The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease
Abstract
1. Introduction
2. Biosynthesis and Pharmacology of Cannabidiol
2.1. Structure and Biosynthesis
2.2. Mechanism of Action
2.3. Pharmacokinetics
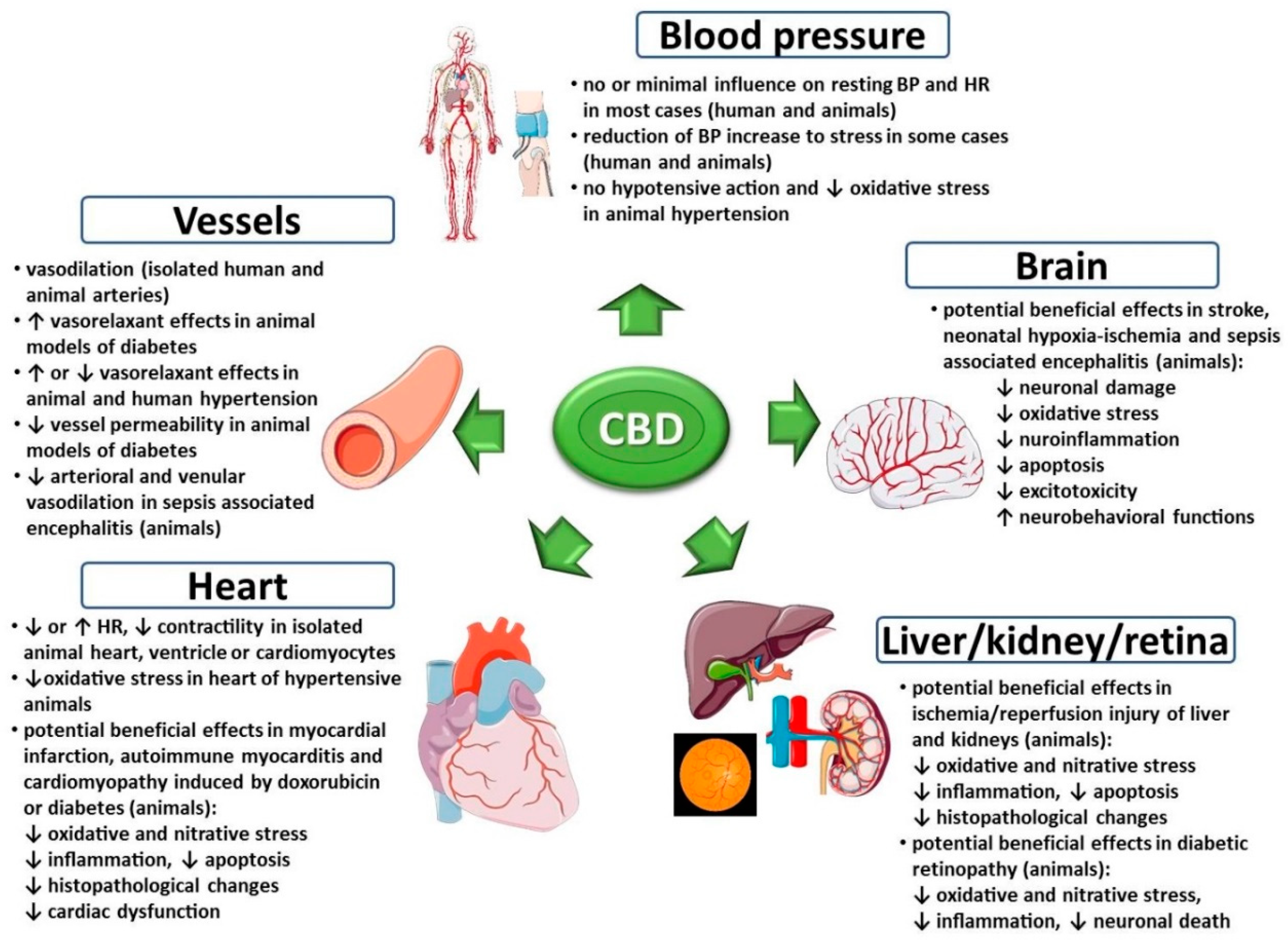
3. Effects of Cannabidiol on the Cardiovascular System under Physiological Conditions
| Species | Organ/Cells | Concentration | Effects 2 | References |
|---|---|---|---|---|
| Human 3 | Isolated mesenteric arteries (pre-constricted with U46619 4 and endothelin-1) | 0.1–100 μmol/L |
| [56] |
| 10 μmol/L (time-dependent effect) |
| |||
| Human 5 | Isolated pulmonary arteries (pre-constricted with U46619 4) | 0.1–30 μmol/L |
| [48] |
| 10 μmol/L (time-dependent effect) |
| |||
| Human | Human aortic endothelialcells (HAEC) | 0.1–30 μmol/L |
| [56] |
| Human | Human umbilical artery smoothmuscle cells (HUASMC) | 0.1–10 μmol/L |
| [107] |
| Rat | Isolated aorta (pre-constricted with U46619 4 and metoxamine 6) | 10 μmol/L (time-dependent effect) |
| [106] |
| Rat | Isolated small mesenteric arteries (pre-constricted with phenylephrine 6) | 0.1–30 μmol/L |
| [48] |
| Rat | Isolated perfused heart | 30 μmol/L |
| [108] |
| Rat | Isolated perfused heart | 9–100 μmol/L |
| [110] |
| Rat | Isolated left atrium | 0.001–30 μmol/L |
| [52] |
| Rat | Isolated ventricular cardiomyocytes | 0.01–10 μmol/L |
| [109] |
4. Effects of Cannabidiol on the Cardiovascular System under Pathological Conditions
4.1. Stress-Induced Cardiovascular Changes
4.2. Arterial Hypertension
4.3. Myocardial Ischemia/Infarction, Cardiomyopathies, Myocarditis
4.4. Stroke, Neonatal Hypoxic-Ischemic Encephalopathy, Sepsis-Associated Encephalitis
4.5. Renal and Hepatic Ischemia/Reperfusion Injury
4.6. Cardiovascular Complications of Diabetes
| Species | Experimental Model/Conditions | Dosage or Concentration | Effects 1 | References |
|---|---|---|---|---|
| 1. Stress-induced cardiovascular changes | ||||
| Human | Simulated public speaking | 300 mg; p.o. |
| [80] |
| Human | Simulated public speaking in patients with social anxiety disorder | 600 mg; p.o. |
| [112] |
| Human | Mental stress (mental arithmetic test), exercise stress (isometric hand-grip test) or cold stress (cold pressor test) | 600 mg; p.o. |
(just before and/or during and/or after the stress test) | [99] |
| Human | Exercise stress (isometric hand-grip test) | 600 mg; p.o. |
| [97] |
| 600 mg; for 7 days; p.o. |
| |||
| Rat | Contextual conditioned fear | 10 mg/kg; i.p. |
| [82] |
| Rat | Acute restraint stress | 1; 10; 20 mg/kg; i.p. |
| [55] |
| Rat | Acute restraint stress | 15; 30; 60 nmol; i.c. |
| [87] |
| Rat | Contextual conditioned fear | 15; 30; 60 nmol; into BNST |
| [90] |
| Rat | Acute restraint stress | 15; 30; 60 nmol; into BNST |
| [92] |
| 2. Arterial hypertension | ||||
| Human | Hypertensive patients 2; isolated mesenteric arteries (pre-constricted with U46619 3 and endothelin-1) | 0.1–100 μmol/L |
| [56] |
| Human | Hypertensive patients 4; isolated pulmonary arteries (pre-constricted with U46619 3) | 0.1–30 μmol/L |
| [48] |
| Rat | SHR (model of primary hypertension); conscious | 10 mg/kg; i.p. |
| [52] |
| SHR (model of primary hypertension); urethane anaesthetised, pithed and vagotmised | 1; 3; 30 mg/kg; i.v. |
(comparable with normotensive control) | ||
| SHR (model of primary hypertension); urethane anaesthetised | 3; 10; 30 mg/kg; i.v. (rapid) |
| ||
| SHR (model of primary hypertension); isolated left atrium | 0.001–30 μmol/L |
| ||
| Rat | SHR (model of primary hypertension) | 10 mg/kg; for 14 days; i.p. |
| [96] |
| DOCA-salt (model of secondary hypertension) |
| |||
| Rat | SHR (model of primary hypertension); isolated small mesenteric arteries (pre-constricted with phenylephrine) | 0.1–30 μmol/L |
| [48] |
| DOCA-salt (model of secondary hypertension); isolated small mesenteric arteries (pre-constricted with phenylephrine) |
| |||
| 3. Myocardial ischemia/infarction, cardiomyopathies, myocarditis | ||||
| Rabbit | LCx occlusion (90 min) + reperfusion (30 h); model of myocardial ischemia/infarction | 0.1 mg/kg; 10 min before occlusion and 10 min before reperfusion; i.v. |
| [116] |
| Rat | LAD occlusion (30 min) + reperfusion (7 days); model of myocardial ischemia/infarction | 5 mg/kg; before occlusion and every 24 h thereafter for 7 days; i.p. |
| [115] |
| LAD occlusion in isolated heart (45 min) + reperfusion (45 min); model of myocardial ischemia/infarction | 5 mg/kg; 24 h and 1 h before heart isolation; i.p. |
| ||
| Rat | LAD occlusion (30 min) + reperfusion (2 h); model of myocardial ischemia/infarction | 10 or 50 µg/kg; 10 min before occlusion; i.v. |
| [105] |
| 50 µg/kg; 10 min before reperfusion; i.v. |
| |||
| Rat | LAD occlusion (6 min) + reperfusion (6 min); model of myocardial ischemia/infarction | 50 µg/kg; 10 min before occlusion; i.v. |
| [49] |
| Rat | Doxorubicin-induced cardiomyopathy | 5 mg/kg; for 4 weeks; i.p. |
| [117] |
| Mouse | Doxorubicin-induced cardiomyopathy | 10 mg/kg; for 5 days; i.p. |
| [118] |
| Mouse | Experimental autoimmune myocarditis | 10 mg/kg; for 46 days; i.p. |
| [119] |
| 4. Stroke, neonatal hypoxic-ischemic encephalopathy, sepsis-related encephalitis | ||||
| Piglet (newborn) | Hypoxia and carotid arteries occlusion (20 min) + post-HI period (6 h); model of neonatal HIE | 0.1 mg/kg; 15 min and 240 min after HI; i.v. |
| [127] |
| Piglet (newborn) | Hypoxia and carotid arteries occlusion (40 min) + post-HI period (6 h); model of neonatal HIE | 1 mg/kg; 30 min after HI; i.v. |
(effects are dependent on CB2 and 5-HT1A) | [54] |
| Piglet (newborn) | Hypoxia and carotid arteries occlusion (20 min) + post-HI period (6 or 72 h); model of neonatal HIE | 0.1 mg/kg; 15 min and 240 min after HI; i.v. |
| [128] |
| Piglet (newborn) | Hypoxia and carotid arteries occlusion (40 min) + post-HI period (6 h); model of neonatal HIE | 1 mg/kg; 30 min after HI; i.v. |
| [129] |
| Piglet (newborn) | Hypoxia + post-hypoxic period (9,5 h); model of neonatal HIE | 1 mg/kg; 30 min after hypoxia; i.v. |
| [137] |
| Piglet(newborn) | Hypoxia + post-hypoxic period (6 h); model of neonatal HIE | 1 mg/kg; 30 min after hypoxia; i.v. |
