Effect of Oral Losartan on Orthobiologics: Implications for Platelet-Rich Plasma and Bone Marrow Concentrate—A Rabbit Study
Abstract
1. Introduction
2. Results
2.1. Whole Blood Complete Blood Count Results
2.2. LP-PRP Complete Blood Count and TGF-β1 Results
2.3. Comparison of TGF-β1 Expression Level in Whole Blood and LP-PRP
2.4. Bone Marrow Aspirate Complete Blood Count Results
2.5. Bone Marrow Concentrate Complete Blood Count Results
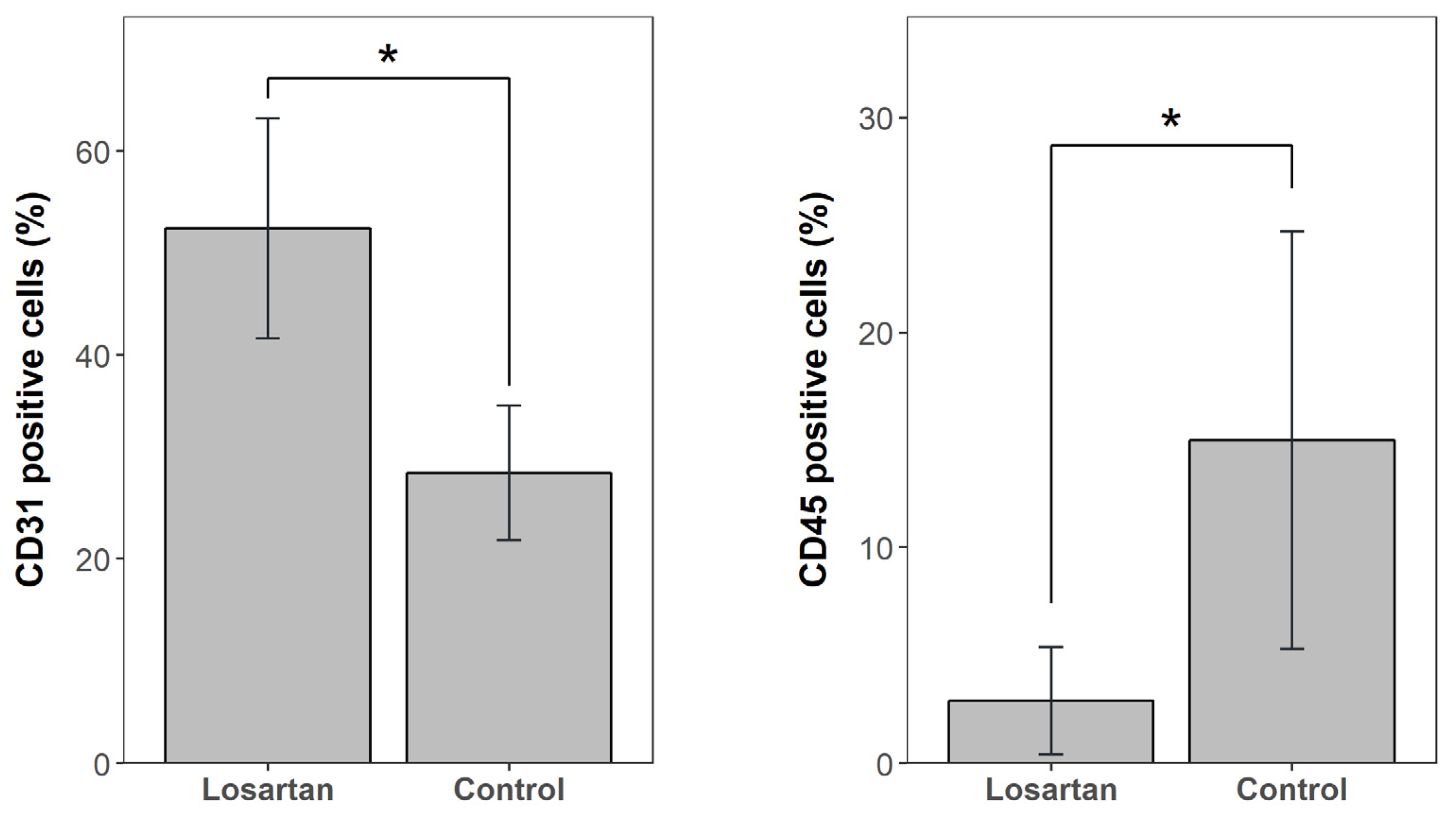
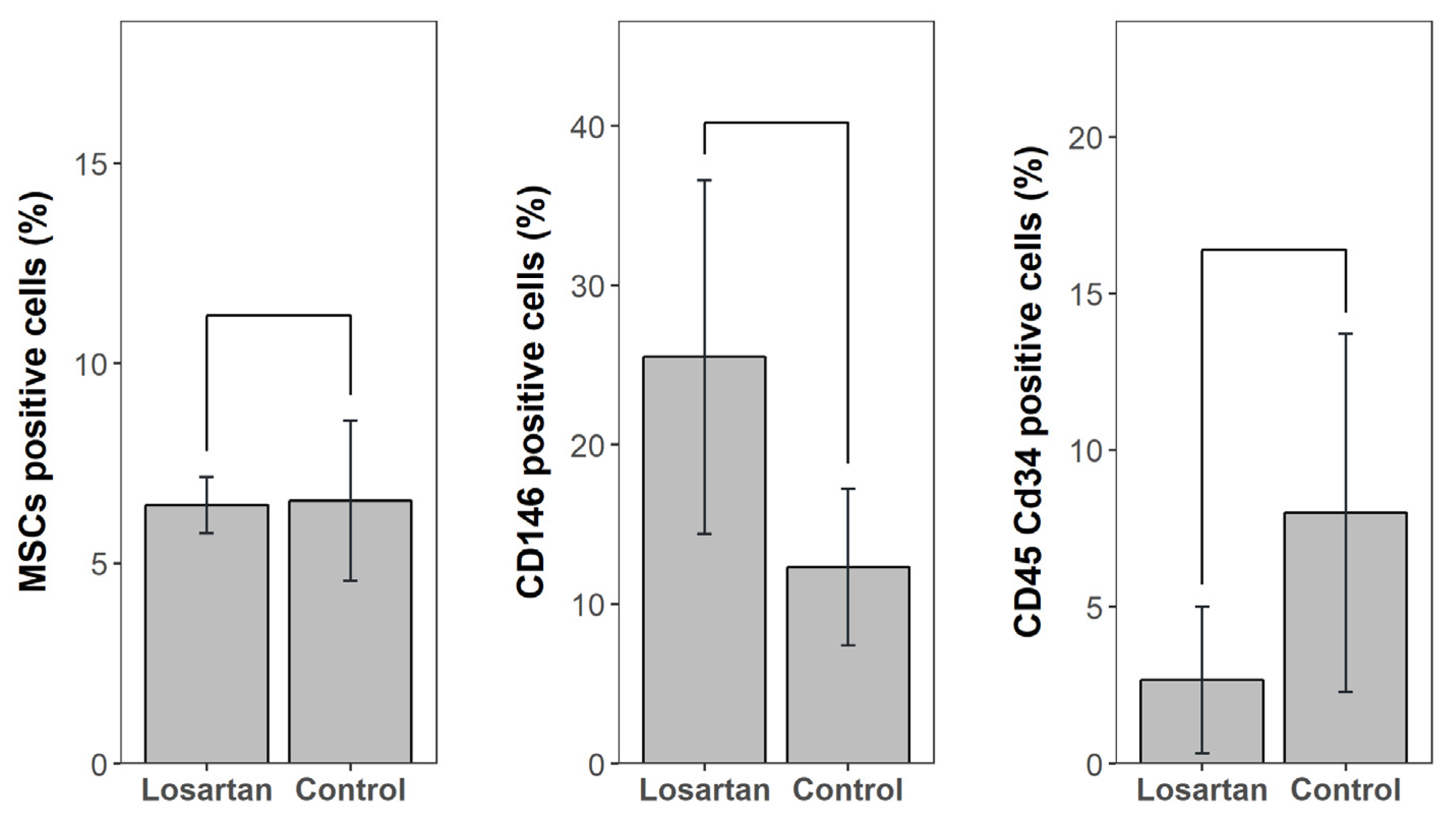
2.6. Bone Marrow Concentrate Flow Cytometry Results
3. Discussion
4. Material and Methods
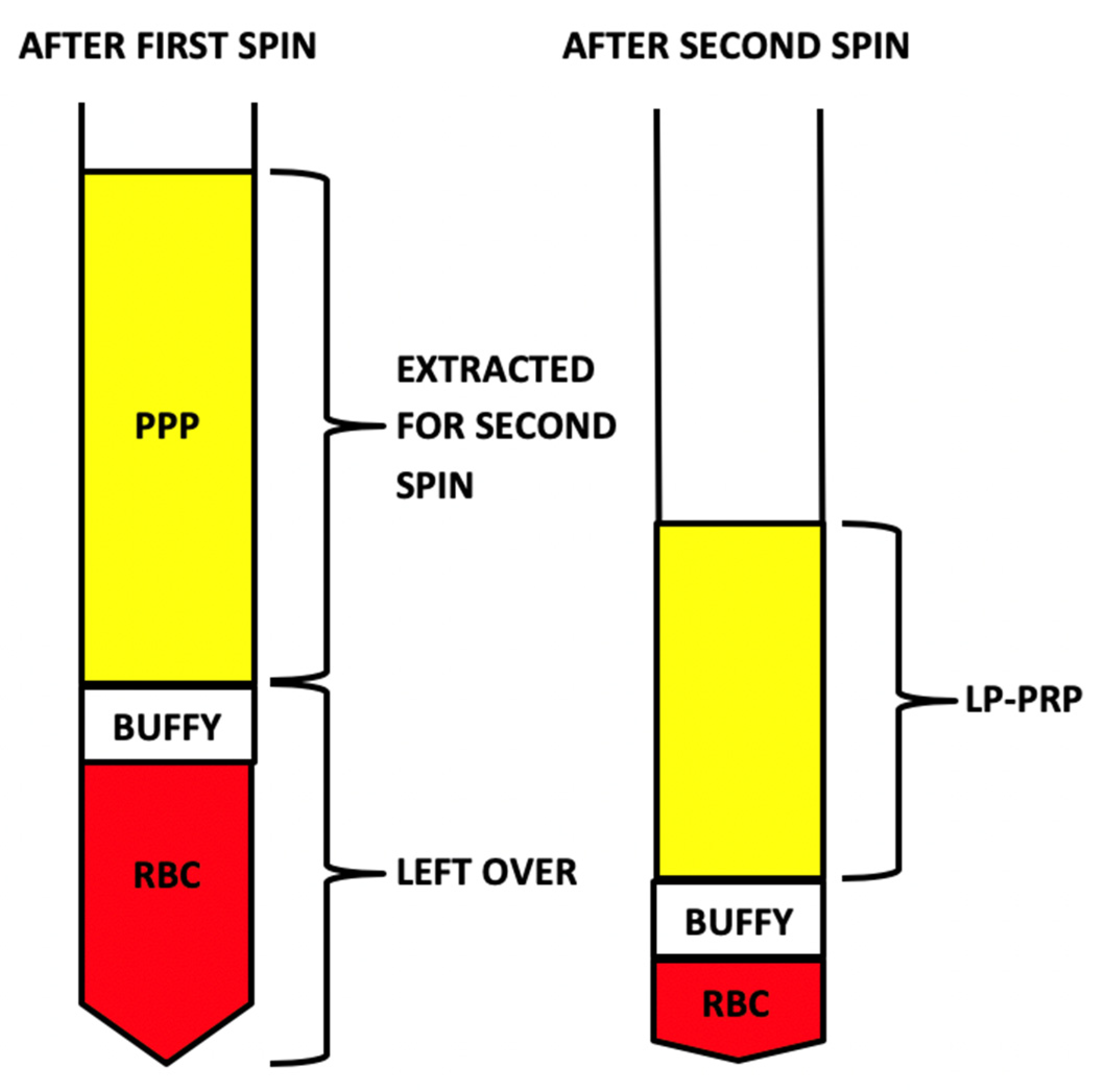
4.1. LP-PRP: Collection and Processing
4.2. Bone Marrow Concentrate: Collection and Processing
4.3. Hematology Assessment
4.4. Whole Blood and LP-PRP Multiplex Assay and Analysis
4.5. Bone Marrow Flow Cytometry and Mononuclear Cell Isolation
4.5.1. Flow Cytometry Analysis
4.5.2. Mononuclear Cell Isolation by Density Gradient Centrifugation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMA | Bone marrow aspirate |
| BMC | Bone marrow concentrate |
| CBC | Complete blood count |
| EndMT | Endothelial mesenchymal transition |
| IQR | Interquartile range |
| LP-PRP | Leukocyte-poor platelet-rich plasma |
| MSCs | Mesenchymal stem cells |
| PRP | Platelet-rich plasma |
| TGF-β1 | Transforming growth factor-beta 1 |
| WB | Whole blood |
References
- Blaney Davidson, E.N.; van der Kraan, P.M.; van den Berg, W.B. TGF-beta and osteoarthritis. Osteoarthr. Cartil. 2007, 15, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Gonzalez, Z.N.; Baily, J.; Dobie, R.; Wallace, R.J.; MacKinnon, A.C.; Smith, J.R.; Greenhalgh, S.N.; Thompson, A.I.; Conroy, K.P.; et al. Alphav integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat. Commun. 2017, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Masta, S.; Meyers, D.; Narayanan, A.S. Collagen synthesis by normal and fibrotic human lung fibroblasts and the effect of transforming growth factor? Am. Rev. Respir. Dis. 1989, 140, 95–100. [Google Scholar] [CrossRef]
