Influence of Sex on Urinary Organic Acids: A Cross-Sectional Study in Children
Abstract
1. Introduction
2. Results
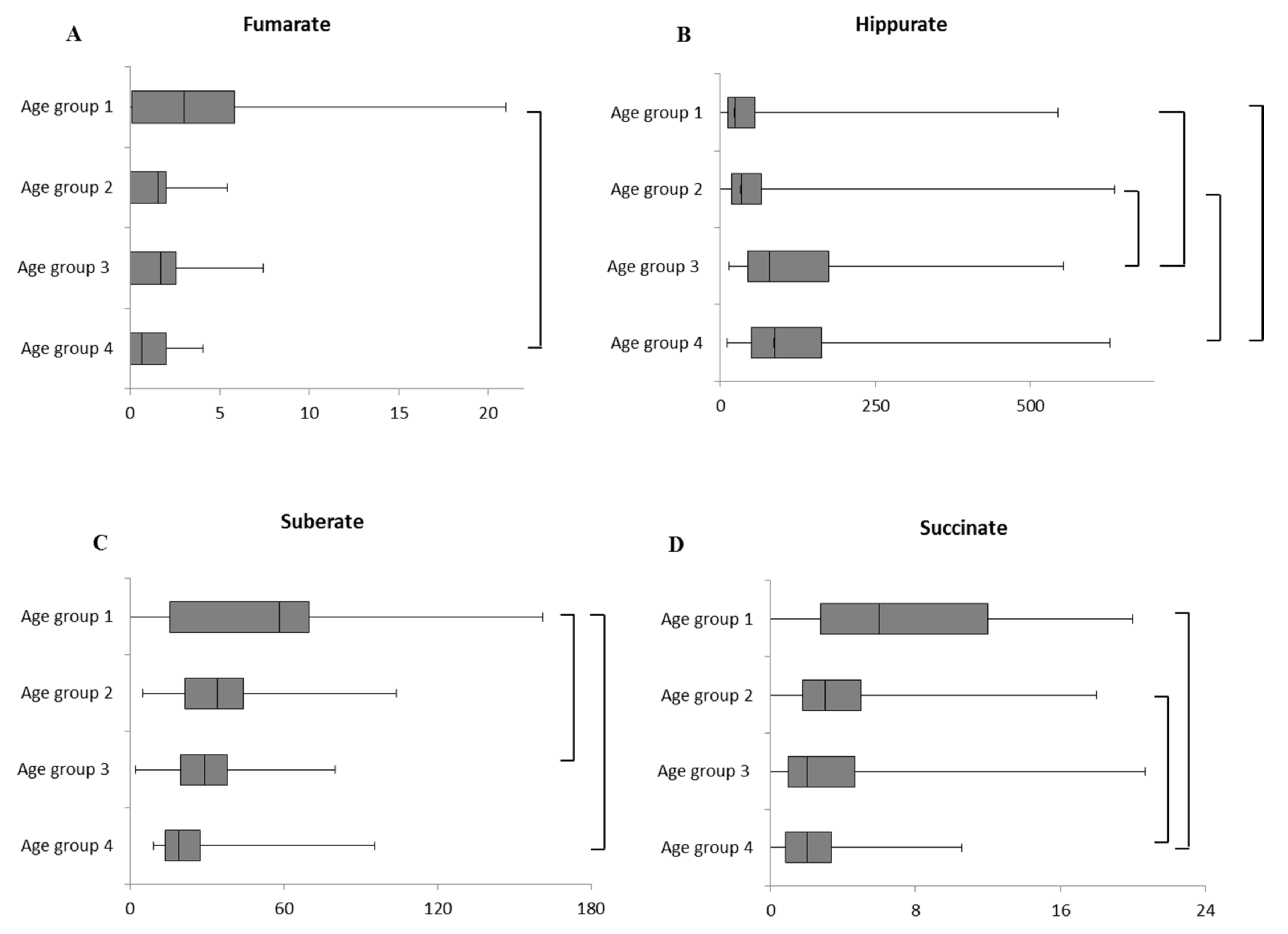
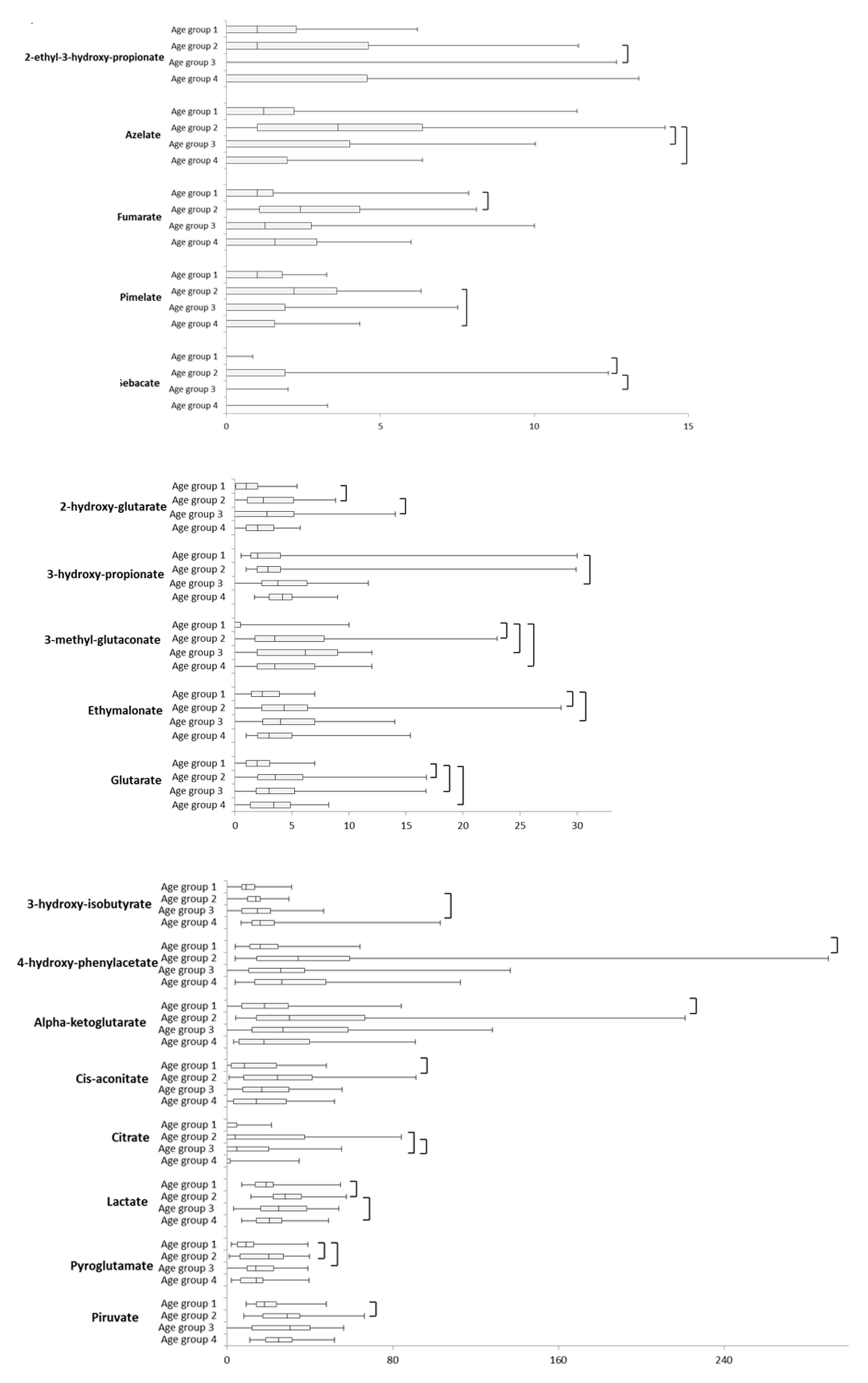
2.1. Intra-Sex Analysis in Male Cohort of Different Ages
2.2. Intra-Sex Analysis in Female Cohort of Different Ages
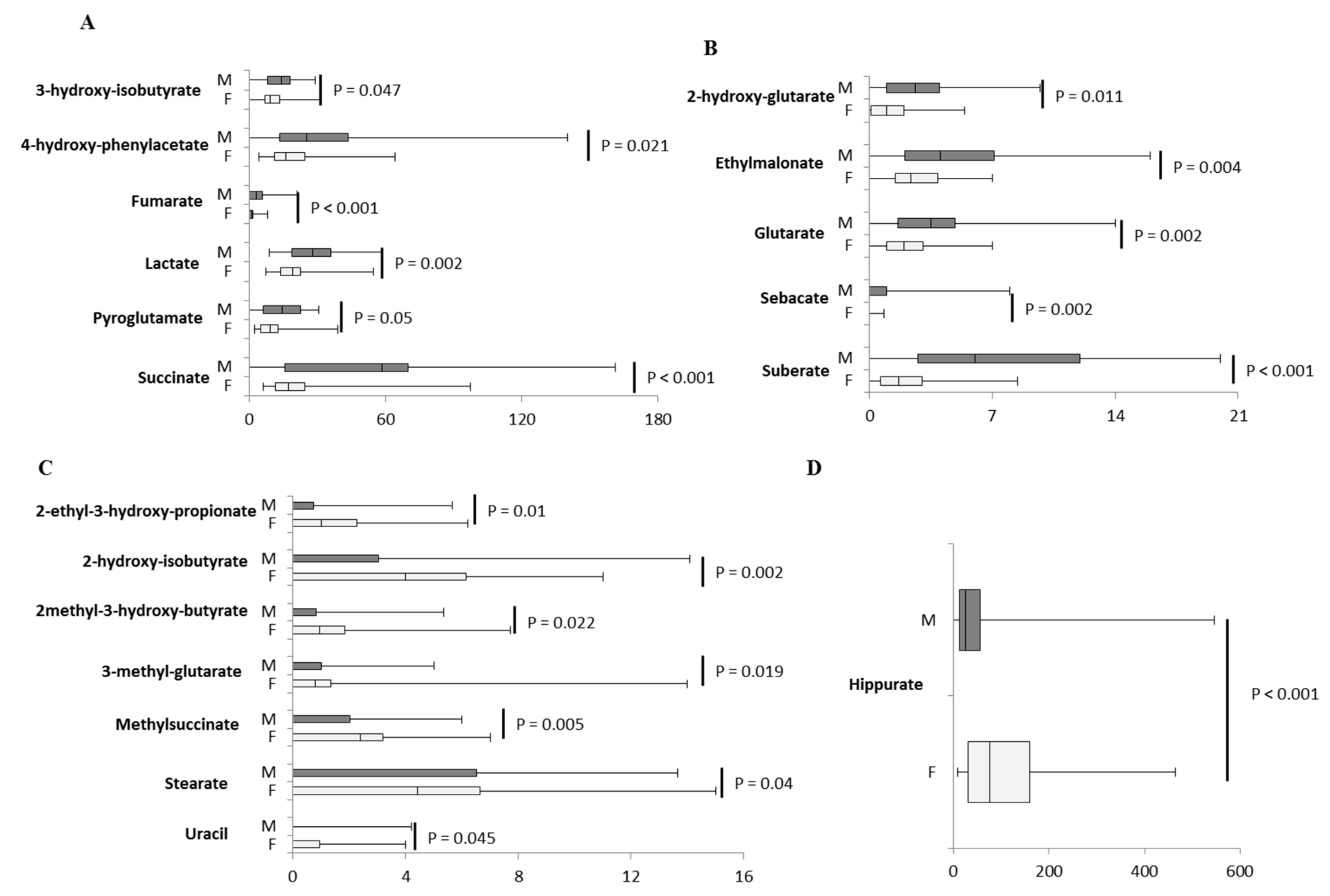
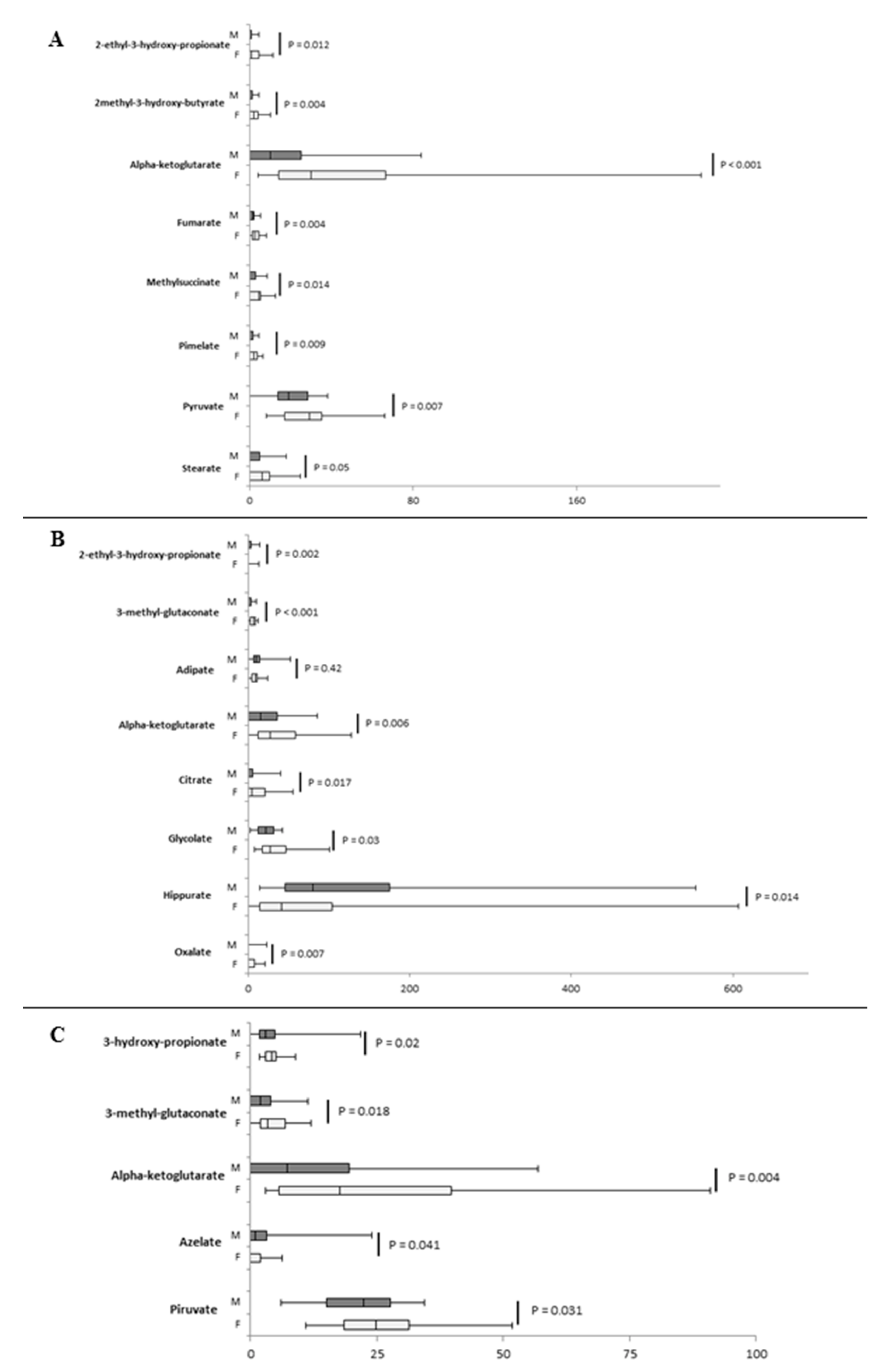
2.3. Inter-Sex Analysis
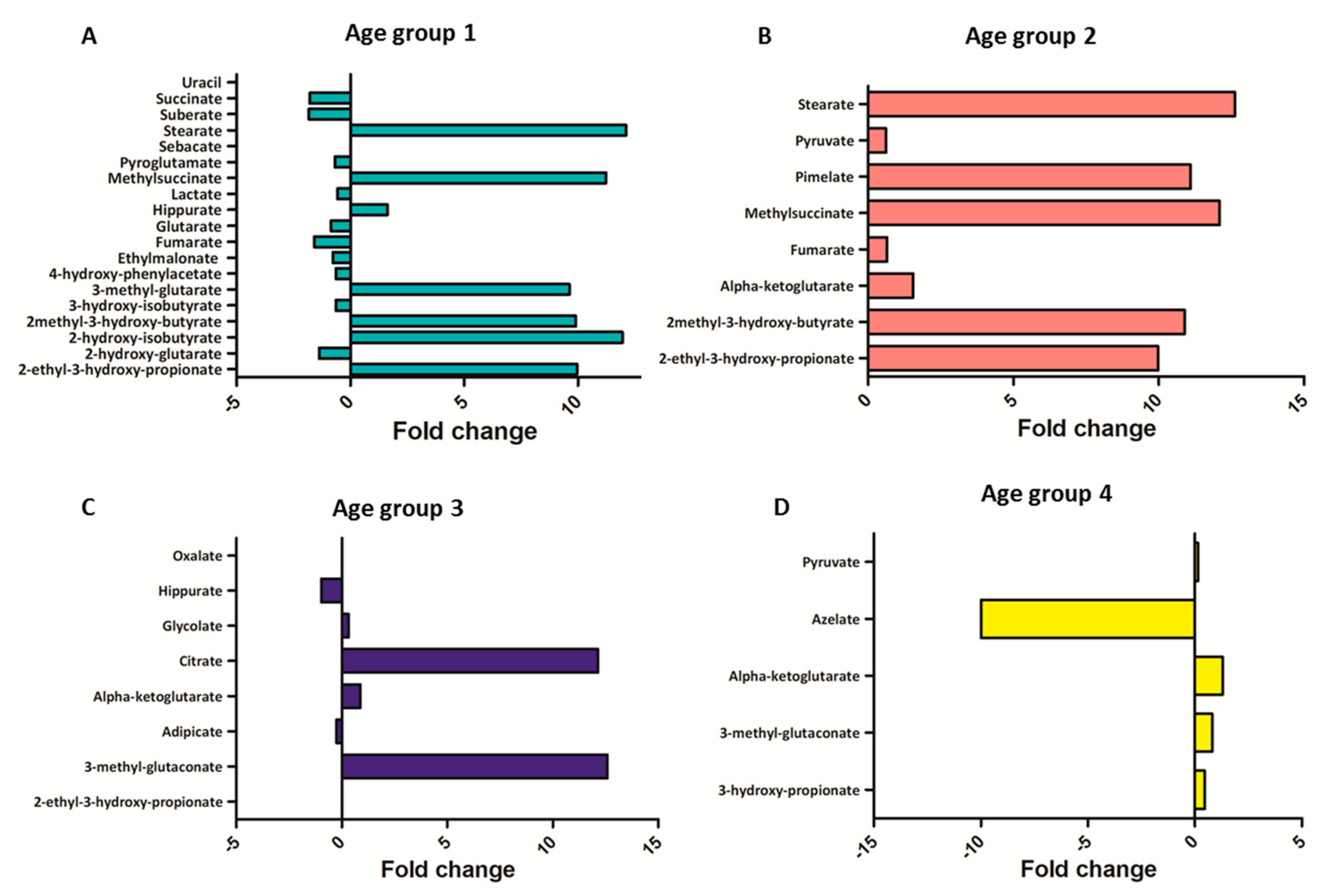
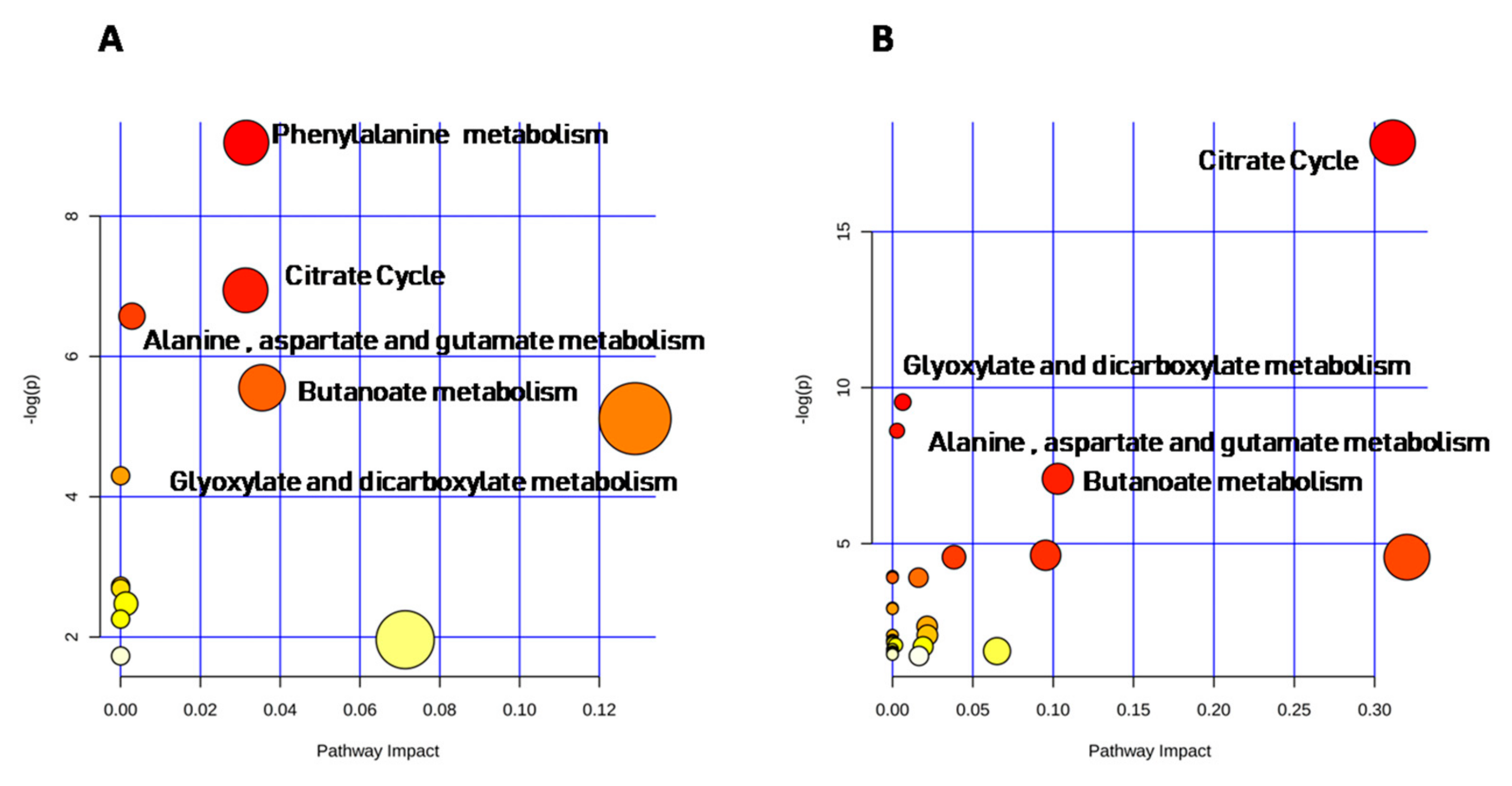
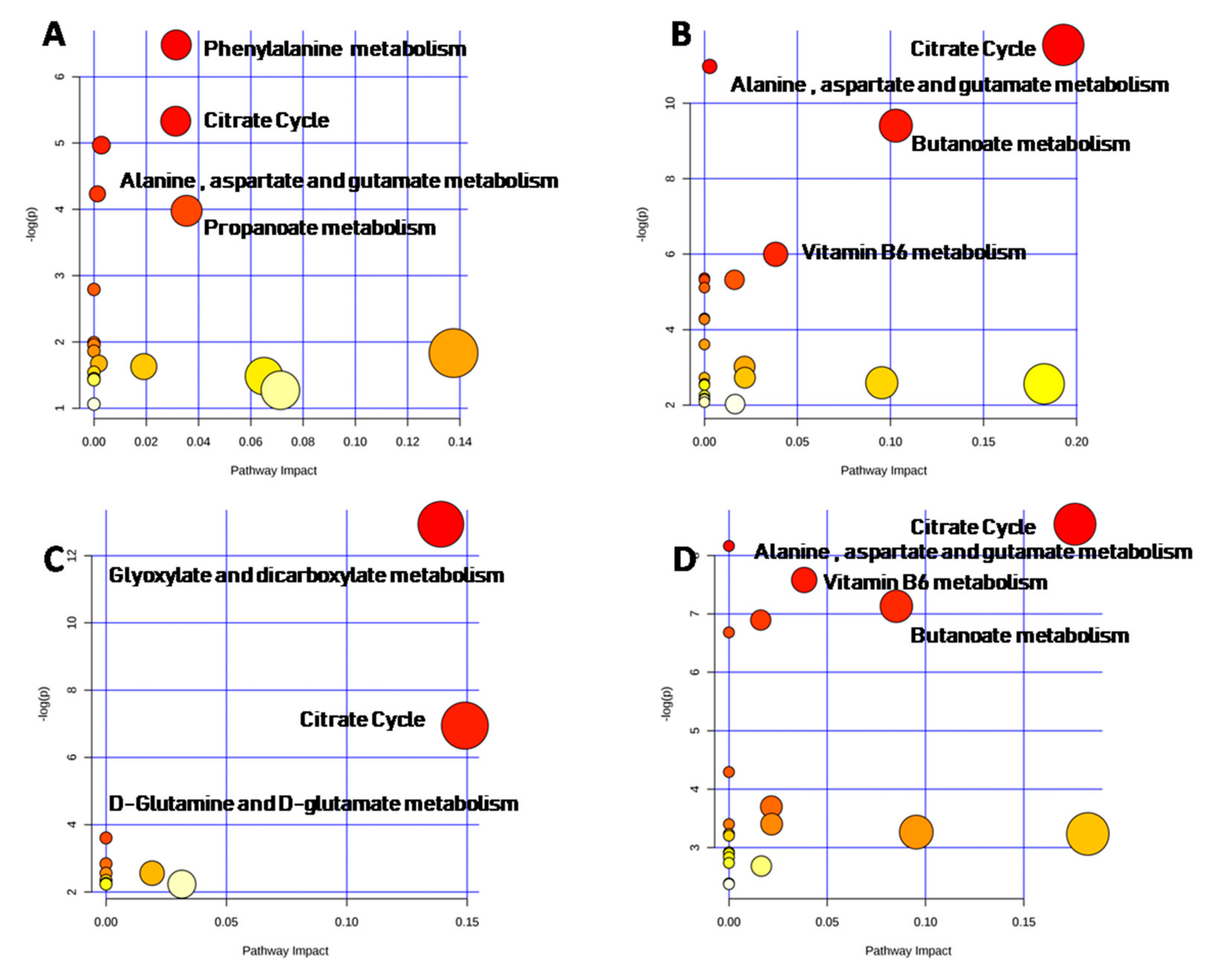
2.4. Metabolomic Intra-Sex Analysis in Male and Female Subjects of Different Ages
2.5. Metabolomic Inter-Sex Analysis
3. Discussion
4. Methods
4.1. Populations
4.2. Urine Collection
4.3. Preparation of Samples for GC-MS Analysis of Metabolites
4.4. GC-MS Analysis
4.5. Pathway Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FDR | false discovery rate |
| IQR | inter-quartile ranges |
| MetPA | metabolic pathway analysis |
| MSEA | metabolite set enrichment analysis |
References
- Sun, J.; Beger, R.D.; Schnackenberg, L.K. Metabolomics as a tool for personalizing medicine: 2012 update. Pers. Med. 2013, 10, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Scolamiero, E.; Cozzolino, C.; Albano, L.; Ansalone, A.; Caterino, M.; Corbo, G.; di Girolamo, M.G.; Di Stefano, C.; Durante, A.; Franzese, G.; et al. Targeted metabolomics in the expanded newborn screening for inborn errors of metabolism. Mol. Biosyst. 2015, 11, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Langridge, J. An emerging role for metabolomics in nutrition science. J. Nutr. Nutr. 2013, 6, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.M.; Bennett, M.J. Urine organic acid analysis for inherited metabolic disease using gas chromatography-mass spectrometry. Methods Mol. Biol. 2010, 603, 423–431. [Google Scholar] [PubMed]
- Tsoukalas, D.; Alegakis, A.; Fragkiadaki, P.; Papakonstantinou, E.; Nikitovic, D.; Karataraki, A.; Nosyrev, A.E.; Papadakis, E.G.; Spandidos, D.A.; Drakoulis, N.; et al. Application of metabolomics: Focus on the quantification of organic acids in healthy adults. Int. J. Mol. Med. 2017, 40, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Barshop, B.A. Metabolomic approaches to mitochondrial disease: Correlation of urine organic acids. Mitochondrion 2004, 4, 521–527. [Google Scholar] [CrossRef]
