Neurogenesis and Specification of Retinal Ganglion Cells
Abstract
1. Introduction
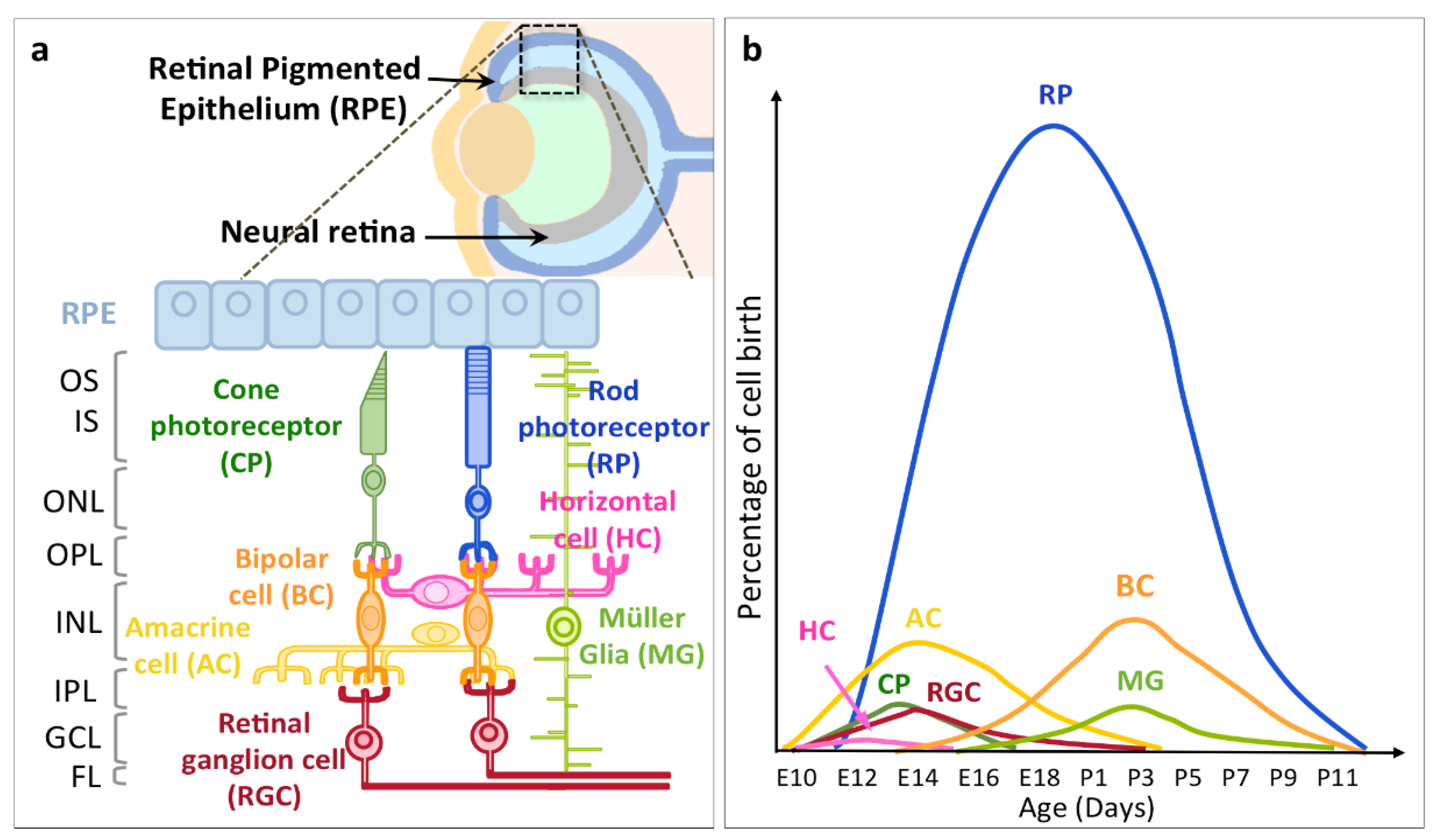
2. Timing of RGC Neurogenesis
3. Extracellular Differentiation Signals
3.1. Notch
3.2. Sonic Hedgehog
3.3. Fibroblast Growth Factors
4. Competence and Stochastic Model of Retinal Cell Fate
5. Factors Controlling the Competence of Progenitors
6. Intrinsic Control of RGC Specification
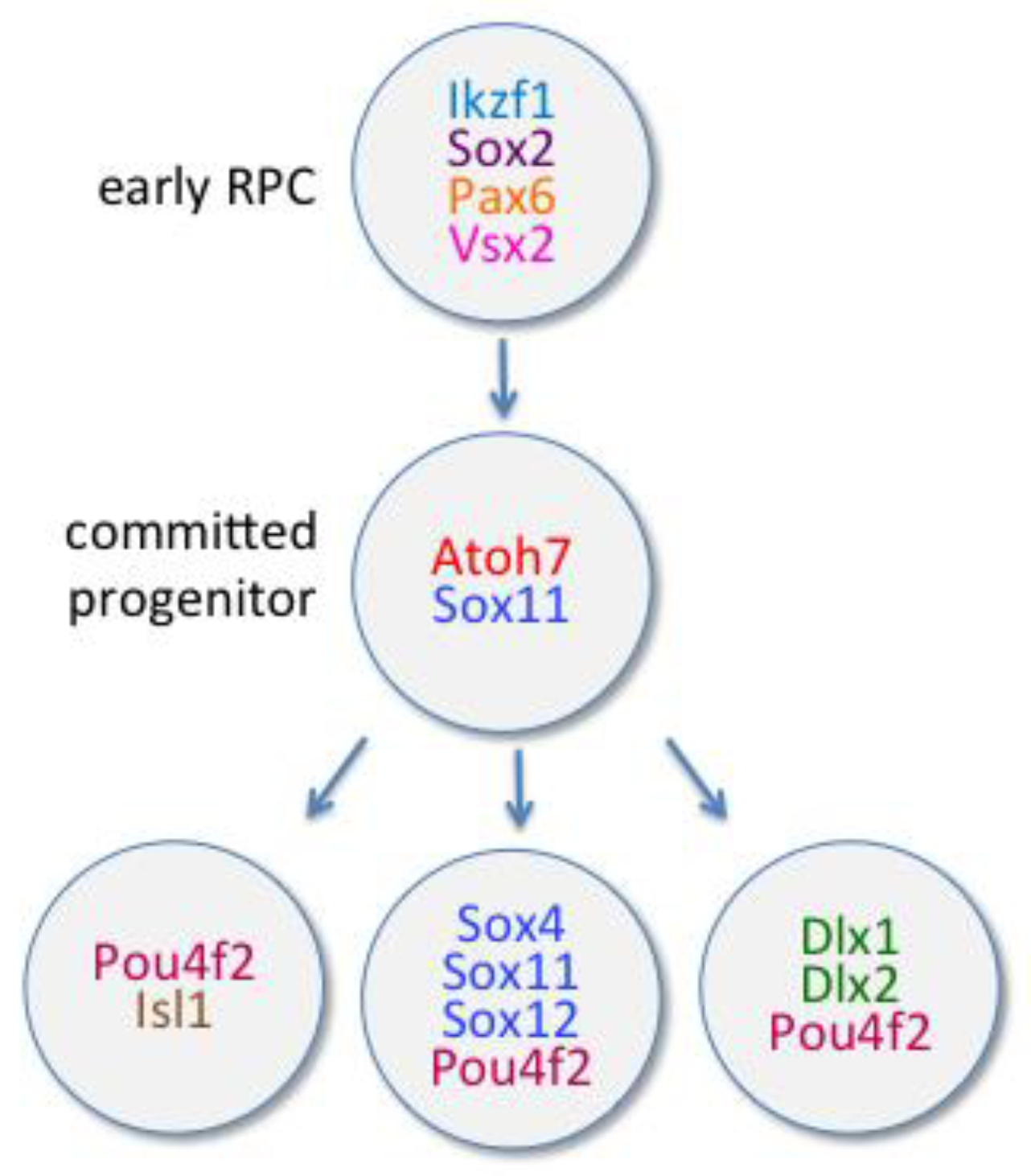
6.1. Transcription Factors
6.1.1. Atoh7
6.1.2. POU Domain, Class 4, Transcription Factors
6.1.3. Dlx Family of Transcription Factors
6.1.4. SoxC Family of Transcription Factors
6.1.5. Other Regulators
6.2. MicroRNA
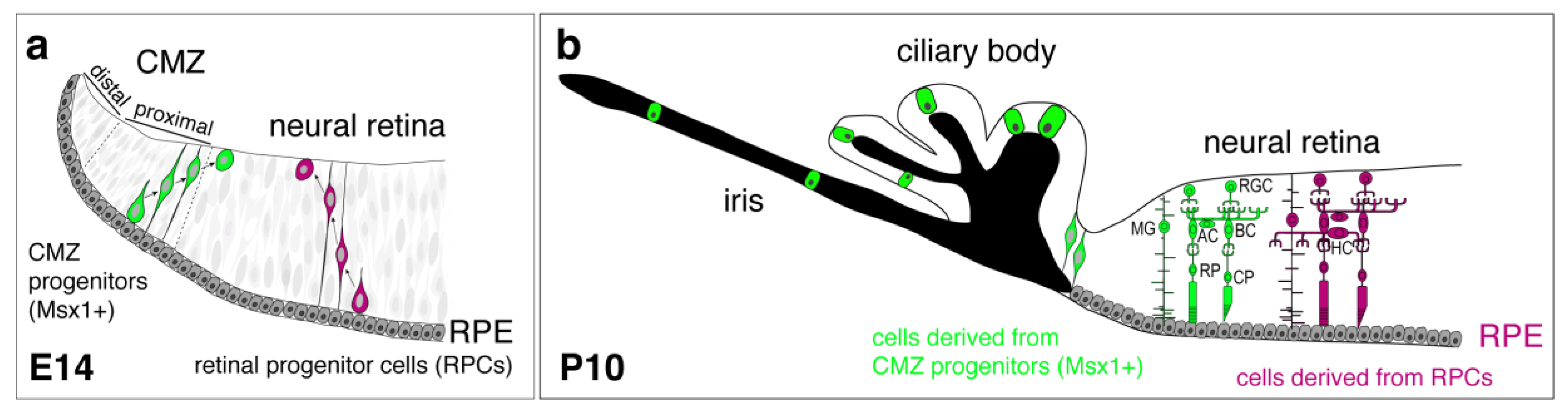
7. The Ciliary Marginal Zone, a Source of Neurogenesis in Mammals
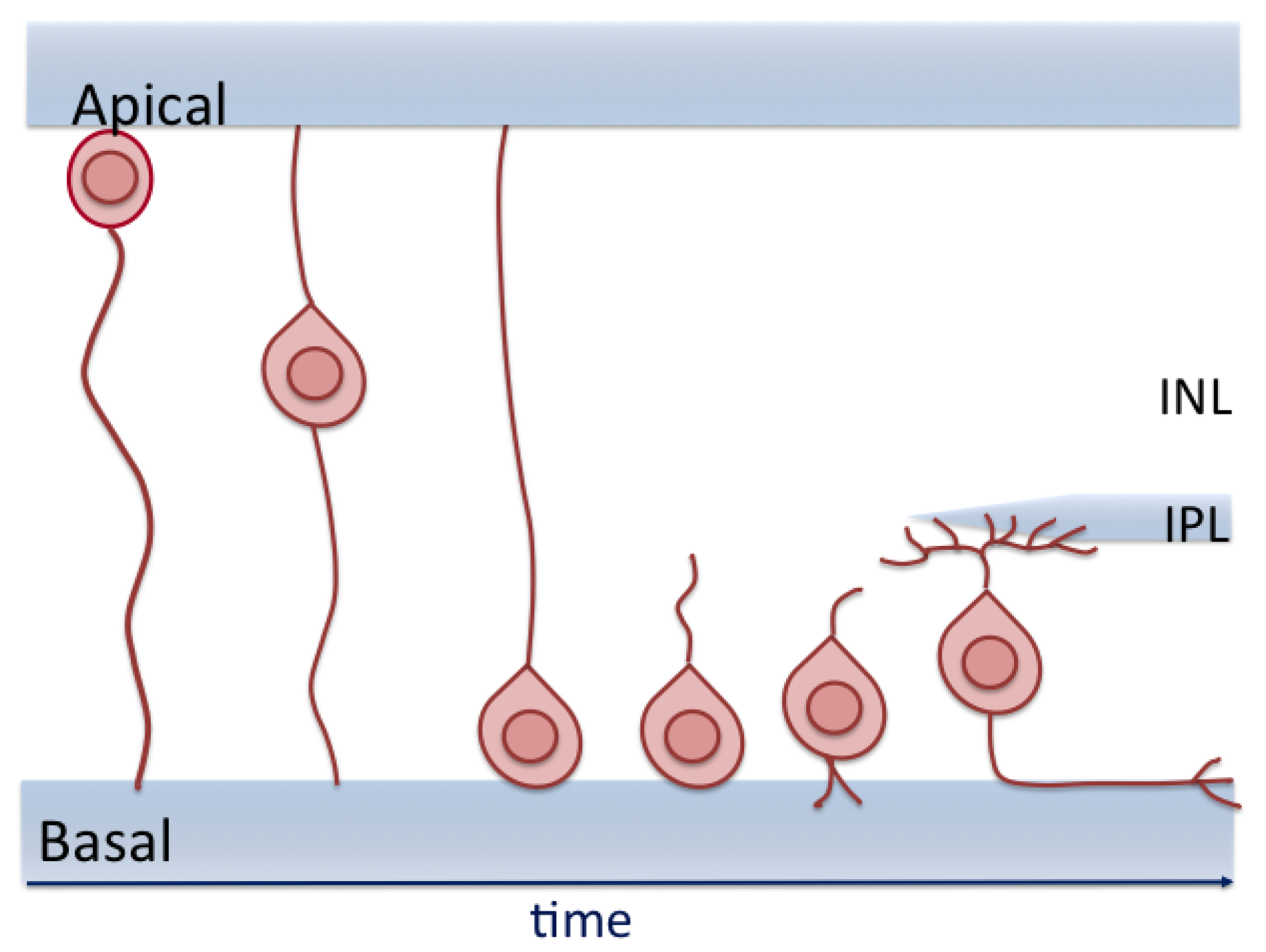
8. RGC Migration
9. Classification of RGC Subtypes
9.1. Morphological Criteria
9.2. Functional Criteria
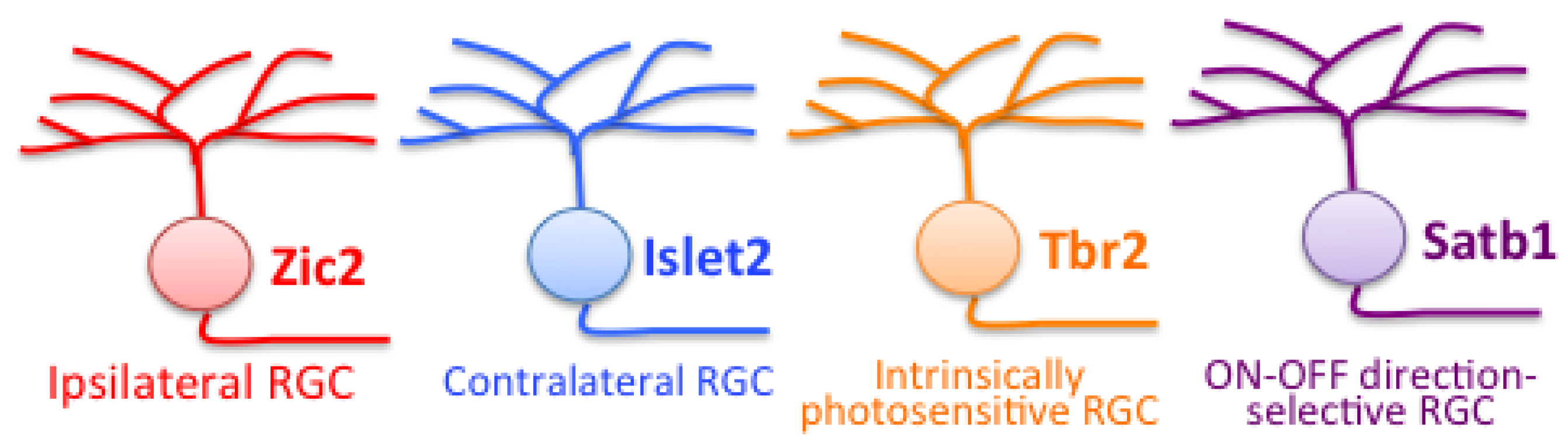
9.3. Molecular Criteria
9.4. Brain Targeting and Pathfinding at the Optic Chiasm
9.4.1. Brain Targeting
9.4.2. Pathfinding at the Optic Chiasm
10. Altered Neurogenesis in Albinism
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| bHLH | basic helic-loop-helix |
| ccnd2 | Cyclin-d2 |
| cKO CMZ | conditional knock-out ciliary marginal zone |
| FGF/Fgf | fibroblast growth factor |
| GCL | ganglion cell layer |
| Hh | hedgehog |
| INL | inner nuclear layer |
| MG | Müller glial cells |
| NGF | nerve growth factor |
| ONL | outer nuclear layer |
| Ptc | Patched |
| RGC | retinal ganglion cell |
| RPC | retinal progenitor cell |
| RPE | retinal pigmented epithelium |
| Shh | Sonic hedgehog |
| Smo | Smoothened |
| TF | transcription factor |
References
- Sanes, J.R.; Masland, R.H. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef]
- Rheaume, B.A.; Jereen, A.; Bolisetty, M.; Sajid, M.S.; Yang, Y.; Renna, K.; Sun, L.; Robson, P.; Trakhtenberg, E.F. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat. Commun. 2018, 9, 2759. [Google Scholar] [CrossRef]
- Turner, D.L.; Cepko, C.L. A common progenitor for neurons and glia persists in rat retina late in development. Nature 1987, 328, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.E.; Bertsch, T.W.; Ellis, H.M.; Harris, W.A. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron 1988, 1, 15–26. [Google Scholar] [CrossRef]
- Wetts, R.; Fraser, S.E. Multipotent precursors can give rise to all major cell types of the frog retina. Science 1988, 239, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Snyder, E.Y.; Cepko, C.L. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 1990, 4, 833–845. [Google Scholar] [CrossRef]
- Fekete, D.M.; Perez-Miguelsanz, J.; Ryder, E.F.; Cepko, C.L. Clonal Analysis in the Chicken Retina Reveals Tangential Dispersion of Clonally Related Cells. Dev. Biol. 1994, 166, 666–682. [Google Scholar] [CrossRef]
- Reh, T.; Kljavin, I. Age of differentiation determines rat retinal germinal cell phenotype: Induction of differentiation by dissociation. J. Neurosci. 1989, 9, 4179–4189. [Google Scholar] [CrossRef]
- Brzezinski, J.A.; Kim, E.J.; Johnson, J.E.; Reh, T.A. Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development 2011, 138, 3519–3531. [Google Scholar] [CrossRef]
- Sidman, R. Histogenesis of mouse retina studied with thymidine-H3. In The Structure of the Eye; Smelser, G., Ed.; Academic Press: New York, NY, USA, 1961; pp. 487–506. [Google Scholar]
- Carter-Dawson, L.D.; Lavail, M.M. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J. Comp. Neurol. 1979, 188, 263–272. [Google Scholar] [CrossRef]
- Young, R.W. Cell differentiation in the retina of the mouse. Anat. Rec. 1985, 212, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Prada, C.; Puga, J.; Perez-Mendez, L.; Lopez, R.; Ramirez, G. Spatial and Temporal Patterns of Neurogenesis in the Chick Retina. Eur. J. Neurosci. 1991, 3, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Easter, S.S. Retinal neurogenesis: The formation of the initial central patch of postmitotic cells. Dev. Biol. 1999, 207, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M. Retinal Ganglion Cells: Specification of Central Connections in Larval Xenopus laevis. Science 1967, 155, 1106–1108. [Google Scholar] [CrossRef]
- Drager, U.C. Birth Dates of Retinal Ganglion Cells Giving Rise to the Crossed and Uncrossed Optic Projections in the Mouse. Proc. R. Soc. B Biol. Sci. 1985, 224, 57–77. [Google Scholar]
- la Vail, M.M.; Rapaport, D.H.; Rakic, P. Cytogenesis in the monkey retina. J. Comp. Neurol. 1991, 309, 86–114. [Google Scholar] [CrossRef]
- Hoshino, A.; Ratnapriya, R.; Brooks, M.J.; Chaitankar, V.; Wilken, M.S.; Zhang, C.; Starostik, M.R.; Gieser, L.; La Torre, A.; Nishio, M.; et al. Molecular Anatomy of the Developing Human Retina. Dev. Cell 2017, 43, 763–779. [Google Scholar] [CrossRef]
- Cepko, C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat. Rev. Neurosci. 2014, 15, 615–627. [Google Scholar] [CrossRef]
- Austin, C.P.; Feldman, D.E.; Ida, J.A.; Cepko, C.L. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development 1995, 121, 3637–3650. [Google Scholar]
- Adler, R.; Hatlee, M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science 1989, 243, 391–393. [Google Scholar] [CrossRef]
- Watanabe, T.; Raff, M.C. Diffusible rod-promoting signals in the developing rat retina. Development 1992, 114, 899–906. [Google Scholar] [PubMed]
- Masai, I.; Stemple, D.L.; Okamoto, H.; Wilson, S.W. Midline Signals Regulate Retinal Neurogenesis in Zebrafish. Neuron 2000, 27, 251–263. [Google Scholar] [CrossRef]
- Belliveau, M.J.; Cepko, C.L. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 1999, 126, 555–566. [Google Scholar] [PubMed]
