25-Hydroxycholesterol Inhibits Adipogenic Differentiation of C3H10T1/2 Pluripotent Stromal Cells
Abstract
1. Introduction
2. Results
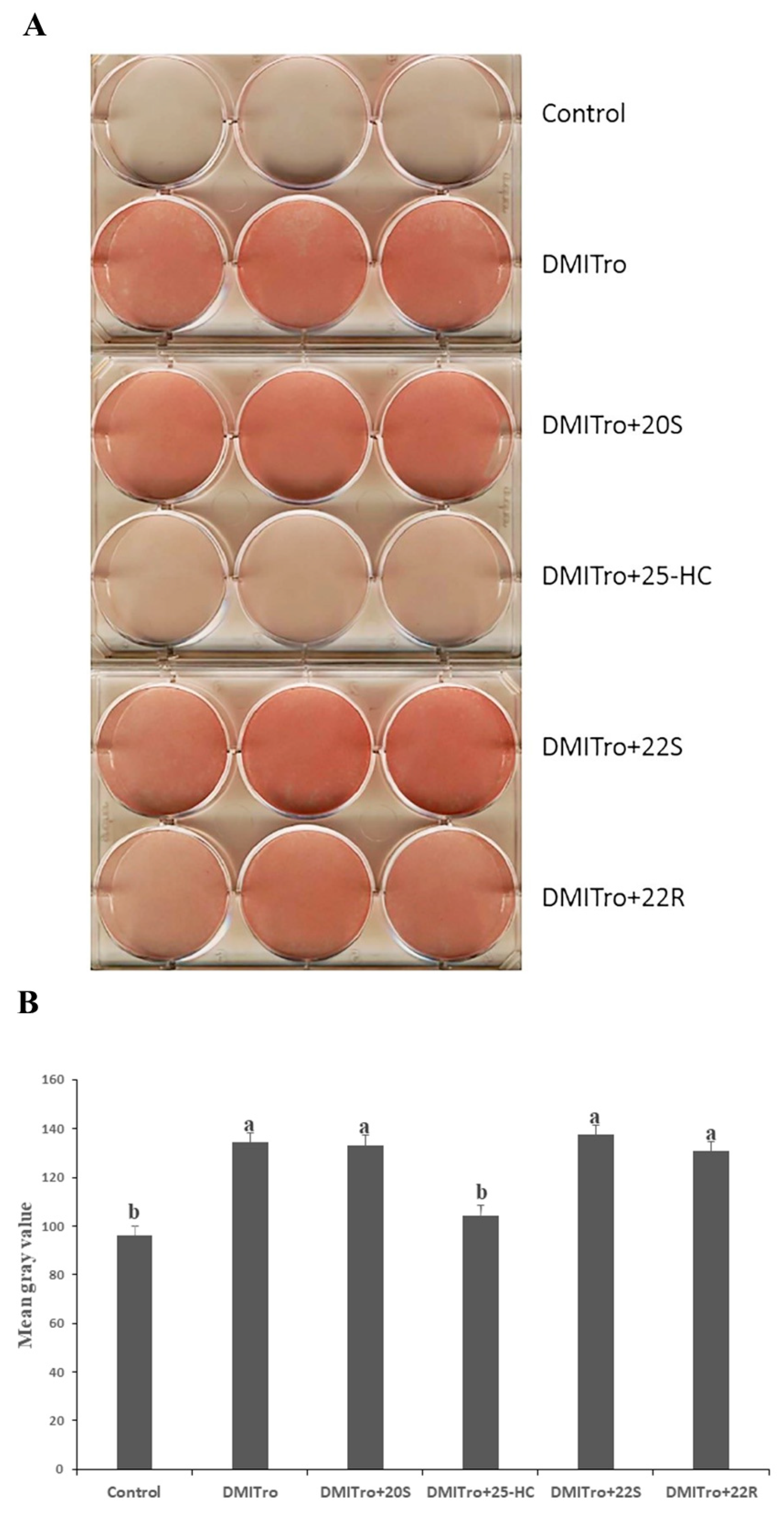
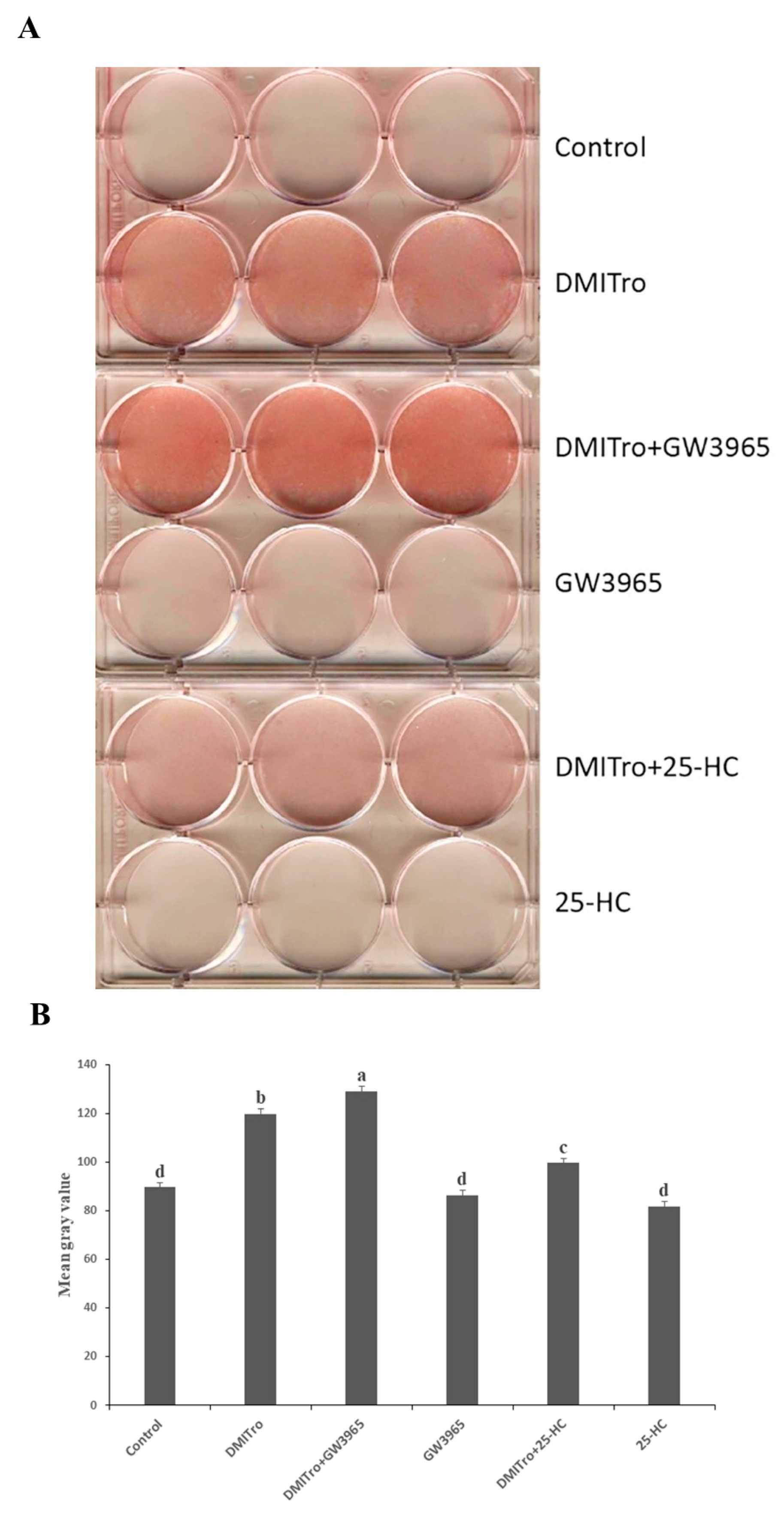
2.1. Effects of Different Oxysterols on Lipid Accumulation in C3H10T1/2 Cells
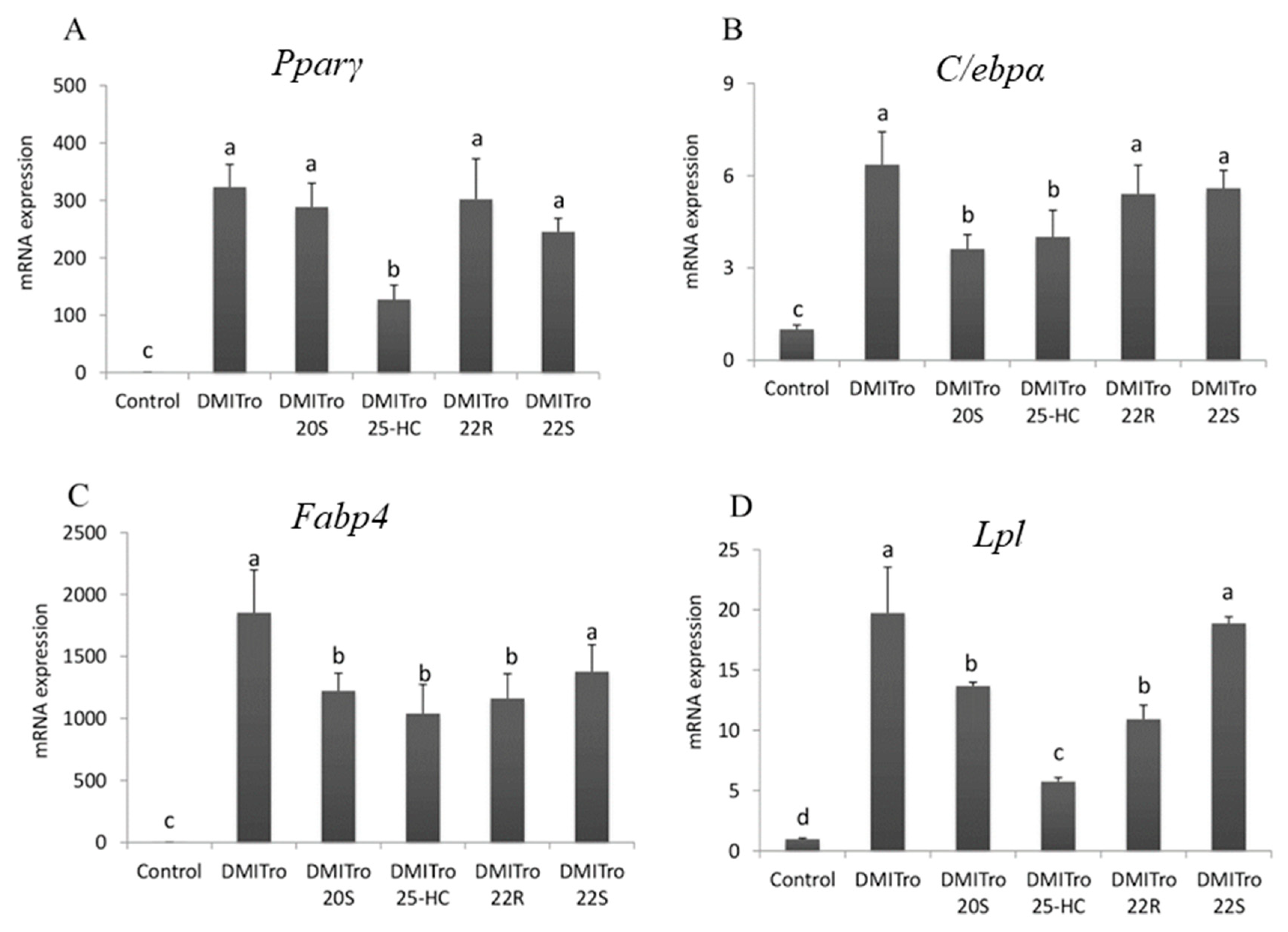
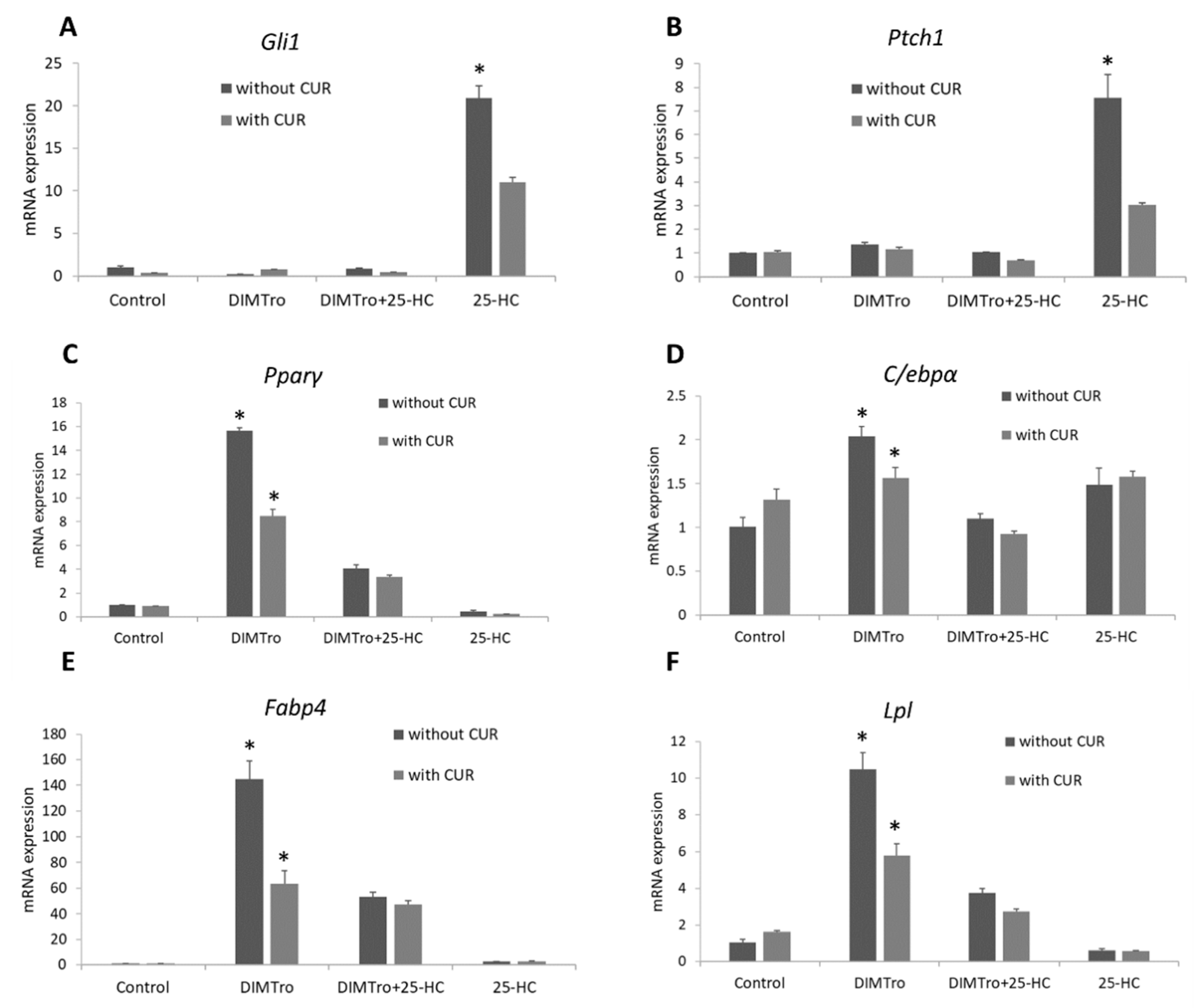
2.2. Effect of Oxysterols on Expression of Key Adipogenic Genes
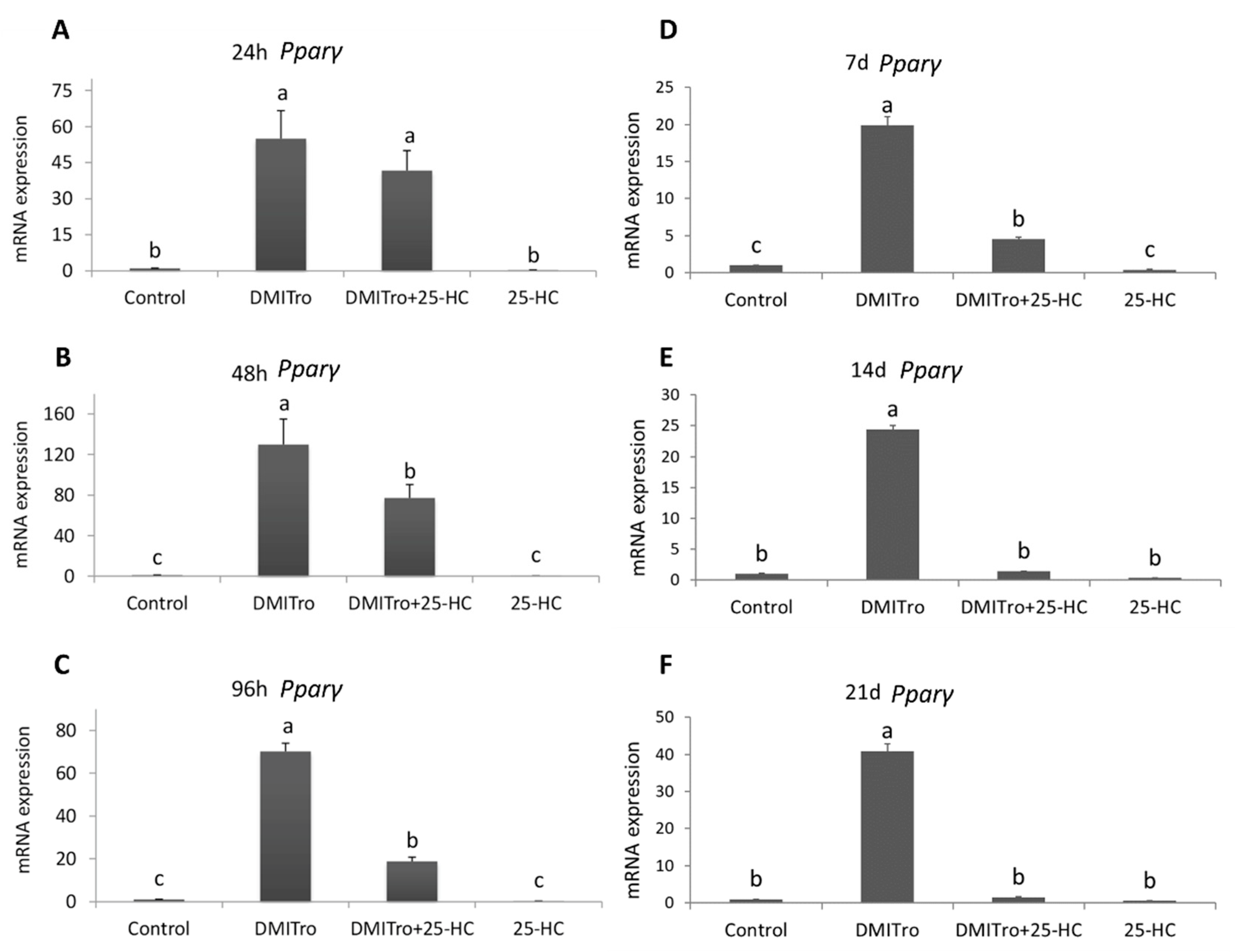
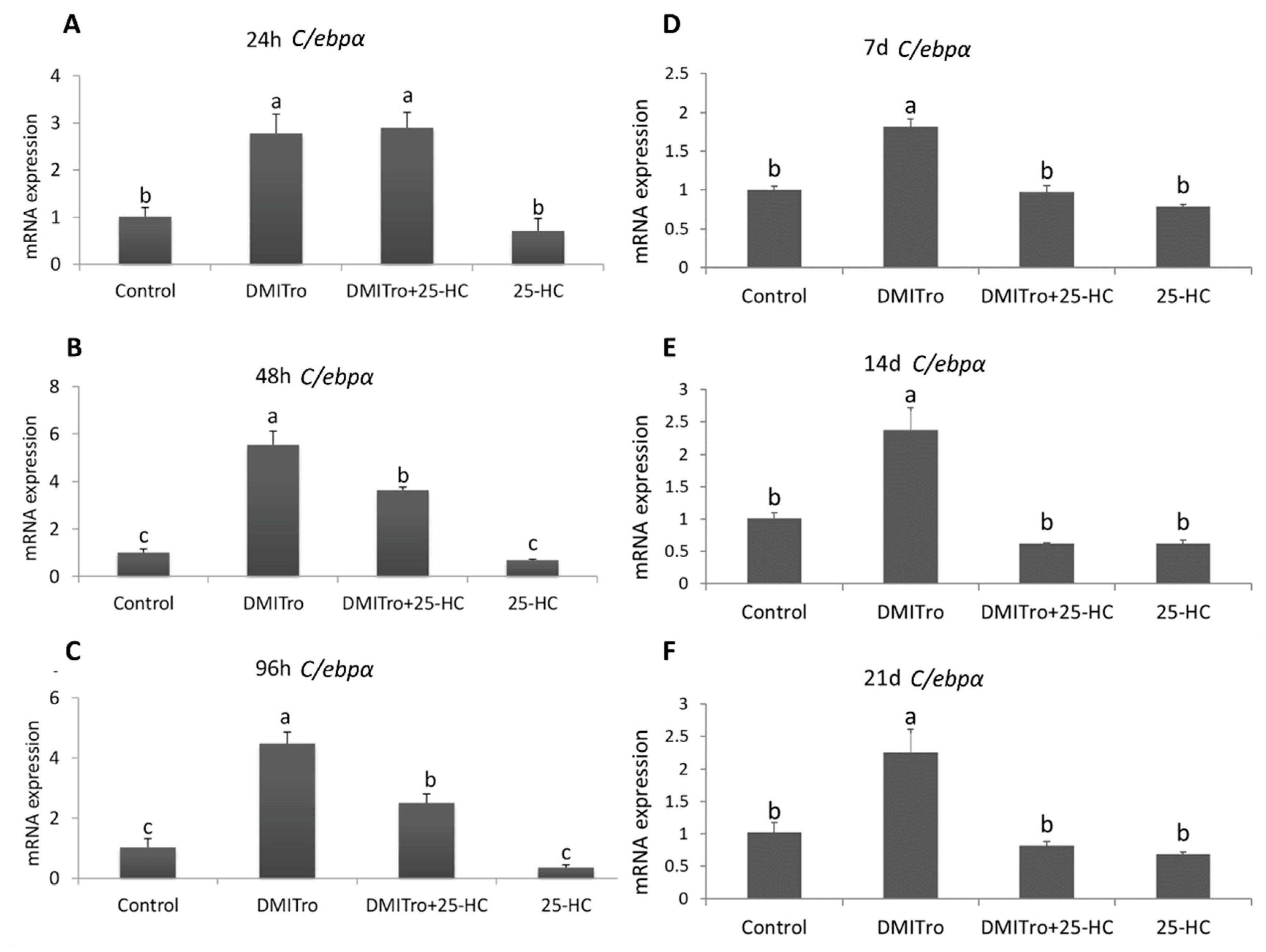
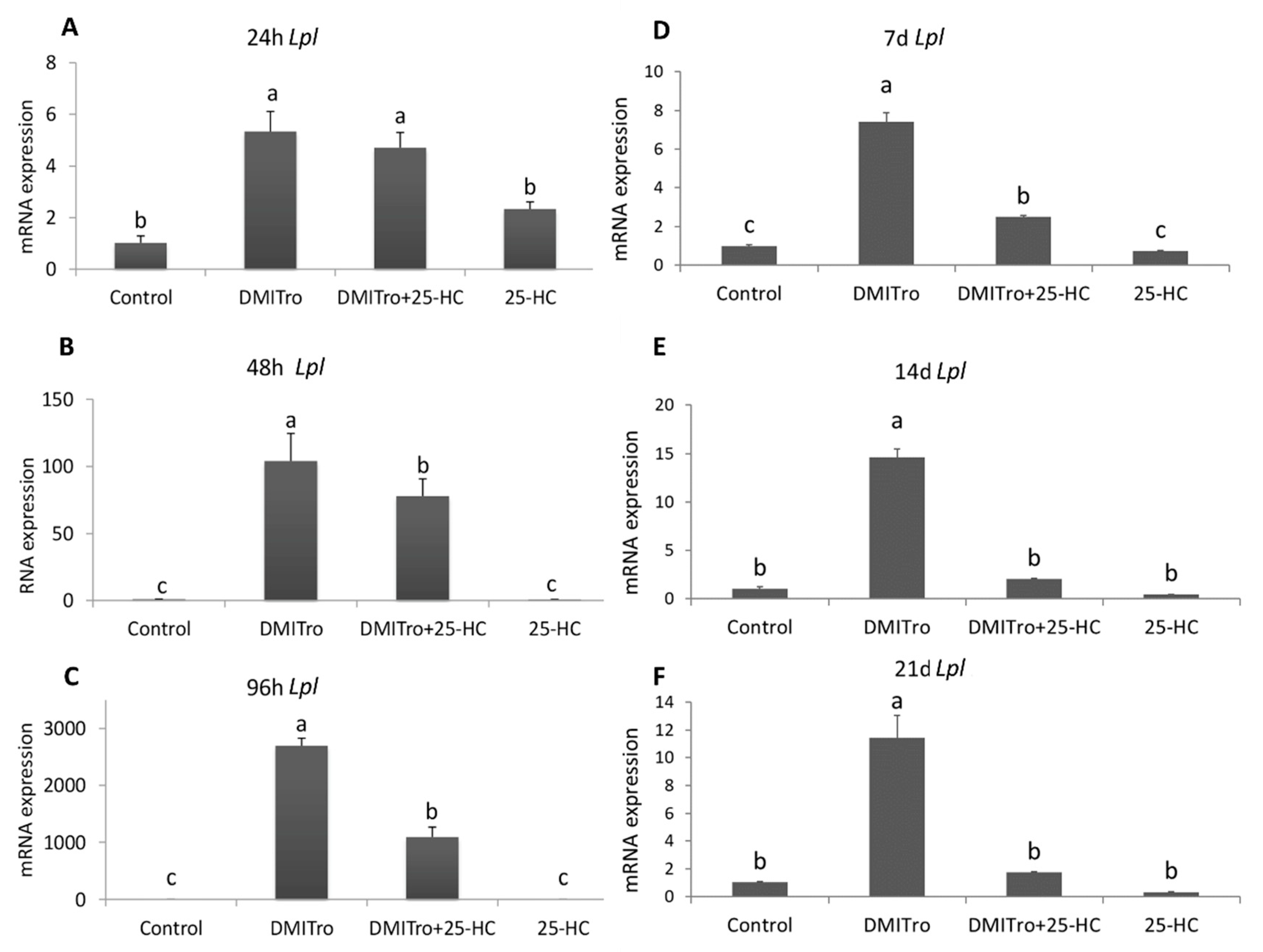
2.3. Effect of 25 Hydroxycholesterol at Different Time Points
2.4. Role of Hedgehog Signalling on the Anti-Adipogenic Effects of 25-Hydroxycholesterol
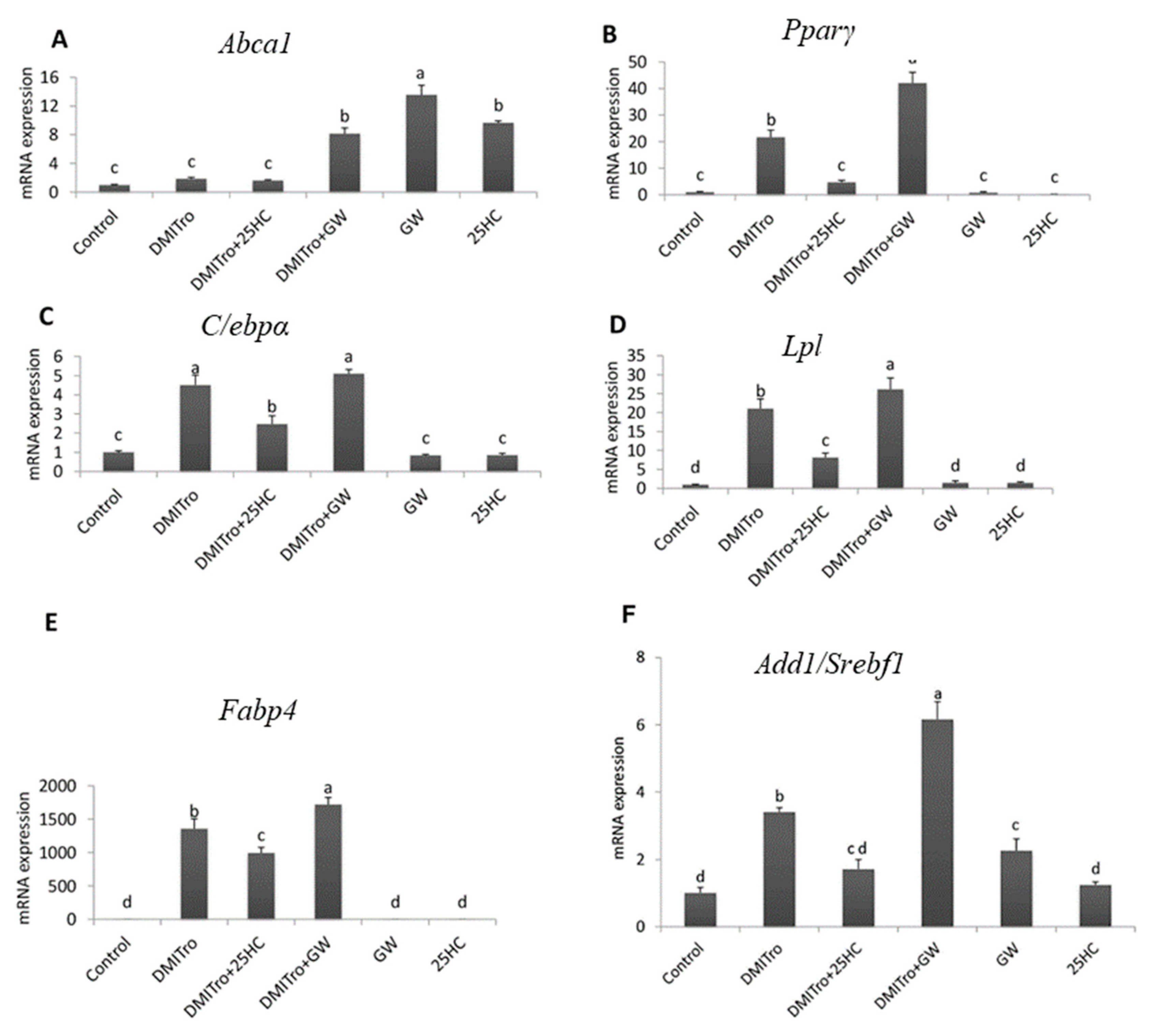
2.5. Role of Liver X Receptor on the Anti-Adipogenic Effects of 25-Hydroxycholesterol
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell culture
4.3. Oil Red Staining
4.4. RNA Extraction and Quantitative Real Time PCR (qRT-PCR)
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abca1 | ATP-binding cassette sub-family A, member 1 |
| Add1/Srebf1 | Adipocyte differentiation and determination factor 1/sterol regulatory |
| C/ebpα | CCAAT/enhancer binding protein α |
| Fabp4 | Fatty acid binding protein 4 |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| Lpl | Lipoprotein lipase |
| LXR | Liver X Receptor |
| MSC | Mesenchymal stromal cells |
| Pparγ | Peroxisome proliferator-activated receptor γ |