- Kasemkijwattana, C.; Menetrey, J.; Somogyl, G.; Moreland, M.S.; Fu, F.H.; Buranapanitkit, B.; Watkins, S.C.; Huard, J. Development of approaches to improve the healing following muscle contusion. Cell Transpl. 1998, 7, 585–598. [Google Scholar] [CrossRef]
- Okada, M.; Payne, T.R.; Drowley, L.; Jankowski, R.J.; Momoi, N.; Beckman, S.; Chen, C.-W.; Keller, B.B.; Tobita, K.; Huard, J. Human skeletal muscle cells with a slow adhesion rate after isolation and an enhanced stress resistance improve function of ischemic hearts. Mol. Ther. 2012, 20, 138–145. [Google Scholar] [CrossRef]
- Unterhauser, F.N.; Bosch, U.; Zeichen, J.; Weiler, A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Arch. Orthop. Trauma Surg. 2004, 124, 585–591. [Google Scholar] [CrossRef]
- Leask, A. Getting to the heart of the matter: New insights into cardiac fibrosis. Circ. Res. 2015, 116, 1269–1276. [Google Scholar] [CrossRef]
- Wu, M.; Peng, Z.; Zu, C.; Ma, J.; Lu, S.; Zhong, J.; Zhang, S. Losartan attenuates myocardial endothelial-to-mesenchymal transition in spontaneous hypertensive rats via inhibiting TGF-beta/smad signaling. PLoS ONE 2016, 11, e0155730. [Google Scholar]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1479. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Boil. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Uehara, K.; Ota, S.; Tobita, K.; Ambrosio, F.; Cummins, J.H.; Terada, S.; Fu, F.H.; Huard, J. The timing of administration of a clinically relevant dose of losartan influences the healing process after contusion induced muscle injury. J. Appl. Physiol. 2013, 114, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Ota, S.; Kobayashi, M.; Kobayashi, T.; Mifune, Y.; Takayama, K.; Witt, M.; Vadalà, G.; Oyster, N.; Otsuka, T.; et al. Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J. Bone Jt. Surg. Am. 2013, 95, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Bedair, H.S.; Karthikeyan, T.; Quintero, A.; Li, Y.; Huard, J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am. J. Sports Med. 2008, 36, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ota, S.; Terada, S.; Kawakami, Y.; Otsuka, T.; Fu, F.H.; Huard, J. The combined use of losartan and muscle-derived stem cells significantly improves the functional recovery of muscle in a young mouse model of contusion injuries. Am. J. Sports Med. 2016, 44, 3252–3261. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Li, D. Losartan reduces myocardial interstitial fibrosis in diabetic cardiomyopathy rats by inhibiting JAK/STAT signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 466–473. [Google Scholar]
- Xu, F.; Mao, C.; Liu, Y.; Wu, L.; Xu, Z.; Zhang, L. Losartan chemistry and its effects via AT1 mechanisms in the kidney. Curr. Med. Chem. 2009, 16, 3701–3715. [Google Scholar] [CrossRef][Green Version]
- Bartko, P.E.; Dal-Bianco, J.P.; Guerrero, J.L.; Beaudoin, J.; Szymanski, C.; Kim, D.-H.; Seybolt, M.M.; Handschumacher, M.; Sullivan, S.; Garcia, M.L.; et al. Effect of losartan on mitral valve changes after myocardial infarction. J. Am. Coll. Cardiol. 2017, 70, 1232–1244. [Google Scholar] [CrossRef]
- Garg, K.; Corona, B.T.; Walters, J.T. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front. Pharmacol. 2015, 6, 87. [Google Scholar] [CrossRef]
- Li, H.; Hicks, J.J.; Wang, L.; Oyster, N.; Philippon, M.J.; Hurwitz, S.; Hohan, M.V.; Huard, J. Customized platelet-rich plasma with transforming growth factor beta1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials 2016, 87, 147–156. [Google Scholar] [CrossRef]
- Whitney, K.E.; Liebowitz, A.; Bolia, I.K.; Chahla, J.; Ravuri, S.K.; Evans, T.A.; Philippon, M.J.; Huard, J. Current perspectives on biological approaches for osteoarthritis. Ann. N. Y. Acad. Sci. 2017, 1410, 26–43. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of platelet-rich plasma: History, biology, mechanism of action, and classification. Skin Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Engebretsen, L.; Steffen, K.; Alsousou, J.; Anitua, E.; Bachl, N.; Devilee, R.; Everts, P.; Hamilton, B.; Huard, J.; Jenoure, P.; et al. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br. J. Sports Med. 2010, 44, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Jalaluddin, M.; Mahesh, J.; Mahesh, R.; Jayanti, I.; Faizuddin, M.; Kripal, K.; Nazeer, N. Effectiveness of platelet rich plasma and bone graft in the treatment of intrabony defects: A clinico-radiographic study. Open Dent. J. 2018, 12, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Cielo, A.; Bonanome, L.; Rastelli, C.; Derla, C.; Corpaci, F.; Falisi, G. Comparison between PRP, PRGF and PRF: Lights and shadows in three similar but different protocols. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 927–930. [Google Scholar] [PubMed]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr. Rev. Musculoskelet Med. 2018, 11, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Braun, H.J.; Durham, J.L.; Ridley, B.A.; Odegaard, J.I.; Luong, R.; Arnoczky, S.P. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am. J. Sports Med. 2012, 40, 1274–1281. [Google Scholar] [CrossRef]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Fortier, L.; Cole, B.J. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2016, 44, 792–800. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Bone marrow takes center stage in cardiovascular disease. Circ. Res. 2016, 119, 701–703. [Google Scholar] [CrossRef]
- Jones, C.P.; Rankin, S. Bone marrow-derived stem cells and respiratory Disease. Chest 2011, 140, 205–211. [Google Scholar] [CrossRef]
- Li, J.R.; Qu, T.T. Into the eyes of bone marrow-derived mesenchymal stem cells therapy for myocardial infarction and other diseases. Stem Cell Investig. 2017, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Alland, J.A.; Verma, N.N. Bone marrow aspirate concentrate for orthopaedic use. Orthop. Nurs. 2018, 37, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Chahla, J.; LaPrade, R.F.; Pascual-Garrido, C. Biologic options for articular cartilage wear (platelet-rich plasma, stem cells, bone marrow aspirate concentrate). Clin. Sports Med. 2017, 36, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Dean, C.S.; Moatshe, G.; Pascual-Garrido, C.; Cruz, R.S.; LaPrade, R.F. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: A systematic review of outcomes. Orthop. J. Sports Med. 2016, 4, 2325967115625481. [Google Scholar] [CrossRef]
