The Commensal Microbiota Enhances ADP-Triggered Integrin αIIbβ3 Activation and von Willebrand Factor-Mediated Platelet Deposition to Type I Collagen
Abstract
1. Introduction
2. Results
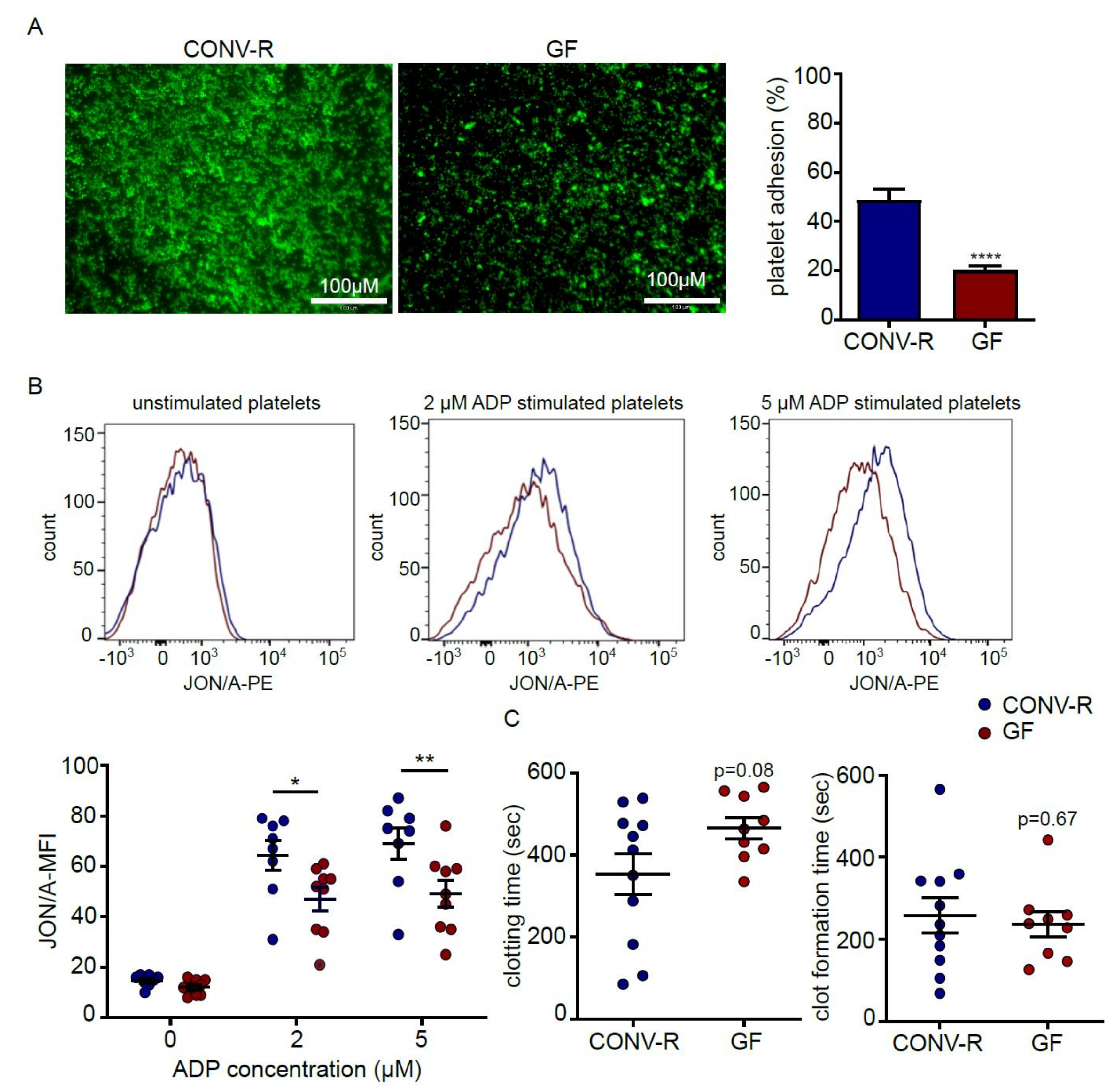
2.1. Platelets from Germ-Free Mice Show Reduced Deposition to Type I Collagen and Diminished ADP-Induced αIIbβ3 Integrin Activation
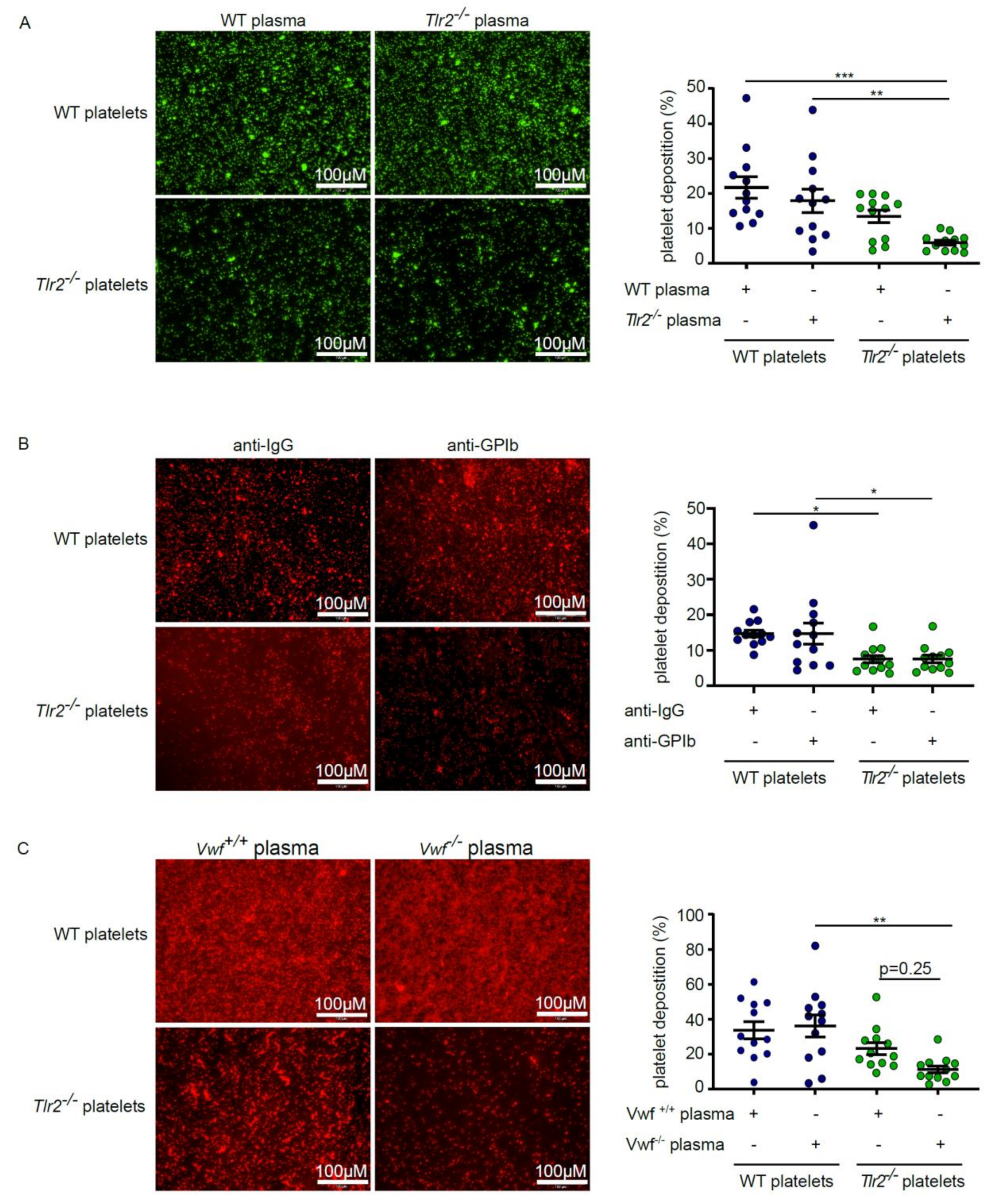
2.2. Deficiency in TLR2 Results in Impaired VWF-Mediated Platelet Deposition to Type I Collagen
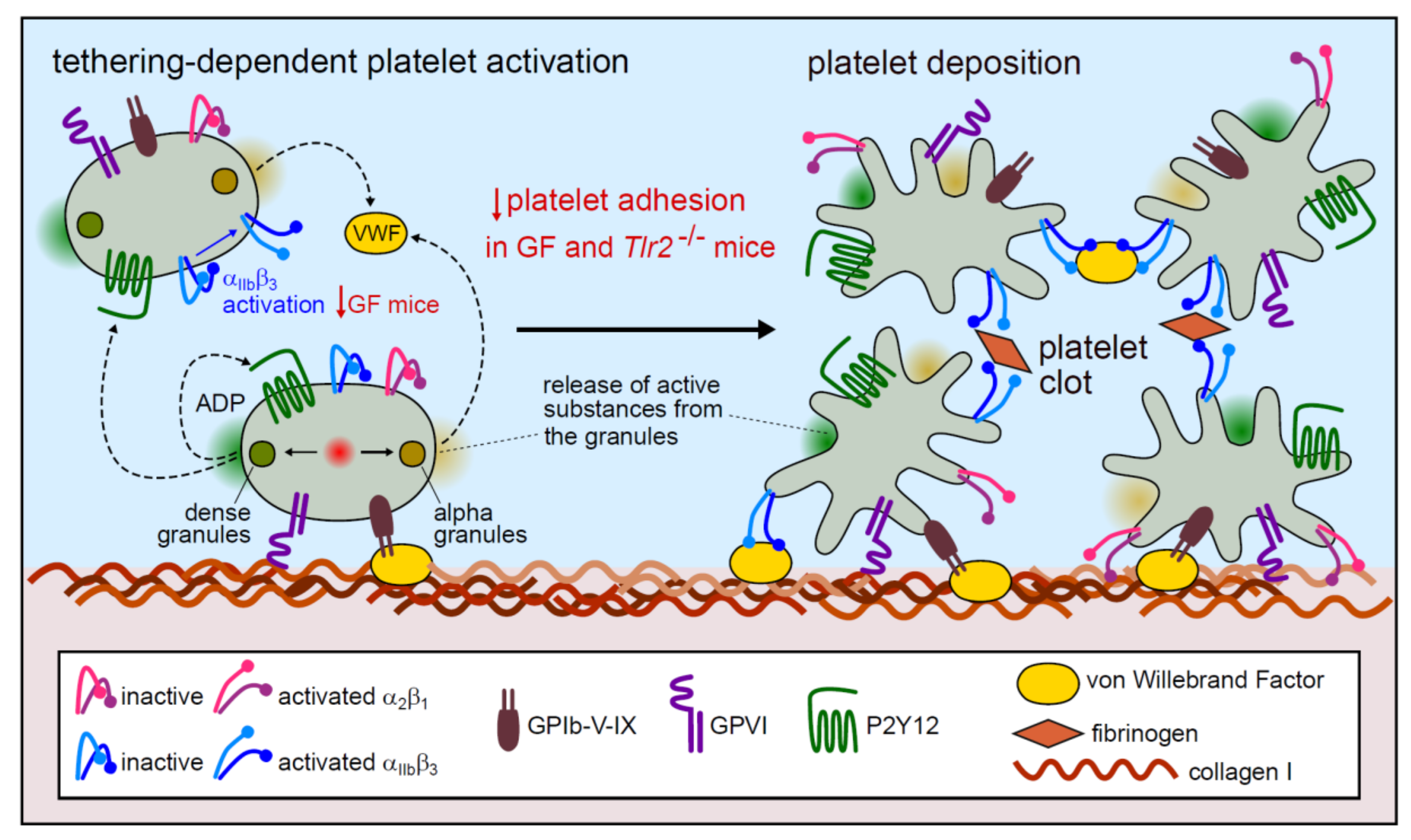
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Blood Collection and Platelet Adhesion Under Static Conditions
4.3. Flow Cytometric Analysis of Platelet Surface Receptors
4.4. Thromboelastometry
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| CONV-R | Conventionally raised |
| CVD | Cardiovascular disease |
| GF | Germ-free |
| GP | Glycoprotein |
| Ldlr | Low-density lipoprotein-receptor gene |
| MAMPs | Microbial-associated molecular patterns |
| MFI | Mean Fluorescence Intensity |
| NOD | Nucleotide-binding oligomerization domain |
| TLRs | Toll-like receptors |
| TLR2 | Toll-like receptor 2 |
| Tlr2 | Toll-like receptor 2 gene |
| VWF | Von Willebrand factor |
| WT | Wild-type |
References
- Mannhalter, C. Biomarkers for arterial and venous thrombotic disorders. Hamostaseologie 2014, 34, 115–120, 122–126, 128–130, passim. [Google Scholar] [CrossRef] [PubMed]
- Ascher, S.; Reinhardt, C. The gut microbiota: An emerging risk factor for cardiovascular and cerebrovascular disease. Eur. J. Immunol. 2018, 48, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 852. [Google Scholar] [PubMed]
- Reinhardt, C.; Reigstad, C.S.; Backhed, F. Intestinal microbiota during infancy and its implications for obesity. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Ståhlman, M.; Ilkayeva, O.; Arnemo, J.M.; Kindberg, J.; Josefsson, J.; Newgard, C.B.; Fröbert, O.; Bäckhed, F. The gut microbiota modulates energy metabolism in the hibernating brown bear ursus arctos. Cell Rep. 2016, 14, 1655–1661. [Google Scholar] [CrossRef]