| [130] |
| Piglet (newborn) | Hypoxia + post-hypoxic period (9,5 h); model of neonatal HIE | 50 mg/kg 5; 30 min after hypoxia; i.v. over 15 min. |
| [132] |
| Piglet(newborn) | Hypoxia and carotid arteries occlusion (20 min) + post-HI period (54 h); model of neonatal HIE | 1 mg/kg; 0.5, 24 and 48 h after HI; i.v. |
| [131] |
| Gerbil | Carotid arteries occlusion (10 min) + reperfusion (7 days); model of stroke | 1.25; 2.5; 5; 10 or 20 mg/kg; 5 min after occlusion; i.p. |
| [125] |
| Rat | MCA occlusion (90 min) + reperfusion (2 days); model of stroke | 5 mg/kg; at the onset of occlusion; i.v. + 20 mg/kg; 12 h after occlusion; i.p. |
| [120] |
| Rat (newborn) | Hypoxia (120 min) and left carotid artery electrocoagulation + post-HI period (7 or 30 days); model of neonatal HIE | 1 mg/kg; 10 min after hypoxia; s.c. |
| [134] |
| Rat (newborn) | MCA occlusion (3 h) + reperfusion (1 week or 1 month); model of neonatal stroke | 5 mg/kg; 15 min after occlusion; i.p. |
| [123] |
| Rat | MCA occlusion (1 h) + reperfusion (1 day); model of stroke | 50, 100 or 200 ng; for 5 days before occlusion; i.c.v. |
| [122] |
| Rat (newborn) | Hypoxia (112 min) and left carotid artery electrocoagulation + post-HI period (30 days); model of neonatal HIE | 1 mg/kg; 10 min after hypoxia; s.c. |
| [135] |
| Mouse | MCA occlusion (4 h) + reperfusion (20 h); model of stroke | 0.1; 1; 3 or 10 mg/kg; immediately before occlusion and 3 h after onset of the occlusion; i.p. |
| [53] |
| Mouse | MCA occlusion (4 h) + reperfusion (20 h or 3 days); model of stroke | 3 mg/kg; immediately before occlusion and 3 h after onset of the occlusion; i.p. |
| [50] |
| MCA occlusion (4 h) + reperfusion (20 h); model of stroke | 0.1; 1 or 3 mg/kg; immediately before occlusion and 3 h after onset of the occlusion; i.p. |
| ||
| 3 mg/kg; immediately before occlusion or 3, 4, 5, 6 h after onset of the occlusion; i.p. |
| |||
| Mouse | MCA occlusion (4 h) + reperfusion (20 h); model of stroke | 0.1; 1 or 3 mg/kg; immediately before occlusion and 3 h after onset of the occlusion; i.p. |
| [50] |
| 3 mg/kg; for 14 days before occlusion + immediately before occlusion and 3 h after onset of the occlusion; i.p. |
(effects are comparable to those observed in the group not treated with CBD for 14 days)
| |||
| Mouse | MCA occlusion (4 h) + reperfusion (20 h); model of stroke | 0.1; 1 or 3 mg/kg; immediately before occlusion and 3 h after onset of the occlusion; i.p. |
| [121] |
| MCA occlusion (4 h) + reperfusion (3 days); model of stroke | 3 mg/kg; immediately before occlusion and 3 h after onset of the occlusion; i.p. |
| ||
| Mouse | MCA occlusion (4 h) + reperfusion (14 days); model of stroke | 3 mg/kg; for 14, 12 or 10 days from day 1, 3 or 5, respectively; i.p. |
(effects for CBD administered from day 1 and 3, but not from day 5) | [124] |
| Mouse(newborn) | Forebrain slices underwent oxygen and glucose deprivation;in vitro model of neonatal HIE | 100 μmol/L |
(effects are dependent on CB2 and A2; independent on CB1; excitotoxicity is also A1 dependent) | [136] |
| Mouse | Lipopolysaccharide-induced encephalitis;model of sepsis-related encephalitis | 3 mg/kg; i.v. |
| [138] |
| Mouse | Carotid arteries occlusion (17 min) + reperfusion (7 days); model of stroke | 3, 10 or 30 mg/kg; 30 min before and 3, 24 and 48 h after occlusion; i.p. |
| [126] |
| Mouse(newborn) | Hypoxia (90 min) and left carotid artery electrocoagulation + post-HI period (7 days); model of neonatal HIE | 1 mg/kg; 15 min, 1, 3, 6, 12, 18 or 24 h after HI; s.c. |
(effects for CBD administered up to 18 h after HI)
(effects for CBD administered 24 h after HI) | [133] |
| 5. Renal and hepatic ischemia/reperfusion injury | ||||
| Human | Human liver sinusoidal endothelial cells (HLSEC) stimulated with TNF-α | 1 μmol/L |
(effects are independent on CB1 and CB2) | [141] |
| Rat | Pedicle of the left hepatic lobe occlusion (30 min) + reperfusion (72 h) | 5 mg/kg; 1 h after occlusion and every 24 h thereafter for 2 days; i.v. |
| [140] |
| Rat | Renal vascular pedicles occlusion (30 min) + reperfusion (24 h) | 5 mg/kg; 1 h before and 12 h after occlusion; i.v. |
| [139] |
| Mouse | Hepatic artery and portal vein occlusion (1 h) + reperfusion (2, 6 or 24 h) | 3 or 10 mg/kg; 2 h before or 90 min after occlusion; i.p. |
| [141] |
| 6. Diabetes and its cardiovascular complications | ||||
| Human | Human coronary artery endothelial cells (HCAEC) exposed to high glucose | 1.5–6 μmol/L; 48 h |
(above effects are independent on CB1 and CB2)
| [146] |
| Human | Human cardiomyocytes exposed to high glucose | 4 μmol/L; 48 h |
| [143] |
| Human | Type 2 diabetic patients 2; isolated mesenteric arteries (pre-constricted with U46619 3 and endothelin-1) | 0.1–100 μmol/L |
| [56] |
| Human | Type 2 diabetic patients | 100 mg; twice a day; for 13 weeks; p.o. |
| [145] |
| Human | Type 2 diabetic patients 4; isolated pulmonary arteries (pre-constricted with U46619 3) | 0.1–30 μmol/L |
| [48] |
| Rat | Streptozotocin-induced diabetes (model of type 1 diabetes) | 10 mg/kg (every 2 days); for 1, 2 or 4 weeks; i.p. |
| [142] |
| Rat | ZDF (model of type 2 diabetes); isolated aorta and femoral artery | 10 µmol/L; 2 h |
| [147] |
| Rat | ZDF (model of type 2 diabetes); isolated femoral artery | 10 μmol/L; 2 h |
| [57] |
| Rat | ZDF (model of type 2 diabetes) | 10 mg/kg; for 7 days; i.p. |
| [144] |
| Mouse | Streptozotocin-induced diabetes (model of type 1 diabetes) | 1, 10 or 20 mg/kg; for 4 or 11 weeks; i.p. |
| [143] |
5. Effects of Abnormal-Cannabidiol on the Cardiovascular System
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| 5-, 15-LOX | 5-, 15-Lipooxygenase |
| 5-HT1A/2A/3 | Serotonin receptors type 1A,2A, 3 |
| 7-COOH-, 7-OH-CBD | 7-Carboxy-, 7-hydroxycannabidiol |
| A1/2 | Adenosine receptor type 1, 2 |
| Abn-CBD | Abnormal-cannabidiol |
| AEA | Anandamide |
| Akt | Protein kinase B |
| BNST | Bed nucleus of the stria terminalis |
| BP | Blood pressure |
| CB1, 2 | Cannabinoid receptor type 1, 2 |
| CBC | Cannabichromene |
| CBCA | Cannabichromenic acid |
| CBD | Cannabidiol |
