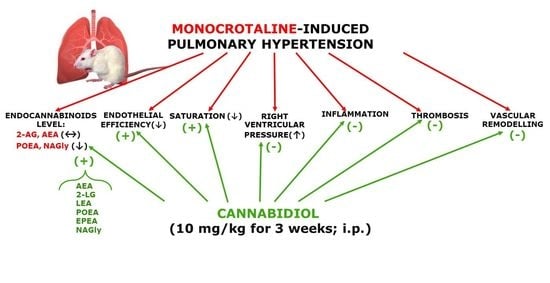
Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats
Abstract
1. Introduction
2. Results
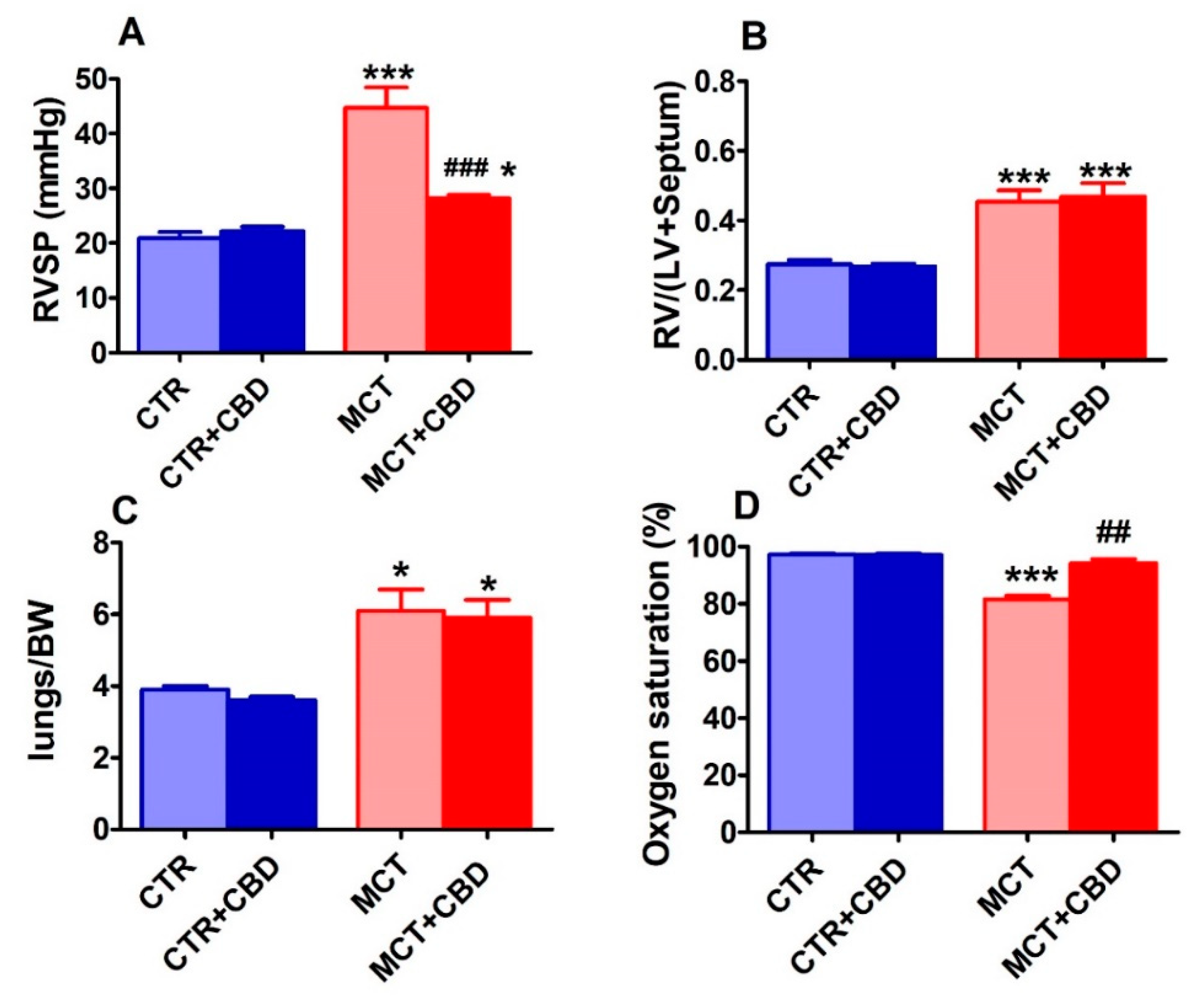
2.1. Effect of PH and Chronic Administration of CBD on RVSP, the Fulton Index, Lung/Weight Ratio and Blood Oxygen Saturation
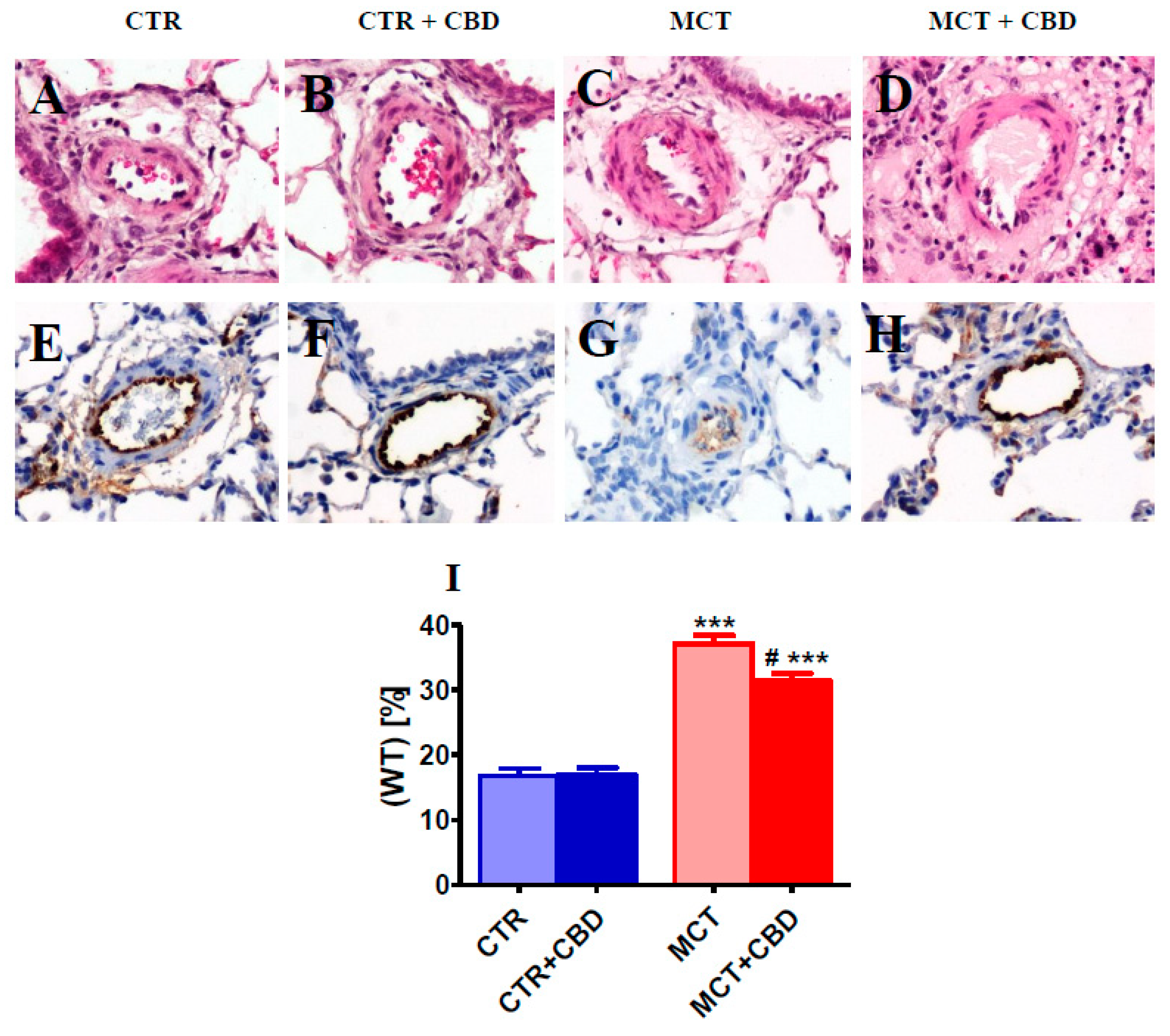
2.2. Effect of PH and Chronic Administration of CBD on Pulmonary Artery Remodeling
2.3. Effect of PH and Chronic Administration of CBD on Pulmonary Artery Vasoreactivity
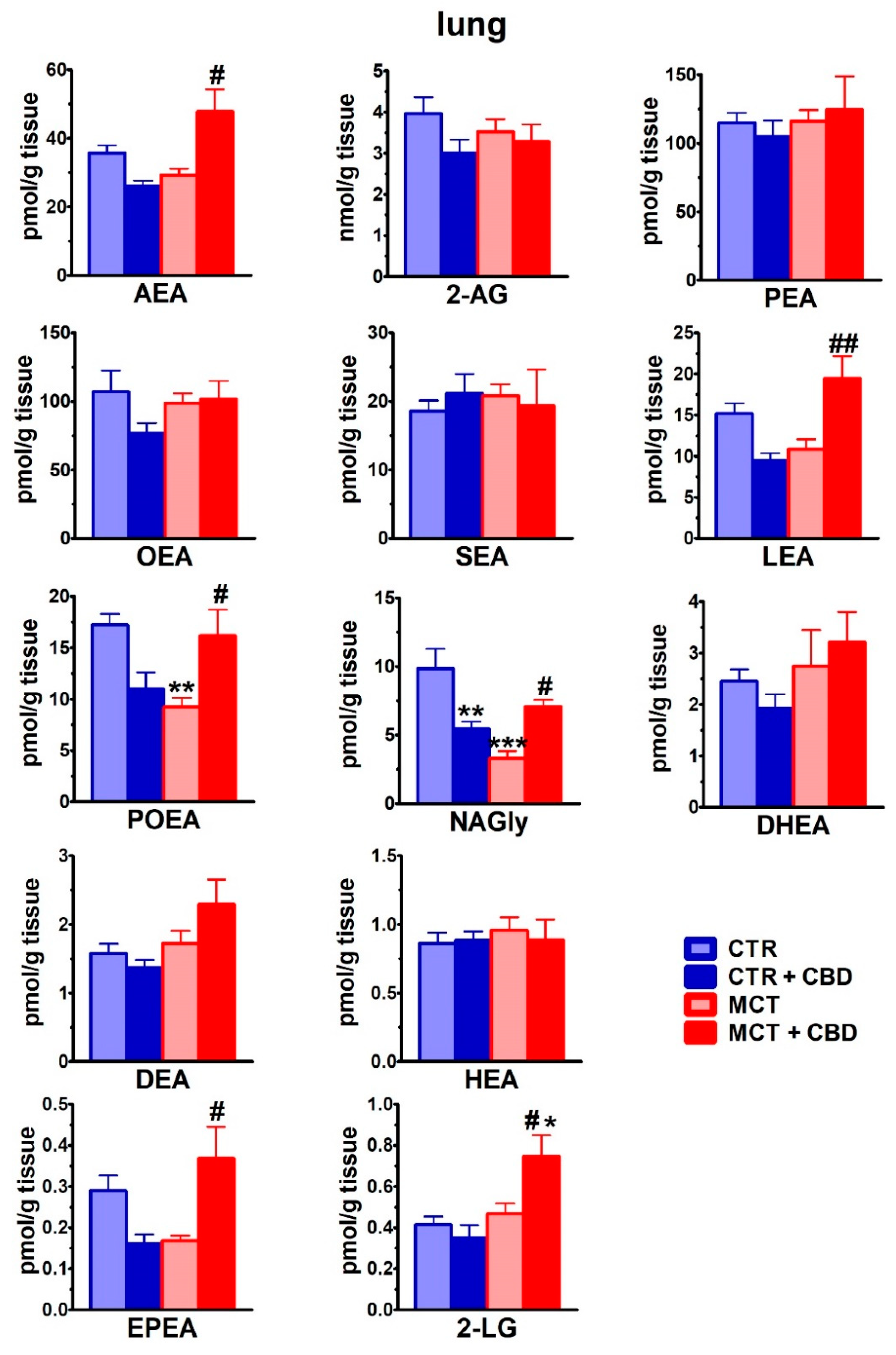
2.4. Effect of PH and Chronic Administration of CBD on the Lung Levels of Endocannabinoids and Endocannabinoid-Related Lipids
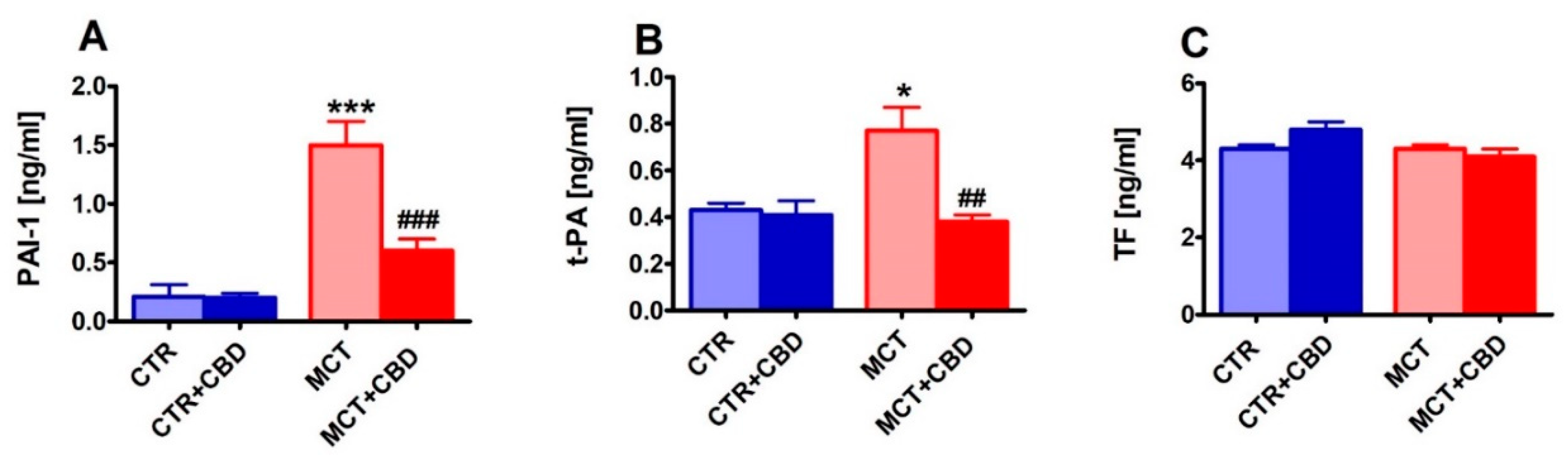
2.5. Effect of PH and Chronic Administration of CBD on Hemostatic Parameters and Blood Morphology
3. Discussion
3.1. Changes Related to PH
3.2. Influence of CBD on the MCT-Induced PH
3.3. Limitations
4. Materials and Methods
4.1. Animals
4.2. Monocrotaline and CBD Treatment
4.3. Blood Oxygen Saturation Measurements
4.4. Hemodynamic Parameters
4.4.1. Measurements of BP
4.4.2. Right Ventricular Systolic Pressure Measurements
4.5. Template Bleeding Time
4.6. Platelet Adhesion to Fibrillar Collagen Ex Vivo
4.7. Hemostatic Parameters and Blood Morphology
4.8. Measurement of Organ Weight
4.9. Pulmonary Artery Preparation
Functional Studies of rPAs
4.10. Morphometric Analysis of rPAs
4.11. Immunohistochemistry
4.12. Quantification of Endocannabinoids and Endocannabinoid-Related Lipids
4.13. Drugs
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | acetonitrile |
| AEA | anandamide |
| 2-AG | 2-arachidonoylglycerol |
| ANOVA | analysis of variance |
| BP | blood pressure |
| BT | bleeding time |
| BW | body weight |
| CBD | cannabidiol |
| CRC | concentration response curve |
| CTR | control |
| DEA | docosatetraenoyl ethanolamide |
| DHEA | docosahexaenoyl ethanolamide |
| EPEA | eicosapentaenoyl ethanolamide |
| FAAH | fatty acid amide hydrolase |
| HEA | homo-γ-linolenyl ethanolamide |
| HR | heart rate |