| T2DM | Type 2 diabetes mellitus |
References
- Haslam, D.; James, W. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Lehrke, M.; Lazar, M.A. The many faces of PPARγ. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Siersbæk, R.; Nielsen, R.; Mandrup, S. PPARγ in adipocyte differentiation and metabolism–Novel insights from genome-wide studies. FEBS Lett. 2010, 584, 3242–3249. [Google Scholar] [CrossRef]
- Couillard, C.; Mauriege, P.; Imbeault, P.; Prud’homme, D.; Nadeau, A.; Tremblay, A.; Bouchard, C.; Despres, J. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int. J. Obes. 2000, 24, 782. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.-H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [PubMed]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Zhao, G.; Li, B.; Li, Y.; Cawthorn, W.P.; MacDougald, O.A.; Franceschi, R.T. Genetic inhibition of PPARγ S112 phosphorylation reduces bone formation and stimulates marrow adipogenesis. Bone 2018, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Ge, K. Transcriptional and epigenetic regulation of PPARγ expression during adipogenesis. Cell Biosci. 2014, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-C.; Cao, Z.; Classon, M.; McKnight, S.L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995, 9, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-d.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Konieczny, S.F.; Emerson, C.P., Jr. 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: Evidence for regulatory genes controlling determination. Cell 1984, 38, 791–800. [Google Scholar] [CrossRef]