| CBF | Cerebral blood flow |
| CBGA | Cannabigerolic acid |
| COX-1, -2 | Cyclooxygenase 1, 2 |
| CREB | cAMP response element-binding protein |
| CYP | Cytochrome P450 |
| D2 | Dopamine receptor type 2 |
| DBP | Diastolic blood pressure |
| DMAPP | Dimethylallyl diphosphate |
| DOCA-salt | Deoxycorticosterone acetate-salt (model of hypertension) |
| EMT | Endocannabinoid membrane transporter |
| EP1, 4 | Prostaglandin E receptor 1, 4 |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| FAAH | Fatty acid amide hydrolase |
| FABP-3, -5, -7 | Fatty acid binding protein 3, 5, 7 |
| GABAA | γ-Aminobutyric acid receptor type A |
| GPP | Geranyl diphosphate |
| GPR3, 6, 12, 18, 55 | G-protein coupled receptor 3, 6, 12, 18, 55 |
| HIE | Hypoxic-ischemic encephalopathy |
| HR | Heart rate |
| i.p. | Intraperitoneally |
| i.v. | Intravenously |
| ICAM-1 | Intercellular adhesion molecule 1 |
| INR | International normalized ratio |
| IP | Prostacyclin receptor |
| IPP | Isopentenyl diphosphate |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinases |
| MBP | Mean blood pressure |
| MCA | Middle cerebral artery |
| MEP | 2-Methylerythritol 4-phosphate |
| MEV | Mevalonic acid |
| NF-кB | Nuclear factor κB |
| OA | Olivetoleic acid |
| p.o. | Per os, orally |
| p70S6K | Ribosomal protein S6 kinase |
| PGE | Prostaglandin E |
| PLA2 | Phospholipase A2 |
| PPAR-γ | Peroxisome proliferator-activated receptor γ |
| RVLM | Rostral ventrolateral medulla |
| SBP | Systolic blood pressure |
| SHR | Spontaneously hypertensive rat |
| STAT5 | Signal transducer and activator of transcription 5 |
| THC | Δ9-Tetrahydrocannabinol |
| THCA | Δ9-Tetrahydrocannabinolic acid |
| TNF-α | Tumour necrosis factor α |
| TP | Thromboxane receptor |
| TRP | Transient receptor potential |
| TRPA1 | Transient receptor potential ankyrin subfamily member 1 |
| TRPM8 | Transient receptor potential melastatin subfamily member 8 |
| TRPV1-4 | Transient receptor potential vanilloid subfamily members 1-4 |
| VCAM-1 | Vascular cell adhesion protein 1 |
| VEGF | Vascular endothelial growth factor |
| ZDF | Zucker Diabetic Fatty rat |
| α1-, α1β-, α3-GlyR | α1, α1β-, α3-Glycine receptor |
| α1-AR | α1-Adrenergic receptor |
| δ-, μ-OR | δ-, μ-Opioid receptor |
References
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa. L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Henschke, P. Cannabis: An ancient friend or foe? What works and doesn’t work. Semin. Fetal. Neonatal. Med. 2019, 24, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. The Plant of the Thousand and One Molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Pina, S.D.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Solowij, N.; Broyd, S.; Greenwood, L.M.; van Hell, H.; Martelozzo, D.; Rueb, K.; Todd, J.; Liu, Z.; Galettis, P.; Martin, J.; et al. A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: Acute intoxication effects. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 17–35. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Laun, A.S.; Shrader, S.H.; Brown, K.J.; Song, Z.H. GPR3, GPR6, and GPR12 as novel molecular targets: Their biological functions and interaction with cannabidiol. Acta. Pharmacol. Sin. 2019, 40, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Leishman, E.; Manchanda, M.; Thelen, R.; Miller, S.; Mackie, K.; Bradshaw, H.B. Cannabidiol’s Upregulation of N-acyl Ethanolamines in the Central Nervous System Requires N-acyl Phosphatidyl Ethanolamine-Specific Phospholipase D. Cannabis Cannabinoid Res. 2018, 3, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, 94. [Google Scholar] [CrossRef]
- Ligresti, A.; Schiano Moriello, A.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef]
- Long, L.E.; Chesworth, R.; Huang, X.F.; McGregor, I.S.; Arnold, J.C.; Karl, T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int. J. Neuropsychopharmacol. 2010, 13, 861–876. [Google Scholar] [CrossRef]
- Malinowska, B.; Baranowska-Kuczko, M.; Schlicker, E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012, 165, 2073–2088. [Google Scholar] [CrossRef]
- Malinowska, M.; Toczek, M.; Pędzińska-Betiuk, A.; Schlicker, E. Cannabinoids in arterial, pulmonary and portal hypertension–Mechanisms of action and potential therapeutic significance. Br. J. Pharmacol. 2019, 176, 1395–1411. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ(9)-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.R.; Millar, S.A.; England, T.J.; O’Sullivan, S.E. A Systematic Review and Meta-Analysis of the Haemodynamic Effects of Cannabidiol. Front. Pharmacol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Sultan, S.R.; Millar, S.A.; O’Sullivan, S.E.; England, T.J. A Systematic Review and Meta-Analysis of the In Vivo Haemodynamic Effects of ∆9-Tetrahydrocannabinol. Pharmaceuticals 2018, 11, 13. [Google Scholar] [CrossRef]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef]
- Franco, V.; Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 2019, 79, 1435–1454. [Google Scholar] [CrossRef]
- Bedrocan (Producer of Legal Medicinal Cannabis) Website. Available online: https://bedrocan.com (accessed on 18 May 2020).
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Laezza, C.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol-from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef] [PubMed]
- Community Register of Orphan Medicinal Products (European Commision). Available online: https://ec.europa.eu/health/documents/community-register/html/reg_od_act.htm?sort=a (accessed on 18 May 2020).