- Zhu, J.; Ide, H.; Fu, Y.Y.; Teichert, A.M.; Kato, H.; Weisel, R.D.; Maynes, J.T.; Coles, J.G.; Caldarone, C.A. Losartan ameliorates “upstream” pulmonary vein vasculopathy in a piglet model of pulmonary vein stenosis. J. Thorac. Cardiovasc. Surg. 2014, 148, 2550–2557. [Google Scholar] [CrossRef]
- Piling, D.; Fan, T.; Huang, D.; Kaul, B.; Gomer, R.H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 2009, 4, e7475. [Google Scholar] [CrossRef]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb. Haemost. 2009, 101, 613–618. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Abreu, R.D.; Vilar-Pereira, G.; Degrave, W.; Meuser-Batista, M.; Ferreira, N.V.C.; Moreira, O.D.; Gomes, N.L.D.; de Souza, E.M.; Ramos, I.P.; et al. TGF-beta inhibitor therapy decreases fibrosis and stimulates cardiac improvement in a pre-clinical study of chronic Chagas’ heart disease. PLoS Negl. Trop. Dis. 2019, 13, e0007602. [Google Scholar] [CrossRef]
- Pohlers, D.; Brenmoehl, J.; Löffler, I.; Müller, C.K.; Leipner, C.; Schultze-Mosgau, S.; Stallmach, A.; Kinne, R.W.; Wolf, G. TGF-beta and fibrosis in different organs-molecular pathway imprints. Biochim. Biophys. Acta 2009, 1792, 746–756. [Google Scholar] [CrossRef]
- Gay-Jordi, G.; Guash, E.; Benito, B.; Brugada, J.; Nattel, S.; Mont, L.; Serrano-Mollar, A. Losartan prevents heart fibrosis induced by long-term intensive exercise in an animal model. PLoS ONE 2013, 8, e55427. [Google Scholar] [CrossRef]
- Wylie-Sears, J.; Levine, R.A.; Bischoff, J. Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-beta-induced phosphorylation of ERK. Biochem. Biophys. Res. Commun. 2014, 446, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Carrasco, J.L.; Beaumont, J.; José, G.S.; Moreno, M.U.; López, B.; González, A.; Zalba, G.; Díez, J.; Fortuño, A.; Ravassa, S. Mechanisms underlying the cardiac antifibrotic effects of losartan metabolites. Sci. Rep. 2017, 7, 41865. [Google Scholar] [CrossRef] [PubMed]
- Campistol, J.M.; Iñigo, P.; Jimenez, W.; Lario, S.; Clesca, P.H.; Oppenheimer, F.; Rivera, F. Losartan decreases plasma levels of TGF-beta1 in transplant patients with chronic allograft nephropathy. Kidney Int. 1999, 56, 714–719. [Google Scholar] [CrossRef] [PubMed]