- Reh, T. Cell-specific regulation of neuronal production in the larval frog retina. J. Neurosci. 1987, 7, 3317–3324. [Google Scholar] [CrossRef]
- Waid, D.K.; McLoon, S.C. Ganglion cells influence the fate of dividing retinal cells in culture. Development 1998, 125, 1059–1066. [Google Scholar]
- Gonzalez-Hoyuela, M.; Barbas, J.A.; Rodriguez-Tebar, A. The autoregulation of retinal ganglion cell number. Development 2001, 128, 117–124. [Google Scholar]
- Frade, J.M.; Rodríguez-Tébar, A.; Barde, Y.A. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature 1996, 383, 166–168. [Google Scholar] [CrossRef]
- Harada, C.; Harada, T.; Nakamura, K.; Sakai, Y.; Tanaka, K.; Parada, L.F. Effect of p75NTR on the regulation of naturally occurring cell death and retinal ganglion cell number in the mouse eye. Dev. Biol. 2006, 290, 57–65. [Google Scholar] [CrossRef]
- Poggi, L.; Vitorino, M.; Masai, I.; Harris, W.A. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J. Cell Biol. 2005, 171, 991–999. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef]
- Louvi, A.; Artavanis-Tsakonas, S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006, 7, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Treisman, J.E. Retinal differentiation in Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, U.M.; Arias, A.M. Cell and molecular biology of Notch. J. Endocrinol. 2007, 194, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X.G. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef]
- Kageyama, R.; Ohtsuka, T.; Kobayashi, T. Roles of Hes genes in neural development. Dev. Growth Differ. 2008, 50, 97–103. [Google Scholar] [CrossRef]
- Nelson, B.R.; Hartman, B.H.; Ray, C.A.; Hayashi, T.; Bermingham-McDonogh, O.; Reh, T.A. Acheate-scute like 1 (Ascl1) is required for normal delta-like (Dll) gene expression and notch signaling during retinal development. Dev. Dyn. 2009, 238, 2163–2178. [Google Scholar] [CrossRef]
- Rocha, S.F.; Lopes, S.S.; Gossler, A.; Henrique, D. Dll1 and Dll4 function sequentially in the retina and pV2 domain of the spinal cord to regulate neurogenesis and create cell diversity. Dev. Biol. 2009, 328, 54–65. [Google Scholar] [CrossRef]
- Luo, H.; Jin, K.; Xie, Z.; Qiu, F.; Li, S.; Zou, M.; Cai, L.; Hozumi, K.; Shima, D.T.; Xiang, M. Forkhead box N4 (Foxn4) activates Dll4-Notch signaling to suppress photoreceptor cell fates of early retinal progenitors. Proc. Natl. Acad. Sci. USA 2012, 109, E553–E562. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Mason, H.A.; Cepko, C.L. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development 2006, 133, 913–923. [Google Scholar] [CrossRef]
- Yaron, O.; Farhy, C.; Marquardt, T.; Applebury, M.; Ashery-Padan, R. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development 2006, 133, 1367–1378. [Google Scholar] [CrossRef]
- Riesenberg, A.N.; Liu, Z.; Kopan, R.; Brown, N.L. Rbpj cell autonomous regulation of retinal ganglion cell and cone photoreceptor fates in the mouse retina. J. Neurosci. 2009, 29, 12865–12877. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.H.; Shi, M.; Pei, Z.; Gao, F.; Han, H.; Ding, Y.Q. The transcription factor RBP-J is essential for retinal cell differentiation and lamination. Mol. Brain 2009, 2, 38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomita, K.; Ishibashi, M.; Nakahara, K.; Ang, S.L.; Nakanishi, S.; Guillemot, F.; Kageyama, R. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron 1996, 16, 723–734. [Google Scholar] [CrossRef]
- Furukawa, T.; Mukherjee, S.; Bao, Z.Z.; Morrow, E.M.; Cepko, C.L. rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron 2000, 26, 383–394. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Cho, S.H.; Cepko, C.L. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc. Natl. Acad. Sci. USA 2006, 103, 18998–19003. [Google Scholar] [CrossRef]
- Henrique, D.; Hirsinger, E.; Adam, J.; Le Roux, I.; Pourquié, O.; Ish-Horowicz, D.; Lewis, J. Maintenance of neuroepithelial progenitor cells by Delta–Notch signalling in the embryonic chick retina. Curr. Biol. 1997, 7, 661–670. [Google Scholar] [CrossRef]
- Bao, Z.Z.; Cepko, C.L. The Expression and Function of Notch Pathway Genes in the Developing Rat Eye. J. Neurosci. 1997, 17, 1425–1434. [Google Scholar] [CrossRef]
- Ha, T.; Moon, K.H.; Dai, L.; Hatakeyama, J.; Yoon, K.; Park, H.S.S.; Kong, Y.Y.Y.; Shimamura, K.; Kim, J.W. The Retinal Pigment Epithelium Is a Notch Signaling Niche in the Mouse Retina. Cell Rep. 2017, 19, 351–363. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Gasperowicz, M.; Chow, R.L. The expression of NOTCH2, HES1 and SOX9 during mouse retinal development. Gene Expr. Patterns 2013, 13, 78–83. [Google Scholar] [CrossRef]
- Riesenberg, A.N.; Brown, N.L. Cell autonomous and nonautonomous requirements for Delltalike1 during early mouse retinal neurogenesis. Dev. Dyn. 2016, 245, 631–640. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, M.; Tonou-Fujimori, N.; Komori, A.; Maeda, R.; Nojima, Y.; Li, H.; Okamoto, H.; Masai, I. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways Masahiro. Development 2005, 132, 3027–3043. [Google Scholar] [CrossRef] [PubMed]
- Seritrakul, P.; Gross, J.M. Tet-mediated DNA hydroxymethylation regulates retinal neurogenesis by modulating cell-extrinsic signaling pathways. PLoS Genet. 2017, 13, e1006987. [Google Scholar] [CrossRef] [PubMed]
- Das, A.V.; James, J.; Bhattacharya, S.; Imbalzano, A.N.; Antony, M.L.; Hegde, G.; Zhao, X.; Mallya, K.; Ahmad, F.; Knudsen, E.; et al. SWI/SNF Chromatin Remodeling ATPase Brm Regulates the Differentiation of Early Retinal Stem Cells/Progenitors by Influencing Brn3b Expression and Notch Signaling. J. Biol. Chem. 2007, 282, 35187–35201. [Google Scholar] [CrossRef] [PubMed]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef]