| LEA | linolenoyl ethanolamide |
| 2-LG | linoleoylglycerol |
| LV | left ventricle |
| MCT | monocrotaline |
| NAGly | N-arachidonoyl glycine |
| OEA | oleoyl ethanolamide |
| PAH | pulmonary arterial hypertension |
| PH | pulmonary hypertension |
| PAI-1 | plasminogen activator inhibitor type 1 |
| PEA | palmitoyl ethanolamide |
| POEA | palmitoleoyl ethanolamide |
| rPAs | rat pulmonary arteries |
| RVSP | right ventricular systolic pressure |
| RV | right ventricle |
| SBP | systolic blood pressure |
| SEA | stearoyl ethanolamide |
| TF | tissue factor |
| t-PA | tissue plasminogen activator |
| vWF | von Willebrand factor |
References
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef] [PubMed]
- Cassano, T.; Villani, R.; Pace, L.; Carbone, A.; Bukke, V.N.; Orkisz, S.; Avolio, C.; Serviddio, G. From Cannabis sativa to cannabidiol: Promising therapeutic candidate for the treatment of neurodegenerative diseases. Front. Pharmacol. 2020, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol improves vasorelaxation in Zucker diabetic fatty rats through cyclooxygenase activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Kozłowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: Modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef]
- Hložek, U.L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F.; Kuchař, M.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; MacLean, M.R.; Kozłowska, H.; Malinowska, B. Endothelium-dependent mechanisms of the vasodilatory effect of the endocannabinoid, anandamide, in the rat pulmonary artery. Pharmacol. Res. 2012, 66, 251–259. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kozłowski, M.; Schlicker, E.; Kloza, M.; Surażyński, A.; Grzęda, E.; Malinowska, B. Mechanisms of endothelium-dependent relaxation evoked by anandamide in isolated human pulmonary arteries. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 477–486. [Google Scholar] [CrossRef]
