Identification of Acute Myeloid Leukemia Bone Marrow Circulating MicroRNAs
Abstract
1. Introduction
2. Results
2.1. Bone Marrow Circulating miRNAs Expression Profile in Newly Diagnosed AML Patients
2.2. Functional Analysis of the Pathways of AML Bone Marrow Circulating miRNAs
2.3. Prognosis Value of Bone Marrow Circulating miRNAs in AML Based on Genetic Risk.
2.4. Correlation between Bone Marrow Circulating miRNA Expression Profiles and Overall Survival Rate of Patients with AML
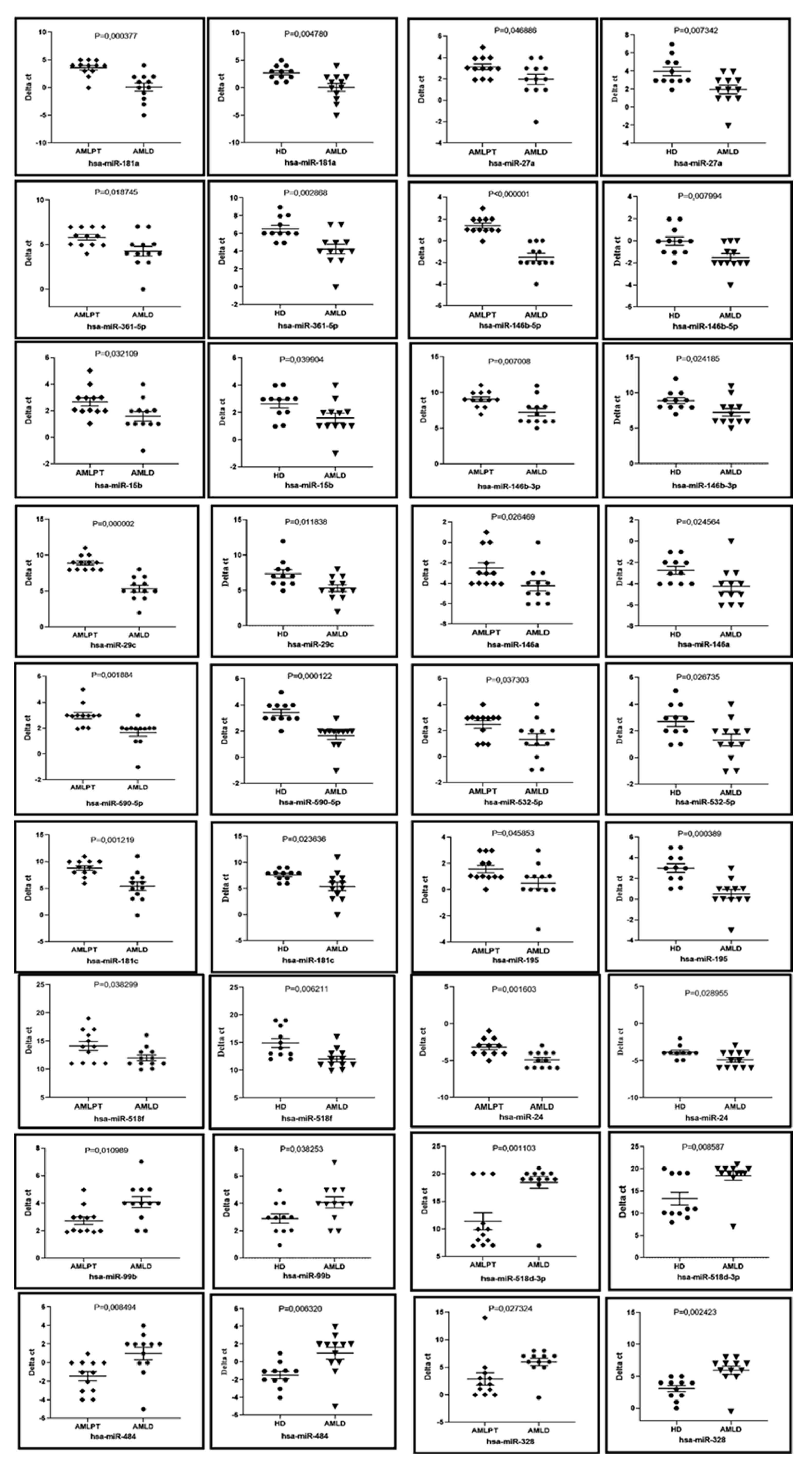
2.5. Bone Marrow Circulating miRNAs Profile of First Complete Remission AML Patients (AMLPT)
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Bone Marrow Aspiration Sampling and RNA Extraction.