- Reznikoff, C.A.; Brankow, D.W.; Heidelberger, C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973, 33, 3231–3238. [Google Scholar]
- Pinney, D.F.; Emerson, C.P., Jr. 10T1/2 cells: An in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ. Health Perspect. 1989, 80, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Meliton, V.; Bourquard, N.; Hahn, T.J.; Parhami, F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J. Cell. Biochem. 2010, 111, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Kha, H.T.; Basseri, B.; Shouhed, D.; Richardson, J.; Tetradis, S.; Hahn, T.J.; Parhami, F. Oxysterols regulate differentiation of mesenchymal stem cells: Pro-bone and anti-fat. J. Bone Miner. Res. 2004, 19, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; De Melo, T.C.; Ruiz, J.L.; Bydlowski, S.P. Oxysterols and mesenchymal stem cell biology. Chem. Phys. Lipids 2017, 207, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Diczfalusy, U. Oxysterols: Friends, foes, or just fellow passengers? Arterioscler. Thromb. Vasc. Biol. 2002, 22, 734–742. [Google Scholar] [CrossRef]
- Van Reyk, D.M.; Brown, A.J.; Hult’en, L.M.; Dean, R.T.; Jessup, W. Oxysterols in biological systems: Sources, metabolism and pathophysiological relevance. Redox Rep. 2006, 11, 255–262. [Google Scholar] [CrossRef]