- Sierra, S.; Luquin, N.; Navarro-Otano, J. The Endocannabinoid System in Cardiovascular Function: Novel Insights and Clinical Implications. Clin. Auton. Res. 2018, 28, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Hunt, M.; Clark, J.H. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Jacob, A.; Todd, A.R. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol. J. Chem. Soc. 1940, 649–653. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shvo, Y. Hashish–I. The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. The absolute configuration of Δ1-tetra-hydrocannabinol, the major active constituent of hashish. Tetrahedron. Lett. 1967, 12, 1109–1111. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content During Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Degenhardt, F.; Stehle, F.; Kayser, O. The Biosynthesis of Cannabinoids. In Handbook of Cannabis and Related Pathologies, 1st ed.; Preedy, R.V., Ed.; Elsevier: London, UK, 2017; pp. 13–23. [Google Scholar]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; El-Sohly, M.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; Piscitelli, F.; Gatta, L.; Vita, D.; De Petrocellis, L.; Palazzo, E.; de Novellis, V.; Di Marzo, V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br. J. Pharmacol. 2011, 162, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Bick-Sander, A.; Fabel, K.; Leal-Galicia, P.; Tauber, S.; Ramirez-Rodriguez, G.; Müller, A.; Melnik, A.; Waltinger, T.P.; Ullrich, O.; et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 2010, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.H.; Crestani, C.C.; Gomes, F.V.; Guimarães, F.S.; Correa, F.M.; Resstel, L.B. Cannabidiol injected into the bed nucleus of the stria terminalis modulates baroreflex activity through 5-HT1A receptors. Pharmacol. Res. 2010, 62, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Kozłowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries-modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Gonca, E.; Darici, F. The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias: The role of adenosine A1 receptors. J. Cardiovasc. Pharmacol Ther. 2015, 20, 76–83. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Hazekawa, M.; Irie, K.; Fujioka, M. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J. Neurochem. 2007, 102, 1488–1496. [Google Scholar] [CrossRef]
- Járai, Z.; Wagner, J.A.; Varga, K.; Lake, K.D.; Compton, D.R.; Martin, B.R.; Zimmer, A.M.; Bonner, T.I.; Buckley, N.E.; Mezey, E.; et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 14136–14141. [Google Scholar] [CrossRef]
- Kossakowski, R.; Schlicker, E.; Toczek, M.; Weresa, J.; Malinowska, B. Cannabidiol affects the Bezold-Jarisch reflex via TRPV1 and 5-HT3 receptors and his periperial sympathomimetic effects in spontaneously hypertensive and normotensive rats. Front. Pharmacol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Mishima, K.; Hayakawa, K.; Abe, K.; Ikeda, T.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine 1A receptor-dependent mechanism. Stroke 2005, 36, 1077–1082. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: Role of 5HT(1A) and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Resstel, L.B.N.; Tavares, R.F.; Lisboa, S.F.S.; Joca, S.R.L.; Corrêa, F.M.A.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol improves vasorelaxation in Zucker diabetic fatty rats through cyclooxygenase activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Watanabe, K.; Itokawa, Y.; Yamaori, S.; Funahashi, T.; Kimura, T.; Toshiyuki, K.; Usami, N.; Yamamoto, I. Conversion of cannabidiol to D9-tetrahydrocannabinol and related cannabinoids in artificial gastric juice, and their pharmacological effects in mice. Forensic Toxicol. 2007, 25, 16–21. [Google Scholar] [CrossRef]
- Merrick, J.; Lane, B.; Sebree, T.; Yaksh, T.; O’Neill, C.; Banks, S.L. Identification of Psychoactive Degradants of Cannabidiol in Simulated Gastric and Physiological Fluid. Cannabis Cannabinoid Res. 2016, 1, 102–112. [Google Scholar] [CrossRef]
- Grotenhermen, F.; Russo, E.; Waldo-Zuardi, A. Even High Doses of Oral Cannabidol Do Not Cause THC-Like Effects in Humans: Comment on Merrick et al. Cannabis and Cannabinoid Research 2016;1(1):102-112; doi:10.1089/can.2015.0004. Cannabis Cannabinoid. Res. 2017, 2, 1–4. [Google Scholar] [CrossRef]
- Nahler, G.; Grotenhermen, F.; Waldo-Zuardi, A.; Crippa, J.A.S. A Conversion of Oral Cannabidiol to ∆9-Tetrahydrocannabinol Seems Not to Occur in Humans. Cannabis Cannabinoid Res. 2017, 2, 81–86. [Google Scholar] [CrossRef]
- Hložek, T.; Uttl, L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC + CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef]
- Palazzoli, F.; Citti, C.; Licata, M.; Vilella, A.; Manca, L.; Zoli, M.; Vandelli, M.A.; Forni, F.; Cannazza, G. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J. Pharm. Biomed. Anal. 2018, 150, 25–32. [Google Scholar] [PubMed]
- Wray, L.; Stott, C.; Jones, N.; Wright, S. Cannabidiol Does Not Convert to D9-Tetrahydrocannabinol in an In Vivo Animal Model. Cannabis Cannabinoid Res. 2017, 2, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Grayson, L.; Vines, B.; Nichol, K.; Szaflarski, J.P. UAB CBD Program. An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav. Case. Rep. 2017, 9, 10–11. [Google Scholar] [CrossRef]
- Stanley, C.; O’Sullivan, S.E. Vascular targets for cannabinoids: Animal and human studies. Br. J. Pharmacol. 2014, 171, 1361–1378. [Google Scholar] [CrossRef]
- Richter, J.S.; Quenardelle, V.; Rouyer, O.; Raul, J.S.; Beaujeux, R.; Gény, B.; Wolff, V. A Systematic Review of the Complex Effects of Cannabinoids on Cerebral and Peripheral Circulation in Animal Models. Front. Physiol. 2018, 9, 622. [Google Scholar] [CrossRef]
- Bondarenko, A.I. Endothelial atypical cannabinoid receptor: Do we have enough evidence? Br. J. Pharmacol. 2014, 171, 5573–5588. [Google Scholar] [CrossRef]
- Graham, J.D.P.; Li, D.M.F. Cardiovascular and respiratory effects of cannabis in cat and rat. Br. J. Pharmacol. 1973, 49, 1–10. [Google Scholar] [CrossRef]
- Belgrave, B.E.; Bird, K.D.; Chesher, G.B.; Jackson, D.M.; Lubbe, K.E.; Starmer, G.A.; Teo, R.K.C. The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacology 1979, 64, 243–246. [Google Scholar] [CrossRef]
- Cunha, J.M.; Carlini, E.A.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 1980, 21, 175–185. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Shirakawa, I.; Finkelfarb, E.; Karniol, I.G. Action of cannabidiol on the anxiety and other effects produced by ∆9-THC in normal subjects. Psychopharmacology 1982, 76, 245–250. [Google Scholar] [CrossRef]