- Marigo, V.; Davey, R.A.; Zuo, Y.; Cunningham, J.M.; Tabin, C.J. Biochemical evidence that Patched is the Hedgehog receptor. Nature 1996, 384, 176–179. [Google Scholar] [CrossRef]
- van den Heuvel, M.; Ingham, P.W. Smoothening the path for hedgehogs. Trends Cell Biol. 1996, 6, 451–453. [Google Scholar] [CrossRef]
- Murone, M.; Rosenthal, A.; de Sauvage, F.J. Sonic hedgehog signaling by the Patched–Smoothened receptor complex. Curr. Biol. 1999, 9, 76–84. [Google Scholar] [CrossRef]
- Dakubo, G.D.; Wang, Y.P.; Mazerolle, C.; Campsall, K.; McMahon, A.P.; Wallace, V.A. Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development. Development 2003, 130, 2967–2980. [Google Scholar] [CrossRef]
- Jensen, A.M.; Wallace, V.A. Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development 1997, 124, 363–371. [Google Scholar]
- Wang, Y.P.; Dakubo, G.; Howley, P.; Campsall, K.D.; Mazarolle, C.J.; Shiga, S.A.; Lewis, P.M.; McMahon, A.P.; Wallace, V.A. Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat. Neurosci. 2002, 5, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camacho, C.; Bovolenta, P. Autonomous and non-autonomous Shh signalling mediate the in vivo growth and guidance of mouse retinal ganglion cell axons. Development 2008, 135, 3531–3541. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.J.; Nuesslain-Volhard, C. Patterning of the Zebrafish Retina by a Wave of Sonic Hedgehog Activity. Science 2000, 289, 2137–2139. [Google Scholar] [CrossRef] [PubMed]
- Perron, M. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development 2003, 130, 1565–1577. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.M.M.; Yang, X.J.J. Regulation of retinal ganglion cell production by Sonic hedgehog. Development 2001, 128, 943–957. [Google Scholar]
- Levine, E.M.; Roelink, H.; Turner, J.; Reh, T.A. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J. Neurosci. 1997, 17, 6277–6288. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Dakubo, G.D.; Thurig, S.; Mazerolle, C.J.; Wallace, V.A. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development 2005, 132, 5103–5113. [Google Scholar] [CrossRef]
- Stenkamp, D.L.; Frey, R.A. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev. Biol. 2003, 258, 349–363. [Google Scholar] [CrossRef]
- Stenkamp, D.L.; Frey, R.A.; Prabhudesai, S.N.; Raymond, P.A. Function for Hedgehog Genes in Zebrafish Retinal Development. Dev. Biol. 2000, 220, 238–252. [Google Scholar] [CrossRef]
- Dakubo, G.D.; Wallace, V.A. Hedgehogs and retinal ganglion cells: Organizers of the mammalian retina. Neuroreport 2004, 15, 479–482. [Google Scholar] [CrossRef]
- McCabe, K.L.; Gunther, E.C.; Reh, T.A. The development of the pattern of retinal ganglion cells in the chick retina: Mechanisms that control differentiation. Development 1999, 126, 5713–5724. [Google Scholar] [PubMed]
- Patel, A.; McFarlane, S. Overexpression of FGF-2 alters cell fate specification in the developing retina of Xenopus laevis. Dev. Biol. 2000, 222, 170–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Morales, J.R.; Del Bene, F.; Nica, G.; Hammerschmidt, M.; Bovolenta, P.; Wittbrodt, J. Differentiation of the Vertebrate Retina Is Coordinated by an FGF Signaling Center. Dev. Cell 2005, 8, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Shkumatava, A.; Fischer, S.; Müller, F.; Strahle, U.; Neumann, C.J. Sonic hedgehog, secreted by amacrine cells, acts as a short-range signal to direct differentiation and lamination in the zebrafish retina. Development 2004, 131, 3849–3858. [Google Scholar] [CrossRef] [PubMed]
- Cepko, C.C.L.; Austin, C.P.; Yang, X.; Alexiades, M.; Ezzeddine, D. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA 1996, 93, 589–595. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Desplan, C. Temporal Patterning of Neural Progenitors in Drosophila. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 105, pp. 69–96. [Google Scholar]
- Watanabe, T.; Raff, M.C. Rod photoreceptor development in vitro: Intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron 1990, 4, 461–467. [Google Scholar] [CrossRef]
- Belliveau, M.J.; Young, T.L.; Cepko, C.L. Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis. J. Neurosci. 2000, 20, 2247–2254. [Google Scholar] [CrossRef]
- Gomes, F.L.A.F.; Zhang, G.; Carbonell, F.; Correa, J.A.; Harris, W.A.; Simons, B.D.; Cayouette, M. Reconstruction of rat retinal progenitor cell lineages in vitro reveals a surprising degree of stochasticity in cell fate decisions. Development 2011, 138, 227–235. [Google Scholar] [CrossRef]
- Cayouette, M.; Barres, B.A.; Raff, M. Importance of Intrinsic Mechanisms in Cell Fate Decisions in the Developing Rat Retina. Neuron 2003, 40, 897–904. [Google Scholar] [CrossRef]
- He, J.; Zhang, G.; Almeida, A.D.; Cayouette, M.; Simons, B.D.D.; Harris, W.A. How Variable Clones Build an Invariant Retina. Neuron 2012, 75, 786–798. [Google Scholar] [CrossRef]
- Elliott, J.; Jolicoeur, C.; Ramamurthy, V.; Cayouette, M. Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron 2008, 60, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Tarchini, B.; Jolicoeur, C.; Cayouette, M. In vivo evidence for unbiased Ikaros retinal lineages using an Ikaros-Cre mouse line driving clonal recombination. Dev. Dyn. 2012, 241, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Mattar, P.; Ericson, J.; Blackshaw, S.; Cayouette, M. A Conserved Regulatory Logic Controls Temporal Identity in Mouse Neural Progenitors. Neuron 2015, 85, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Boije, H.; MacDonald, R.B.; Harris, W.A. Reconciling competence and transcriptional hierarchies with stochasticity in retinal lineages. Curr. Opin. Neurobiol. 2014, 27, 68–74. [Google Scholar] [CrossRef]