- Esser, D.; Lange, J.; Marinos, G.; Sieber, M.; Best, L.; Prasse, D.; Bathia, J.; Rühlemann, M.C.; Boersch, K.; Jaspers, C.; et al. Functions of the microbiota for the physiology of animal metaorganisms. J. Innate. Immun. 2019, 11, 393–404. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Kiouptsi, K.; Reinhardt, C. Contribution of the commensal microbiota to atherosclerosis and arterial thrombosis. Br. J. Pharmacol. 2018, 175, 4439–4449. [Google Scholar] [CrossRef]
- Kinross, J.M.; Alkhamesi, N.; Barton, R.H.; Silk, D.B.; Yap, I.K.; Darzi, A.W.; Holmes, E.; Nicholson, J.K. Global metabolic phenotyping in an experimental laparotomy model of surgical trauma. J. Proteome. Res. 2011, 10, 277–287. [Google Scholar] [CrossRef]
- Wilmanski, T.; Rappaport, N.; Earls, J.C.; Magis, A.T.; Manor, O.; Lovejoy, J.; Omenn, G.S.; Hood, L.; Gibbons, S.M.; Price, N.D. Blood metabolome predicts gut microbiome alpha-diversity in humans. Nat. Biotechnol. 2019, 37, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, N.; Brandao, I.; Jäckel, S.; Ens, N.; Lillich, M.; Walter, U.; Reinhardt, C. Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa. PLoS ONE 2014, 9, e113080. [Google Scholar] [CrossRef]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.L.; Slack, E.; de Gottardi, A.; Lawson, M.A.; Hapfelmeier, S.; Miele, L.; Grieco, A.; Van Vlierberghe, H.; Fahrner, R.; Patuto, N.; et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci. Transl. Med. 2014, 6, 237ra66. [Google Scholar] [CrossRef]
- Reinhardt, C.; Bergentall, M.; Greiner, T.U.; Schaffner, F.; Ostergren-Lundén, G.; Petersen, L.C.; Ruf, W.; Bäckhed, F. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 2012, 483, 627–631. [Google Scholar] [CrossRef]