- Carraro, S.; Giordano, G.; Reniero, F.; Perilongo, G.; Baraldi, E. Metabolomics: A new frontier for research in pediatrics. J. Pediatr. 2009, 154, 638–644. [Google Scholar] [CrossRef]
- Mittelstrass, K.; Ried, J.S.; Yu, Z.; Krumsiek, J.; Gieger, C.; Prehn, C.; Roemisch-Margl, W.; Polonikov, A.; Peters, A.; Theis, F.J.; et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet 2011, 7, e1002215. [Google Scholar] [CrossRef]
- Ruoppolo, M.; Campesi, I.; Scolamiero, E.; Pecce, R.; Caterino, M.; Cherchi, S.; Mercuro, G.; Tonolo, G.; Franconi, F. Serum metabolomic profiles suggest influence of sex and oral contraceptive use. Am. J. Transl. Res. 2014, 6, 614–624. [Google Scholar]
- Ruoppolo, M.; Scolamiero, E.; Caterino, M.; Mirisola, V.; Franconi, F.; Campesi, I. Female and male human babies have distinct blood metabolomic patterns. Mol. Biosyst. 2015, 11, 2483–2492. [Google Scholar] [CrossRef]
- Bertram, H.C.; Duus, J.O.; Petersen, B.O.; Hoppe, C.; Larnkjaer, A.; Schack-Nielsen, L.; Molgaard, C.; Michaelsen, K.F. Nuclear magnetic resonance-based metabonomics reveals strong sex effect on plasma metabolism in 17-year-old Scandinavians and correlation to retrospective infant plasma parameters. Metabolism 2009, 58, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stuckler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Kruger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yeon, A.; Shahid, M.; Anger, J.T.; Eilber, K.S.; Fiehn, O.; Kim, J. Sex-associated differences in baseline urinary metabolites of healthy adults. Sci. Rep. 2018, 8, 11883. [Google Scholar] [CrossRef] [PubMed]
- Darst, B.F.; Koscik, R.L.; Hogan, K.J.; Johnson, S.C.; Engelman, C.D. Longitudinal plasma metabolomics of aging and sex. Aging Albany NY 2019, 11, 1262–1282. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.; Singh, A.; Ramji, S.; Shoemaker, J.D.; Kapoor, S. Urinary organic acids quantitated in a healthy north Indian pediatric population. Indian J. Clin. Biochem. 2015, 30, 221–229. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Yeh, K.W.; Lin, G.; Chiang, M.H.; Yang, S.C.; Chao, W.J.; Yao, T.C.; Tsai, M.H.; Hua, M.C.; Liao, S.L.; et al. Metabolomics reveals dynamic metabolic changes associated with age in early childhood. PLoS ONE 2016, 11, e0149823. [Google Scholar] [CrossRef]
