphonic acid benzyl chitosan |
| OCV | Open circuit voltage |
| PA | Phosphonic acid |
| PCILs | Proton-conducting ionic liquids |
| PDDA | Poly(diallyldimethylammonium chloride) |
| PEM | Proton exchange membrane |
| PEMFCs | Polymer electrolyte membrane fuel cells |
| PES | Polyethersulphone |
| PGO | Phosphorylated graphene oxide |
| PHMSS | Phosphorylated hollow mesoporous silica sub-microspheres |
| PMA | Phosphomolybdic acid |
| PSF | Polysulphone |
| PTA | Phosphotungstic acid |
| PVA | Poly(vinyl alcohol) |
| PVDF | Poly(vinylidene fluoride) |
| QPIENPC | Quaternized poly[O-(2-imidazolyethylene)-N-picolylchitosan |
| RH | Relative humidity |
| SCNTs | Silica-coated carbon nanotubes |
| SDBS | Sodium salts of dodecylbenzene sulphonic acid |
| SSA | Sulphosuccinic acid |
| SWCNTs | Single-walled carbon nanotubes |
| TCNTs | Titania-coated carbon nanotubes |
| TPTZ | 2,3,5-triphenyltetrazolium chloride |
References
- Kim, H.-J.; Lim, S.J.; Lee, J.W.; Min, I.-G.; Lee, S.-Y.; Cho, E.; Oh, I.-H.; Lee, J.H.; Oh, S.-C.; Lim, T.-W. Development of shut-down process for a proton exchange membrane fuel cell. J. Power Sources 2008, 180, 814–820. [Google Scholar] [CrossRef]
- Rueda, D.; Secall, T.; Bayer, R. Differences in the interaction of water with starch and chitosan films as revealed by infrared spectroscopy and differential scanning calorimetry. Carbohydr. Polym. 1999, 40, 49–56. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Song, C.; Zhang, J.; Wang, H. PEM fuel cell open circuit voltage (OCV) in the temperature range of 23 C to 120 C. J. Power Sources 2006, 163, 532–537. [Google Scholar] [CrossRef]
- Wu, P.; Imai, M. Novel Biopolymer Composite Membrane Involved with Selective Mass Transfer and Excellent Water Permeability; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Ikram, S.; Ahmed, S.; Ali, S.W.; Agarwal, H. Chitosan-based polymer electrolyte membranes for fuel cell applications. In Organic-Inorganic Composite Polymer Electrolyte Membranes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 381–398. [Google Scholar]
- Yamada, M.; Honma, I. Anhydrous proton conductive membrane consisting of chitosan. Electrochim. Acta 2005, 50, 2837–2841. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Swami, B.L.; Ikram, S. Preparation and characterization of antibacterial thiosemicarbazide chitosan as efficient Cu (II) adsorbent. Carbohydr. Polym. 2015, 132, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Khatoon, F.; Ahmed, S. An overview on wounds, their issue s and natural remedies for wound healing. Biochem. Physiol. 2015, 4, 2. [Google Scholar]
- Ramirez-Salgado, J. Study of basic biopolymer as proton membrane for fuel cell systems. Electrochim. Acta 2007, 52, 3766–3778. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Berenjian, A.; Anarjan, N. Recent advances in application of chitosan in fuel cells. Sustain. Chem. Process. 2013, 1, 1. [Google Scholar] [CrossRef]
- Varshney, P.K.; Gupta, S. Natural polymer-based electrolytes for electrochemical devices: A review. Ionics 2011, 17, 479–483. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Kumar, M.; Shahi, V.K. Chitosan Based Membranes for Separation, Pervaporation and Fuel Cell Applications: Recent Developments; INTECH Open Access Publisher: London, UK, 2010. [Google Scholar]
- Zhang, Y.; Cui, Z.; Liu, C.; Xing, W.; Zhang, J. Implantation of Nafion® ionomer into polyvinyl alcohol/chitosan composites to form novel proton-conducting membranes for direct methanol fuel cells. J. Power Sources 2009, 194, 730–736. [Google Scholar] [CrossRef]
- de Moraes, M.A.; Cocenza, D.S.; da Cruz Vasconcellos, F.; Fraceto, L.F.; Beppu, M.M. Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides. J. Environ. Manag. 2013, 131, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Mat, N.; Liong, A. Chitosan-poly (vinyl alcohol) and calcium oxide composite membrane for direct methanol fuel cell applications. Eng. Lett. 2009, 116, 1017–1029. [Google Scholar]
- Tripathi, B.P.; Shahi, V.K. Organic–inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Zheng, X.; Jiang, Z.; Li, J. Zeolite beta-filled chitosan membrane with low methanol permeability for direct methanol fuel cell. J. Power Sources 2008, 183, 454–463. [Google Scholar] [CrossRef]
- Di Noto, V.; Boaretto, N.; Negro, E.; Pace, G. New inorganic–organic proton conducting membranes based on Nafion and hydrophobic fluoroalkylated silica nanoparticles. J. Power Sources 2010, 195, 7734–7742. [Google Scholar] [CrossRef]
- Dupuis, A.-C. Proton exchange membranes for fuel cells operated at medium temperatures: Materials and experimental techniques. Prog. Mater. Sci. 2011, 56, 289–327. [Google Scholar] [CrossRef]
- Albu, A.-M.; Maior, I.; Nicolae, C.A.; Bocăneală, F.L. Novel PVA proton conducting membranes doped with polyaniline generated by in-situ polymerization. Electrochim. Acta 2016, 211, 911–917. [Google Scholar] [CrossRef]
- Ublekov, F.; Penchev, H.; Georgiev, V.; Radev, I.; Sinigersky, V. Protonated montmorillonite as a highly effective proton-conductivity enhancer in p-PBI membranes for PEM fuel cells. Mater. Lett. 2014, 135, 5–7. [Google Scholar] [CrossRef]
- Yang, J.; Shen, P.K.; Varcoe, J.; Wei, Z. Nafion/polyaniline composite membranes specifically designed to allow proton exchange membrane fuel cells operation at low humidity. J. Power Sources 2009, 189, 1016–1019. [Google Scholar] [CrossRef]
- Wu, H.; Shen, X.; Cao, Y.; Li, Z.; Jiang, Z. Composite proton conductive membranes composed of sulfonated poly (ether ether ketone) and phosphotungstic acid-loaded imidazole microcapsules as acid reservoirs. J. Membr. Sci. 2014, 451, 74–84. [Google Scholar] [CrossRef]
- Gupta, D.; Madhukar, A.; Choudhary, V. Effect of functionality of polyhedral oligomeric silsesquioxane [POSS] on the properties of sulfonated poly (ether ether ketone) [SPEEK] based hybrid nanocomposite proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 12817–12829. [Google Scholar] [CrossRef]
- Chen, P.; Hao, L.; Wu, W.; Li, Y.; Wang, J. Polymer-inorganic hybrid proton conductive membranes: Effect of the interfacial transfer pathways. Electrochim. Acta 2016, 212, 426–439. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Khastgir, D. Hybrid composite membranes of chitosan/sulfonated polyaniline/silica as polymer electrolyte membrane for fuel cells. Carbohydr. Polym. 2018, 179, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.P.; Shahi, V.K. Functionalized organic− inorganic nanostructured N-p-carboxy benzyl chitosan−silica−PVA hybrid polyelectrolyte complex as proton exchange membrane for DMFC applications. J. Phys. Chem. B 2008, 112, 15678–15690. [Google Scholar] [CrossRef] [PubMed]
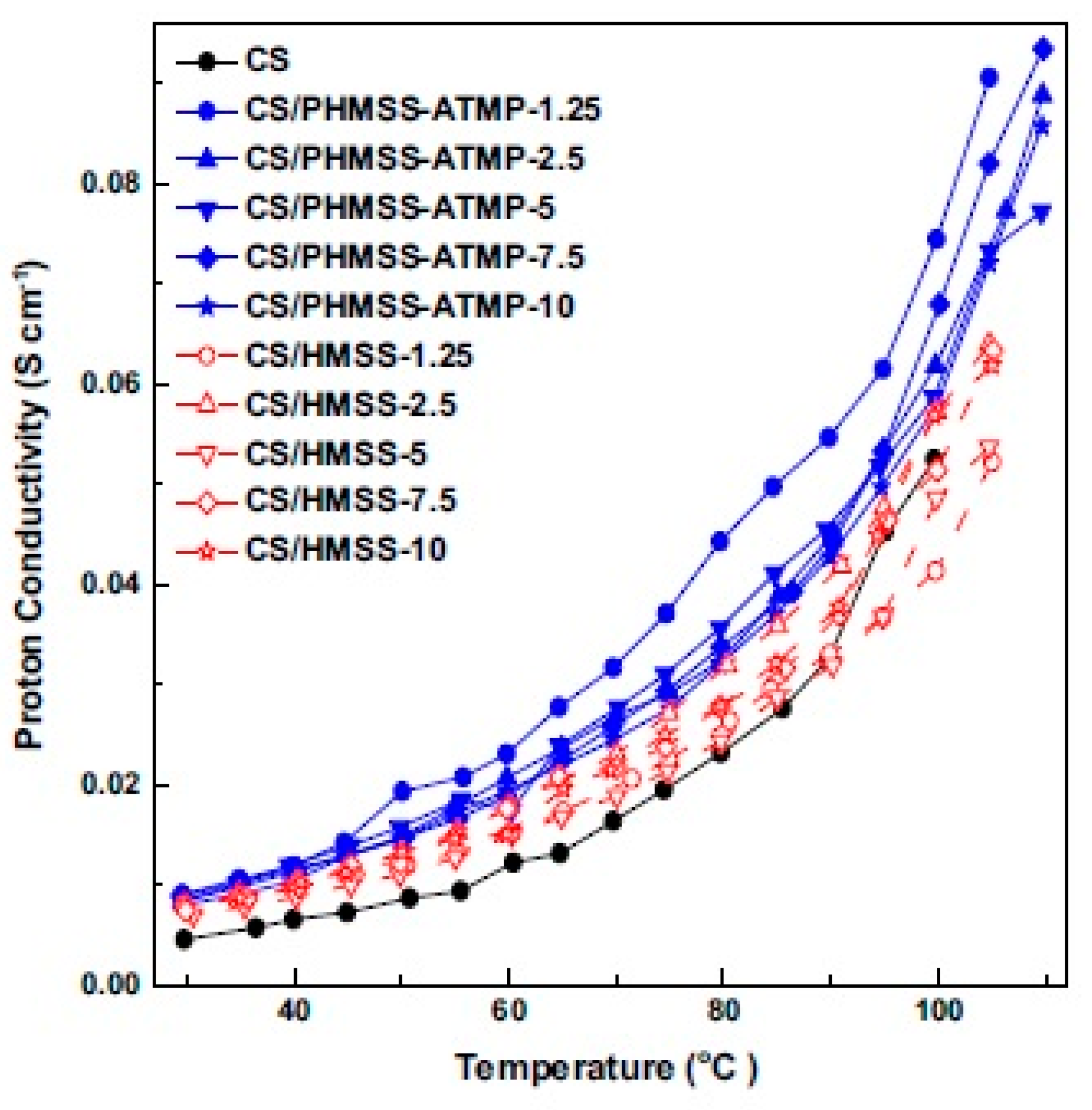
- Zhao, Y.; Yang, H.; Wu, H.; Jiang, Z. Enhanced proton conductivity of hybrid membranes by incorporating phosphorylated hollow mesoporous silica submicrospheres. J. Membr. Sci. 2014, 469, 418–427. [Google Scholar] [CrossRef]
- Jin, Y.G.; Qiao, S.Z.; Xu, Z.P.; Yan, Z.; Huang, Y.; da Costa, J.C.D.; Lu, G.Q. Phosphonic acid functionalized silicas for intermediate temperature proton conduction. J. Mater. Chem. 2009, 19, 2363–2372. [Google Scholar] [CrossRef]