- Kozłowska, H.; Baranowska, M.; Schlicker, E.; Kozłowski, M.; Laudański, J.; Malinowska, B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J. Hypertens. 2007, 25, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, H.; Baranowska, M.; Schlicker, E.; Kozłowski, M.; Laudański, J.; Malinowska, B. Virodhamine relaxes the human pulmonary artery through the endothelial cannabinoid receptor and indirectly through a COX product. Br. J. Pharmacol. 2008, 155, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, O.; Baranowska-Kuczko, M.; Kloza, M.; Ambrożewicz, E.; Kozłowski, T.; Kasacka, I.; Malinowska, B.; Kozłowska, H. Activation of CB1 receptors by 2-arachidonoylglycerol attenuates vasoconstriction induced by U46619 and angiotensin II in human and rat pulmonary arteries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R883–R893. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, O.; Baranowska-Kuczko, M.; Malinowska, B.; Kloza, M.; Kusaczuk, M.; Gęgotek, A.; Golec, P.; Kasacka, I.; Kozłowska, H. Mechanisms of l-alpha-lysophosphatidylinositol-induced relaxation in human pulmonary arteries. Life Sci. 2018, 192, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hornig, B. Endothelial vasodilatory cannabinoid receptor in the human pulmonary artery: A future option in the therapy of pulmonary hypertension? J. Hypertens. 2007, 25, 2202–2203. [Google Scholar] [CrossRef]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic cannabidiol administration fails to diminish blood pressure in rats with primary and secondary hypertension despite its effects on cardiac and plasma endocannabinoid system, oxidative stress and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–40. [Google Scholar] [CrossRef]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; Martín de Llano, J.J.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid system: A target for cancer treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Formukong, E.A.; Evans, A.T.; Evans, F.J. The inhibitory effects of cannabinoids, the active constituents of Cannabis sativa L. on human and rabbit platelet aggregation. J. Pharm. Pharmacol. 1989, 41, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Shih, H.J.; Huang, I.T.; Tsai, P.S.; Chen, K.J.; Huang, H.J. Magnesium sulfate mitigates the progression of monocrotaline pulmonary hypertension in rats. Int. J. Mol. Sci. 2019, 20, 4622. [Google Scholar] [CrossRef]