- El-Agroudy, A.E.; Hassan, N.A.; Foda, M.A.; Ismail, A.M.; El-Sawy, E.A.; Mousa, O.; Ghoneim, M.A. Effect of angiotensin II receptor blocker on plasma levels of TGF-beta 1 and interstitial fibrosis in hypertensive kidney transplant patients. Am. J. Nephrol. 2003, 23, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Gao, X.; Deng, Z.; Cheng, H.; Nakama, G.; Scibetta, A.C.; Ravuri, S.K.; Goldman, J.L.; Lowe, W.R.; Rodkey, W.G.; et al. Biologically regulated marrow stimulation by blocking TGF-beta1 with losartan oral administration results in hyaline-like cartilage repair: A rabbit osteochondral defect model. Am. J. Sports Med. 2020, 48, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Ziegler, C.G.; Van Sloun, R.; Gonzalez, S.; Whitney, K.E.; DePhillipo, N.N.; Kennedy, M.I.; Dornan, G.J.; Evans, T.A.; Huard, J.; Laprade, R.F. Characterization of growth factors, cytokines, and chemokines in bone marrow concentrate and platelet-rich plasma: A prospective analysis. Am. J. Sports Med. 2019, 47, 2174–2187. [Google Scholar] [CrossRef]
- Kennedy, M.I.; Whitney, K.; Evans, T.; Laprade, R.F. Platelet-rich plasma and cartilage repair. Curr. Rev. Musculoskelet. Med. 2018, 11, 573–582. [Google Scholar] [CrossRef]


| Control | Losartan | p Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Red blood cells | 7.81 | 7.42–8.12 | 9.92 | 9.87–12.31 | 0.42 |
| White blood cells | 1.75 | 1.13–2.26 | 1.13 | 0.74–1.52 | 0.22 |
| Platelets | 1.00 | 0.63–1.47 | 2.12 | 1.41–2.36 | 0.10 |
| Whole Blood | LP-PRP | p Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Red blood cells | 9.00 | 7.42–12.31 | 0.07 | 0.01–0.22 | <0.01 |
| White blood cells | 1.33 | 0.96–2.22 | 0.77 | 0.29–1.88 | 0.20 |
| Platelets | 1.44 | 1.00–2.12 | 6.63 | 4.77–12.63 | <0.01 |
| TGF-β1, pg/mL | 1630.5 | 1273.5–2677.0 | 18122.0 | 8577.5–27575.0 | 0.01 |
| Control | Losartan | p-Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Red blood cells | 3.97 | 3.94–4.09 | 4.32 | 4.16–4.77 | 0.15 |
| White blood cells | 8.85 | 7.98–10.02 | 8.49 | 6.82–11.83 | 0.99 |
| Platelets | 93.0 | 46.0–101.0 | 20.0 | 12.0–38.0 | 0.15 |
| Control | Losartan | p-Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Red blood cells | 7.81 | 7.42–8.12 | 9.92 | 9.87–12.31 | 0.42 |