- Chiba, Y.; Tominaga, Y. Poly (ethylene-co-vinyl alcohol)/sulfonated mesoporous organosilicate composites as proton-conductive membranes. J. Power Sources 2012, 203, 42–47. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, T.; Shen, X.; Wu, H.; Jiang, Z. Fabrication of chitosan/zwitterion functionalized titania–silica hybrid membranes with improved proton conductivity. J. Membr. Sci. 2014, 469, 355–363. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Y.; Hu, B.; Lü, C. Quaternized graphene oxide modified ionic cross-linked sulfonated polymer electrolyte composite proton exchange membranes with enhanced properties. Solid State Ion. 2016, 294, 43–53. [Google Scholar] [CrossRef]
- Gong, C.; Zheng, X.; Liu, H.; Wang, G.; Cheng, F.; Zheng, G.; Wen, S.; Law, W.-C.; Tsui, C.-P.; Tang, C.-Y. A new strategy for designing high-performance sulfonated poly (ether ether ketone) polymer electrolyte membranes using inorganic proton conductor-functionalized carbon nanotubes. J. Power Sources 2016, 325, 453–464. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, B.; Zheng, X.; Wang, J.; Yuan, W.; Jiang, Z. Surface-modified Y zeolite-filled chitosan membrane for direct methanol fuel cell. J. Power Sources 2007, 173, 842–852. [Google Scholar] [CrossRef]
- Yuan, W.; Wu, H.; Zheng, B.; Zheng, X.; Jiang, Z.; Hao, X.; Wang, B. Sorbitol-plasticized chitosan/zeolite hybrid membrane for direct methanol fuel cell. J. Power Sources 2007, 172, 604–612. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Wu, H.; Zheng, B.; Jiang, Z.; Hao, X.; Wang, B. Effect of zeolites on chitosan/zeolite hybrid membranes for direct methanol fuel cell. J. Power Sources 2008, 178, 9–19. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Li, H.; Yang, D. Chitosan membranes filled by GPTMS-modified zeolite beta particles with low methanol permeability for DMFC. Chem. Eng. Process. Process. Intensif. 2010, 49, 278–285. [Google Scholar] [CrossRef]
- Holmberg, B.A.; Hwang, S.-J.; Davis, M.E.; Yan, Y. Synthesis and proton conductivity of sulfonic acid functionalized zeolite BEA nanocrystals. Microporous Mesoporous Mater. 2005, 80, 347–356. [Google Scholar] [CrossRef]
- Ranjani, M.; Pannipara, M.; Al-Sehemi, A.G.; Vignesh, A. Chitosan/sulfonated graphene oxide/silica nanocomposite membranes for direct methanol fuel cells. Solid State Ion. 2019, 338, 153–160. [Google Scholar] [CrossRef]
- Indrasti, N.; Ismayana, A.; Damayanti, R.; Maddu, A.; Aryanto, A. Utilization of Nanosilica from Boiler Ash Sugar Cane Industry for Filler of Chitosan Sulfate Membrane on Direct Methanol Fuel Cell; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 012016. [Google Scholar]
- Kalaiselvimary, J.; Sundararajan, M.; Prabhu, M.R. Preparation and characterization of chitosan-based nanocomposite hybrid polymer electrolyte membranes for fuel cell application. Ionics 2018, 24, 3555–3571. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Jiang, Z.; Yang, X.; Xiao, L. Tuning the performance of direct methanol fuel cell membranes by embedding multifunctional inorganic submicrospheres into polymer matrix. J. Power Sources 2009, 188, 64–74. [Google Scholar] [CrossRef]
- Wu, H.; Hou, W.; Wang, J.; Xiao, L.; Jiang, Z. Preparation and properties of hybrid direct methanol fuel cell membranes by embedding organophosphorylated titania submicrospheres into a chitosan polymer matrix. J. Power Sources 2010, 195, 4104–4113. [Google Scholar] [CrossRef]
- Arata, K. Solid superacids. Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1990; Volume 37, pp. 165–211. [Google Scholar]
- Yamaguchi, T. Recent progress in solid superacid. Appl. Catal. 1990, 61, 1–25. [Google Scholar] [CrossRef]
- Thanganathan, U. Structural study on inorganic/organic hybrid composite membranes. J. Mater. Chem. 2011, 21, 456–465. [Google Scholar] [CrossRef]
- Sauk, J.; Byun, J.; Kim, H. Composite Nafion/polyphenylene oxide (PPO) membranes with phosphomolybdic acid (PMA) for direct methanol fuel cells. J. Power Sources 2005, 143, 136–141. [Google Scholar] [CrossRef]
- Aparicio, M.; Mosa, J.; Etienne, M.; Durán, A. Proton-conducting methacrylate–silica sol–gel membranes containing tungstophosphoric acid. J. Power Sources 2005, 145, 231–236. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Wang, Y. Novel proton conducting composite membranes for direct methanol fuel cell. Mater. Lett. 2003, 57, 1406–1410. [Google Scholar] [CrossRef]
- Nakamura, O.; Ogino, I. Electrical conductivities of some hydrates of dodecamolybdophosphoric acid and dodecatungstophosphoric acid and their mixed crystals. Mater. Res. Bull. 1982, 17, 231–234. [Google Scholar] [CrossRef]
- Cui, Z.; Xing, W.; Liu, C.; Liao, J.; Zhang, H. Chitosan/heteropolyacid composite membranes for direct methanol fuel cell. J. Power Sources 2009, 188, 24–29. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, H.; Ma, C.; Liu, J.; Cao, S.; Zhang, X. Enhancement of proton conductivity of chitosan membrane enabled by sulfonated graphene oxide under both hydrated and anhydrous conditions. J. Power Sources 2014, 269, 898–911. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, H.; Cui, Z.; Li, X.; Na, H.; Xing, W. Highly conductive, methanol resistant fuel cell membranes fabricated by layer-by-layer self-assembly of inorganic heteropolyacid. J. Power Sources 2009, 194, 168–174. [Google Scholar] [CrossRef]
- Shakeri, S.E.; Ghaffarian, S.R.; Tohidian, M.; Bahlakeh, G.; Taranejoo, S. Polyelectrolyte nanocomposite membranes, based on chitosan-phosphotungstic acid complex and montmorillonite for fuel cells applications. J. Macromol. Sci. Part B 2013, 52, 1226–1241. [Google Scholar] [CrossRef]
- Majid, S.R.; Arof, A.K. Conductivity studies and performance of chitosan based polymer electrolytes in H2/air fuel cell. Polym. Advanced Technologies 2009, 20, 524–528. [Google Scholar] [CrossRef]
- Pecoraro, C.; Santamaria, M.; Bocchetta, P.; Di Quarto, F. Influence of synthesis conditions on the performance of chitosan–heteropolyacid complexes as membranes for low temperature H2–O2 fuel cell. Int. J. Hydrogen Energy 2015, 40, 14616–14626. [Google Scholar] [CrossRef]
- Santamaria, M.; Pecoraro, C.; Di Franco, F.; Di Quarto, F.; Gatto, I.; Saccà, A. Improvement in the performance of low temperature H2–O2 fuel cell with chitosan–phosphotungstic acid composite membranes. Int. J. Hydrogen Energy 2016, 41, 5389–5395. [Google Scholar] [CrossRef]
- Santamaria, M.; Pecoraro, C.; Di Franco, F.; Di Quarto, F. Phosphomolybdic acid and mixed phosphotungstic/phosphomolybdic acid chitosan membranes as polymer electrolyte for H2/O2 fuel cells. Int. J. Hydrogen Energy 2017, 42, 6211–6219. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Raj, V. Cesium-substituted mesoporous phosphotungstic acid embedded chitosan hybrid polymer membrane for direct methanol fuel cells. Ionics 2018, 24, 1–15. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Fabrication and comprehensive investigation of physicochemical and electrochemical properties of chitosan-silica supported silicotungstic acid nanocomposite membranes for fuel cell applications. Energy 2018, 142, 313–330. [Google Scholar]