- Swaroop, A.; Kim, D.; Forrest, D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci. 2010, 11, 563–576. [Google Scholar] [CrossRef]
- Marquardt, T.; Ashery-Padan, R.; Andrejewski, N.; Scardigli, R.; Guillemot, F.; Gruss, P. Pax6 Is Required for the Multipotent State of Retinal Progenitor Cells Genesis, RPCs Appear to Be Retained in a Progenitor State by the Action of Notch-Delta Signaling and Down-Stream Effectors of Notch, like the bHLH Transcription. Cell 2001, 105, 43–45. [Google Scholar]
- Burmeister, M.; Novak, J.; Liang, M.Y.; Basu, S.; Ploder, L.; Hawes, N.L.; Vidgen, D.; Hoover, F.; Goldman, D.; Kalnins, V.I.; et al. Ocular retardation mouse caused by Chx10 homeobox null allele: Impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 1996, 12, 376–384. [Google Scholar] [CrossRef]
- Vitorino, M.; Jusuf, P.R.; Maurus, D.; Kimura, Y.; Higashijima, S.I.; Harris, W.A. Vsx2 in the zebrafish retina: Restricted lineages through derepression. Neural Dev. 2009, 4, 14. [Google Scholar] [CrossRef]
- Brown, N.L.; Kanekar, S.; Vetter, M.L.; Tucker, P.K.; Gemza, D.L.; Glaser, T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development 1998, 125, 4821–4833. [Google Scholar]
- Wang, S.W.; Kim, B.S.; Ding, K.; Wang, H.; Sun, D.; Johnson, R.L.; Klein, W.H.; Gan, L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001, 15, 24–29. [Google Scholar] [CrossRef]
- Brown, N.L.; Patel, S.; Brzezinski, J.; Glaser, T. Math5 is required for retinal ganglion cell and optic nerve formation. Development 2001, 128, 2497–2508. [Google Scholar]
- Le, T.T.; Wroblewski, E.; Patel, S.; Riesenberg, A.N.; Brown, N.L. Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev. Biol. 2006, 295, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.N.; Finger-Baier, K.C.; Roeser, T.; Staub, W.; Baier, H. Retinal ganglion cell genesis requires lakritz, a zebrafish atonal homolog. Neuron 2001, 30, 725–736. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, K.; Pan, L.; Deng, M.; Gan, L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev. Biol. 2003, 264, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xie, Z.; Ding, Q.; Xie, X.; Libby, R.T.; Gan, L. MATH5 controls the acquisition of multiple retinal cell fates. Mol. Brain 2010, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, J.A.; Prasov, L.; Glaser, T. Math5 defines the ganglion cell competence state in a subpopulation of retinal progenitor cells exiting the cell cycle. Dev. Biol. 2012, 365, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Matter-Sadzinski, L.; Skowronska-Krawczyk, D.; Chiodini, F.; Alliod, C.; Ballivet, M.; Matter, J.M. Highly conserved sequences mediate the dynamic interplay of basic helix-loop-helix proteins regulating retinogenesis. J. Biol. Chem. 2007, 282, 37894–37905. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mo, Z.; Xiang, M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc. Natl. Acad. Sci. USA 2001, 98, 1649–1654. [Google Scholar] [CrossRef]
- Pan, L.; Yang, Z.; Feng, L.; Gan, L. Functional equivalence of Brn3 POU-domain transcription factors in mouse retinal neurogenesis. Development 2005, 132, 703–712. [Google Scholar] [CrossRef][Green Version]
- Gan, L.; Xiang, M.; Zhou, L.; Wagner, D.S.; Klein, W.H.; Nathans, J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1996, 93, 3920–3925. [Google Scholar] [CrossRef]
- Xiang, M.; Zhou, L.; Macke, J.P.; Yoshioka, T.; Hendry, S.H.C.; Eddy, R.L.; Shows, T.B.; Nathans, J. The Brn-3 family of POU-domain factors: Primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J. Neurosci. 1995, 15, 4762–4785. [Google Scholar] [CrossRef]
- Erkman, L.; McEvilly, R.J.; Luo, L.; Ryan, A.K.; Hooshmand, F.; O’Connell, S.M.; Keithley, E.M.; Rapaport, D.H.; Ryan, A.F.; Rosenfeld, M.G. Role of transcription factors a Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 1996, 381, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wang, S.W.; Huang, Z.; Klein, W.H. POU Domain Factor Brn-3b Is Essential for Retinal Ganglion Cell Differentiation and Survival but Not for Initial Cell Fate Specification. Dev. Biol. 1999, 210, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Beremand, P.D.; Zhao, S.; Pershad, R.; Sun, H.; Scarpa, A.; Liang, S.; Thomas, T.L.; Klein, W.H. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development 2004, 131, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Jiang, H.; Xiang, M. A comprehensive negative regulatory program controlled by Brn3b to ensure ganglion cell specification from multipotential retinal precursors. J. Neurosci. 2008, 28, 3392–3403. [Google Scholar] [CrossRef]
- Xiang, M. Requirement for Brn-3b in early differentiation of postmitotic retinal ganglion cell precursors. Dev. Biol. 1998, 197, 155–169. [Google Scholar] [CrossRef]
- Wang, S.W.; Mu, X.; Bowers, W.J.; Kim, D.S.; Plas, D.J.; Crair, M.C.; Federoff, H.J.; Gan, L.; Klein, W.H.; Wang, S.W.; et al. Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth. Development 2002, 129, 467–477. [Google Scholar]
- Badea, T.C.; Cahill, H.; Ecker, J.; Hattar, S.; Nathans, J. Distinct Roles of Transcription Factors Brn3a and Brn3b in Controlling the Development, Morphology, and Function of Retinal Ganglion Cells. Neuron 2009, 61, 852–864. [Google Scholar] [CrossRef]
- Mao, C.A.A.; Wang, S.W.; Pan, P.; Klein, W.H. Rewiring the retinal ganglion cell gene regulatory network: Neurod1 promotes retinal ganglion cell fate in the absence of Math5. Development 2008, 135, 3379–3388. [Google Scholar] [CrossRef]