- Gu, H.; Pan, Z.; Xi, B.; Hainline, B.E.; Shanaiah, N.; Asiago, V.; Gowda, G.A.; Raftery, D. 1H NMR metabolomics study of age profiling in children. NMR Biomed. 2009, 22, 826–833. [Google Scholar] [CrossRef]
- Psihogios, N.G.; Gazi, I.F.; Elisaf, M.S.; Seferiadis, K.I.; Bairaktari, E.T. Gender-related and age-related urinalysis of healthy subjects by NMR-based metabonomics. NMR Biomed. 2008, 21, 195–207. [Google Scholar] [CrossRef]
- Slupsky, C.M.; Rankin, K.N.; Wagner, J.; Fu, H.; Chang, D.; Weljie, A.M.; Saude, E.J.; Lix, B.; Adamko, D.J.; Shah, S.; et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal. Chem. 2007, 79, 6995–7004. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, W.; Hong, Q.; Xiao, C.; Yang, L.; Ma, Z.; Wang, Y.; Tan, H.; Tang, X.; Gao, Y. Rapid comparison of metabolites in humans and rats of different sexes using untargeted UPLC-TOFMS and an in-house software platform. Eur. J. Mass Spectrom. Chichester 2015, 21, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Brennan, L.; Price, R.K.; Wallace, J.M.; Strain, J.J.; Gibney, M.J.; Shewry, P.R.; Ward, J.L.; Garg, L.; Welch, R.W. Using NMR-based metabolomics to evaluate postprandial urinary responses following consumption of minimally processed wheat bran or wheat aleurone by men and women. Nutrients 2016, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, M.E.; Paiva, M.J. Hippuric acid in urine: Reference values. Revista de Saúde Pública 2002, 36, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yde, C.C.; Arnberg, K.; Molgaard, C.; Michaelsen, K.F.; Larnkjaer, A.; Bertram, H.C. NMR-based metabolomic profiling of overweight adolescents: An elucidation of the effects of inter-/intraindividual differences, gender, and pubertal development. Biomed Res. Int. 2014, 2014, 537157. [Google Scholar] [CrossRef]
- Gallagher, R.C.; Pollard, L.; Scott, A.I.; Huguenin, S.; Goodman, S.; Sun, Q. Laboratory analysis of organic acids, 2018 update: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2018, 20, 683–691. [Google Scholar] [CrossRef]