- Liu, X.; He, S.; Song, G.; Jia, H.; Shi, Z.; Liu, S.; Zhang, L.; Lin, J.; Nazarenko, S. Proton conductivity improvement of sulfonated poly (ether ether ketone) nanocomposite membranes with sulfonated halloysite nanotubes prepared via dopamine-initiated atom transfer radical polymerization. J. Membr. Sci. 2016, 504, 206–219. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. A No-sulphonic acid benzyl chitosan (NSBC) and N, N-dimethylene phosphonic acid propylsilane graphene oxide (NMPSGO) based multi-functional polymer electrolyte membrane with enhanced water retention and conductivity. RSC Adv. 2014, 4, 57200–57209. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Gashoul, F. Comprehensive investigation of physicochemical and electrochemical properties of sulfonated poly (ether ether ketone) membranes with different degrees of sulfonation for proton exchange membrane fuel cell applications. Energy 2017, 125, 614–628. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Lee, Y.M. Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J. Membr. Sci. 2004, 240, 37–48. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, C.; Lu, T.; Xing, W. Polyelectrolyte complexes of chitosan and phosphotungstic acid as proton-conducting membranes for direct methanol fuel cells. J. Power Sources 2007, 167, 94–99. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, Y.; Xiu, R.; Lu, S. Development of cesium phosphotungstate salt and chitosan composite membrane for direct methanol fuel cells. Carbohydr. Polym. 2013, 98, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.; Pecoraro, C.; Di Quarto, F.; Bocchetta, P. Chitosan–phosphotungstic acid complex as membranes for low temperature H2–O2 fuel cell. J. Power Sources 2015, 276, 189–194. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Lu, S.; Xu, X.; Liang, D.; Xiang, Y. Novel methanol-blocking proton exchange membrane achieved via self-anchoring phosphotungstic acid into chitosan membrane with submicro-pores. J. Membr. Sci. 2016, 500, 203–210. [Google Scholar] [CrossRef]
- Kalaiselvimary, J.; Prabhu, M.R. Fabrications and investigation of physicochemical and electrochemical properties of heteropoly acid-doped sulfonated chitosan-based polymer electrolyte membranes for fuel cell applications. Polym. Bull. 2019, 76, 1401–1422. [Google Scholar] [CrossRef]
- Jesuraj, K.; Manimuthu, R.P. Preparation and Characterization of Hybrid Chitosan/PEO–Silica Membrane Doped with Phosphotungstic Acid for PEM Fuel Cell Application. Polym. Plast. Technol. Mater. 2019, 58, 14–30. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes: Preparation and Properties; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- José-Yacamán, M.; Miki-Yoshida, M.; Rendon, L.; Santiesteban, J. Catalytic growth of carbon microtubules with fullerene structure. Appl. Phys. Lett. 1993, 62, 657–659. [Google Scholar] [CrossRef]
- Chico, L.; Crespi, V.H.; Benedict, L.X.; Louie, S.G.; Cohen, M.L. Pure carbon nanoscale devices: Nanotube heterojunctions. Phys. Rev. Lett. 1996, 76, 971. [Google Scholar] [CrossRef]
- Ajayan, P.; Ebbesen, T. Nanometre-size tubes of carbon. Rep. Prog. Phys. 1997, 60, 1025. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G. Crystalline ropes of metallic carbon nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V. Carbon nanotubes and its applications: A review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Hou, P.; Bai, S.; Yang, Q.; Liu, C.; Cheng, H. Multi-step purification of carbon nanotubes. Carbon 2002, 40, 81–85. [Google Scholar] [CrossRef]
- Dong, B.; Wang, C.; He, B.; Li, H. Preparation and tribological properties of poly (methyl methacrylate)/styrene/MWNTs copolymer nanocomposites. J. Appl. Polym. Sci. 2008, 108, 1675–1679. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Tan, L.S.; Baek, J.B. Nanocomposites derived from in situ grafting of linear and hyperbranched poly (ether-ketone) s containing flexible oxyethylene spacers onto the surface of multiwalled carbon nanotubes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3471–3481. [Google Scholar] [CrossRef]
- Appenzeller, J.; Martel, R.; Derycke, V.; Radosavljević, M.; Wind, S.; Neumayer, D.; Avouris, P. Carbon nanotubes as potential building blocks for future nanoelectronics. Microelectron. Eng. 2002, 64, 391–397. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Ou, Y.; Tsen, W.C.; Gong, C.; Wang, J.; Liu, H.; Zheng, G.; Qin, C.; Wen, S. Chitosan-based composite membranes containing chitosan-coated carbon nanotubes for polymer electrolyte membranes. Polym. Adv. Technol. 2018, 29, 612–622. [Google Scholar] [CrossRef]
- Kannan, R.; Kakade, B.A.; Pillai, V.K. Polymer electrolyte fuel cells using Nafion-based composite membranes with functionalized carbon nanotubes. Angew. Chem. Int. Ed. 2008, 47, 2653–2656. [Google Scholar] [CrossRef]
- Kannan, R.; Kagalwala, H.N.; Chaudhari, H.D.; Kharul, U.K.; Kurungot, S.; Pillai, V.K. Improved performance of phosphonated carbon nanotube–polybenzimidazole composite membranes in proton exchange membrane fuel cells. J. Mater. Chem. 2011, 21, 7223–7231. [Google Scholar] [CrossRef]
- Liu, H.; Gong, C.; Wang, J.; Liu, X.; Liu, H.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C.; Wen, S. Chitosan/silica coated carbon nanotubes composite proton exchange membranes for fuel cell applications. Carbohydr. Polym. 2016, 136, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Ramesh, P.; Bekyarova, E.; Tian, X.; Wang, F.; Itkis, M.E.; Haddon, R.C. Functionalized single-walled carbon nanotube-based fuel cell benchmarked against US DOE 2017 technical targets. Sci. Rep. 2013, 3, 2257. [Google Scholar] [CrossRef] [PubMed]
- Maiti, J.; Kakati, N.; Lee, S.H.; Jee, S.H.; Yoon, Y.S. PVA nano composite membrane for DMFC application. Solid State Ion. 2011, 201, 21–26. [Google Scholar] [CrossRef]
- Majedi, F.S.; Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Haghighi, A.H.; Bertsch, A.; Moaddel, H.; Renaud, P. Polybenzimidazole-decorated carbon nanotube: A high-performance proton conductor. Rapid Res. Lett. 2012, 6, 318–320. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Shi, Z.; Holdcroft, S. Transport properties of composite membranes containing silicon dioxide and Nafion®. J. Membr. Sci. 2008, 325, 346–356. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, T.; Wang, J.; Pei, F.; He, Y.; Liu, J. Enhanced proton conductivity of sulfonated poly (ether ether ketone) membrane embedded by dopamine-modified nanotubes for proton exchange membrane fuel cell. Fuel Cells 2013, 13, 1155–1165. [Google Scholar] [CrossRef]
- Ou, Y.; Tsen, W.-C.; Jang, S.-C.; Chuang, F.-S.; Wang, J.; Liu, H.; Wen, S.; Gong, C. Novel composite polymer electrolyte membrane using solid superacidic sulfated zirconia-Functionalized carbon nanotube modified chitosan. Electrochim. Acta 2018, 264, 251–259. [Google Scholar] [CrossRef]