- Jäckel, S.; Kiouptsi, K.; Lillich, M.; Hendrikx, T.; Khandagale, A.; Kollar, B.; Hörmann, N.; Reiss, C.; Subramaniam, S.; Wilms, E.; et al. Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via Toll-like receptor-2. Blood 2017, 130, 542–553. [Google Scholar] [CrossRef]
- D’ Atri, L.P.; Schattner, M. Platelet toll-like receptors in thromboinflammation. Front. Biosci. 2017, 22, 1867–1883. [Google Scholar]
- Reinhardt, C. The involvement of Toll-like receptor-2 in arterial thrombus formation. Hamostaseologie 2018, 38, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.; Curtiss, L.K. Thematic review series: The immune system and atherogenesis. Paying the price for pathogen protection: Toll receptors in atherogenesis. J. Lipid Res. 2005, 46, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ascher, S.; Wilms, E.; Pontarollo, G.; Formes, H.; Bayer, F.; Müller, M.; Malinarich, F.; Grill, A.; Bosmann, M.; Saffarzadeh, M.; et al. Gut microbiota restricts NETosis in acute mesenteric ischemia-reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Hu, L.; Zhai, L.; Xue, R.; Ye, J.; Chen, L.; Cheng, G.; Mruk, J.; Kunapuli, S.P.; et al. Nucleotide-binding oligomerization domain 2 receptor is expressed in platelets and enhances platelet activation and thrombosis. Circulation 2015, 131, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Stepankova, R.; Tonar, Z.; Bartova, J.; Nedorost, L.; Rossman, P.; Poledne, R.; Schwarzer, M.; Tlaskalova-Hogenova, H. Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J. Atheroscler. Thromb. 2010, 17, 796–804. [Google Scholar] [CrossRef]
- Lindskog Jonsson, A.; Caesar, R.; Akrami, R.; Reinhardt, C.; Fåk Hållenius, F.; Borén, J.; Bäckhed, F. Impact of gut microbiota and diet on the development of atherosclerosis in Apoe −/− mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2318–2326. [Google Scholar] [CrossRef]