- Fiehn, O.; Spranger, J. Use of metabolomics to discover metabolic patterns associated with human disease. Pulm. Circ. 2014, 3, 417–423. [Google Scholar]
- Hosten, A.O. BUN and Creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Franconi, F.; Campesi, I.; Colombo, D.; Antonini, P. Sex-gender variable: Methodological recommendations for increasing scientific value of clinical studies. Cells 2019, 8, 476. [Google Scholar] [CrossRef]
- Duke-Margolis Center for Health Policy. Biologic Variability to Drug Response: Sex Differences in Clinical Trials; Duke-Margolis Center for Health Policy: Washington, DC, USA, 2016. [Google Scholar]
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Penta, L.; Esposito, S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front. Endocrinol. Lausanne 2018, 9, 410. [Google Scholar] [CrossRef]
- Boulat, O.; Gradwohl, M.; Matos, V.; Guignard, J.P.; Bachmann, C. Organic acids in the second morning urine in a healthy Swiss paediatric population. Clin. Chem. Lab. Med. 2003, 41, 1642–1658. [Google Scholar] [CrossRef]
- Guneral, F.; Bachmann, C. Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin. Chem. 1994, 40, 862–866. [Google Scholar] [CrossRef]
- Diaz, S.O.; Pinto, J.; Barros, A.S.; Morais, E.; Duarte, D.; Negrao, F.; Pita, C.; Almeida Mdo, C.; Carreira, I.M.; Spraul, M.; et al. Newborn urinary metabolic signatures of prematurity and other disorders: A case control study. J. Proteome Res. 2016, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Scalabre, A.; Jobard, E.; Demede, D.; Gaillard, S.; Pontoizeau, C.; Mouriquand, P.; Elena-Herrmann, B.; Mure, P.Y. Evolution of newborns’ urinary metabolomic profiles according to age and growth. J. Proteome Res. 2017, 16, 3732–3740. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.D.; Roth, R.H. Metabolism of gamma-hydroxy-[1-14C] butyrate by rat brain: Relationship to the Krebs cycle and metabolic compartmentation of amino acids. J. Neurochem. 1978, 30, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- LeBeau, M.A.; Montgomery, M.A.; Morris-Kukoski, C.; Schaff, J.E.; Deakin, A.; Levine, B. A comprehensive study on the variations in urinary concentrations of endogenous gamma-hydroxybutyrate (GHB). J. Anal. Toxicol. 2006, 30, 98–105. [Google Scholar] [CrossRef] [PubMed]