- Venkatesan, P.N.; Dharmalingam, S. Characterization and performance study on chitosan-functionalized multi walled carbon nano tube as separator in microbial fuel cell. J. Membr. Sci. 2013, 435, 92–98. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wen, S.; Gong, C.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C. Composite membranes of chitosan and titania-coated carbon nanotubes as promising materials for new proton-exchange membranes. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Wang, J.; Gong, C.; Wen, S.; Liu, H.; Qin, C.; Xiong, C.; Dong, L. Proton exchange membrane based on chitosan and solvent-free carbon nanotube fluids for fuel cells applications. Carbohydr. Polym. 2018, 186, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; He, X.; Lu, Z. Studies on a novel anion-exchange membrane based on chitosan and ionized organic compounds with multiwalled carbon nanotubes for alkaline fuel cells. J. Appl. Polym. Sci. 2018, 135, 46323. [Google Scholar] [CrossRef]
- Wang, W.; Shan, B.; Zhu, L.; Xie, C.; Liu, C.; Cui, F. Anatase titania coated CNTs and sodium lignin sulfonate doped chitosan proton exchange membrane for DMFC application. Carbohydr. Polym. 2018, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ali, M.; Cai, Y.; Lu, Y.; Ahmad, Z.; Khannal, S.; Xu, S. Novel sulfonated multi-walled carbon nanotubes filled chitosan composite membrane for fuel-cell applications. J. Appl. Polym. Sci. 2019, 136, 47603. [Google Scholar] [CrossRef]
- Wang, J.; Gong, C.; Wen, S.; Liu, H.; Qin, C.; Xiong, C.; Dong, L. A facile approach of fabricating proton exchange membranes by incorporating polydopamine-functionalized carbon nanotubes into chitosan. Int. J. Hydrogen Energy 2019, 44, 6909–6918. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. Sulfonated graphene oxide–silica for highly selective Nafion-based proton exchange membranes. J. Mater. Chem. A 2014, 2, 16083–16092. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Manthiram, A. Sulfonated poly (ether ether ketone) membranes with sulfonated graphene oxide fillers for direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 5875–5884. [Google Scholar] [CrossRef]
- He, Y.; Tong, C.; Geng, L.; Liu, L.; Lü, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Membr. Sci. 2014, 458, 36–46. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouk, M.; Scott, K. Sulfonated polyether ether ketone–sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv. 2014, 4, 617–623. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Ye, Y.S.; Cheng, M.Y.; Kao, K.Y.; Shen, W.C.; Rick, J.; Chen, J.C.; Hwang, B.J. Sulfonated polyimide proton exchange membranes with graphene oxide show improved proton conductivity, methanol crossover impedance, and mechanical properties. Adv. Energy Mater. 2011, 1, 1220–1224. [Google Scholar] [CrossRef]
- Shukla, G.; Pandey, R.P.; Shahi, V.K. Temperature resistant phosphorylated graphene oxide-sulphonated polyimide composite cation exchange membrane for water desalination with improved performance. J. Membr. Sci. 2016, 520, 972–982. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Fu, Y.; Manthiram, A. Composite membranes based on sulfonated poly (ether ether ketone) and SDBS-adsorbed graphene oxide for direct methanol fuel cells. J. Mater. Chem. 2012, 22, 24862–24869. [Google Scholar] [CrossRef]
- Lee, D.; Yang, H.; Park, S.; Kim, W. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Membr. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.-M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Chang, X.; Zhang, Y.; Huang, Y.; Yao, Y.; Guo, Z. Graphene oxide cross-linked chitosan nanocomposite membrane. Appl. Surf. Sci. 2013, 280, 989–992. [Google Scholar] [CrossRef]
- Shirdast, A.; Sharif, A.; Abdollahi, M. Effect of the incorporation of sulfonated chitosan/sulfonated graphene oxide on the proton conductivity of chitosan membranes. J. Power Sources 2016, 306, 541–551. [Google Scholar] [CrossRef]
- Kalaiselvimary, J.; Prabhu, M.R. Influence of sulfonated GO/sulfonated biopolymer as polymer electrolyte membrane for fuel cell application. J. Mater. Sci. Mater. Electron. 2018, 29, 5525–5535. [Google Scholar] [CrossRef]
- Kang, M.-S.; Lee, M.-J. Anhydrous solid proton conductors based on perfluorosulfonic ionomer with polymeric solvent for polymer electrolyte fuel cell. Electrochem. Commun. 2009, 11, 457–460. [Google Scholar] [CrossRef]
- Bai, H.; Li, Y.; Zhang, H.; Chen, H.; Wu, W.; Wang, J.; Liu, J. Anhydrous proton exchange membranes comprising of chitosan and phosphorylated graphene oxide for elevated temperature fuel cells. J. Membr. Sci. 2015, 495, 48–60. [Google Scholar] [CrossRef]
- Weber, J.; Kreuer, K.D.; Maier, J.; Thomas, A. Proton conductivity enhancement by nanostructural control of poly (benzimidazole)-phosphoric acid adducts. Adv. Mater. 2008, 20, 2595–2598. [Google Scholar] [CrossRef]
- Chung, S.; Bajue, S.; Greenbaum, S. Mass transport of phosphoric acid in water: A 1 H and 31 P pulsed gradient spin-echo nuclear magnetic resonance study. J. Chem. Phys. 2000, 112, 8515–8521. [Google Scholar] [CrossRef]
- Yadav, M.; Ahmad, S. Montmorillonite/graphene oxide/chitosan composite: Synthesis, characterization and properties. Int. J. Biol. Macromol. 2015, 79, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P.; Kulshrestha, V. Synthesis of highly stable and high water retentive functionalized biopolymer-graphene oxide modified cation exchange membranes. RSC Adv. 2015, 5, 56498–56506. [Google Scholar] [CrossRef]
- Holder, S.L.; Lee, C.-H.; Popuri, S.R. Simultaneous wastewater treatment and bioelectricity production in microbial fuel cells using cross-linked chitosan-graphene oxide mixed-matrix membranes. Environ. Sci. Pollut. Res. 2017, 24, 13782–13796. [Google Scholar] [CrossRef] [PubMed]
- Correlo, V.; Boesel, L.; Bhattacharya, M.; Mano, J.; Neves, N.; Reis, R. Properties of melt processed chitosan and aliphatic polyester blends. Mater. Sci. Eng. A 2005, 403, 57–68. [Google Scholar] [CrossRef]
- Singh, D.K.; Ray, A.R. Radiation-induced grafting of N, N′-dimethylaminoethylmethacrylate onto chitosan films. J. Appl. Polym. Sci. 1997, 66, 869–877. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Takayama, T.; Tsubokawa, N. Grafting reaction of living polymer cations with amino groups on chitosan powder. J. Appl. Polym. Sci. 1998, 68, 1883–1889. [Google Scholar] [CrossRef]
- Fazli, N.; Nasir, M.; Norita, M.Z.; Raha, M.G.; Nahrizul Adib, K. Characterization of Chitosan-Poly (Ethylene Oxide) Blends as Haemodialysis Membrane. American J. Applied Sci. 2005, 2, 1578–1583. [Google Scholar]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.; Peppley, B.; Bui, V.T. Chitosan-based electrolyte composite membranes: II. Mechanical properties and ionic conductivity. J. Membr. Sci. 2006, 284, 331–338. [Google Scholar] [CrossRef]
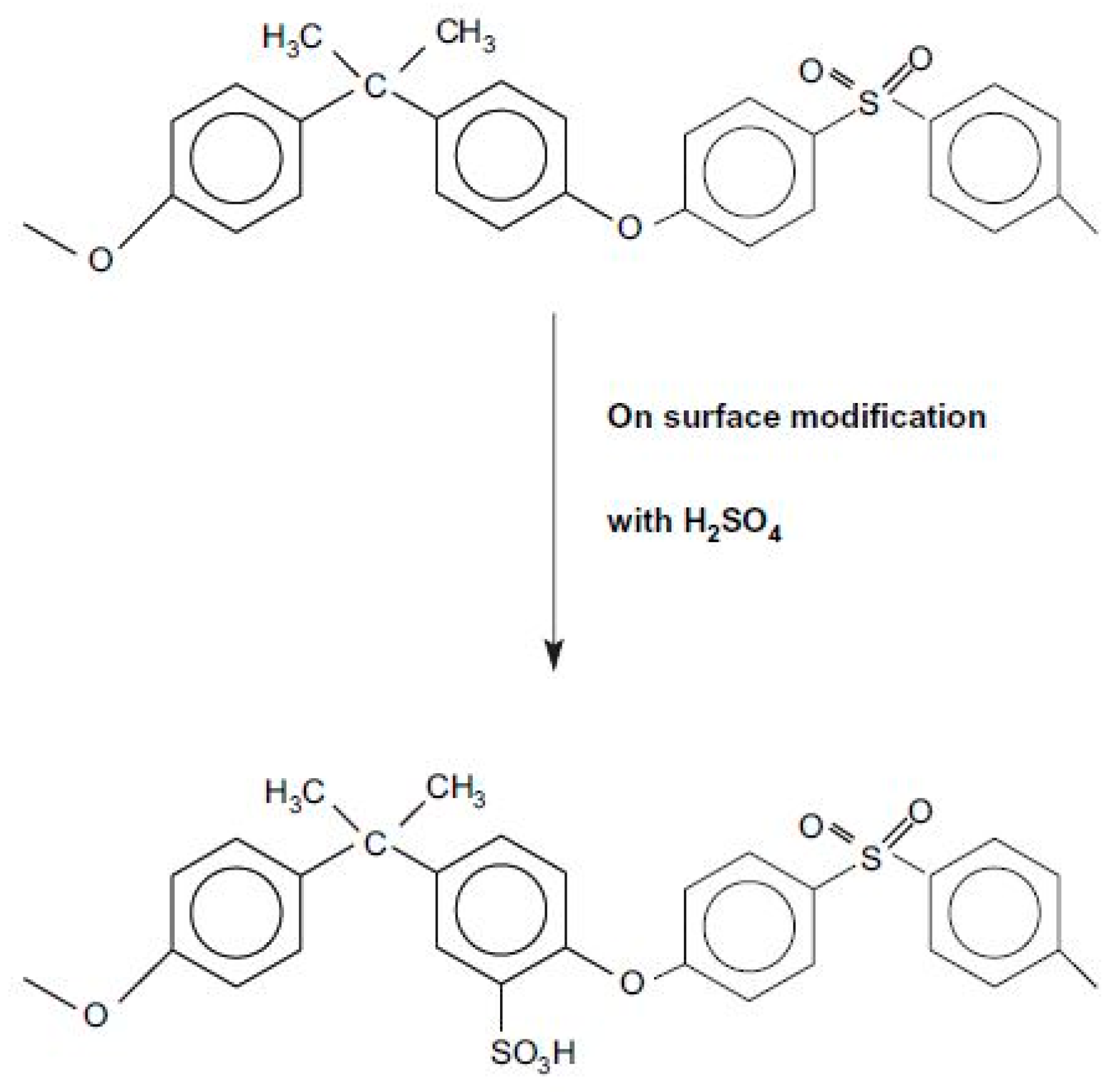
- Bunn, A.; Rose, J.B. Sulphonation of poly (phenylene ether sulphone) s containing hydroquinone residues. Polymer 1993, 34, 1114–1116. [Google Scholar] [CrossRef]
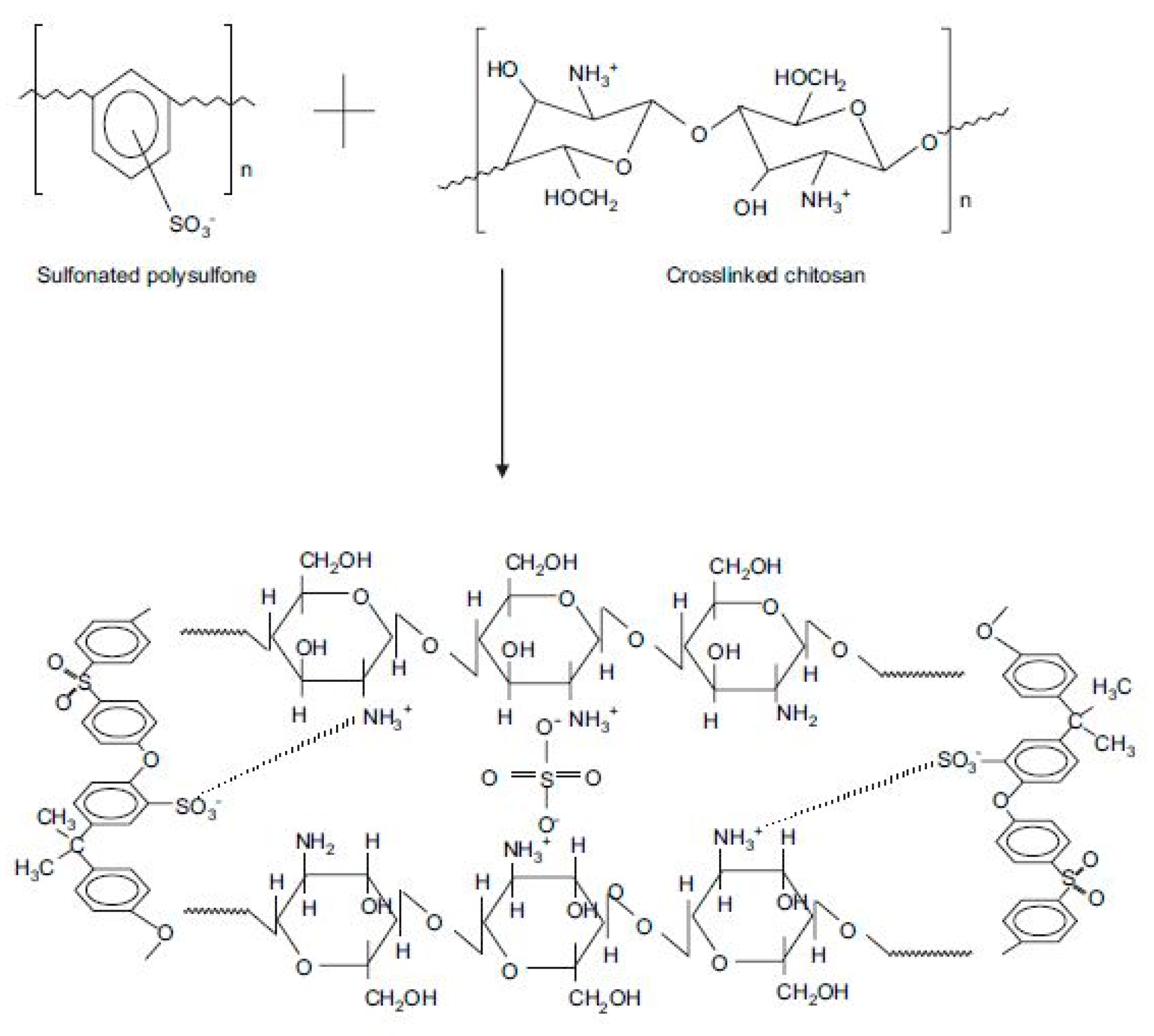
- Smitha, B.; Devi, D.A.; Sridhar, S. Proton-conducting composite membranes of chitosan and sulfonated polysulfone for fuel cell application. Int. J. Hydrogen Energy 2008, 33, 4138–4146. [Google Scholar] [CrossRef]
- Bao, C.; Ouyang, M.; Yi, B. Analysis of the water and thermal management in proton exchange membrane fuel cell systems. Int. J. Hydrogen Energy 2006, 31, 1040–1057. [Google Scholar] [CrossRef]
- Padaki, M.; Isloor, A.M.; Wanichapichart, P. Polysulfone/N-phthaloylchitosan novel composite membranes for salt rejection application. Desalination 2011, 279, 409–414. [Google Scholar] [CrossRef]