- Kmecova, Z.; Veteskova, J.; Lelkova-Zirova, K.; Bies Pivackova, L.; Doka, G.; Malikova, E.; Paulis, L.; Krenek, P.; Klimas, J. Disease severity-related alterations of cardiac microRNAs in experimental pulmonary hypertension. J. Cell. Mol. Med. 2020, 24, 6943–6951. [Google Scholar] [CrossRef]
- Hill, N.S.; Gillespie, M.N.; McMurtry, I.F. Fifty years of monocrotaline-induced pulmonary hypertension: What has it meant to the field? Chest 2017, 152, 1106–1108. [Google Scholar] [CrossRef]
- Hester, J.; Ventetuolo, C.; Lahm, T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr. Physiol. 2019, 10, 125–170. [Google Scholar] [CrossRef]
- Fowler, E.D.; Hauton, D.; Boyle, J.; Egginton, S.; Steele, D.S.; White, E. Energy metabolism in the failing right ventricle: Limitations of oxygen delivery and the creatine kinase system. Int. J. Mol. Sci. 2019, 20, 1805. [Google Scholar] [CrossRef]
- Christou, H.; Ossama, M.; Reslan, O.M.; Mam, V.; Tanbe, A.F.; Vitali, S.H.; Marlin Touma, M.; Arons, E.; Mitsialis, S.A.; Kourembanas Khalil, R.A. Improved pulmonary vascular reactivity and decreased hypertrophic remodeling during nonhypercapnic acidosis in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L875–L890. [Google Scholar] [CrossRef]
- Iyinikkel, J.; Murray, F. GPCRs in pulmonary arterial hypertension: Tipping the balance. Br. J. Pharmacol. 2018, 175, 3063–3079. [Google Scholar] [CrossRef]
- Berger, G.; Azzam, Z.S.; Hoffman, R.; Yigla, M. Coagulation and anticoagulation in pulmonary arterial hypertension. ISRAEL Med. Assoc. J. 2009, 11, 376–379. [Google Scholar]