- La Du, B.N. Alkaptonuria. In The Metabolic Molecular Bases of Inherited Disease; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 2109–2123. [Google Scholar]
- Ostergaard, J.; Larsen, C. Bioreversible derivatives of phenol. 2. Reactivity of carbonate esters with fatty acid-like structures towards hydrolysis in aqueous solutions. Molecules 2007, 12, 2396–2412. [Google Scholar] [CrossRef]
- Tonin, A.M.; Amaral, A.U.; Busanello, E.N.; Grings, M.; Castilho, R.F.; Wajner, M. Long-chain 3-hydroxy fatty acids accumulating in long-chain 3-hydroxyacyl-CoA dehydrogenase and mitochondrial trifunctional protein deficiencies uncouple oxidative phosphorylation in heart mitochondria. J. Bioenerg. Biomembr. 2013, 45, 47–57. [Google Scholar] [CrossRef]
- Kirejczyk, J.K.; Porowski, T.; Konstantynowicz, J.; Kozerska, A.; Nazarkiewicz, A.; Hoppe, B.; Wasilewska, A. Urinary citrate excretion in healthy children depends on age and gender. Pediatr. Nephrol. 2014, 29, 1575–1582. [Google Scholar] [CrossRef]
- Eshima, J.; Ong, S.; Davis, T.J.; Miranda, C.; Krishnamurthy, D.; Nachtsheim, A.; Stufken, J.; Plaisier, C.; Fricks, J.; Bean, H.D.; et al. Monitoring changes in the healthy female metabolome across the menstrual cycle using GCxGC-TOFMS. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2019, 1121, 48–57. [Google Scholar] [CrossRef]
- Heller, H.J.; Sakhaee, K.; Moe, O.W.; Pak, C.Y. Etiological role of estrogen status in renal stone formation. J. Urol. 2002, 168, 1923–1927. [Google Scholar] [CrossRef]
- Vicente-Munoz, S.; Morcillo, I.; Puchades-Carrasco, L.; Paya, V.; Pellicer, A.; Pineda-Lucena, A. Nuclear magnetic resonance metabolomic profiling of urine provides a noninvasive alternative to the identification of biomarkers associated with endometriosis. Fertil. Steril. 2015, 104, 1202–1209. [Google Scholar] [CrossRef]
- Frezza, C. Mitochondrial metabolites: Undercover signalling molecules. Interface Focus 2017, 7, 20160100. [Google Scholar] [CrossRef] [PubMed]
- Borras, C.; Sastre, J.; Garcia-Sala, D.; Lloret, A.; Pallardo, F.V.; Vina, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef]