- Padaki, M.; Isloor, A.M.; Wanichapichart, P.; Ismail, A.F. Preparation and characterization of sulfonated polysulfone and N-phthloyl chitosan blend composite cation-exchange membrane for desalination. Desalination 2012, 298, 42–48. [Google Scholar] [CrossRef]
- Yang, J.M.; Chiu, H.C. Preparation and characterization of polyvinyl alcohol/chitosan blended membrane for alkaline direct methanol fuel cells. J. Membr. Sci. 2012, 419, 65–71. [Google Scholar] [CrossRef]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Abraham, A.; Soloman, P.; Rejini, V. Preparation of chitosan-polyvinyl alcohol blends and studies on thermal and mechanical properties. Procedia Technol. 2016, 24, 741–748. [Google Scholar] [CrossRef]
- Gopi, K.H.; Dhavale, V.M.; Bhat, S.D. Development of polyvinyl alcohol/chitosan blend anion exchange membrane with mono and di quaternizing agents for application in alkaline polymer electrolyte fuel cells. Mater. Sci. Energy Technol. 2019, 2, 194–202. [Google Scholar]
- Ghasemi, M.; Daud, W.R.W.; Alam, J.; Ilbeygi, H.; Sedighi, M.; Ismail, A.F.; Yazdi, M.H.; Aljlil, S.A. Treatment of two different water resources in desalination and microbial fuel cell processes by poly sulfone/Sulfonated poly ether ether ketone hybrid membrane. Energy 2016, 96, 303–313. [Google Scholar] [CrossRef]
- Saleem, A.; Frormann, L.; Iqbal, A. High performance thermoplastic composites: Study on the mechanical, thermal, and electrical resistivity properties of carbon fiber-reinforced polyetheretherketone and polyethersulphone. Polym. Compos. 2007, 28, 785–796. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Xu, Y.; Wang, X.; Sun, Y.; Wang, R.; Wang, J.; Gao, X.; Gao, J. Developing homogeneous ion exchange membranes derived from sulfonated polyethersulfone/N-phthaloyl-chitosan for improved hydrophilic and controllable porosity. Korean J. Chem. Eng. 2018, 35, 1716–1725. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Neelakandan, S.; Kanagaraj, P.; Nagendran, A. Synthesis and properties of novel proton exchange membranes based on sulfonated polyethersulfone and N-phthaloyl chitosan blends for DMFC applications. Renew. Energy 2016, 86, 922–929. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Chitosan/partially sulfonated poly (vinylidene fluoride) blends as polymer electrolyte membranes for direct methanol fuel cell applications. Cellulose 2018, 25, 661–681. [Google Scholar] [CrossRef]
- Wootthikanokkhan, J.; Seeponkai, N. Methanol permeability and properties of DMFC membranes based on sulfonated PEEK/PVDF blends. J. Appl. Polym. Sci. 2006, 102, 5941–5947. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly (vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kundu, P.P. Effect of the presence of partially sulfonated polyaniline on the proton and methanol transport behavior of partially sulfonated PVdF membrane. Polym. J. 2016, 48, 301. [Google Scholar] [CrossRef]
- Farrokhzad, H.; Darvishmanesh, S.; Genduso, G.; Van Gerven, T.; Van der Bruggen, B. Development of bivalent cation selective ion exchange membranes by varying molecular weight of polyaniline. Electrochim. Acta 2015, 158, 64–72. [Google Scholar] [CrossRef]
- Farrokhzad, H.; Kikhavani, T.; Monnaie, F.; Ashrafizadeh, S.; Koeckelberghs, G.; Van Gerven, T.; Van der Bruggen, B. Novel composite cation exchange films based on sulfonated PVDF for electromembrane separations. J. Membr. Sci. 2015, 474, 167–174. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in vitro antioxidant activities of 6-amino-6-deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef]
- Jung, B.-O.; Na, J.; Kim, C.H. Synthesis of chitosan derivatives with anionic groups and its biocompatibility in vitro. J. Ind. Eng. Chem. 2007, 13, 772–776. [Google Scholar]
- Lima, P.H.; Pereira, S.V.; Rabello, R.B.; Rodriguez-Castellón, E.; Beppu, M.M.; Chevallier, P.; Mantovani, D.; Vieira, R.S. Blood protein adsorption on sulfonated chitosan and κ-carrageenan films. Colloids Surf. B Biointerfaces 2013, 111, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Degoutin, S.; Tabary, N.; Cazaux, F.; Maton, M.; Gaucher, V.; Janus, L.; Neut, C.; Chai, F.; Blanchemain, N. Triclosan loaded electrospun nanofibers based on a cyclodextrin polymer and chitosan polyelectrolyte complex. Int. J. Pharm. 2016, 513, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, J.; Cao, L.; Yu, Y.; Liu, C. Enhanced osteogenesis of bone morphology protein-2 in 2-N, 6-O-sulfated chitosan immobilized PLGA scaffolds. Colloids Surf. B Biointerfaces 2014, 122, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
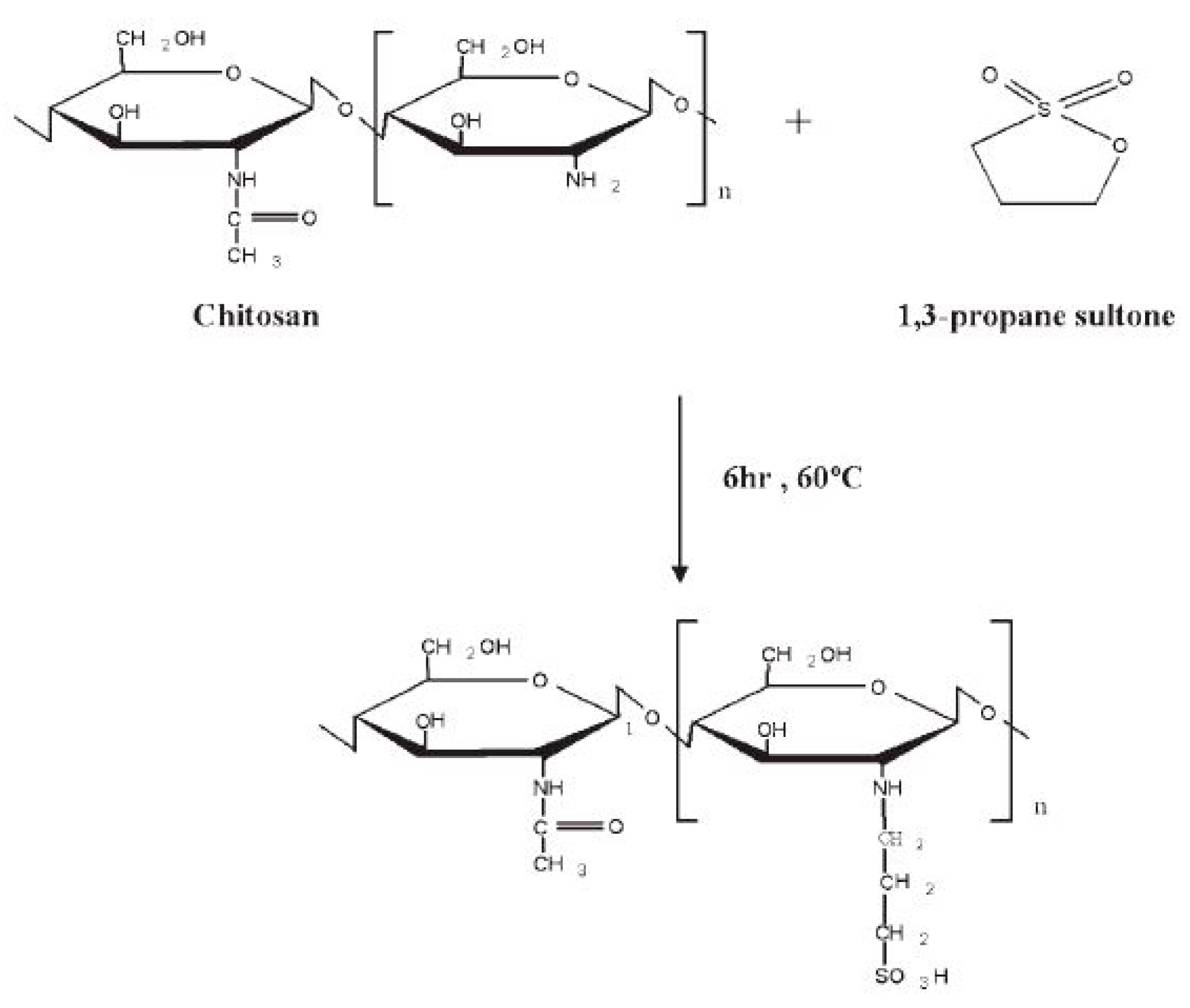
- Tsai, H.S.; Wang, Y.Z.; Lin, J.J.; Lien, W.F. Preparation and properties of sulfopropyl chitosan derivatives with various sulfonation degree. J. Appl. Polym. Sci. 2010, 116, 1686–1693. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Jayakumar, R.; Reis, R.; Mano, J. Chemistry and applications of phosphorylated chitin and chitosan. e-Polymers 2006, 6. [Google Scholar] [CrossRef]
- Nishi, N.; Ebina, A.; Nishimura, S.-i.; Tsutsumi, A.; Hasegawa, O.; Tokura, S. Highly phosphorylated derivatives of chitin, partially deacetylated chitin and chitosan as new functional polymers: Preparation and characterization. Int. J. Biol. Macromol. 1986, 8, 311–317. [Google Scholar] [CrossRef]
- Jayakumar, R.; Selvamurugan, N.; Nair, S.; Tokura, S.; Tamura, H. Preparative methods of phosphorylated chitin and chitosan—An overview. Int. J. Biol. Macromol. 2008, 43, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Horikoshi, T.; Nakajima, A. Adsorption of uranium by chitin phosphate and chitosan phosphate. Agric. Biol. Chem. 1981, 45, 2191–2195. [Google Scholar] [CrossRef]
- Ramos, V.M.; Rodríguez, M.S.; Agulló, E.; Rodríguez, N.M.; Heras, A. Chitosan with phosphonic and carboxylic group: New multidentate ligands. Int. J. Polym. Mater. 2002, 51, 711–720. [Google Scholar] [CrossRef]
- Jung, B.O.; Kim, C.H.; Choi, K.S.; Lee, Y.M.; Kim, J.J. Preparation of amphiphilic chitosan and their antimicrobial activities. J. Appl. Polym. Sci. 1999, 72, 1713–1719. [Google Scholar] [CrossRef]
- Palma, G.; Casals, P.; Cardenas, G. Synthesis and characterization of new chitosan-O-ethyl phosphonate. J. Chil. Chem. Soc. 2005, 50, 719–724. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Rinaudo, M. Synthesis and characterization of chitosan alkyl phosphate. J. Chil. Chem. Soc. 2006, 51, 815–820. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.; Peppley, B.; Bui, V.T. Synthesis, characterization and ionic conductive properties of phosphorylated chitosan membranes. Macromol. Chem. Phys. 2003, 204, 850–858. [Google Scholar] [CrossRef]
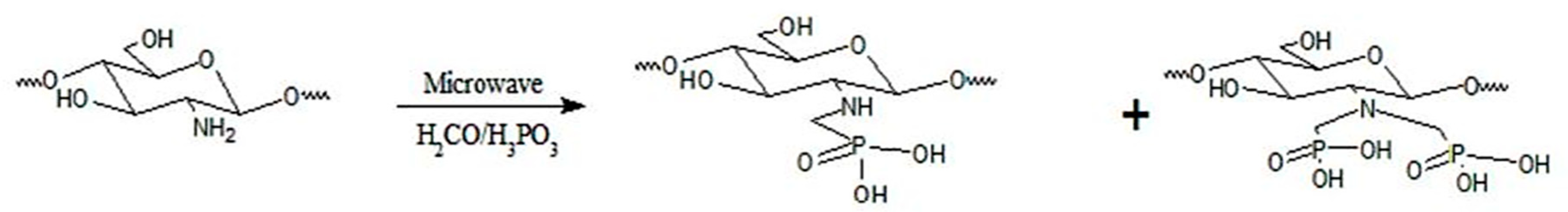
- Dadhich, P.; Das, B.; Dhara, S. Microwave assisted rapid synthesis of N-methylene phosphonic chitosan via Mannich-type reaction. Carbohydr. Polym. 2015, 133, 345–352. [Google Scholar] [CrossRef]
- Moedritzer, K.; Irani, R.R. The direct synthesis of α-aminomethylphosphonic acids. Mannich-type reactions with orthophosphorous acid. J. Org. Chem. 1966, 31, 1603–1607. [Google Scholar] [CrossRef]