- Hosokawa, S.; Haraguchi, G.; Sasaki, A.; Arai, H.; Muto, S.; Itai, A.; Doi, S.; Mizutani, S.; Isobe, M. Pathophysiological roles of nuclear factor kappaB (NF-κB) in pulmonary arterial hypertension: Effects of synthetic selective NF-κB inhibitor IMD-0354. Cardiovasc. Res. 2013, 99, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; Wang, J.; Pang, B.; Huang, X.; Burg, E.D.; Yuan, J.X.; Wang, C. Combination of sildenafil and simvastatin ameliorates monocrotaline-induced pulmonary hypertension in rats. Pulm. Pharmacol. Ther. 2010, 23, 4564. [Google Scholar] [CrossRef] [PubMed]
- Wahn, H.; Wolf, J.; Kram, F.; Frantz, S.; Wagner, J.A. The endocannabinoid arachidonyl ethanolamide (anandamide) increases pulmonary arterial pressure via cyclooxygenase-2 products in isolated rabbit lungs. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2491–H2496. [Google Scholar] [CrossRef] [PubMed]
- Avraham, Y.; Magen, I.; Zolotarev, O.; Vorobiav, L.; Nachmias, A.; Pappo, O.; Ilan, Y.; Berry, E.M.; Ackerman, Z. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist, in various rat tissues during the evolution of experimental cholestatic liver disease. Prostaglandins Leukot. Essent. Fatty Acids 2008, 79, 35–40. [Google Scholar] [CrossRef]
- Nomura, D.K.; Hudak, C.S.; Ward, A.M.; Burston, J.J.; Issa, R.S.; Fisher, K.J.; Abood, M.E.; Wiley, J.L.; Lichtman, A.H.; Casida, J.E. Monoacylglycerol lipase regulates 2-arachidonoylglycerol action and arachidonic acid levels. Bioorg. Med. Chem. Lett. 2008, 18, 5875–5878. [Google Scholar] [CrossRef]
- Wenzel, D.; Matthey, M.; Bindila, L.; Lerner, R.; Lutz, B.; Zimmer, A.; Fleischmann, B.K. Endocannabinoid anandamide mediates hypoxic pulmonary vasoconstriction. Proc. Natl. Acad. Sci. USA 2013, 110, 18710–18715. [Google Scholar] [CrossRef]
- Schwartz, M.; Böckmann, S.; Hinz, B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features by cannabidiol in vascular smooth muscle cells. Oncotarget 2018, 9, 34595–34616. [Google Scholar] [CrossRef]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In vivo cannabidiol treatment improves endothelium-dependent vasorelaxation in mesenteric arteries of zucker diabetic fatty rats. Front. Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Malinowska, B. Chronic cannabidiol treatment improves vascular function of hypertensive DOCA-salt rats in vascular bed specific manner. In Proceedings of the 29th Annual Symposium of the International Cannabinoid Research Society, Bethesda, MD, USA, 30 June–3 July 2019. [Google Scholar]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the hemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef]
- Kossakowski, R.; Schlicker, E.; Toczek, M.; Weresa, J.; Malinowska, B. Cannabidiol affects the Bezold-Jarisch reflex via TRPV1 and 5-HT3 receptors and has peripheral sympathomimetic effects in spontaneously hypertensive and normotensive rats. Front. Pharmacol. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Al Suleimani, Y.M.; Al Mahruqi, A.S. The endogenous lipid N-arachidonoyl glycine is hypotensive and nitric oxide-cGMP-dependent vasorelaxant. Eur. J. Pharmacol. 2017, 794, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Mermer, P.; Goldenberg, A.; Pfeil, U.; Paddenberg, R.; Weissmann, N.; Lochnit, G.; Kummer, W. TASK-1 potassium channel is not critically involved in mediating hypoxic pulmonary vasoconstriction of murine intra-pulmonary arteries. PLoS ONE 2017, 12, e0174071. [Google Scholar] [CrossRef] [PubMed]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, I. Endocannabinoids as guardians of metastasis. Int. J. Mol. Sci. 2016, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants (Basel) 2019, 9, 21. [Google Scholar] [CrossRef]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A(2A) receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef]
- Shayesteh, M.R.H.; Haghi-Aminjan, H.; Mousavi, M.J.; Momtaz, S.; Abdollahi, M. The protective mechanism of cannabidiol in cardiac injury: A systematic review of non-clinical studies. Curr. Pharm. Des. 2019, 25, 2499–2507. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Clozel, M.; Hess, P.; Rey, M.; Iglarz, M.; Binkert, C.; Qiu, C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp. Biol. Med. (Maywood) 2006, 231, 967–973. [Google Scholar]