- Brown, A.; Jessup, W. Oxyster Ols Atherosclerosis. Atherosclerosis 1999, 142, 1–28. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Béaslas, O.; Nissilä, E. Oxysterols and their cellular effectors. Biomolecules 2012, 2, 76–103. [Google Scholar] [CrossRef]
- Kim, W.K.; Meliton, V.; Amantea, C.M.; Hahn, T.J.; Parhami, F. 20 (S)-hydroxycholesterol inhibits PPARγ expression and adipogenic differentiation of bone marrow stromal cells through a hedgehog-dependent mechanism. J. Bone Miner. Res. 2007, 22, 1711–1719. [Google Scholar] [CrossRef]
- Amantea, C.M.; Kim, W.K.; Meliton, V.; Tetradis, S.; Parhami, F. Oxysterol-induced osteogenic differentiation of marrow stromal cells is regulated by Dkk-1 inhibitable and PI3-kinase mediated signaling. J. Cell. Biochem. 2008, 105, 424–436. [Google Scholar] [CrossRef]
- Kim, W.K.; Meliton, V.; Tetradis, S.; Weinmaster, G.; Hahn, T.J.; Carlson, M.; Nelson, S.F.; Parhami, F. Osteogenic oxysterol, 20 (S)-hydroxycholesterol, induces notch target gene expression in bone marrow stromal cells. J. Bone Miner. Res. 2010, 25, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.A.; Kast, H.R.; Anisfeld, A.M. BAREing it all: The adoption of LXR and FXR and their roles in lipid homeostasis. J. Lipid Res. 2002, 43, 2–12. [Google Scholar] [PubMed]
- Peet, D.J.; Janowski, B.A.; Mangelsdorf, D.J. The LXRs: A new class of oxysterol receptors. Curr. Opin. Genet. Dev. 1998, 8, 571–575. [Google Scholar] [CrossRef]