- Binsu, V.; Nagarale, R.; Shahi, V.K.; Ghosh, P. Studies on N-methylene phosphonic chitosan/poly (vinyl alcohol) composite proton-exchange membrane. React. Funct. Polym. 2006, 66, 1619–1629. [Google Scholar] [CrossRef]
- Datta, P.; Dhara, S.; Chatterjee, J. Hydrogels and electrospun nanofibrous scaffolds of N-methylene phosphonic chitosan as bioinspired osteoconductive materials for bone grafting. Carbohydr. Polym. 2012, 87, 1354–1362. [Google Scholar] [CrossRef]
- Saxena, A.; Kumar, A.; Shahi, V.K. Preparation and characterization of N-methylene phosphonic and quaternized chitosan composite membranes for electrolyte separations. J. Colloid Interface Sci. 2006, 303, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Holder, S.L.; Lee, C.-H.; Popuri, S.R.; Zhuang, M.-X. Enhanced surface functionality and microbial fuel cell performance of chitosan membranes through phosphorylation. Carbohydr. Polym. 2016, 149, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Du, Y.; Yang, J.; Fan, L. Influence of functional groups on the in vitro anticoagulant activity of chitosan sulfate. Carbohydr. Res. 2003, 338, 483–489. [Google Scholar] [CrossRef]
- Polnok, A.; Borchard, G.; Verhoef, J.; Sarisuta, N.; Junginger, H. Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2004, 57, 77–83. [Google Scholar] [CrossRef]
- Spinelli, V.A.; Laranjeira, M.C.; Favere, V.T. Preparation and characterization of quaternary chitosan salt: Adsorption equilibrium of chromium (VI) ion. React. Funct. Polym. 2004, 61, 347–352. [Google Scholar] [CrossRef]
- Ignatova, M.; Starbova, K.; Markova, N.; Manolova, N.; Rashkov, I. Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly (vinyl alcohol). Carbohydr. Res. 2006, 341, 2098–2107. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, J.W.; Chun, H.J.; Choi, K.S. Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym. Bull. 1997, 38, 387–393. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 2001, 333, 1–6. [Google Scholar] [CrossRef]
- Uragami, T.; Aketa, T.; Gobodani, S.; Sugihara, M. Studies of syntheses and permeabilities of special polymer membranes. Polym. Bull. 1986, 15, 101–106. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Loubaki, E.; Ourevitch, M.; Sicsic, S. Chemical modification of chitosan by glycidyl trimethylammonium chloride. Characterization of modified chitosan by 13C-and 1H-NMR spectroscopy. Eur. Polym. J. 1991, 27, 311–317. [Google Scholar] [CrossRef]
- Wan, Y.; Peppley, B.; Creber, K.A.; Bui, V.T.; Halliop, E. Quaternized-chitosan membranes for possible applications in alkaline fuel cells. J. Power Sources 2008, 185, 183–187. [Google Scholar] [CrossRef]
- Sumner, J.; Creager, S.; Ma, J.; DesMarteau, D. Proton conductivity in Nafion® 117 and in a novel bis [(perfluoroalkyl) sulfonyl] imide ionomer membrane. J. Electrochem. Soc. 1998, 145, 107–110. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, C.; Chen, J.; Ren, X. Synthesis and characterization of cross-linked quaternized chitosan/poly (diallyldimethylammonium chloride) blend anion-exchange membranes. Ionics 2018, 24, 1173–1180. [Google Scholar] [CrossRef]
- Wang, J.; He, R.; Che, Q. Anion exchange membranes based on semi-interpenetrating polymer network of quaternized chitosan and polystyrene. J. Colloid Interface Sci. 2011, 361, 219–225. [Google Scholar] [CrossRef]
- Liao, G.-M.; Yang, C.-C.; Hu, C.-C.; Teng, L.-W.; Hsieh, C.-H.; Lue, S.J. Optimal loading of quaternized chitosan nanofillers in functionalized polyvinyl alcohol polymer membrane for effective hydroxide ion conduction and suppressed alcohol transport. Polymer 2018, 138, 65–74. [Google Scholar] [CrossRef]
- Lue, S.J.; Lee, D.-T.; Chen, J.-Y.; Chiu, C.-H.; Hu, C.-C.; Jean, Y.; Lai, J.-Y. Diffusivity enhancement of water vapor in poly (vinyl alcohol)–fumed silica nano-composite membranes: Correlation with polymer crystallinity and free-volume properties. J. Membr. Sci. 2008, 325, 831–839. [Google Scholar] [CrossRef]
- Lue, S.J.; Mahesh, K.; Wang, W.-T.; Chen, J.-Y.; Yang, C.-C. Permeant transport properties and cell performance of potassium hydroxide doped poly (vinyl alcohol)/fumed silica nanocomposites. J. Membr. Sci. 2011, 367, 256–264. [Google Scholar] [CrossRef]
- Ryu, J.; Seo, J.Y.; Choi, B.N.; Kim, W.-J.; Chung, C.-H. Quaternized chitosan-based anion exchange membrane for alkaline direct methanol fuel cells. J. Ind. Eng. Chem. 2019, 73, 254–259. [Google Scholar] [CrossRef]
- Chávez, E.L.; Oviedo-Roa, R.; Contreras-Pérez, G.; Martínez-Magadán, J.M.; Castillo-Alvarado, F. Theoretical studies of ionic conductivity of crosslinked chitosan membranes. Int. J. Hydrogen Energy 2010, 35, 12141–12146. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Cui, Z.; Xiang, Y.; Si, J.; Yang, M.; Zhang, Q.; Zhang, T. Ionic interactions between sulfuric acid and chitosan membranes. Carbohydr. Polym. 2008, 73, 111–116. [Google Scholar] [CrossRef]
- Fideles, T.B.; Santos, J.L.; Tomás, H.; Furtado, G.T.; Lima, D.B.; Borges, S.M.; Fook, M.V. Characterization of Chitosan Membranes Crosslinked by Sulfuric Acid. Open Access Libr. J. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Du, J.; Bai, Y.; Chu, W.; Qiao, L. The structure and electric characters of proton-conducting chitosan membranes with various ammonium salts as complexant. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 880–885. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y.; Buchheit, R.G. A high performance direct borohydride fuel cell employing cross-linked chitosan membrane. J. Power Sources 2011, 196, 8257–8264. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J. Membr. Sci. 2017, 523, 45–59. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Ghica, M.E.; Fatibello-Filho, O.; Brett, C.M. Electrochemical impedance studies of chitosan-modified electrodes for application in electrochemical sensors and biosensors. Electrochim. Acta 2010, 55, 6239–6247. [Google Scholar] [CrossRef]
- Zeng, X.; Ruckenstein, E. Control of pore sizes in macroporous chitosan and chitin membranes. Ind. Eng. Chem. Res. 1996, 35, 4169–4175. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Hasani-Sadrabadi, M.M.; Moaddel, H. Structural modification of chitosan biopolymer as a novel polyelectrolyte membrane for green power generation. Polym. Adv. Technol. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Mokarram, N.; Majedi, F.S.; Jacob, K.I. Triple-layer proton exchange membranes based on chitosan biopolymer with reduced methanol crossover for high-performance direct methanol fuel cells application. Polymer 2012, 53, 2643–2651. [Google Scholar] [CrossRef]
- Srinophakun, P.; Thanapimmetha, A.; Plangsri, S.; Vetchayakunchai, S.; Saisriyoot, M. Application of modified chitosan membrane for microbial fuel cell: Roles of proton carrier site and positive charge. J. Clean. Prod. 2017, 142, 1274–1282. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhang, H.; Wu, W.; Liu, J.; Wang, J. Constructing proton-conductive highways within an ionomer membrane by embedding sulfonated polymer brush modified graphene oxide. J. Power Sources 2015, 286, 445–457. [Google Scholar] [CrossRef]