- Straface, E.; Vona, R.; Campesi, I.; Franconi, F. Mitochondria can orchestrate sex differences in cell fate of vascular smooth muscle cells from rats. Biol. Sex Differ. 2015, 6, 34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. Lond 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; D’Agostino, D.P.; Katz, H.E.; Roth, J.P.; Meuillet, E.J.; Somlyai, G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med. Hypotheses 2016, 87, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Somlyai, G.; Jancso, G.; Jakli, G.; Vass, K.; Barna, B.; Lakics, V.; Gaal, T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993, 317, 1–4. [Google Scholar] [CrossRef]
- Sobczyk, L.; Obrzud, M.; Filarowski, A. H/D isotope effects in hydrogen bonded systems. Molecules 2013, 18, 4467–4476. [Google Scholar] [CrossRef]
- Lehninger, A.L. Lehninger Principles of Biochemistry, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Del Chierico, F.; Abbatini, F.; Russo, A.; Quagliariello, A.; Reddel, S.; Capoccia, D.; Caccamo, R.; Ginanni Corradini, S.; Nobili, V.; De Peppo, F.; et al. Gut microbiota markers in obese adolescent and adult patients: Age-dependent differential patterns. Front. Microbiol. 2018, 9, 1210. [Google Scholar] [CrossRef]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef]
- Mair, R.D.; Sirich, T.L.; Plummer, N.S.; Meyer, T.W. Characteristics of colon-derived uremic solutes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1398–1404. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.L.; Ruthven, C.R.; Sandler, M. Gut flora and the origin of some urinary aromatic phenolic compounds. Biochem. Pharmacol. 1994, 47, 2294–2297. [Google Scholar] [CrossRef]
- Williams, H.R.; Cox, I.J.; Walker, D.G.; Cobbold, J.F.; Taylor-Robinson, S.D.; Marshall, S.E.; Orchard, T.R. Differences in gut microbial metabolism are responsible for reduced hippurate synthesis in Crohn’s disease. BMC Gastroenterol. 2010, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Lees, H.J.; Swann, J.R.; Wilson, I.D.; Nicholson, J.K.; Holmes, E. Hippurate: The natural history of a mammalian-microbial cometabolite. J. Proteome Res. 2013, 12, 1527–1546. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Hong, H.; Chang, C.W.; Guo, L.W.; Lyn-Cook, B.; Shi, L.; Ning, B. Sex differences in the expression of drug-metabolizing and transporter genes in human liver. J. Drug Metab. Toxicol. 