- Gong, H.; Tashkin, D.P.; Simmons, M.S.; Calvarese, B.; Shapiro, B.J. Acute and subacute bronchial effects of oral cannabinoids. Clin. Pharmacol. Ther. 1984, 35, 26–32. [Google Scholar] [CrossRef]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimarães, F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993, 7, 82–88. [Google Scholar] [CrossRef]
- McQueen, D.S.; MBond, S.; Smith, P.J.W.; Balali-Mood, K.; Smart, D. Cannabidiol lacks the vanilloid VR1-mediated vasorespiratory effects of capsaicin and anandamide in anaesthetised rats. Eur. J. Pharmacol. 2004, 491, 181–189. [Google Scholar] [CrossRef]
- Resstel, L.B.; Joca, S.R.; Moreira, F.A.; Corrêa, F.M.; Guimarães, F.S. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav. Brain. Res. 2006, 172, 294–298. [Google Scholar] [CrossRef]
- Borgwardt, S.J.; Allen, P.; Bhattacharyya, S.; Fusar-Poli, P.; Crippa, J.A.; Seal, M.L.; Fraccaro, V.; Atakan, Z.; Martin-Santos, R.; O’Carroll, C.; et al. Neural basis of Δ-9-tetrahydrocannabinol and cannabidiol: Effects during response inhibition. Biol. Psychiatry 2008, 64, 966–973. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Fusar-Poli, P.; Borgwardt, S.; Martin-Santos, R.; Nosarti, C.; O’Carroll, C.; Allen, P.; Seal, M.L.; Fletcher, P.C.; Crippa, J.A.; et al. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol: A neural basis for the effects of Cannabis sativa on learning and psychosis. Arch. Gen. Psychiatry 2009, 66, 442–451. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Crippa, J.A.; Bhattacharyya, S.; Borgwardt, S.J.; Allen, P.; Martin-Santos, R.; Seal, M.; Surguladze, S.A.; O’Carrol, C.; Atakan, Z.; et al. Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry 2009, 66, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’Carroll, C.M.; Seal, M.; Allen, P.; et al. Opposite effects of Δ-9-tetrahydrocannainol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Granjeiro, E.M.; Gomes, F.V.; Guimarães, F.S.; Fernando, M.A.; Corrêa, F.M.A.; Leonardo, B.M.; Resstel, L.B.M. Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol. Biochem. Behav. 2011, 99, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Hallak, J.E.; Dursun, S.M.; Bosi, D.C.; de Macedo, L.R.; Machado-de-Sousa, J.P.; Abrão, J.; Crippa, J.A.S.; McGuire, P.; Krystal, J.H.; Baker, G.B.; et al. The interplay of cannabinoid and NMDA glutamate receptor systems in humans: Preliminary evidence of interactive effects of cannabidiol and ketamine in healthy human subjects. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Winton-Brown, T.T.; Allen, P.; Bhattacharyya, S.; Borgwardt, S.J.; Fusar-Poli, P.; Crippa, J.A.; Seal, M.L.; Martin-Santos, R.; Ffytche, D.; Zuardi, A.W.; et al. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: An FMRI study. Neuropsychopharmacology 2011, 36, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Reis, D.G.; Alves, F.H.; Corrêa, F.M.; Guimarães, F.S.; Resstel, L.B. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J. Psychopharmacol. 2012, 26, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Martin-Santos, R.; Crippa, J.A.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; Farre, M.; et al. Acute effects of a single, oral dose of d9- tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef]
- Gomes, F.V.; Alves, F.H.; Guimarães, F.S.; Correa, F.M.; Resstel, L.B.; Crestani, C.C. Cannabidiol administration into the bed nucleus of the stria terminalis alters cardiovascular responses induced by acute restraint stress through 5-HT₁A receptor. Eur. Neuropsychopharmacol. 2013, 23, 1096–1104. [Google Scholar] [CrossRef]
- Haney, M.; Malcolm, R.J.; Babalonis, S.; Nuzzo, P.A.; Cooper, Z.D.; Bedi, G.; Gray, K.M.; McRae-Clark, A.; Lofwall, M.R.; Sparenborg, S.; et al. Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology 2016, 41, 1974–1982. [Google Scholar] [CrossRef]
- Patrician, A.; Versic-Bratincevic, M.; Mijacika, T.; Banic, I.; Marendic, M.; Sutlović, D.; Dujić, Ž.; Ainslie, P.N. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv. Ther. 2019, 36, 3196–3210. [Google Scholar] [CrossRef] [PubMed]
- Kayser, R.R.; Haney, M.; Raskin, M.; Arout, C.; Simpson, H.B. Acute effects of cannabinoids on symptoms of obsessive-compulsive disorder: A human laboratory study. Depress. Anxiety 2020, 37, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef]
- Tomida, I.; Azuara-Blanco, A.; House, H.; Flint, M.; Pertwee, R.G.; Robson, P.J. Effect of sublingual application of cannabinoids on intraocular pressure: A pilot study. J. Glaucoma 2006, 15, 349–353. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef]
- Consroe, P.; Sandyk, R.; Snider, S.R. Open label evaluation of cannabidiol in dystonic movement disorders. Int. J. Neurosci. 1986, 30, 277–282. [Google Scholar] [CrossRef]
- Crippa, J.A.; Zuardi, A.W.; Garrido, G.E.; Wichert-Ana, L.; Guarnieri, R.; Ferrari, L.; Azevedo-Marques, P.M.; Hallak, J.E.C.; McGuire, P.K.; Busatto, G.F. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 2004, 29, 417–426. [Google Scholar] [CrossRef]
- Bright, T.P.; Farber, M.O.; Brown, D.J.; Lewis, S.C.; Forney, R.B. Cardiopulmonary effects of cannabidiol in anesthetized mongrel dogs. Toxicol. Appl. Pharmacol. 1975, 31, 520–526. [Google Scholar] [CrossRef]
- Borgen, L.A.; Davis, W.M. Cannabidiol (CBD) attenuation of effects of ∆9-THC. Pharmacologist 1973, 15, 201. [Google Scholar]
- Walsh, S.K.; Hepburn, C.Y.; Keown, O.; Åstrand, A.; Lindblom, A.; Ryberg, E.; Hjorth, S.; Leslie, S.J.; Greasley, P.J.; Wainwright, C.L. Pharmacological profiling of the hemodynamic effects of cannabinoid ligands: A combined in vitro and in vivo approach. Pharmacol. Res. Perspect. 2015, 3, e00143. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Böckmann, S.; Hinz, B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features by cannabidiol in vascular smooth muscle cells. Oncotarget 2018, 9, 34595–34616. [Google Scholar] [CrossRef] [PubMed]
- Smiley, K.A.; Karler, R.; Turkanis, S.A. Effects of cannabinoids on the perfused rat heart. Res. Commun. Chem. Pathol. Pharmacol. 1976, 14, 659–675. [Google Scholar]
- Ali, R.M.; Al Kury, L.T.; Yang, K.H.; Qureshi, A.; Rajesh, M.; Galadari, S.; Shuba, Y.M.; Howarth, F.C.; Oz, M. Effects of cannabidiol on contractions and calcium signaling in rat ventricular myocytes. Cell. Calcium. 2015, 57, 290–299. [Google Scholar] [CrossRef]