- Mu, X.; Fu, X.; Beremand, P.D.; Thomas, T.L.; Klein, W.H. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc. Natl. Acad. Sci. USA 2008, 105, 6942–6947. [Google Scholar] [CrossRef]
- Pan, L.; Deng, M.; Xie, X.; Gan, L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development 2008, 135, 1981–1990. [Google Scholar] [CrossRef]
- Elshatory, Y.; Deng, M.; Xie, X.; Gan, L. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J. Comp. Neurol. 2007, 503, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Elshatory, Y.; Everhart, D.; Deng, M.; Xie, X.; Barlow, R.B.; Gan, L. Islet-1 Controls the Differentiation of Retinal Bipolar and Cholinergic Amacrine Cells. J. Neurosci. 2007, 27, 12707–12720. [Google Scholar] [CrossRef] [PubMed]
- Erkman, L.; Yates, P.A.; McLaughlin, T.; McEvilly, R.J.; Whisenhunt, T.; O’Connell, S.M.; Krones, A.I.; Kirby, M.A.; Rapaport, D.H.; Bermingham, J.R.; et al. A POU Domain Transcription Factor-Dependent Program Regulates Axon Pathfinding in the Vertebrate Visual System. Neuron 2000, 28, 779–792. [Google Scholar] [CrossRef]
- Wu, F.; Kaczynski, T.J.; Sethuramanujam, S.; Li, R.; Jain, V.; Slaughter, M.; Mu, X. Two transcription factors, Pou4f2 and Isl1, are sufficient to specify the retinal ganglion cell fate. Proc. Natl. Acad. Sci. USA 2015, 112, E1559–E1568. [Google Scholar] [CrossRef]
- Del Bene, F.; Ettwiller, L.; Skowronska-Krawczyk, D.; Baier, H.; Matter, J.M.; Birney, E.; Wittbrodt, J. In Vivo Validation of a Computationally Predicted Conserved Ath5 Target Gene Set. PLoS Genet. 2007, 3, e159. [Google Scholar] [CrossRef]
- de Melo, J.; Du, G.; Fonseca, M.; Gillespie, L.A.; Turk, W.J.; Rubenstein, J.L.R.; Eisenstat, D.D. Dlx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development 2005, 132, 311–322. [Google Scholar] [CrossRef]
- Zhang, Q.; Zagozewski, J.; Cheng, S.; Dixit, R.; Zhang, S.; de Melo, J.; Mu, X.; Klein, W.H.; Brown, N.L.; Wigle, J.T.; et al. Regulation of Brn3b by DLX1 and DLX2 is required for retinal ganglion cell differentiation in the vertebrate retina. Development 2017, 144, 1698–1711. [Google Scholar] [CrossRef]
- Usui, A.; Mochizuki, Y.; Iida, A.; Miyauchi, E.; Satoh, S.; Sock, E.; Nakauchi, H.; Aburatani, H.; Murakami, A.; Wegner, M.; et al. The early retinal progenitor-expressed gene Sox11 regulates the timing of the differentiation of retinal cells. Development 2013, 140, 740–750. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, Q.; Xie, X.; Libby, R.T.; Lefebvre, V.; Gan, L. Transcription Factors SOX4 and SOX11 Function Redundantly to Regulate the Development of Mouse Retinal Ganglion Cells. J. Biol. Chem. 2013, 288, 18429–18438. [Google Scholar] [CrossRef]
- Gao, Z.; Mao, C.A.; Pan, P.; Mu, X.; Klein, W.H. Transcriptome of Atoh7 retinal progenitor cells identifies new Atoh7-dependent regulatory genes for retinal ganglion cell formation. Dev. Neurobiol. 2014, 74, 1123–1140. [Google Scholar] [CrossRef]
- Clark, B.S.; Stein-O’Brien, G.L.; Shiau, F.; Cannon, G.H.; Davis-Marcisak, E.; Sherman, T.; Santiago, C.P.; Hoang, T.V.; Rajaii, F.; James-Esposito, R.E.; et al. Single-Cell RNA-Seq Analysis of Retinal Development Identifies NFI Factors as Regulating Mitotic Exit and Late-Born Cell Specification. Neuron 2019, 102, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, Q.; Leleu, M.; La Manno, G.; Fabre, P.J. Single-cell transcriptional logic of cell-fate specification and axon guidance in early born retinal neurons. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, X.; Hu, B.; Mao, Y.; Chen, Y.; Yan, L.; Yong, J.; Dong, J.; Wei, Y.; Wang, W.; et al. Dissecting the transcriptome landscape of the human fetal neural retina and retinal pigment epithelium by single-cell RNA-seq analysis. PLoS Biol. 2019, 17, e3000365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lufkin, T.; Gelb, B.D. Expression of Tfap2d, the gene encoding the transcription factor Ap-2δ, during mouse embryogenesis. Gene Expr. Patterns 2003, 3, 213–217. [Google Scholar] [CrossRef]
- Reichman, S.; Terray, A.; Slembrouck, A.; Nanteau, C.; Orieux, G.; Habeler, W.; Nandrot, E.F.; Sahel, J.A.; Monville, C.; Goureau, O. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc. Natl. Acad. Sci. USA 2014, 111, 8518–8523. [Google Scholar] [CrossRef] [PubMed]
- Reh, T.A.; Hindges, R. MicroRNAs in Retinal Development. Annu. Rev. Vis. Sci. 2018, 4, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, N.A.; Hindges, R. Non-Coding RNAs in Retinal Development. Int. J. Mol. Sci. 2012, 13, 558–578. [Google Scholar] [CrossRef]
- Decembrini, S.; Andreazzoli, M.; Barsacchi, G.; Cremisi, F. Dicer inactivation causes heterochronic retinogenesis in Xenopus laevis. Int. J. Dev. Biol. 2008, 52, 1099–1103. [Google Scholar] [CrossRef]
- Georgi, S.A.; Reh, T.A. Dicer Is Required for the Transition from Early to Late Progenitor State in the Developing Mouse Retina. J. Neurosci. 2010, 30, 4048–4061. [Google Scholar] [CrossRef]
- Georgi, S.A.; Reh, T.A. Dicer is required for the maintenance of notch signaling and gliogenic competence during mouse retinal development. Dev. Neurobiol. 2011, 71, 1153–1169. [Google Scholar] [CrossRef]