- Edwards, P.A.; Kennedy, M.A.; Mak, P.A. LXRs: Oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vasc. Pharmacol. 2002, 38, 249–256. [Google Scholar] [CrossRef]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.-C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell. Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef]
- Davis, R.L.; Weintraub, H.; Lassar, A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987, 51, 987–1000. [Google Scholar] [CrossRef]
- Christy, R.; Yang, V.; Ntambi, J.; Geiman, D.; Landschulz, W.; Friedman, A.D.; Nakabeppu, Y.; Kelly, T.; Lane, M. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989, 3, 1323–1335. [Google Scholar] [CrossRef]
- Samulin, J.; Berget, I.; Lien, S.; Sundvold, H. Differential gene expression of fatty acid binding proteins during porcine adipogenesis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 147–152. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C.; Zhang, C.; Wang, H.; Lu, Y.; Yao, J.; Wei, Q.; Xing, G.; Cao, X. 25-hydroxycholesterol down-regulates oxysterol binding protein like 2 (OSBPL2) via the p53/SREBF2/NFYA signaling pathway. J. Steroid Biochem. Mol. Biol. 2019, 187, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Son, K.M.; Park, H.C.; Kim, N.R.; Lee, I.-S.; Yang, H.-C. Enhancement of the ALP activity of C3H10T1/2 cells by the combination of an oxysterol and apatite. Biomed. Mater. 2010, 5, 044107. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, C.; Cousin, W.; Plaisant, M.; Dani, C.; Peraldi, P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells 2008, 26, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bao, C.; Kim, J.T.; Cho, J.S.; Qiu, S.; Lee, H.J. Sulforaphene Inhibition of Adipogenesis via Hedgehog Signaling in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2018, 66, 11926–11934. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Guicherit, O.M.; Zaharian, B.I.; Xu, Y.; Chai, L.; Wichterle, H.; Kon, C.; Gatchalian, C.; Porter, J.A.; Rubin, L.L. Identification of a small molecule inhibitor of the hedgehog signaling pathway: Effects on basal cell carcinoma-like lesions. Proc. Natl. Acad. Sci. USA 2003, 100, 4616–4621. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.M.; Gao, X.; McKay, J.; McKay, R.; Salo, Z.; Graff, J.M. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006, 3, 25–34. [Google Scholar] [CrossRef]
- Cousin, W.; Dani, C.; Peraldi, P. Inhibition of the anti-adipogenic Hedgehog signaling pathway by cyclopamine does not trigger adipocyte differentiation. Biochem. Biophys. Res. Commun. 2006, 349, 799–803. [Google Scholar] [CrossRef]
- Teperino, R.; Amann, S.; Bayer, M.; McGee, S.L.; Loipetzberger, A.; Connor, T.; Jaeger, C.; Kammerer, B.; Winter, L.; Wiche, G. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell 2012, 151, 414–426. [Google Scholar] [CrossRef]