- Nahas, G.; Trouve, R. Effects and interactions of natural cannabinoids on the isolated heart. Proc. Soc. Exp. Biol. Med. 1985, 180, 312–316. [Google Scholar] [CrossRef]
- Karschner, E.L.; Darwin, W.D.; McMahon, R.P.; Liu, F.; Wright, S.; Goodwin, R.S.; Huestis, M.A. Subjective and physiological effects after controlled Sativex and oral THC administration. Clin. Pharmacol. Ther. 2011, 89, 400–407. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.; Chagas, M.H.; de Oliveira, D.C.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef]
- Biernacki, M.; Łuczaj, W.; Jarocka-Karpowicz, I.; Ambrożewicz, E.; Toczek, M.; Skrzydlewska, E. The Effect of Long-Term Administration of Fatty Acid Amide Hydrolase Inhibitor URB597 on Oxidative Metabolism in the Heart of Rats with Primary and Secondary Hypertension. Molecules 2018, 23, 2350. [Google Scholar] [CrossRef]
- Biernacki, M.; Malinowska, B.; Timoszuk, M.; Toczek, M.; Jastrząb, A.; Remiszewski, P.; Skrzydlewska, E. Hypertension and chronic inhibition of endocannabinoid degradation modify the endocannabinoid system and redox balance in rat heart and plasma. Prostaglandins Other Lipid Mediat. 2018, 138, 54–63. [Google Scholar] [CrossRef]
- Durst, R.; Danenberg, H.; Gallily, R.; Mechoulam, R.; Meir, K.; Grad, E.; Beeri, R.; Pugatsch, T.; Tarsish, E.; Lotan, C. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am J. Physiol. Heart. Circ. Physiol. 2007, 293, 3602–3607. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, F.; Yin, T.; Xia, Q.; Liu, Y.; Huang, G.; Zhang, J.; Oyen, R.; Ni, Y.J. Pharmacologic Effects of Cannabidiol on Acute Reperfused Myocardial Infarction in Rabbits: Evaluated with 3.0T Cardiac Magnetic Resonance Imaging and Histopathology. J. Cardiovasc. Pharmacol. 2015, 66, 354–363. [Google Scholar] [CrossRef]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Erdelyi, K.; Matyas, C.; Mukhopadhyay, P.; Varga, Z.V.; Liaudet, L.; Haskú, G.; Čiháková, D.; Mechoulam, R.; Pacher, P. Cannabidiol Limits T Cell-Mediated Chronic Autoimmune Myocarditis: Implications to Autoimmune Disorders and Organ Transplantation. Mol. Med. 2016, 22, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Lolic, M.; Wink, D.; Rosenthal, R.; Axelrod, J. Neuroprotective antioxidants from marijuana. Ann. N. Y. Acad. Sci. 2000, 899, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Mishima, K.; Irie, K.; Hazekawa, M.; Mishima, S.; Fujioka, M.; Orito, K.; Egashira, N.; Katsurabayashi, S.; Takasaki, K.; et al. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology 2008, 55, 1280–1286. [Google Scholar] [CrossRef]
- Khaksar, S.; Bigdeli, M.R. Correlation Between Cannabidiol-Induced Reduction of Infarct Volume and Inflammatory Factors Expression in Ischemic Stroke Model. Basic. Clin. Neurosci. 2017, 8, 139–146. [Google Scholar] [PubMed]
- Ceprián, M.; Jiménez-Sánchez, L.; Vargas, C.; Barata, L.; Hind, W.; Martínez-Orgado, J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology 2017, 116, 151–159. [Google Scholar] [CrossRef]
- Hayakawa, K.; Irie, K.; Sano, K.; Watanabe, T.; Higuchi, S.; Enoki, M.; Nakano, T.; Harada, K.; Ishikane, S.; Ikeda, T.; et al. Therapeutic time window of cannabidiol treatment on delayed ischemic damage via high-mobility group box1-inhibiting mechanism. Biol. Pharm. Bull. 2009, 32, 1538–1544. [Google Scholar] [CrossRef]
- Braida, D.; Pegorini, S.; Arcidiacono, M.V.; Consalez, G.G.; Croci, L.; Sala, M. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci. Lett. 2003, 346, 61–64. [Google Scholar] [CrossRef]
- Schiavon, A.P.; Soares, L.M.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; Weffort de Oliveira, R.M. Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox. Res. 2014, 26, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Lafuente, H.; Rey-Santano, M.C.; Mielgo, V.E.; Gastiasoro, E.; Rueda, M.; Pertwee, R.G.; Castillo, A.I.; Romero, J.; Martínez-Orgado, J. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr. Res. 2008, 64, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, H.; Alvarez, F.J.; Pazos, M.R.; Alvarez, A.; Rey-Santano, M.C.; Mielgo, V.; Murgia-Esteve, X.; Hilario, E.; Martinez-Orgado, J. Cannabidiol reduces brain damage and improves functional recovery after acute hypoxia-ischemia in newborn pigs. Pediatr. Res. 2011, 70, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, H.; Pazos, M.R.; Alvarez, A.; Mohammed, N.; Santos, M.; Arizti, M.; Alvarez, F.J.; Martinez-Orgado, J.A. Effects of Cannabidiol and Hypothermia on Short-Term Brain Damage in New-Born Piglets after Acute Hypoxia-Ischemia. Front. Neurosci. 2016, 10, 323. [Google Scholar] [CrossRef]
- Arruza, L.; Pazos, M.R.; Mohammed, N.; Escribano, N.; Lafuente, H.; Santos, M.; Alvarez-Díaz, F.J.; Hind, W.; Martínez-Orgado, J. Cannabidiol reduces lung injury induced by hypoxic-ischemic brain damage in newborn piglets. Pediatr. Res. 2017, 82, 79–86. [Google Scholar] [CrossRef]
- Barata, L.; Arruza, L.; Rodríguez, M.J.; Aleo, E.; Vierge, E.; Criado, E.; Sobrino, E.; Vargas, C.; Ceprián, M.; Gutiérrez-Rodríguez, A.; et al. Neuroprotection by cannabidiol and hypothermia in a piglet model of newborn hypoxic-ischemic brain damage. Neuropharmacology 2019, 146, 1–11. [Google Scholar] [CrossRef]
- Garberg, H.T.; Solberg, R.; Barlinn, J.; Martinez-Orgado, J.; Løberg, E.M.; Saugstad, O.D. High-Dose Cannabidiol Induced Hypotension after Global Hypoxia-Ischemia in Piglets. Neonatology 2017, 112, 143–149. [Google Scholar] [CrossRef]