- Ma, Z.; Mao, L.; Rajagopal, S. Hemodynamic characterization of rodent models of pulmonary arterial hypertension. J. Vis. Exp. 2016, 11, 53335. [Google Scholar] [CrossRef] [PubMed]
- Gromotowicz, A.; Szemraj, J.; Stankiewicz, A.; Zakrzeska, A.; Mantur, M.; Jaroszewicz, E.; Rogowski, F.; Chabielska, E. Study of the mechanisms of aldosterone prothrombotic effect in rats. J. Renin-Angiotensin-Aldosterone Syst. 2011, 12, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zakrzeska, A.; Gromotowicz-Popławska, A.; Szemraj, J.; Szoka, P.; Kisiel, W.; Purta, T.; Kasacka, I.; Chabielska, E. Eplerenone reduces arterial thrombosis in diabetic rats. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Kloza, M.; Baranowska-Kuczko, M.; Toczek, M.; Kusaczuk, M.; Sadowska, O.; Kasacka, I.; Kozłowska, H. Modulation of cardiovascular function in primary hypertension in rat by SKA-31, an activator of KCa2.x and KCa3.1 channels. Int. J. Mol. Sci. 2019, 20, 4118. [Google Scholar] [CrossRef] [PubMed]
- Luque-Córdoba, D.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Study of sample preparation for determination of endocannabinoids and analogous compounds in human serum by LC-MS/MS in MRM mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef] [PubMed]

| Group | CTR | CTR+CBD | MCT | MCT+CBD |
|---|---|---|---|---|
| Ach | (7) | (7) | (7) | (7) |
| pEC50 | 6.0 ± 0.1 | 6.0 ± 0.1 | 5.3 ± 0.1 *** | 5.4 ± 0.1 ** |
| Emax (%) | 72.7 ± 2.9 | 69.0 ± 7.4 | 43.1 ± 3.9 * | 77.3 ± 8.0 ## |
| SNP | (8) | (8) | (8) | (8) |
| pEC50 | 6.9 ± 0.1 | 7.0 ± 0.1 | 6.2 ± 0.1 *** | 6.7 ± 0.1 ## |
| Emax (%) | 90.8 ± 6 | 90.9 ± 7.0 | 70.8 ± 3.9 * | 101.4 ± 5.9 ## |
| PHE | (7) | (7) | (7) | (7) |
| pEC50 | 7.1 ± 0.2 | 7.0 ± 0.1 | 6.8 ± 0.1 | 6.8 ± 0.1 |
| Emax (%) | 40.5 ± 9.3 | 41.8 ± 5.1 | 71.4 ± 7.0 | 53.4 ± 3.6 |
| U46619 | (8) | (8) | (8) | (8) |
| pEC50 | 7.0 ± 0.1 | 6.9 ± 0.1 | 7.2 ± 0.1 | 6.9 ± 0.1 |
| Emax (%) | 85.6 ± 7.1 | 98.5 ± 9.0 | 118.6 ± 7.8 * | 85.5 ± 6.9 # |
| Parameter | n | CTR | CTR+CBD | MCT | MCT+CBD |
|---|---|---|---|---|---|