- Kiouptsi, K.; Jäckel, S.; Pontarollo, G.; Grill, A.; Kuijpers, M.J.E.; Wilms, E.; Weber, C.; Sommer, F.; Nagy, M.; Neideck, C.; et al. The microbiota promotes arterial thrombosis in low-density lipoprotein receptor-deficient mice. mBio 2019, 10, e02298-19. [Google Scholar] [CrossRef]
- Kiouptsi, K.; Pontarollo, G.; Todorov, H.; Braun, J.; Jäckel, S.; Koeck, T.; Bayer, F.; Karwot, C.; Karpi, A.; Gerber, S.; et al. Germ-free housing conditions do not affect aortic root and aortic arch lesion size of late atherosclerotic low-density lipoprotein receptor-deficient mice. Gut Microbes 2020, 11, 1809–1823. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Kuijpers, M.J.E.; Schulte, V.; Bergmeier, W.; Lindhout, T.; Brakebusch, C.; Offermanns, S.; Fässler, R.; Heemskerk, J.W.M.; Nieswandt, B. Complementary roles of glycoprotein VI and alpha2beta1 integrin in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003, 17, 685–687. [Google Scholar] [CrossRef]
- Lecut, C.; Schoolmeester, A.; Kuijpers, M.J.E.; Broers, J.L.V.; van Zandvoort, M.A.M.J.; Vanhoorelbeke, K.; Deckmyn, H.; Jandrot-Perrus, M.; Heemskerk, J.W.M. Principal role of glycoprotein VI in alpha2beta1 and alphaIIbbeta3 activation during collagen-induced thrombus formation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1727–1733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion mechanisms in platelet function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Savage, B.; Almus-Jacobs, F.; Ruggeri, Z.M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 1998, 94, 657–666. [Google Scholar] [CrossRef]
- Piétu, G.; Ribba, A.S.; Chérel, G.; Meyer, D. Epitope mapping by cDNA expression of a monoclonal antibody which inhibits the binding of von Willebrand factor to platelet glycoprotein IIb/IIIa. Biochem. J. 1992, 284, 711–715. [Google Scholar] [CrossRef]