- Mohammed, N.; Ceprián, M.; Jimenez, L.; Pazos, M.R.; Martínez-Orgado, J. Neuroprotective Effects of Cannabidiol in Hypoxic Ischemic Insult. The Therapeutic Window in Newborn Mice. CNS Neurol. Disord. Drug Targets 2017, 16, 102–108. [Google Scholar] [CrossRef]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Ceprián, M.; Vargas, C.; García-Toscano, L.; Penna, F.; Jiménez-Sánchez, L.; Achicallende, S.; Elezgarai, I.; Grandes, P.; Hind, W.; Pazos, M.R.; et al. Cannabidiol Administration Prevents Hypoxia-Ischemia-Induced Hypomyelination in Newborn Rats. Front. Pharmacol. 2019, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- Garberg, H.T.; Huun, M.U.; Escobar, J.; Martinez-Orgado, J.; Løberg, E.M.; Solberg, R.; Didrik-Saugstad, O. Short-term effects of cannabidiol after global hypoxia-ischemia in newborn piglets. Pediatr. Res. 2016, 80, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas, L.; Martínez-Orgado, J.A.; Benito, C.; Millán, A.; Tolón, R.M.; Romero, J. Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: An intravital microscopy study. J. Neuroinflammation. 2011, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Al-Mulhim, A.S.; Jresat, I. Cannabidiol treatment ameliorates ischemia/reperfusion renal injury in rats. Life Sci. 2012, 91, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Jresat, I. Therapeutic potential of cannabidiol against ischemia/reperfusion liver injury in rats. Eur. J. Pharmacol. 2011, 670, 216–223. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In Vivo Cannabidiol Treatment Improves Endothelium-Dependent Vasorelaxation in Mesenteric Arteries of Zucker Diabetic Fatty Rats. Front. Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef]
- Stanley, C.P.; Wheal, A.J.; Randall, M.D.; O’Sullivan, S.E. Cannabinoids alter endothelial function in the Zucker rat model of type 2 diabetes. Eur. J. Pharmacol. 2013, 720, 376–382. [Google Scholar] [CrossRef]
- Adams, M.D.; Earnhardt, J.T.; Martin, B.R.; Harris, L.S.; Dewey, W.L.; Razdan, R.K. A cannabinoid with cardiovascular activity but no overt behavioral effects. Experientia 1977, 33, 1204–1205. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Johns, D.; Behm, D.J.; Walker, D.J.; Ao, Z.; Shapland, E.M.; Daniels, D.A.; Riddick, M.; Dowell, S.; Staton, P.C.; Green, P.; et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 2007, 152, 825–831. [Google Scholar]
- Ho, W.S.; Hiley, C.R. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br. J. Pharmacol. 2003, 138, 1320–1332. [Google Scholar]
- Offertáler, L.; Mo, F.M.; Bátkai, S.; Liu, J.; Begg, M.; Razdan, R.K.; Martin, B.R.; Bukoski, R.D.; Kunos, G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003, 63, 699–705. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; MacLean, M.R.; Kozłowska, H.; Malinowska, B. Endothelium-dependent mechanisms of the vasodilatory effect of the endocannabinoid, anandamide, in the rat pulmonary artery. Pharmacol. Res. 2012, 66, 251–259. [Google Scholar] [CrossRef]
- Su, J.Y.; Vo, A.C. 2-Arachidonylglyceryl ether and abnormal cannabidiol-induced vascular smooth muscle relaxation in rabbit pulmonary arteries via receptor-pertussis toxin sensitive G proteins-ERK1/2 signaling. Eur. J. Pharmacol. 2007, 559, 189–195. [Google Scholar] [CrossRef]
- Kozłowska, H.; Baranowska, M.; Schlicker, E.; Kozłowski, M.; Laudański, J.; Malinowska, B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J. Hypertens. 2007, 25, 2240–2248. [Google Scholar] [CrossRef]
- McHugh, D. GPR18 in microglia: Implications for the CNS and endocannabinoid system signalling. Br. J. Pharmacol. 2012, 167, 1575–1582. [Google Scholar] [CrossRef]
- Bondarenko, A.I.; Panasiuk, O.; Drachuk, K.; Montecucco, F.; Brandt, K.J.; Mach, F. The quest for endothelial atypical cannabinoid receptor: BKCa channels act as cellular sensors for cannabinoids in in vitro and in situ endothelial cells. Vascul. Pharmacol. 2018, 102, 44–55. [Google Scholar] [CrossRef]
- Penumarti, A.; Abdel-Rahman, A.A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J. Pharmacol. Exp. Ther. 2014, 349, 29–38. [Google Scholar] [CrossRef]
- Matouk, A.I.; Taye, A.; El-Moselhy, M.A.; Heeba, G.H.; Abdel-Rahman, A.A. The Effect of Chronic Activation of the Novel Endocannabinoid Receptor GPR18 on Myocardial Function and Blood Pressure in Conscious Rats. J. Cardiovasc. Pharmacol. 2017, 69, 23–33. [Google Scholar] [CrossRef]
- Matouk, A.I.; Taye, A.; El-Moselhy, M.A.; Heeba, G.H.; Abdel-Rahman, A.A. Abnormal cannabidiol confers cardioprotection in diabetic rats independent of glycemic control. Eur. J. Pharmacol. 2018, 820, 256–264. [Google Scholar] [CrossRef]
- McKillop, A.M.; Moran, B.M.; Abdel-Wahab, Y.H.; Flatt, P.R. Evaluation of the insulin releasing and antihyperglycaemic activities of GPR55 lipid agonists using clonal beta-cells, isolated pancreatic islets and mice. Br. J. Pharmacol. 2013, 170, 978–990. [Google Scholar] [CrossRef]
- McKillop, A.M.; Moran, B.M.; Abdel-Wahab, Y.H.; Gormley, N.M.; Flatt, P.R. Metabolic effects of orally administered small-molecule agonists of GPR55 and GPR119 in multiple low-dose streptozotocin-induced diabetic and incretin-receptor-knockout mice. Diabetologia 2016, 59, 2674–2685. [Google Scholar] [CrossRef]
- Vong, C.T.; Tseng, H.H.L.; Kwan, Y.W.; Lee, S.M.; Hoi, M.P.M. G-protein coupled receptor 55 agonists increase insulin secretion through inositol trisphosphate-mediated calcium release in pancreatic β-cells. Eur. J. Pharmacol. 2019, 85, 372–379. [Google Scholar] [CrossRef]
- Vong, C.T.; Tseng, H.H.L.; Kwan, Y.W.; Lee, S.M.; Hoi, M.P.M. Novel protective effect of O-1602 and abnormal cannabidiol, GPR55 agonists, on ER stress-induced apoptosis in pancreatic β-cells. Biomed. Pharmacother. 2019, 111, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
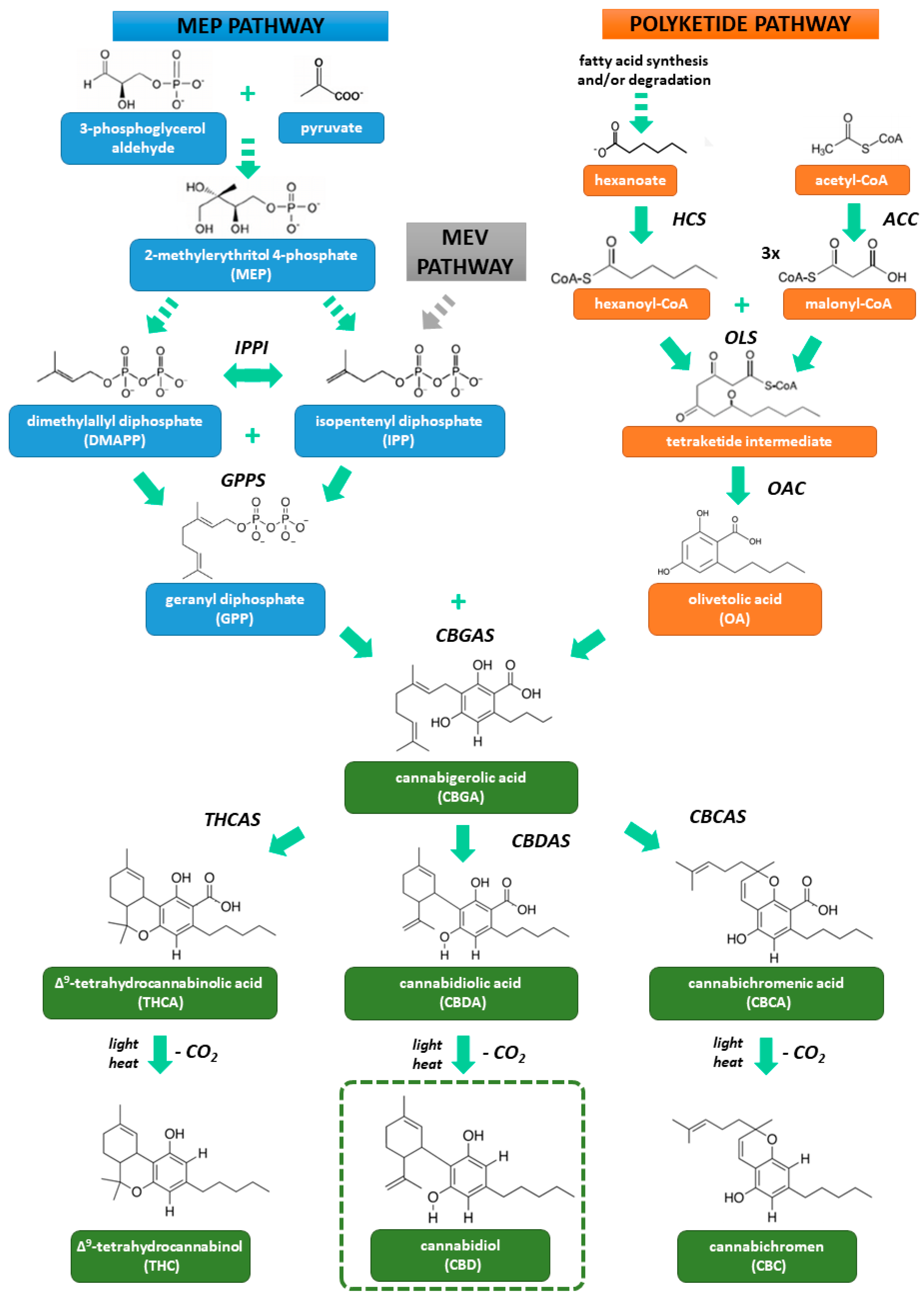
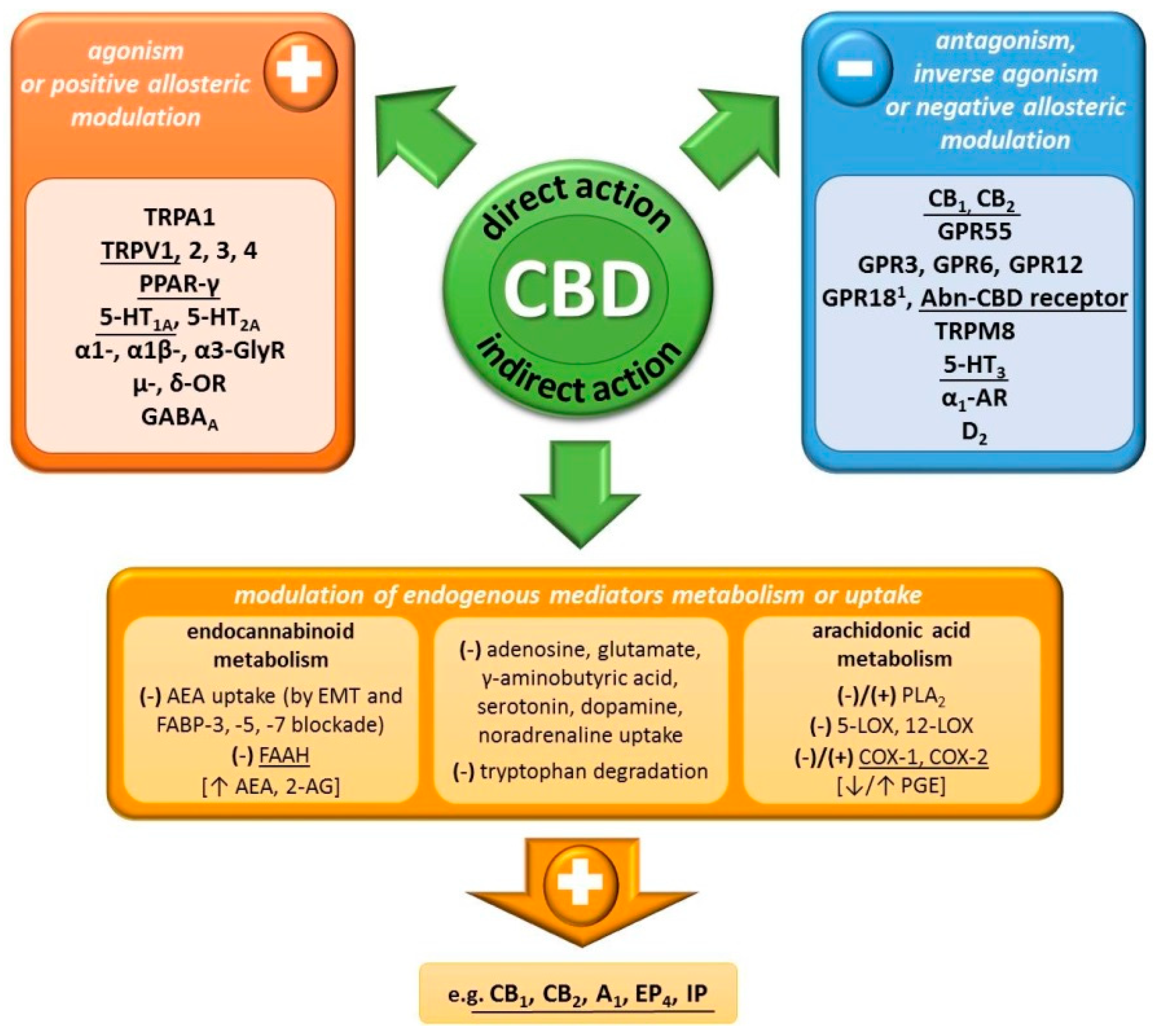
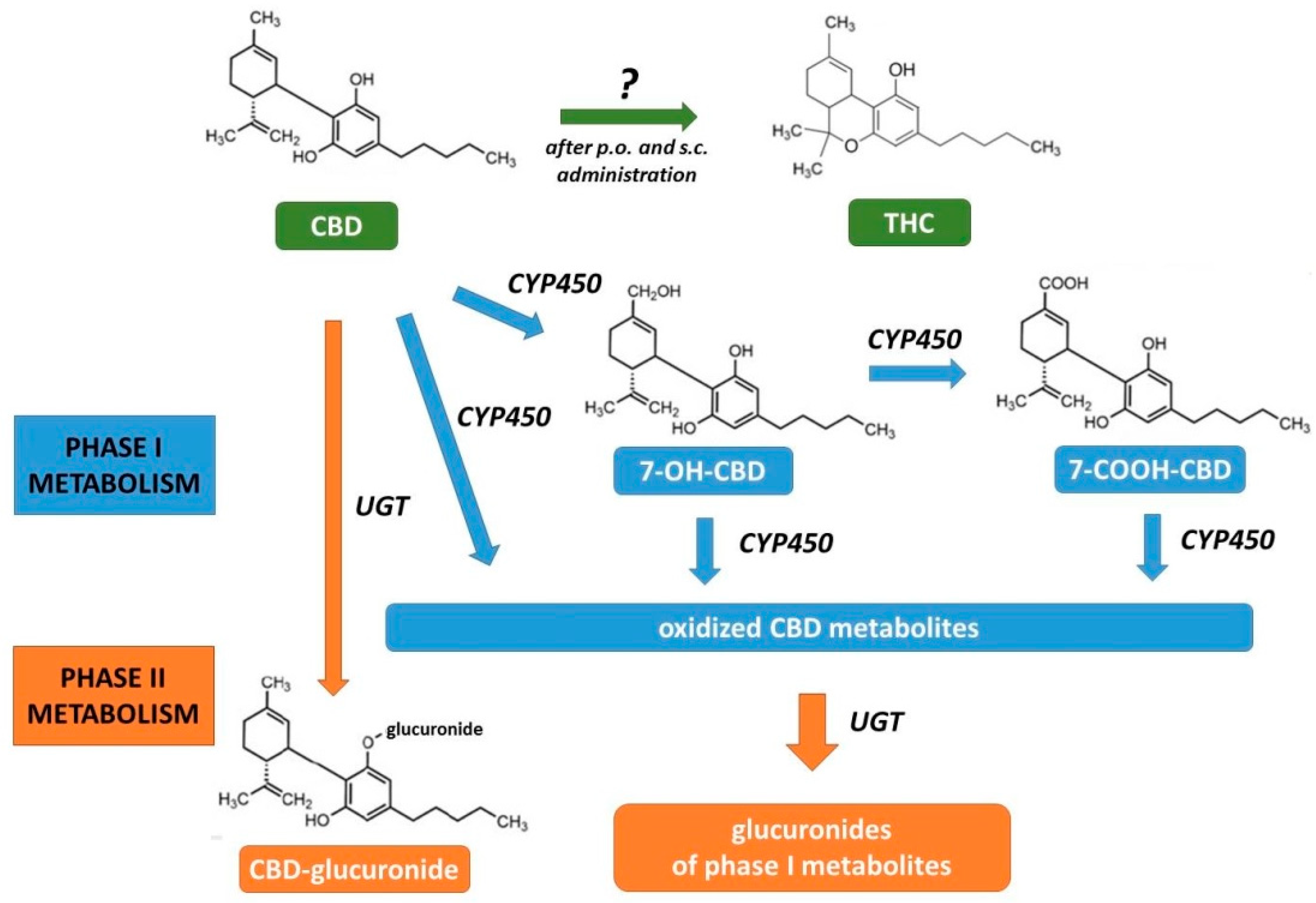
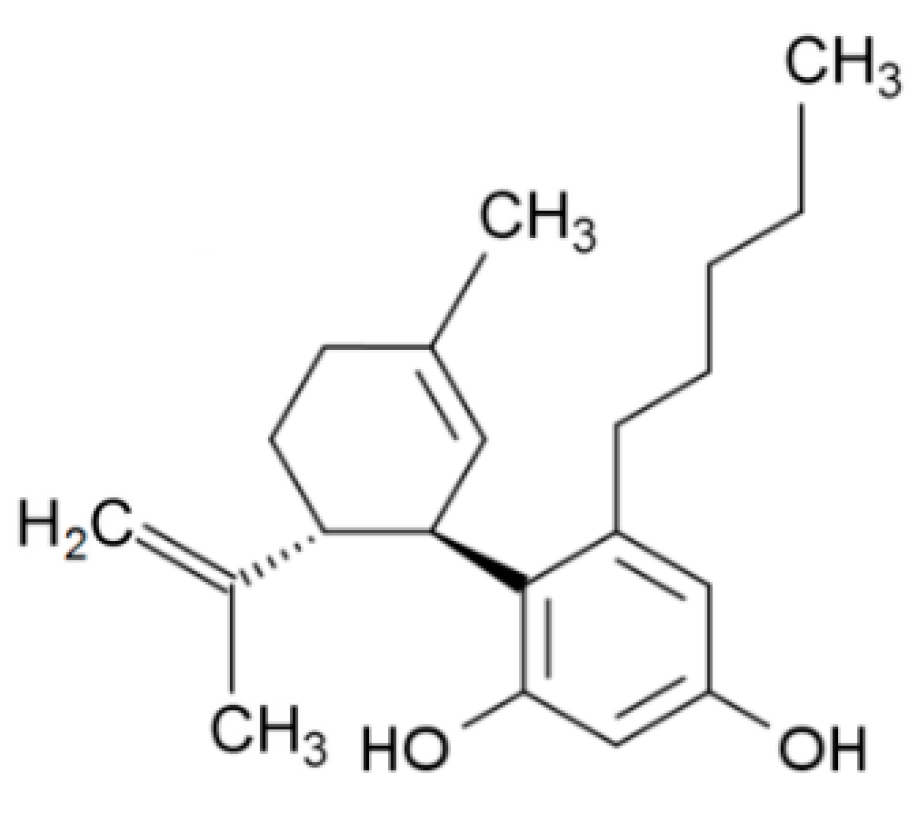
| Cannabidiol (CBD) | Δ9-Tetrahydrocannabinol (THC) | |
|---|---|---|
| Structure and IUPAC name | 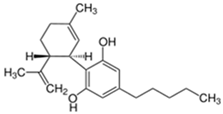 2-[(1R,6R)-3-Methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol | 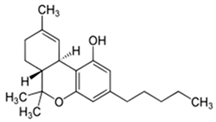 (6aR,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c]chromen-1-ol |
| Psychoactive properties | Psychoactive 1 but non-intoxicating; does not produce cannabinoid tetrad 2 | Psychoactive and intoxicating (‘high’, euphoria, sensations of pleasure and relaxation, psychomotor and cognition impairment); produces cannabinoid tetrad 2 |