- Davis, N.; Mor, E.; Ashery-Padan, R. Roles for Dicer1 in the patterning and differentiation of the optic cup neuroepithelium. Development 2011, 138, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Shinoe, T.; Baba, Y.; Mano, H.; Watanabe, S. Dicer plays essential roles for retinal development by regulation of survival and differentiation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Georgi, S.; Reh, T.A. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, V.A.; Sreekanth, S.; Dhanesh, S.B.; Divya, M.S.; Divya, T.S.; Akhila, P.K.; Subashini, C.; Chandrika Sivakumar, K.; Das, A.V.; James, J. Developmental wave of Brn3b expression leading to RGC fate specification is synergistically maintained by miR-23a and miR-374. Dev. Neurobiol. 2014, 74, 1155–1171. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Pinter, R.; Hindges, R. Perturbations of MicroRNA Function in Mouse Dicer Mutants Produce Retinal Defects and Lead to Aberrant Axon Pathfinding at the Optic Chiasm. PLoS ONE 2010, 5, e10021. [Google Scholar] [CrossRef]
- Seritrakul, P.; Gross, J.M. Genetic and epigenetic control of retinal development in zebrafish. Curr. Opin. Neurobiol. 2019, 59, 120–127. [Google Scholar] [CrossRef]
- Fischer, A.J.; Reh, T.A. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev. Biol. 2000, 220, 197–210. [Google Scholar] [CrossRef]
- Johns, P.R. Growth of the adult goldfish eye. III. Source of the new retinal cells. J. Comp. Neurol. 1977, 176, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Straznicky, K.; Gaze, R.M. The growth of the retina in Xenopus laevis: An autoradiographic study. J. Embryol. Exp. Morph. 1971, 26, 67–79. [Google Scholar] [PubMed]
- Fischer, A.J.; Bosse, J.L.; El-Hodiri, H.M. The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp. Eye Res. 2013, 116, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, P.; Raymond, P. Retinal regeneration. Trends Neurosci. 1992, 15, 103–108. [Google Scholar] [CrossRef]
- Hollyfield, J.G. Differential growth of the neural retina inXenopus laevis larvae. Dev. Biol. 1971, 24, 264–286. [Google Scholar] [CrossRef]
- Livesey, F.J.; Cepko, C.L.C. Vertebrate neural cell-fate determination: Lessons from the retina. Nat. Rev. Neurosci. 2001, 2, 109–118. [Google Scholar] [CrossRef]
- Davis, N.; Yoffe, C.; Raviv, S.; Antes, R.; Berger, J.; Holzmann, S.; Stoykova, A.; Overbeek, P.A.; Tamm, E.R.; Ashery-Padan, R. Pax6 dosage requirements in iris and ciliary body differentiation. Dev. Biol. 2009, 333, 132–142. [Google Scholar] [CrossRef]
- Marcucci, F.; Murcia-Belmonte, V.; Wang, Q.; Coca, Y.; Ferreiro-Galve, S.; Kuwajima, T.; Khalid, S.; Ross, M.E.; Mason, C.; Herrera, E. The Ciliary Margin Zone of the Mammalian Retina Generates Retinal Ganglion Cells. Cell Rep. 2016, 17, 3153–3164. [Google Scholar] [CrossRef]
- Bélanger, M.C.C.; Robert, B.; Cayouette, M. Msx1-Positive Progenitors in the Retinal Ciliary Margin Give Rise to Both Neural and Non-neural Progenies in Mammals. Dev. Cell 2017, 40, 137–150. [Google Scholar] [CrossRef]
- Morest, D.K. The pattern of neurogenesis in the retina of the rat. Z. Anat. Entwickl. 1970, 131, 45–67. [Google Scholar] [CrossRef]
- Hinds, J.W.; Hinds, P.L. Early ganglion cell differentiation in the mouse retina: An electron microscopic analysis utilizing serial sections. Dev. Biol. 1974, 37, 381–416. [Google Scholar] [CrossRef]
- McLoon, S.C.; Barnes, R.B. Early differentiation of retinal ganglion cells: An axonal protein expressed by premigratory and migrating retinal ganglion cells. J. Neurosci. 1989, 9, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Zolessi, F.R.; Poggi, L.; Wilkinson, C.J.; Chien, C.B.; Harris, W.A. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 2006, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Icha, J.; Kunath, C.; Rocha-Martins, M.; Norden, C. Independent modes of ganglion cell translocation ensure correct lamination of the zebrafish retina. J. Cell Biol. 2016, 215, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Riccomagno, M.M.; Sun, L.O.; Brady, C.M.; Alexandropoulos, K.; Seo, S.; Kurokawa, M.; Kolodkin, A.L. Cas Adaptor Proteins Organize the Retinal Ganglion Cell Layer Downstream of Integrin Signaling. Neuron 2014, 81, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Amini, R.; Rocha-Martins, M.; Norden, C. Neuronal Migration and Lamination in the Vertebrate Retina. Front. Neurosci. 2018, 11, 742. [Google Scholar] [CrossRef]
- Barnstable, C.J.; Dräger, U.C. Thy-1 antigen: A ganglion cell specific marker in rodent retina. Neuroscience 1984, 11, 847–855. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; de Sevilla Müller, L.P.; Brecha, N.C. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef]
- Ramón y Cajal, S. La rétine des vertébrés. Cellule 1892, 1, 119–257. [Google Scholar]
- Baden, T.; Berens, P.; Franke, K.; Román Rosón, M.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef]
- Helmstaedter, M.; Briggman, K.L.; Turaga, S.C.; Jain, V.; Seung, H.S.; Denk, W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 2013, 500, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.; van Der List, D.; Wang, G.Y.; Chalupa, L.M. Morphological properties of mouse retinal ganglion cells. Neuroscience 2006, 140, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.H.; Fish, D.R.; Rockhill, R.L.; Masland, R.H. Diversity of ganglion cells in the mouse retina: Unsupervised morphological classification and its limits. J. Comp. Neurol. 2005, 489, 293–310. [Google Scholar] [CrossRef]
- Badea, T.C.; Nathans, J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J. Comp. Neurol. 2004, 480, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Völgyi, B.; Chheda, S.; Bloomfield, S.A. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J. Comp. Neurol. 2009, 512, 664–687. [Google Scholar] [CrossRef] [PubMed]
- Sümbül, U.; Song, S.; McCulloch, K.; Becker, M.; Lin, B.; Sanes, J.R.; Masland, R.H.; Seung, H.S. A genetic and computational approach to structurally classify neuronal types. Nat. Commun. 2014, 5, 3512. [Google Scholar] [CrossRef]