- Matsushita, K.; Morello, F.; Zhang, Z.; Masuda, T.; Iwanaga, S.; Steffensen, K.R.; Gustafsson, J.-Å.; Pratt, R.E.; Dzau, V.J. Nuclear hormone receptor LXRα inhibits adipocyte differentiation of mesenchymal stem cells with Wnt/beta-catenin signaling. Lab. Investig. 2016, 96, 230. [Google Scholar] [CrossRef]
- Amemiya-Kudo, M.; Shimano, H.; Yoshikawa, T.; Yahagi, N.; Hasty, A.H.; Okazaki, H.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K. Promoter analysis of the mouse sterol regulatory element-binding protein-1c gene. J. Biol. Chem. 2000, 275, 31078–31085. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A.; Ou, J.; Goldstein, J.L.; Brown, M.S. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proc. Natl. Acad. Sci. USA 2001, 98, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997, 272, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 1996, 383, 728. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Wright, H.M.; Wright, M.; Spiegelman, B.M. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4333–4337. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequences (Forward and Reverse) | Accession Number |
|---|---|---|
| Gapdh | 5′-ATGGACTGTGGTCATGAGCC-3′ | NM_001289726.1 |
| 5′-ATTGTCAGCAATGCATCCTG-3′ | ||
| Pparγ | 5′-TGAAACTCTGGGAGATTCTCCTG-3′ | NM_011146.3 |
| 5′-CCATGGTAATTTCTTGTGAAGTGC-3′ | ||
| C/ebpα | 5′-GGACAAGAACAGCAACGAGTACC-3′ | NM_001287514.1 |
| 5′-GGCGGTCATTGTCACTGGTC-3′ | ||
| Fabp4 | 5′-AACACCGAGATTTCCTT-3′ | NM_024406.2 |
| 5′-ACACATTCCACCACCAG-3′ | ||
| Lpl | 5′-AGGACCCCTGAAGACAC-3′ | NM_008509.2 |