- Savage, B.; Shattil, S.J.; Ruggeri, Z.M. Modulation of platelet function through adhesion receptors. A dual role for glycoprotein IIb-IIIa (integrin alpha IIb beta 3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J. Biol. Chem. 1992, 267, 11300–11306. [Google Scholar] [PubMed]
- Stark, K.; Philippi, V.; Stockhausen, S.; Busse, J.; Antonelli, A.; Miller, M.; Schubert, I.; Hoseinpour, P.; Chandraratne, S.; von Brühl, M.L.; et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016, 128, 2435–2449. [Google Scholar] [CrossRef]
- Kainoh, M.; Ikeda, Y.; Nishio, S.; Nakadate, T. Glycoprotein Ia/IIa-mediated activation-dependent platelet adhesion to collagen. Thromb. Res. 1992, 65, 165–176. [Google Scholar] [CrossRef]
- Laduca, F.M.; Bell, W.R.; Bettigole, R.E. Platelet-collagen adhesion enhances platelet aggregation induced by binding of VWF to platelets. Am. J. Physiol 1987, 253, H1208–H1214. [Google Scholar] [CrossRef]
- Kaiser, R.; Tang, L.F.; Taylor, K.E.; Sterba, K.; Nititham, J.; Brown, E.E.; Edberg, J.C.; McGwin, G., Jr.; Alarcon, G.S.; Ramsey-Goldman, R.; et al. A polymorphism in TLR2 is associated with arterial thrombosis in a multiethnic population of patients with systemic lupus erythematosus. Arthritis Rheumatol. 2014, 66, 1882–1887. [Google Scholar] [CrossRef]
- Grüner, S.; Prostredna, M.; Aktas, B.; Moers, A.; Schulte, V.; Krieg, T.; Offermanns, S.; Eckes, B.; Nieswandt, B. Anti–glycoprotein VI treatment severely compromises hemostasis in mice with reduced α2β1 levels or concomitant aspirin therapy. Circulation 2004, 110, 2946–2951. [Google Scholar] [CrossRef]
| Antibody | Clone | Target Molecule | Company |
|---|---|---|---|
| PE-conjugated rat anti-mouse Integrin αIIbβ3 | JON/A | activated mouse integrin alpha IIb beta 3 | Emfret Analytics, Eibelstad, Germany |
| Rat anti-mouse GPIbα | 5A7 | GPIbα | MERU Vasimmune |
| FITC-labeled Rat-antimouse GPIbβ | Xia.C3 | GPIbβ | Emfret Analytics, Eibelstad, Germany |
| FITC-rat anti-mouse GPVI | JAQ1 Rat IgG2A | GPVI | Emfret Analytics, Eibelstad, Germany |