| Potential therapeutic properties 3 | Anti-inflammatory, antioxidant, immunomodulatory, neuroprotective, anticonvulsant, anxiolytic, antipsychotic, antidepressant, procognitive, antiarthritic, analgesic, antiemetic, anticancer, cardioprotective, vasodilatory | Analgesic, antispastic, anti-inflammatory, appetite stimulant, antiemetic, neuroprotective, anxiolytic, antiasthmatic, antiglaucomatous, anticancer |
| Pharmaceutical products | Dried female cannabis flowers (‘medical marijuana’) and their derivatives (oil, granulate) with different THC:CBD ratios (e.g., Bedrocan® products) nabiximols (Sativex®)—cannabis extract containing CBD and THC in a ~1:1 ratio | |
| Cannabis-derived CBD (Epidiolex®) | Dronabinol (Marinol®, Syndros®)—synthetic THC Nabilon (Cesamet®, Canemes®)—synthetic THC analogue | |
| Hypothesized mechanism of action | Affinity for cannabinoid receptors CB1 (Ki = 4350 to >10,000 nM) CB2 (Ki = 2399 to >10,000 nM) Antagonist of CB1/CB2 receptor agonists, negative allosteric modulator of CB1 and inverse agonist of CB2 | Affinity for cannabinoid receptors CB1 (Ki = 5.05–80.03 nM) CB2 (Ki = 3.13–75.3 nM) Partial agonist of CB1 and CB2 |
| Indirect cannabimimetics: ↑AEA, 2-AG (inhibits FAAH and AEA uptake by binding to EMT and FABP-3, -5, -7) | Indirect cannabimimetics: ↑AEA (inhibits AEA re-uptake by binding to FABP-3, -5, -7) | |
| (+) TRPA1, TRPV1–4, PPAR-γ, 5-HT1A, 5-HT2A, α1-, α1β-, α3-GlyR,μ-, δ-OR, GABAA (–) GPR55, GPR3, GPR6, GPR12, GPR18 4,Abn-CBD receptor, TRPM8, 5-HT3, α1-AR, D2 Affects uptake/metabolism of adenosine, glutamate, serotonin, dopamine, γ-aminobutyric acid, noradrenaline, tryptophan, arachidonic acid | (+) GPR55, GPR18, PPAR-γ, TRPA1, TRPV2, 5-HT2A, α1- and α1β1-GlyR (–) 5-HT3, μ- and δ-OR, TRPM8 Affects uptake/metabolism of adenosine, serotonin, γ-aminobutyric acid, dopamine, noradrenaline, arachidonic acid | |
| Influence on cardiovascular system (physiological conditions) | No or slight influence on BP and HR in human (usually) No or slight influence on BP and HR in animals (usually) Vasodilation of isolated vessels | ↑ HR (significant) and ↑ or ↓ BP in human ↓ HR (usually), and ↓ or ↑ or biphasic changes in BP in animals Vasodilation or vasoconstriction of isolated vessels |
| Species | Anaesthesia | Route | Dose | Effects 2 | References |
|---|---|---|---|---|---|
| Single administration | |||||
| human | - | p.o. | 320 µg/kg | ↔ HR | [75] |
| human | - | p.o. | 1 mg/kg | ↔ HR | [77] |
| human | - | p.o. | 100; 600; 1200 mg | ↔ DBP, SBP, HR | [78] |
| human | - | p.o. | 300 mg | ↔ SBP, HR | [80] |
| human | - | p.o. | 400 mg | ↑ CBF (regional) | [101] |
| human 3 | - | s.l. | 20; 40 mg | ↑ SBP ↔ DBP, HR | [98] |
| human | - | p.o. | 600 mg | ↔ SBP, DBP, HR | [83] |
| human | - | p.o. | 600 mg | ↔ BP, HR | [84] |
| human | - | p.o. | 600 mg | ↔ BP, HR | [85] |
| human | - | p.o. | 600 mg | ↔ BP, HR | [86] |
| human | - | p.o. | 600 mg | ↔ DBP, SBP, HR | [88] |
| human | - | p.o. | 600 mg | ↔ BP, HR | [89] |
| human | - | p.o. | 600 mg | ↔ DBP, SBP, HR | [91] |
| human | - | p.o. | 200; 400; 800 mg | ↔ DBP, SBP, HR | [93] |
| human | - | p.o. | 600 mg | ↓ SBP, DBP, MBP, SV, TPR, SBF ↑ HR ↔ CO, EJT | [99] |
| human | - | p.o. | 45; 90 mg | ↔ SBP, DBP, MBP, HR, CBF | [94] |
| 45; 90 mg TurboCBDTM 4 | ↔ SBP, HR ↓ DBP, MBP ↑ CBF | ||||
| human | inhalation (vaporisation) | 400 mg | ↔ HR, SBP, DBP (↑ DBP in frequent cannabis users) | [7] | |
| human 5 | - | inhalation (smoking) | 1/2 of cigarette containing ~800 mg of cannabis (0.4% THC/10.4% CBD) | ↔ SBP, DBP, HR | [95] |
| human | - | p.o. | 600 mg | ↓ MBP ↔ SBP, DBP, HR, CO, SV, EJT, TPR | [97] |
| dog | pentobarbital | i.v. | 0.5; 1 mg/kg | ↑ MBP, HR | [102] |
| rabbit | - | i.v. | 25 mg/kg | ↓ HR | [103] |
| rat | - | i.p. | 10 mg/kg | ↔ MBP, HR | [82] |
| rat | - | i.p. | 1; 10; 20 mg/kg | ↔ MBP, HR | [55] |
| rat | - | i.p. | 10 mg/kg | ↑ (slight) SBP, DBP, HR | [52] |
| rat | urethan | i.v. | 1 mg/kg | ↔ BP, HR | [74] |
| rat | urethan | i.v. (rapid) | 3; 10; 30 mg/kg | ↓ SBP, DBP, HR (Bezold-Jarisch reflex induced via TRPV1) ↓ Bezold-Jarisch reflex induced by 5-HT3 (but not TRPV1) activation | [55] |
| rat 6 | urethane | i.v. | 1; 3; 30 mg/kg | ↑ SBP, HR ↓ DBP | [55] |
| rat | pentobarbital | i.a. or i.v. | 1-2000 µg | ↔ MBP | [81] |
| rat | pentobarbital | i.v. | 10; 50 µg/kg | ↓ MBP ↔ HR | [105] |
| rat | pentobarbital | i.v. | 50 µg/kg | ↓ MBP ↔ HR | [104] |
| rat | thiopental | i.v. | 50 µg/kg | ↔ MBP, HR | [49] |
| rat | - | i.c. | 15; 30; 60 nmol | ↔ MBP, HR | [87] |
| rat | - | into BNST | 15; 30; 60 nmol | ↔ MBP, HR | [90] |
| rat | - | into BNST | 15; 30; 60 nmol | ↔ MBP, HR | [92] |
| rat | - | into BNST | 60 nmol | ↔ MBP, HR ↑ reflex bradycardiac response to BP increase (effect is dependent on 5-HT1A) ↔ reflex tachycardiac response to BP decrease | [47] |
| mouse | ketamine + xylazine | i.v. | 50 µg/kg | ↓ MBP ↔ HR | [104] |
| Chronic administration | |||||
| human | - | p.o. | 3 mg/kg for 30 days | ↔ HR, ECG | [76] |
| human 7 | - | p.o. | 200-300 mg for 4,5 months | ↔ HR, ECG | [76] |
| human | - | p.o. | 1200 mg for 20 days | ↔ DBP, SBP, HR | [78] |
| human 8 | - | p.o. | increasing doses 100-600 mg for 6 weeks | ↓ BP | [100] |
| human 9 | - | p.o. | 10 mg/kg/day over 6 weeks | ↔ MBP, HR | [79] |
| human 10 | - | p.o. | 800 11 mg for 4 weeks | ↔ SBP, DBP, HR | [15] |
| human | - | p.o. | 600 mg for 7 days | ↔ SBP, DBP, MBP, HR ↑ PWV, FMD | [97] |
| rat | - | i.p. | 10 mg/kg for 14 days | ↔ SBP, DBP, HR 11,12↑ oxidative stress markers in plasma (MDA 11, 4-HHE 11,12, 4-HNE 11) and in heart (MDA11, 4-HHE 11, 4-HNE 11) | [96] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. https://doi.org/10.3390/ijms21186740
Kicman A, Toczek M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. International Journal of Molecular Sciences. 2020; 21(18):6740. https://doi.org/10.3390/ijms21186740
Chicago/Turabian StyleKicman, Aleksandra, and Marek Toczek. 2020. "The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease" International Journal of Molecular Sciences 21, no. 18: 6740. https://doi.org/10.3390/ijms21186740
APA StyleKicman, A., & Toczek, M. (2020). The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. International Journal of Molecular Sciences, 21(18), 6740. https://doi.org/10.3390/ijms21186740