- Mondal, A.N.; Tripathi, B.P.; Shahi, V.K. Highly stable aprotic ionic-liquid doped anhydrous proton-conducting polymer electrolyte membrane for high-temperature applications. J. Mater. Chem. 2011, 21, 4117–4124. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Li, Y.; Liu, Y.; Wang, J.; Zhang, B.; Liu, J. Polyelectrolyte microcapsules as ionic liquid reservoirs within ionomer membrane to confer high anhydrous proton conductivity. J. Power Sources 2015, 279, 667–677. [Google Scholar] [CrossRef]
- Sekhon, S.; Park, J.-S.; Baek, J.-S.; Yim, S.-D.; Yang, T.-H.; Kim, C.-S. Small-angle X-ray scattering study of water free fuel cell membranes containing ionic liquids. Chem. Mater. 2009, 22, 803–812. [Google Scholar] [CrossRef]
- Jothi, P.R.; Dharmalingam, S. An efficient proton conducting electrolyte membrane for high temperature fuel cell in aqueous-free medium. J. Membr. Sci. 2014, 450, 389–396. [Google Scholar] [CrossRef]
- Di Noto, V.; Negro, E.; Sanchez, J.-Y.; Iojoiu, C. Structure-relaxation interplay of a new nanostructured membrane based on tetraethylammonium trifluoromethanesulfonate ionic liquid and neutralized nafion 117 for high-temperature fuel cells. J. Am. Chem. Soc. 2010, 132, 2183–2195. [Google Scholar] [CrossRef]
- Xu, C.; Liu, X.; Cheng, J.; Scott, K. A polybenzimidazole/ionic-liquid-graphite-oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2015, 274, 922–927. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Tseng, C.-Y.; Shen, W.-C.; Wang, J.-S.; Chen, K.-J.; Cheng, M.-Y.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J. A new graphene-modified protic ionic liquid-based composite membrane for solid polymer electrolytes. J. Mater. Chem. 2011, 21, 10448–10453. [Google Scholar] [CrossRef]
- Evans, C.M.; Sanoja, G.E.; Popere, B.C.; Segalman, R.A. Anhydrous Proton Transport in Polymerized Ionic Liquid Block Copolymers: Roles of Block Length, Ionic Content, and Confinement. Macromolecules 2015, 49, 395–404. [Google Scholar] [CrossRef]
- Ghenciu, A.F. Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 389–399. [Google Scholar] [CrossRef]
- Chen, G.; Wei, B.; Luo, Y.; Logan, B.E.; Hickner, M.A. Polymer separators for high-power, high-efficiency microbial fuel cells. ACS Appl. Mater. Interfaces 2012, 4, 6454–6457. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Krodkiewska, I.; Drummond, C.J. Protic ionic liquids: Solvents with tunable phase behavior and physicochemical properties. J. Phys. Chem. B 2006, 110, 22479–22487. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 2008, 37, 1709–1726. [Google Scholar] [CrossRef]
- Markusson, H.; Belieres, J.-P.; Johansson, P.; Angell, C.A.; Jacobsson, P. Prediction of macroscopic properties of protic ionic liquids by ab initio calculations. J. Phys. Chem. A 2007, 111, 8717–8723. [Google Scholar] [CrossRef]
- Belieres, J.-P.; Angell, C.A. Protic ionic liquids: Preparation, characterization, and proton free energy level representation. J. Phys. Chem. B 2007, 111, 4926–4937. [Google Scholar] [CrossRef]
- Rana, U.A.; Forsyth, M.; MacFarlane, D.R.; Pringle, J.M. Toward protic ionic liquid and organic ionic plastic crystal electrolytes for fuel cells. Electrochim. Acta 2012, 84, 213–222. [Google Scholar] [CrossRef]
- Yasuda, T.; Watanabe, M. Protic ionic liquids: Fuel cell applications. MRS Bull. 2013, 38, 560–566. [Google Scholar] [CrossRef]
- Hagiwara, R.; Lee, J.S. Ionic liquids for electrochemical devices. Electrochemistry 2007, 75, 23–34. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Kennedy, D.F.; Drummond, C.J. Large aggregated ions found in some protic ionic liquids. J. Phys. Chem. B 2009, 113, 5690–5693. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.; Li, W.L.B. Proton exchange membrane fuel cell working at elevated temperature with ionic liquid as electrolyte. Int. J. Electrochem. Sci. 2011, 6, 6115–6122. [Google Scholar]
- Li, H.; Jiang, F.; Di, Z.; Gu, J. Anhydrous proton-conducting glass membranes doped with ionic liquid for intermediate-temperature fuel cells. Electrochim. Acta 2012, 59, 86–90. [Google Scholar] [CrossRef]
- Luo, J.; Conrad, O.; Vankelecom, I.F. Physicochemical properties of phosphonium-based and ammonium-based protic ionic liquids. J. Mater. Chem. 2012, 22, 20574–20579. [Google Scholar] [CrossRef]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Membr. Sci. 2014, 469, 379–396. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, W.; Zhou, Y.; Zhang, S.; Hua, D.; Zhu, X. Modification of chitosan with carboxyl-functionalized ionic liquid for anion adsorption. Int. J. Biol. Macromol. 2013, 62, 365–369. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Li, C.; Zhang, D.; Xiao, Y.; Guan, G.; Zhu, W. A novel and simple procedure to synthesize chitosan-graft-polycaprolactone in an ionic liquid. Carbohydr. Polym. 2013, 94, 505–510. [Google Scholar] [CrossRef]
- Xu, G.; Deng, L.; Wen, X.; Pi, P.; Zheng, D.; Cheng, J.; Yang, Z. Synthesis and characterization of fluorine-containing poly-styrene-acrylate latex with core–shell structure using a reactive surfactant. J. Coat. Technol. Res. 2011, 8, 401–407. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Huang, L.; Zhai, M.; Peng, J.; Xu, L.; Li, J.; Wei, G. Synthesis of room temperature ionic liquids from carboxymethylated chitosan. Carbohydr. Polym. 2008, 71, 690–693. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhattacharya, B.; Nagarale, R.; Kim, K.-W.; Rhee, H.-W. Synthesis, characterization and application of biopolymer-ionic liquid composite membranes. Synth. Met. 2010, 160, 139–142. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, H.; Wu, C.; Wang, R. Preparation and characterization of conductive chitosan–ionic liquid composite membranes. Polym. Adv. Technol. 2012, 23, 1429–1434. [Google Scholar] [CrossRef]
- Reece, D.; Ralph, S.; Wallace, G. Metal transport studies on inherently conducting polymer membranes containing cyclodextrin dopants. J. Membr. Sci. 2005, 249, 9–20. [Google Scholar] [CrossRef]
- Pawlicka, A.; Danczuk, M.; Wieczorek, W.; Zygadło-Monikowska, E. Influence of plasticizer type on the properties of polymer electrolytes based on chitosan. J. Phys. Chem. A 2008, 112, 8888–8895. [Google Scholar] [CrossRef]
- Orel, B.; Vuk, A.Š.; Ješe, R.; Lianos, P.; Stathatos, E.; Judeinstein, P.; Colomban, P. Development of sol–gel redox I 3−/I− electrolytes and their application in hybrid electrochromic devices. Solid State Ion. 2003, 165, 235–246. [Google Scholar] [CrossRef]
- Leones, R.; Sentanin, F.; Rodrigues, L.; Marrucho, I.; Esperança, J.; Pawlicka, A.; Silva, M. Investigation of polymer electrolytes based on agar and ionic liquids. Express Polym. Lett. 2012, 6, 1007–1016. [Google Scholar] [CrossRef]
- Shamsudin, I.J.; Ahmad, A.; Hassan, N.H. Green Polymer Electrolytes Based on Chitosan and 1-Butyl-3-Methylimidazolium Acetate, AIP Conference Proceedings; AIP: College Park, MD, USA, 2014; pp. 393–398. [Google Scholar]
- Shamsudin, I.; Ahmad, A.; Hassan, N.H.; Kaddami, H. Bifunctional ionic liquid in conductive biopolymer based on chitosan for electrochemical devices application. Solid State Ion. 2015, 278, 11–19. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Rabenau, A.; Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. Engl. 1982, 21, 208–209. [Google Scholar] [CrossRef]