- Weng, S.; Sun, W.; He, S. Identification of ON-OFF direction-selective ganglion cells in the mouse retina. J. Physiol. 2005, 562, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Deng, Q.; Levick, W.R.; He, S. ON direction-selective ganglion cells in the mouse retina. J. Physiol. 2006, 576, 197–202. [Google Scholar] [CrossRef]
- Pang, J.J.; Gao, F.; Wu, S.M. Light-Evoked Excitatory and Inhibitory Synaptic Inputs to ON and OFF α Ganglion Cells in the Mouse Retina. J. Neurosci. 2003, 23, 6063–6073. [Google Scholar] [CrossRef]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Jacoby, J.; Schwartz, G.W. Three Small-Receptive-Field Ganglion Cells in the Mouse Retina Are Distinctly Tuned to Size, Speed, and Object Motion. J. Neurosci. 2017, 37, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, I.J.; Sanes, J.R.; Meister, M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc. Natl. Acad. Sci. USA 2012, 109, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Zhang, Y.; Yamagata, M.; Meister, M.; Sanes, J.R. Molecular identification of a retinal cell type that responds to upward motion. Nature 2008, 452, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Vlasits, A.L.; Euler, T.; Franke, K. Function first: Classifying cell types and circuits of the retina. Curr. Opin. Neurobiol. 2019, 56, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, N.T.; James, K.N.; Nistorica, A.; Lorig-Roach, R.M.; Feldheim, D.A. Expression of transcription factors divides retinal ganglion cells into distinct classes. J. Comp. Neurol. 2019, 527, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Triplett, J.W.; Wei, W.; Gonzalez, C.; Sweeney, N.T.; Huberman, A.D.; Feller, M.B.; Feldheim, D.A. Dendritic and axonal targeting patterns of a genetically-specified class of retinal ganglion cells that participate in image-forming circuits. Neural Dev. 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.R.R.; Tran, N.M.; Krishnaswamy, A.; Kostadinov, D.; Martersteck, E.M.; Sanes, J.R. Satb1 Regulates Contactin 5 to Pattern Dendrites of a Mammalian Retinal Ganglion Cell. Neuron 2017, 95, 869–883. [Google Scholar] [CrossRef]
- Mao, C.A.; Li, H.; Zhang, Z.; Kiyama, T.; Panda, S.; Hattar, S.; Ribelayga, C.P.; Mills, S.L.; Wang, S.W. T-box Transcription Regulator Tbr2 Is Essential for the Formation and Maintenance of Opn4/Melanopsin-Expressing Intrinsically Photosensitive Retinal Ganglion Cells. J. Neurosci. 2014, 34, 13083–13095. [Google Scholar] [CrossRef]
- Sweeney, N.T.; Tierney, H.; Feldheim, D.A. Tbr2 Is Required to Generate a Neural Circuit Mediating the Pupillary Light Reflex. J. Neurosci. 2014, 34, 5447–5453. [Google Scholar] [CrossRef]
- Laboissonniere, L.A.; Goetz, J.J.; Martin, G.M.; Bi, R.; Lund, T.J.S.; Ellson, L.; Lynch, M.R.; Mooney, B.; Wickham, H.; Liu, P.; et al. Molecular signatures of retinal ganglion cells revealed through single cell profiling. Sci. Rep. 2019, 9, 15778. [Google Scholar] [CrossRef]
- Klingler, E.; Prados, J.; Kebschull, J.M.; Dayer, A.; Zador, A.M.; Jabaudon, D. Single-cell molecular connectomics of intracortically-projecting neurons. bioRxiv 2018. [Google Scholar] [CrossRef]
- Morin, L.P.; Studholme, K.M. Retinofugal projections in the mouse. J. Comp. Neurol. 2014, 522, 3733–3753. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Kim, I.J.; Sanes, J.R. Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. J. Comp. Neurol. 2011, 519, 1691–1711. [Google Scholar] [CrossRef]
- Dhande, O.S.; Huberman, A.D. Retinal ganglion cell maps in the brain: Implications for visual processing. Curr. Opin. Neurobiol. 2014, 24, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dhande, O.S.; Stafford, B.K.; Lim, J.H.A.; Huberman, A.D. Contributions of Retinal Ganglion Cells to Subcortical Visual Processing and Behaviors. Annu. Rev. Vis. Sci. 2015, 1, 291–328. [Google Scholar] [CrossRef] [PubMed]
- Osterhout, J.A.; El-Danaf, R.N.; Nguyen, P.L.; Huberman, A.D. Birthdate and Outgrowth Timing Predict Cellular Mechanisms of Axon Target Matching in the Developing Visual Pathway. Cell Rep. 2014, 8, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Prieur, D.S.; Rebsam, A. Retinal axon guidance at the midline: Chiasmatic misrouting and consequences. Dev. Neurobiol. 2017, 77, 844–860. [Google Scholar] [CrossRef]
- Murcia-Belmonte, V.; Erskine, L.; Murcia-Belmonte, V.; Erskine, L. Wiring the Binocular Visual Pathways. Int. J. Mol. Sci. 2019, 20, 3282. [Google Scholar] [CrossRef]
- Rompani, S.B.; Müllner, F.E.; Wanner, A.; Zhang, C.; Roth, C.N.; Yonehara, K.; Roska, B. Different Modes of Visual Integration in the Lateral Geniculate Nucleus Revealed by Single-Cell-Initiated Transsynaptic Tracing. Neuron 2017, 93, 767–776. [Google Scholar] [CrossRef]
- Pak, W.; Hindges, R.; Lim, Y.S.; Pfaff, S.L.; O’leary, D.D.M. Magnitude of Binocular Vision Controlled by Islet-2 Repression of a Genetic Program that Specifies Laterality of Retinal Axon Pathfinding. Cell 2004, 119, 567–578. [Google Scholar] [CrossRef]
- Kuwajima, T.; Soares, C.A.; Sitko, A.A.; Lefebvre, V.; Mason, C. SoxC Transcription Factors Promote Contralateral Retinal Ganglion Cell Differentiation and Axon Guidance in the Mouse Visual System. Neuron 2017, 93, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Kuwajima, T.; Yoshida, Y.; Takegahara, N.; Petros, T.J.; Kumanogoh, A.; Jessell, T.M.; Sakurai, T.; Mason, C. Optic Chiasm Presentation of Semaphorin6D in the Context of Plexin-A1 and Nr-CAM Promotes Retinal Axon Midline Crossing. Neuron 2012, 74, 676–690. [Google Scholar] [CrossRef] [PubMed]