2012, 3, 1000119. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Snowberg, L.K.; Hirsch, P.E.; Lauber, C.L.; Org, E.; Parks, B.; Lusis, A.J.; Knight, R.; Caporaso, J.G.; Svanback, R. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat. Commun. 2015, 5, 4500. [Google Scholar] [CrossRef]
- Campesi, I.; Romani, A.; Franconi, F. The sex-gender effects in the road to tailored botanicals. Nutrients 2019, 11, 1637. [Google Scholar] [CrossRef]
- Sinha, T.; Vich Vila, A.; Garmaeva, S.; Jankipersadsing, S.A.; Imhann, F.; Collij, V.; Bonder, M.J.; Jiang, X.; Gurry, T.; Alm, E.J.; et al. Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes 2019, 10, 358–366. [Google Scholar] [CrossRef]
- Bennett, M.J. The laboratory diagnosis of inborn errors of mitochondrial fatty acid oxidation. Ann. Clin. Biochem. 1990, 27, 519–531. [Google Scholar] [CrossRef]
- Miura, Y. The biological significance of omega-oxidation of fatty acids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 370–382. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wust, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.H.; Walensky, R.P. Where were the women? Gender parity in clinical trials. New Engl. J. Med. 2019, 381, 2491. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Campesi, I. Sex and gender influences on pharmacological response: An overview. Expert Rev. Clin. Pharmacol. 2014, 7, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Fois, M.; Franconi, F. Sex and gender aspects in anesthetics and pain medication. Handb. Exp. Pharmacol. 2012, 214, 265–278. [Google Scholar]
- Basili, S.; Raparelli, V.; Proietti, M.; Napoleone, L.; Ferroni, P.; Franconi, F. Old and new oral anticoagulants in management of atrial fibrillation: A double-edged sword for women. Curr. Vasc. Pharmacol. 2015, 13, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Taylor, S.L.; Ganti, S.; Guo, L.; Osier, M.V.; Weiss, R.H. Urine metabolomic analysis identifies potential biomarkers and pathogenic pathways in kidney cancer. Omics 2011, 15, 293–303. [Google Scholar] [CrossRef]