| WBC (103/µL) | 8 | 2.4 ± 0.2 | 2.5 ± 0.4 | 5.0 ± 0.4 *** | 3.6 ± 0.3 # |
| RBC (106/µL) | 8 | 6.9 ± 0.2 | 6.9 ± 0.2 | 7.2 ± 0.2 | 7.4 ± 0.3 |
| HCT (%) | 8 | 40.5 ± 0.7 | 40.7 ± 1.2 | 42.1 ± 1.3 | 44.1 ± 2.1 |
| HGB (g/dL) | 8 | 13.6 ± 0.2 | 13.7 ± 0.2 | 14.5 ± 0.3 | 15.2 ± 0.6 |
| MCV (fl) | 8 | 58.0 ± 0.7 | 59.0 ± 0.3 | 57.7 ± 0.6 | 60.2 ± 0.8 |
| MCH (pg/cell) | 8 | 19.6 ± 0.3 | 19.9 ± 0.3 | 19.6 ± 0.4 | 20.5 ± 0.4 |
| MCHC (g/dL) | 8 | 33.7 ± 0.2 | 33.8 ± 0.3 | 34.3 ± 0.4 | 34.0 ± 0.3 |
| PLT (103/µL) | 8 | 575.8 ± 20.1 | 620.0 ± 7.8 | 611.9 ± 37.4 | 554.4 ± 22.4 |
| Bleeding time (s) | 8 | 97.6 ± 4.9 | 104.3 ± 4.0 | 101.7 ± 5.2 | 107.3 ± 9.5 |
| Platelet adhesion to collagen (%) | 8 | 29.3 ± 1.5 | 29.2 ± 2.1 | 32.6 ± 2.2 | 30.5 ± 1.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, O.; Baranowska-Kuczko, M.; Gromotowicz-Popławska, A.; Biernacki, M.; Kicman, A.; Malinowska, B.; Kasacka, I.; Krzyżewska, A.; Kozłowska, H. Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. Int. J. Mol. Sci. 2020, 21, 7077. https://doi.org/10.3390/ijms21197077
Sadowska O, Baranowska-Kuczko M, Gromotowicz-Popławska A, Biernacki M, Kicman A, Malinowska B, Kasacka I, Krzyżewska A, Kozłowska H. Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. International Journal of Molecular Sciences. 2020; 21(19):7077. https://doi.org/10.3390/ijms21197077
Chicago/Turabian StyleSadowska, Olga, Marta Baranowska-Kuczko, Anna Gromotowicz-Popławska, Michał Biernacki, Aleksandra Kicman, Barbara Malinowska, Irena Kasacka, Anna Krzyżewska, and Hanna Kozłowska. 2020. "Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats" International Journal of Molecular Sciences 21, no. 19: 7077. https://doi.org/10.3390/ijms21197077
APA StyleSadowska, O., Baranowska-Kuczko, M., Gromotowicz-Popławska, A., Biernacki, M., Kicman, A., Malinowska, B., Kasacka, I., Krzyżewska, A., & Kozłowska, H. (2020). Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. International Journal of Molecular Sciences, 21(19), 7077. https://doi.org/10.3390/ijms21197077