- Noda, A.; Susan, M.A.B.H.; Kudo, K.; Mitsushima, S.; Hayamizu, K.; Watanabe, M. Brønsted acid− base ionic liquids as proton-conducting nonaqueous electrolytes. J. Phys. Chem. B 2003, 107, 4024–4033. [Google Scholar] [CrossRef]
- Kreuer, K.; Fuchs, A.; Ise, M.; Spaeth, M.; Maier, J. Imidazole and pyrazole-based proton conducting polymers and liquids. Electrochim. Acta 1998, 43, 1281–1288. [Google Scholar] [CrossRef]
- Ye, H.; Huang, J.; Xu, J.; Kodiweera, N.; Jayakody, J.; Greenbaum, S. New membranes based on ionic liquids for PEM fuel cells at elevated temperatures. J. Power Sources 2008, 178, 651–660. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
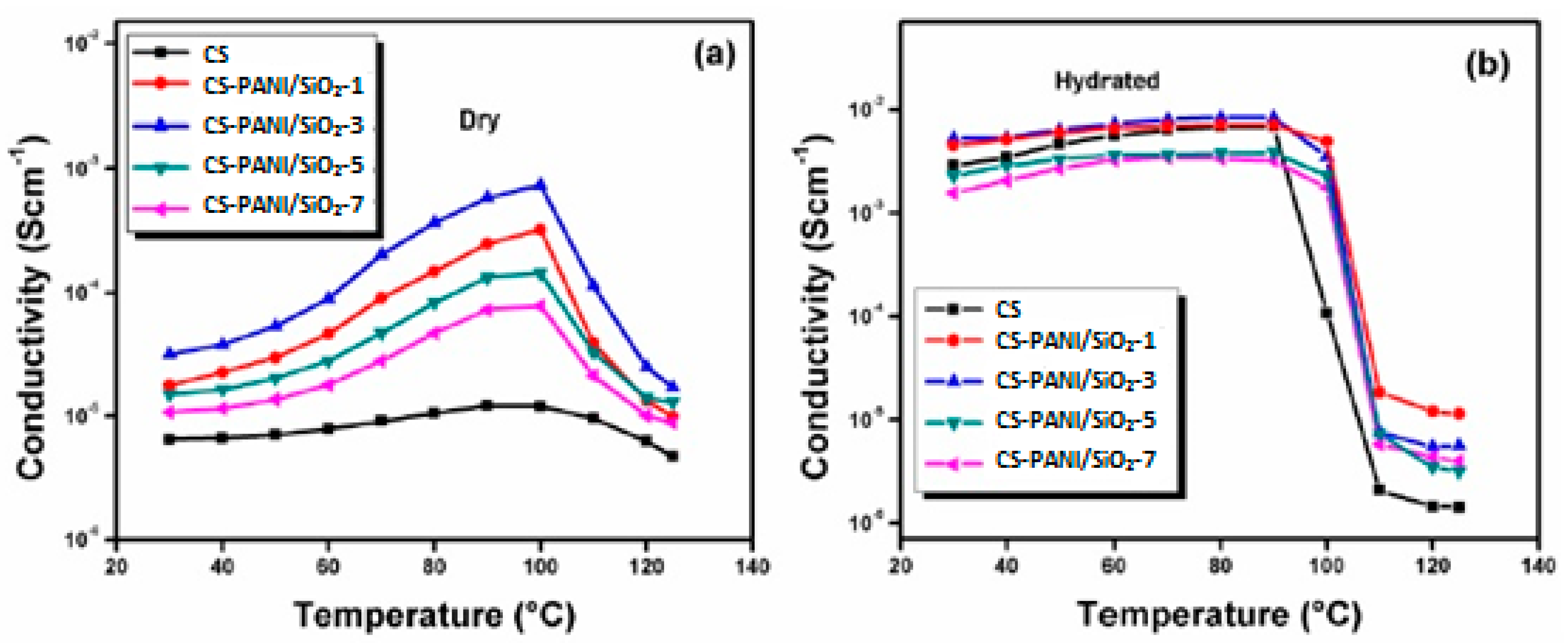
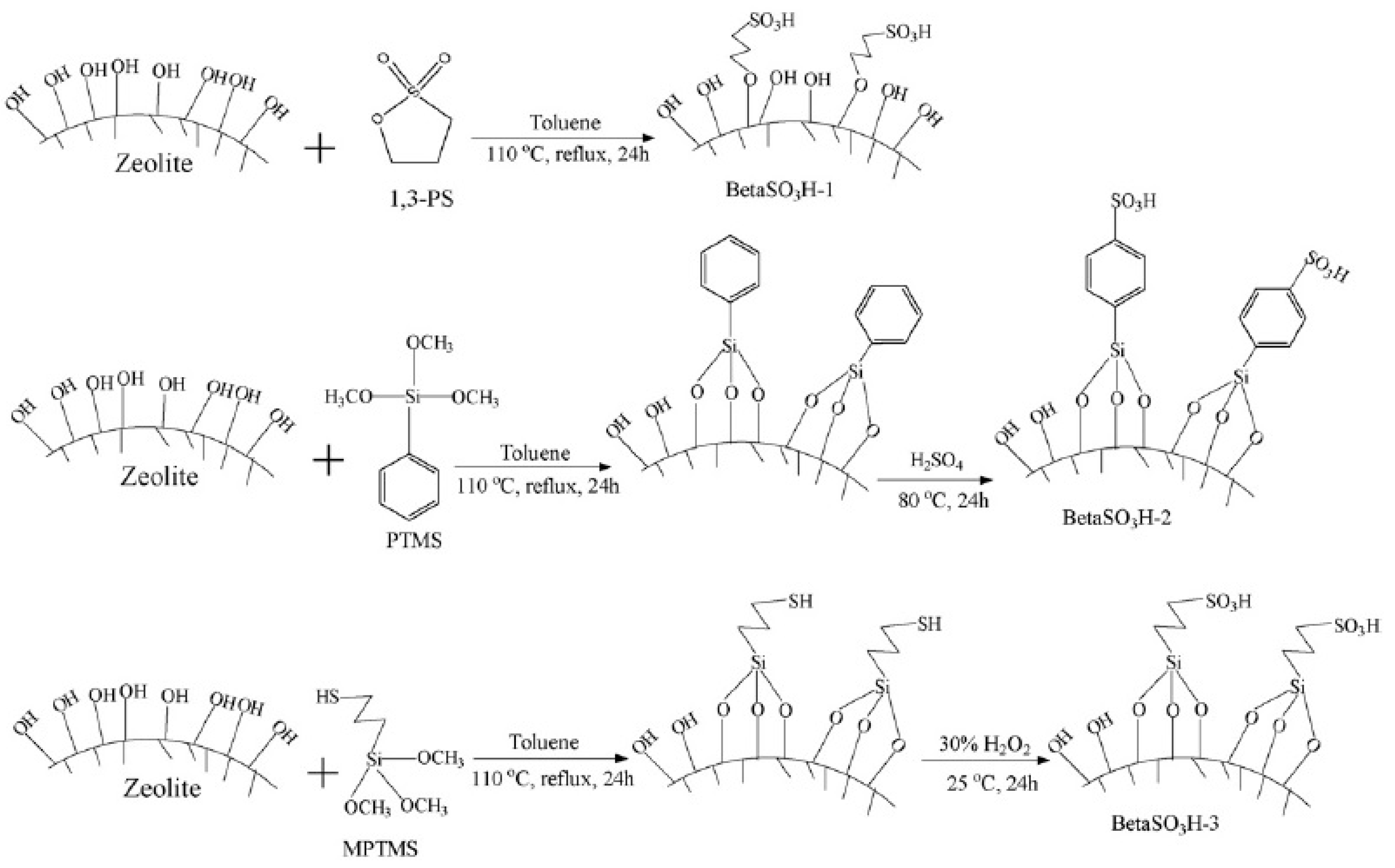
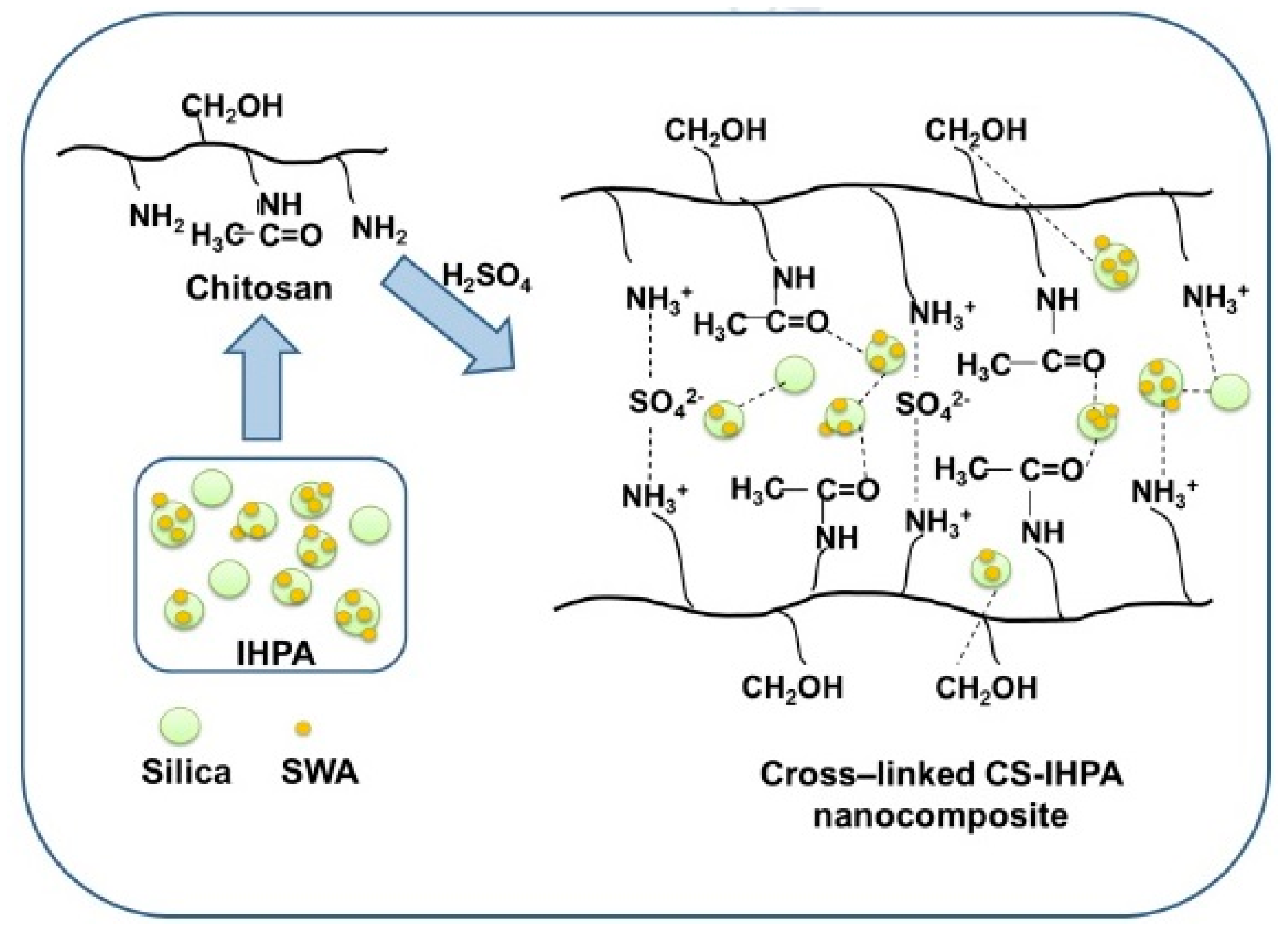
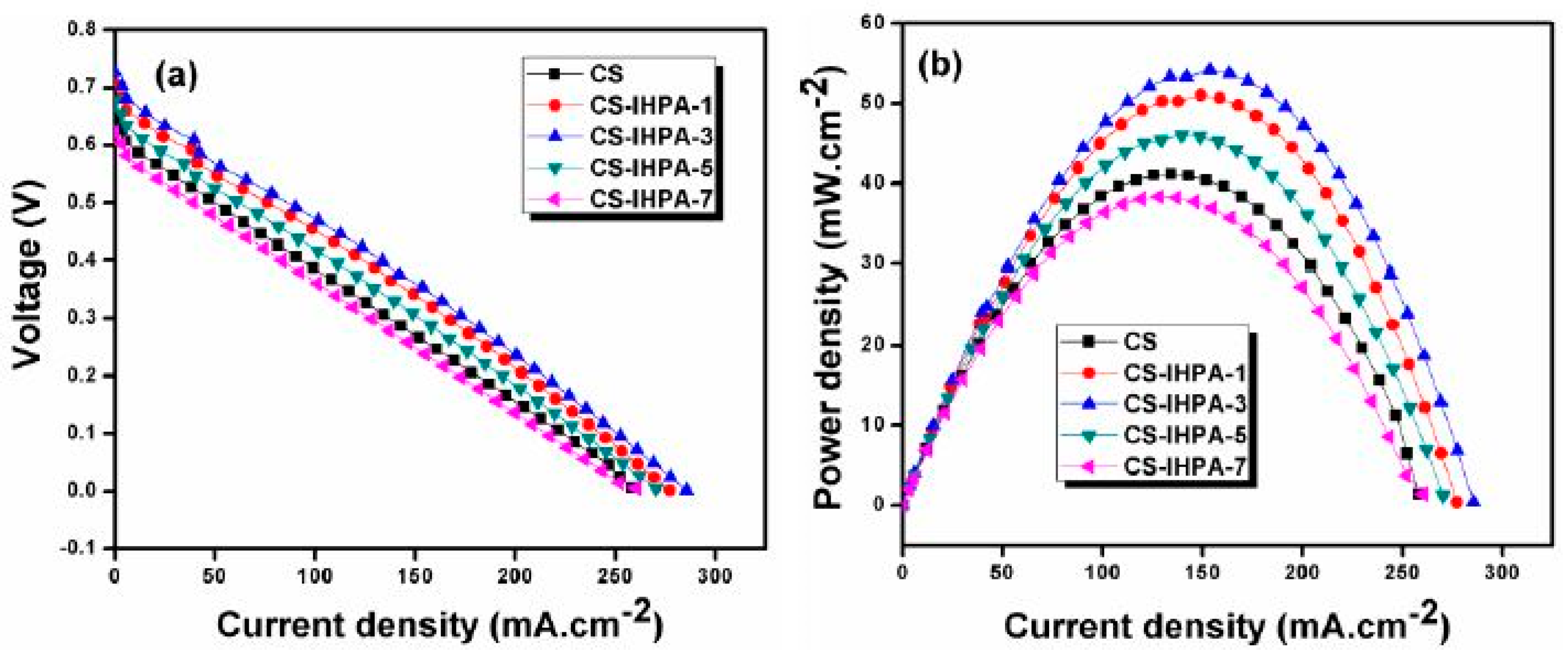


| Membrane | Inorganic Fillers | Synthesis Method | Performance | Application | References |
| CS/sulphonated graphene oxide/silica (CS/sGO/SiO2) | Sulphonated graphene oxide/silica (sGO/SiO2) |
|
| DMFC | [39] |
| CS sulphate | Nanosilica |
|
| DMFC | [40] |
| Sulphonated CS/poly (ethylene oxide)/sulphonated silica (s-CS/PEO/s-SiO2) | Sulphonated silica (s-SiO2) |
|
| PEMFC | [41] |
| CS-polyaniline/nano silica (CS-PAni/SiO2) | Polyaniline/nanosilica (PAni/SiO2) |
|
| DMFC | [26] |
| CS/zwitterion functionalized titania-silica (CS/TiC-SiN) | Zwitterion functionalized titania-silica (TiC-SiN) |
|
| DMFC | [31] |
| CS/phosphorylated hollow mesoporous silica sub-microspheres-amino tris (methylene phosphonic acid) (CS/PHMSS-ATMP) | Phosphorylated hollow mesoporous silica sub-microspheres-amino tris (methylene phosphonic acid) (PHMSS-ATMP) |
|
| DMFC | [28] |
| CS/functionalized silica sub-microspheres | Functionalized silica sub-microspheres |
|
| DMFC | [42] |
| CS-organophosphorylated titania sub-microspheres (CS-OPTi) | Organophosphorylated titania sub-microspheres (CS-OPTi) |
|
| DMFC | [43] |
| Sorbitol-plasticized CS/zeolite hybrid | Zeolite (mordenite) |
|
| DMFC | [35] |
| Surface-modified Y zeolite-filled CS | Modified Y zeolite |
|
| DMFC | [34] |
| CS/zeolite hybrid | Zeolite |
|
| DMFC | [36] |
| CS/zeolite beta hybrid | Zeolite beta |
|
| DMFC | [17] |
| CS/zeolite beta hybrid | Zeolite beta |
|
| DMFC | [37] |
| Membrane | Heteropoly Acids | Proton Conductivity | Performance | Application | References |
| PEC membrane (CS and phosphotungstic acid) | Phosphotungstic acid, H3PW12O40 | 0.024 S cm−1 at 80 °C |
| DMFC | [65] |
| CS/HPA composite membrane | Phosphomolybdic acid, H3PMo12O40; phosphotungstic acid, H3PW12O40; silicotungstic acid, H4SiW12O40 | 0.15 cm−1 at 25 °C |
| DMFC | [51] |
| PEC membrane (CS and phosphotungstic acid with montmorillonite) | Phosphotungstic acid, H3PW12O40 and MMT | 0.030 S cm−1 at 25 °C |
| DMFC | [54] |
| Cesium phosphotungstate salt and CS membrane (CTS/Cs2-PTA) | Cesium phosphotungstate salt, Cs2-PTA | 6.00 × 10−3 S cm−1 at 25 °C and 1.75 × 10−2 S cm−1 at 80 °C |
| DMFC | [66] |
| CS-phosphotungstic acid complex membrane | Phosphotungstic acid, H3PW12O40 | ≈18 mS cm−1 |
| PEMFC | [67] |
| Anodisc-supported CS/phosphotungstic acid membrane | Phosphotungstic acid, H3PW12O40 | ≈14 mS cm−1 |
| PEMFC | [57] |
| Sub-micropore CS/phosphotungstic acid membrane (smpCTS/HPW) | Phosphotungstic acid, H3PW12O40 | 2.90 × 10−2 S cm−1 at 80 °C |
| DMFC | [68] |
| Mixed phosphotungstic/phosphomolybdic acid CS membrane (CS/PMA-PTA) | Phosphomolybdic acid, H3PMo12O40 and phosphotungstic acid, H3PW12O40 | ≈7 mS cm−1 |
| PEMFC | [58] |
| Cesium-substituted mesoporous phosphotungstic acid/CS membrane (CS/m-PTA) | Cesium-substituted mesoporous phosphotungstic acid (m-PTA) | 1.85 × 10−2 S cm−1 |
| DMFC | [59] |
| Sulphonated CS-PEO/HPA membrane | Phosphomolybdic acid, H3PMo12O40; phosphotungstic acid, H3PW12O40 | 9.21 × 10−2 S cm−1 at 80 °C |
| PEMFC | [69] |
| Sulphonated CS-PEO-s-SiO2 doped phosphotungstic acid membrane | Phosphotungstic acid, H3PW12O40 | 1.53 × 10−1 S cm−1 at 25 °C |
| PEMFC | [70] |
| Membrane | Fillers | Proton Conductivity | Performance | Application | References |
| CS-functionalized MWCNTs | MWCNTs | n/a |
| MFC | [94] |
| CS/silica-coated CNTs | Silica-coated CNTs | 0.015–0.025 S cm−1 |
| PEMFC | [86] |
| CS/titania-coated CNTs | Titania-coated CNTs (TCNTs) | 0.016–0.023 S cm−1 |
| PEMFC | [95] |
| CS/CS-coated CNTs | CS-coated CNTs | 9.70 × 10−3 S cm−1 at 20 °C 3.46 × 10−2 S cm−1 at 80 °C |
| DMFC | [83] |
| CS/CNT fluids | Solvent-free CNT fluids | 0.044 S cm−1 at 80 °C |
| DMFC | [96] |
| CS/superacidic sulphated zirconia-coated CNTs | Superacidic sulphated zirconia-coated CNTs | 3.40 × 10−2 S cm−1 at 80 °C |
| DMFC | [93] |
| CS/ionized organic compounds/hydroxylated MWCNTs | N-Benzyl-N,N-dimethyl-3-((2-methyl-1,3-dioxo-2,3-dihydro-1H-benzo[de] isoquinolin-6-yl) amino) propan-1-aminium hydroxide and hydroxylated MWCNTs-OH | 0.83–5.66 × 10−3 S cm−1 |
| PEMFC | [97] |
| Sodium lignin sulphonate (SLS) doped TCNTs CS membrane | Anatase TCNTs and sodium lignin sulphonate | 3.67 × 10−2 S cm−1 at 25 °C 6.47 × 10−2 S cm−1 at 60 °C |
| DMFC | [98] |
| CS/sulphonated MWCNTs | PS@CNT (sulphonated by 1, 3-propane sultone; PS method) and DP@CNT (distillation-precipitation polymerization; DP method) | 0.011–0.026 S cm−1 |
| PEMFC | [99] |
| CS/polydopamine-functionalized CNTs | Polydopamine- functionalized CNTs | 0.028 S cm−1 at 80 °C |
| DMFC | [100] |
| Membrane | Fillers | Proton Conductivity | Performance | Application | References |
| CS/GO nanocomposites | GO | n/a |
| n/a | [109] |
| GO cross-linked CS (CS nanocomposite) | GO | n/a |
| n/a | [110] |
| CS/SGO | SGO | 0.0612 S cm−1 at 85 °C (100% RH) 10.9 mS cm−1 at 120 °C (0% RH) |
| PEMFC | [52] |
| N-o-sulphonic acid benzyl CS/N,N-di-methylene phosphonic acid propylsilane (NSBC/NMPSGO) | NMPSGO | 8.87 × 10−2 S cm−1 at 30 °C (100% RH) |
| DMFC | [62] |
| Montmorillonite/GO/CS composite | GO | n/a |
| n/a | [117] |
| CS/phosphorylated GO | PGO | 63.4 mS cm−1 at 95 °C (100% RH) 5.79 mS cm−1 at 160 °C (0% RH) |
| PEMFC | [114] |
| Modified-sulphonated CS | MGO | 6.77 × 10−2 S cm−1 at 30 °C 11.20 × 10−2 S cm−1 at 90 °C |
| DMFC | [118] |
| CS/SCS/SGO | SCS/SGO | 1.30–7.20 mS cm−1 |
| DMFC | [111] |
| Phosphorylated or sulphurized CS- mixed-matrix composite | GO | n/a |
| MFC | [119] |
| Sulphonated CS/polyethylene oxide/sulphonated GO | SGO | 4.83 × 10−2 S cm−1 at 30 °C 11.11 × 10−2 S cm−1 at 80 °C |
| PEMFC | [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. https://doi.org/10.3390/ijms21020632
Rosli NAH, Loh KS, Wong WY, Yunus RM, Lee TK, Ahmad A, Chong ST. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. International Journal of Molecular Sciences. 2020; 21(2):632. https://doi.org/10.3390/ijms21020632
Chicago/Turabian StyleRosli, Nur Adiera Hanna, Kee Shyuan Loh, Wai Yin Wong, Rozan Mohamad Yunus, Tian Khoon Lee, Azizan Ahmad, and Seng Tong Chong. 2020. "Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids" International Journal of Molecular Sciences 21, no. 2: 632. https://doi.org/10.3390/ijms21020632
APA StyleRosli, N. A. H., Loh, K. S., Wong, W. Y., Yunus, R. M., Lee, T. K., Ahmad, A., & Chong, S. T. (2020). Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. International Journal of Molecular Sciences, 21(2), 632. https://doi.org/10.3390/ijms21020632