| White blood cells | 33.89 | 18.83–41.3 | 25.48 | 9.82–31.2 | 0.42 |
| Platelets | 55.0 | 36.0–69.0 | 15.0 | 9.0–29.0 | 0.04 |
| Processing Steps for LP-PRP | Processing Steps for BMC |
|---|---|
| 1. First spin: 800 RPM for 10 min | 1. First spin: 1500 RPM for 10 min |
| 2. Extract platelet-poor plasma (PPP) without disrupting “buffy coat layer” | 2. Extract PPP and “buffy coat layer” |
| 3. Hard spin: 3300 RPM for 6 min | 3. Second spin: 3300 RPM for 6 min |
| 4. Remove excess plasma to obtain 10% sample | 4. Remove excess plasma discarded and extract “buffy coat layer” to obtain a 10% sample |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakama, G.Y.; Gonzalez, S.; Matre, P.; Mu, X.; Whitney, K.E.; Utsunomiya, H.; Arner, J.W.; Philippon, M.J.; Ravuri, S.; Huard, J. Effect of Oral Losartan on Orthobiologics: Implications for Platelet-Rich Plasma and Bone Marrow Concentrate—A Rabbit Study. Int. J. Mol. Sci. 2020, 21, 7374. https://doi.org/10.3390/ijms21197374
Nakama GY, Gonzalez S, Matre P, Mu X, Whitney KE, Utsunomiya H, Arner JW, Philippon MJ, Ravuri S, Huard J. Effect of Oral Losartan on Orthobiologics: Implications for Platelet-Rich Plasma and Bone Marrow Concentrate—A Rabbit Study. International Journal of Molecular Sciences. 2020; 21(19):7374. https://doi.org/10.3390/ijms21197374
Chicago/Turabian StyleNakama, Gilberto Y., Sabrina Gonzalez, Polina Matre, Xiaodong Mu, Kaitlyn E. Whitney, Hajime Utsunomiya, Justin W. Arner, Marc J. Philippon, Sudheer Ravuri, and Johnny Huard. 2020. "Effect of Oral Losartan on Orthobiologics: Implications for Platelet-Rich Plasma and Bone Marrow Concentrate—A Rabbit Study" International Journal of Molecular Sciences 21, no. 19: 7374. https://doi.org/10.3390/ijms21197374
APA StyleNakama, G. Y., Gonzalez, S., Matre, P., Mu, X., Whitney, K. E., Utsunomiya, H., Arner, J. W., Philippon, M. J., Ravuri, S., & Huard, J. (2020). Effect of Oral Losartan on Orthobiologics: Implications for Platelet-Rich Plasma and Bone Marrow Concentrate—A Rabbit Study. International Journal of Molecular Sciences, 21(19), 7374. https://doi.org/10.3390/ijms21197374