| 5′-GGCACCCAACTCTCATA-3′ | ||
| Add1/Srebf-1 | 5′-CCTCCACTCACCAGGGTCT-3′ | NM_011480.3 |
| 5′-CTCAGCAGCCCCTAGAACAA-3′ | ||
| Abca1 | 5′-CTGTGTTGTGTGGGCTCCTC-3′ | NM_013454.3 |
| 5′-GTCAGCGTGTCACTTTCATGG-3′ | ||
| Gli1 | 5′-GCTTGGATGAAGGACCTTGTG-3′ | NM_010296.2 |
| 5′-GCTGATCCAGCCTAAGGTTCTC-3′ | ||
| Ptch1 | 5′-TTCTGCTGCCTGTCCTCTTATC-3′ | NM_008957.2 |
| 5′-CCTGCTGTGCTTCGTATTGC-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moseti, D.; Regassa, A.; Chen, C.; O, K.; Kim, W.K. 25-Hydroxycholesterol Inhibits Adipogenic Differentiation of C3H10T1/2 Pluripotent Stromal Cells. Int. J. Mol. Sci. 2020, 21, 412. https://doi.org/10.3390/ijms21020412
Moseti D, Regassa A, Chen C, O K, Kim WK. 25-Hydroxycholesterol Inhibits Adipogenic Differentiation of C3H10T1/2 Pluripotent Stromal Cells. International Journal of Molecular Sciences. 2020; 21(2):412. https://doi.org/10.3390/ijms21020412
Chicago/Turabian StyleMoseti, Dorothy, Alemu Regassa, Chongxiao Chen, Karmin O, and Woo Kyun Kim. 2020. "25-Hydroxycholesterol Inhibits Adipogenic Differentiation of C3H10T1/2 Pluripotent Stromal Cells" International Journal of Molecular Sciences 21, no. 2: 412. https://doi.org/10.3390/ijms21020412
APA StyleMoseti, D., Regassa, A., Chen, C., O, K., & Kim, W. K. (2020). 25-Hydroxycholesterol Inhibits Adipogenic Differentiation of C3H10T1/2 Pluripotent Stromal Cells. International Journal of Molecular Sciences, 21(2), 412. https://doi.org/10.3390/ijms21020412