- Jager, K.J.; Zoccali, C.; Macleod, A.; Dekker, F.W. Confounding: What it is and how to deal with it. Kidney Int. 2008, 73, 256–260. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I. Sex impact on biomarkers, pharmacokinetics and pharmacodynamics. Curr. Med. Chem. 2017, 24, 2561–2575. [Google Scholar] [CrossRef]
- World Health Organisation. Multisectoral and Intersectoral Action for Improved Health and Well-Being for All: Mapping of the Who European Region. Governance for a Sustainable Future: Improving Health and Well-Being for All; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Butler, A.R. The Jaffe reaction. Identification of the coloured species. Clin. Chim. Acta 1975, 59, 227–232. [Google Scholar] [CrossRef]
- Rossi, A.; Ruoppolo, M.; Formisano, P.; Villani, G.; Albano, L.; Gallo, G.; Crisci, D.; Moccia, A.; Parenti, G.; Strisciuglio, P.; et al. Insulin-resistance in glycogen storage disease type Ia: Linking carbohydrates and mitochondria? J. Inherit. Metab. Dis. 2018, 41, 985–995. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caterino, M.; Ruoppolo, M.; Villani, G.R.D.; Marchese, E.; Costanzo, M.; Sotgiu, G.; Dore, S.; Franconi, F.; Campesi, I. Influence of Sex on Urinary Organic Acids: A Cross-Sectional Study in Children. Int. J. Mol. Sci. 2020, 21, 582. https://doi.org/10.3390/ijms21020582
Caterino M, Ruoppolo M, Villani GRD, Marchese E, Costanzo M, Sotgiu G, Dore S, Franconi F, Campesi I. Influence of Sex on Urinary Organic Acids: A Cross-Sectional Study in Children. International Journal of Molecular Sciences. 2020; 21(2):582. https://doi.org/10.3390/ijms21020582
Chicago/Turabian StyleCaterino, Marianna, Margherita Ruoppolo, Guglielmo Rosario Domenico Villani, Emanuela Marchese, Michele Costanzo, Giovanni Sotgiu, Simone Dore, Flavia Franconi, and Ilaria Campesi. 2020. "Influence of Sex on Urinary Organic Acids: A Cross-Sectional Study in Children" International Journal of Molecular Sciences 21, no. 2: 582. https://doi.org/10.3390/ijms21020582
APA StyleCaterino, M., Ruoppolo, M., Villani, G. R. D., Marchese, E., Costanzo, M., Sotgiu, G., Dore, S., Franconi, F., & Campesi, I. (2020). Influence of Sex on Urinary Organic Acids: A Cross-Sectional Study in Children. International Journal of Molecular Sciences, 21(2), 582. https://doi.org/10.3390/ijms21020582