| PE-Anti-Mo CD49c | PE-Anti-Mo CD49c | Integrin α2 | eBioscience, San Diego, California |
| FITC-Rat Anti-Mouse CD41 | MWReg30 | Integrin αIIb | BD Pharmigen San Jose, California |
| Anti-Rat IgG -FITC | - | - | Emfret Analytics, Eibelstad, Germany |
| Rat IgG 2aκ | eBR2a | - | eBioscience, San Diego, CA, USA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiouptsi, K.; Jäckel, S.; Wilms, E.; Pontarollo, G.; Winterstein, J.; Karwot, C.; Groß, K.; Jurk, K.; Reinhardt, C. The Commensal Microbiota Enhances ADP-Triggered Integrin αIIbβ3 Activation and von Willebrand Factor-Mediated Platelet Deposition to Type I Collagen. Int. J. Mol. Sci. 2020, 21, 7171. https://doi.org/10.3390/ijms21197171
Kiouptsi K, Jäckel S, Wilms E, Pontarollo G, Winterstein J, Karwot C, Groß K, Jurk K, Reinhardt C. The Commensal Microbiota Enhances ADP-Triggered Integrin αIIbβ3 Activation and von Willebrand Factor-Mediated Platelet Deposition to Type I Collagen. International Journal of Molecular Sciences. 2020; 21(19):7171. https://doi.org/10.3390/ijms21197171
Chicago/Turabian StyleKiouptsi, Klytaimnistra, Sven Jäckel, Eivor Wilms, Giulia Pontarollo, Jana Winterstein, Cornelia Karwot, Kathrin Groß, Kerstin Jurk, and Christoph Reinhardt. 2020. "The Commensal Microbiota Enhances ADP-Triggered Integrin αIIbβ3 Activation and von Willebrand Factor-Mediated Platelet Deposition to Type I Collagen" International Journal of Molecular Sciences 21, no. 19: 7171. https://doi.org/10.3390/ijms21197171
APA StyleKiouptsi, K., Jäckel, S., Wilms, E., Pontarollo, G., Winterstein, J., Karwot, C., Groß, K., Jurk, K., & Reinhardt, C. (2020). The Commensal Microbiota Enhances ADP-Triggered Integrin αIIbβ3 Activation and von Willebrand Factor-Mediated Platelet Deposition to Type I Collagen. International Journal of Molecular Sciences, 21(19), 7171. https://doi.org/10.3390/ijms21197171