- García-Frigola, C.; Carreres, M.I.; Vegar, C.; Mason, C.A.; Herrera, E. Zic2 promotes axonal divergence at the optic chiasm midline by EphB1-dependent and -independent mechanisms. Development 2008, 135, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- García-Frigola, C.; Herrera, E. Zic2 regulates the expression of Sert to modulate eye-specific refinement at the visual targets. EMBO J. 2010, 29, 3170–3183. [Google Scholar] [CrossRef]
- Herrera, E.; Brown, L.; Aruga, J.; Rachel, R.A.; Dolen, G.; Mikoshiba, K.; Brown, S.; Mason, C.A. Zic2 Patterns Binocular Vision by Specifying the Uncrossed Retinal Projection. Cell 2003, 114, 545–557. [Google Scholar] [CrossRef]
- Williams, S.E.; Mann, F.; Erskine, L.; Sakurai, T.; Wei, S.; Rossi, D.J.; Gale, N.W.; Holt, C.E.; Mason, C.A.; Henkemeyer, M. Ephrin-B2 and EphB1 Mediate Retinal Axon Divergence at the Optic Chiasm. Neuron 2003, 39, 919–935. [Google Scholar] [CrossRef]
- Upton, A.L.; Salichon, N.; Lebrand, C.; Ravary, A.; Blakely, R.; Seif, I.; Gaspar, P. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: Possible role of 5-HT uptake in retinal ganglion cells during development. J. Neurosci. 1999, 19, 7007–7024. [Google Scholar] [CrossRef]
- Upton, A.L.; Ravary, A.; Salichon, N.; Moessner, R.; Lesch, K.P.; Hen, R.; Seif, I.; Gaspar, P. Lack of 5-HT1B receptor and of serotonin transporter have different effects on the segregation of retinal axons in the lateral geniculate nucleus compared to the superior colliculus. Neuroscience 2002, 111, 597–610. [Google Scholar] [CrossRef]
- Petros, T.J.; Shrestha, B.R.; Mason, C.A. Specificity and sufficiency of EphB1 in driving the ipsilateral retinal projection. J. Neurosci. 2009, 29, 3463–3474. [Google Scholar] [CrossRef]
- Wang, Q.; Marcucci, F.; Cerullo, I.; Mason, C. Ipsilateral and Contralateral Retinal Ganglion Cells Express Distinct Genes during Decussation at the Optic Chiasm. eNeuro 2016, 3. [Google Scholar] [CrossRef]
- Herrera, E.; Marcus, R.; Li, S.; Williams, S.E.; Erskine, L.; Lai, E.; Mason, C. Foxd1 is required for proper formation of the optic chiasm. Development 2004, 131, 5727–5739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carreres, M.I.; Escalante, A.; Murillo, B.; Chauvin, G.; Gaspar, P.; Vegar, C.; Herrera, E. Transcription Factor Foxd1 Is Required for the Specification of the Temporal Retina in Mammals. J. Neurosci. 2011, 31, 5673–5681. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arrones, L.; Nieto-Lopez, F.; Sanchez-Camacho, C.; Carreres, M.I.; Herrera, E.; Okada, A.; Bovolenta, P. Shh/Boc Signaling Is Required for Sustained Generation of Ipsilateral Projecting Ganglion Cells in the Mouse Retina. J. Neurosci. 2013, 33, 8596–8607. [Google Scholar] [CrossRef] [PubMed]
- Abadi, R.; Pascal, E. The recognition and management of albinism. Ophthalmic Physiol. Opt. 1989, 9, 3–15. [Google Scholar] [CrossRef]
- Apkarian, P. Chiasmal crossing defects in disorders of binocular vision. Eye 1996, 10, 222–232. [Google Scholar] [CrossRef]
- Holder, G.E. Electrophysiological assessment of optic nerve disease. Eye 2004, 18, 1133–1143. [Google Scholar] [CrossRef]
- von dem Hagen, E.A.H.; Hoffmann, M.B.; Morland, A.B. Identifying human albinism: A comparison of VEP and fMRI. Investig. Ophthalmol. Vis. Sci. 2008, 49, 238–249. [Google Scholar] [CrossRef][Green Version]
- Ilia, M.; Jeffery, G. Delayed neurogenesis in the albino retina: Evidence of a role for melanin in regulating the pace of cell generation. Brain Res. Dev. Brain Res. 1996, 95, 176–183. [Google Scholar] [CrossRef]
- Bhansali, P.; Rayport, I.; Rebsam, A.; Mason, C. Delayed neurogenesis leads to altered specification of ventrotemporal retinal ganglion cells in albino mice. Neural Dev. 2014, 9, 11. [Google Scholar] [CrossRef]
- Jeffery, G.; Brem, G.; Montoliu, L. Correction of retinal abnormalities found in albinism by introduction of a functional tyrosinase gene in transgenic mice and rabbits. Dev. Brain Res. 1997, 99, 95–102. [Google Scholar] [CrossRef]
- Averaimo, S.; Assali, A.; Ros, O.; Couvet, S.; Zagar, Y.; Genescu, I.; Rebsam, A.; Nicol, X. A plasma membrane microdomain compartmentalizes ephrin-generated cAMP signals to prune developing retinal axon arbors. Nat. Commun. 2016, 7, 12896. [Google Scholar] [CrossRef] [PubMed]
- Rebsam, A.; Bhansali, P.; Mason, C.A. Eye-Specific Projections of Retinogeniculate Axons Are Altered in Albino Mice. J. Neurosci. 2012, 32, 4821–4826. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Takekoshi, L.; Balasubramanian, R.; Sitko, A.; Khan, R.; Weinreb, S.; Robinson, K.; Mason, C. Activation of Wnt signaling reduces ipsilaterally projecting retinal ganglion cells in pigmented retina. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Llorca, A.; Ciceri, G.; Beattie, R.; Wong, F.K.; Diana, G.; Serafeimidou-Pouliou, E.; Fernández-Otero, M.; Streicher, C.; Arnold, S.J.; Meyer, M.; et al. A stochastic framework of neurogenesis underlies the assembly of neocortical cytoarchitecture. Elife 2019, 8, e51381. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen-Ba-Charvet, K.T.; Rebsam, A. Neurogenesis and Specification of Retinal Ganglion Cells. Int. J. Mol. Sci. 2020, 21, 451. https://doi.org/10.3390/ijms21020451
Nguyen-Ba-Charvet KT, Rebsam A. Neurogenesis and Specification of Retinal Ganglion Cells. International Journal of Molecular Sciences. 2020; 21(2):451. https://doi.org/10.3390/ijms21020451
Chicago/Turabian StyleNguyen-Ba-Charvet, Kim Tuyen, and Alexandra Rebsam. 2020. "Neurogenesis and Specification of Retinal Ganglion Cells" International Journal of Molecular Sciences 21, no. 2: 451. https://doi.org/10.3390/ijms21020451
APA StyleNguyen-Ba-Charvet, K. T., & Rebsam, A. (2020). Neurogenesis and Specification of Retinal Ganglion Cells. International Journal of Molecular Sciences, 21(2), 451. https://doi.org/10.3390/ijms21020451