4.3. miRNA Expression Profile
4.4. TaqMan miRNA Assay for Individual miRNAs
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, D.; Majeti, R. Biology and relevance of human acute myeloid leukemia stem cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef]
- Shysh, A.C.; Nguyen, L.T.; Guo, M.; Vaska, M.; Naugler, C.; Rashid-Kolvear, F. The incidence of acute myeloid leukemia in Calgary, Alberta, Canada: A retrospective cohort study. BMC Public Health 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Z.; Pang, Y.; Cui, L.; Qian, T.; Quan, L.; Zhao, H.; Shi, J.; Ke, X.; Fu, L. Role of microRNAs, circRNAs and long noncoding RNAs in acute myeloid leukemia. J. Hematol. Oncol. 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.; Nimer, S. Recent advances in the understanding and treatment of acute myeloid leukemia. F1000Research 2018, 7, 1196. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Mrozek, K.; Radmacher, M.D.; Garzon, R.; Bloomfield, C.D. The prognostic and functional role of microRNAs in acute myeloid leukemia. Blood 2011, 117, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Luo, B.; Jiang, N.; Liang, Y.; He, Y.; Zeng, J.; Liu, J.; Zheng, X. OncomiR or antioncomiR: Role of miRNAs in Acute Myeloid Leukemia. Leuk. Lymphoma 2019, 60, 284–294. [Google Scholar] [CrossRef]
- Trino, S.; Lamorte, D.; Caivano, A.; Laurenzana, I.; Tagliaferri, D.; Falco, G.; Del Vecchio, L.; Musto, P.; De Luca, L. MicroRNAs as New Biomarkers for Diagnosis and Prognosis, and as Potential Therapeutic Targets in Acute Myeloid Leukemia. Int. J. Mol. Sci 2018, 19, 460. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847. [Google Scholar]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 25. [Google Scholar]
- Slaby, O.; Laga, R.; Sedlacek, O. Therapeutic targeting of non-coding RNAs in cancer. Biochem. J. 2017, 474, 4219–4251. [Google Scholar] [PubMed]
- Ferreira, P.; Roela, R.A.; Lopez, R.V.M.; Del Pilar Estevez-Diz, M. The prognostic role of microRNA in epithelial ovarian cancer: A systematic review of literature with an overall survival. Oncotarget 2020, 11, 1085–1095. [Google Scholar]
- Buhagiar, A.; Borg, J.; Ayers, D. Overview of current microRNA biomarker signatures as potential diagnostic tools for leukaemic conditions. Noncoding RNA Res. 2020, 5, 22–26. [Google Scholar]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar]
- Dufresne, S.; Rebillard, A.; Muti, P.; Friedenreich, C.M.; Brenner, D.R. A Review of Physical Activity and Circulating miRNA Expression: Implications in Cancer Risk and Progression. Cancer Epidemiol. Biomark. Prev. 2018, 27, 11–24. [Google Scholar]
- Yan, J.; Wu, G.; Chen, J.; Xiong, L.; Chen, G.; Li, P. Downregulated miR-217 expression predicts a poor outcome in acute myeloid leukemia. Cancer Biomark. 2018, 22, 73–78. [Google Scholar] [CrossRef]
- Fayyad-Kazan, H.; Bitar, N.; Najar, M.; Lewalle, P.; Fayyad-Kazan, M.; Badran, R.; Hamade, E.; Daher, A.; Hussein, N.; ElDirani, R.; et al. Circulating miR-150 and miR-342 in plasma are novel potential biomarkers for acute myeloid leukemia. J. Transl. Med. 2013, 11, 31. [Google Scholar] [CrossRef]
- Zhi, F.; Cao, X.; Xie, X.; Wang, B.; Dong, W.; Gu, W.; Ling, Y.; Wang, R.; Yang, Y.; Liu, Y. Identification of circulating microRNAs as potential biomarkers for detecting acute myeloid leukemia. PLoS ONE 2013, 8, e56718. [Google Scholar] [CrossRef]
- Tang, X.; Chen, L.; Yan, X.; Li, Y.; Xiong, Y.; Zhou, X. Overexpression of miR-210 is Associated with Poor Prognosis of Acute Myeloid Leukemia. Med. Sci. Monit. 2015, 21, 3427. [Google Scholar]
- Liu, L.; Chen, R.; Zhang, Y.; Fan, W.; Xiao, F.; Yan, X. Low expression of circulating microRNA-328 is associated with poor prognosis in patients with acute myeloid leukemia. Diagn. Pathol. 2015, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, P.; Yun, Y.; Zhang, D.; Ma, H.; He, Q.; Jia, G.; Peng, J. Prognostic value of plasma miR-638 in patients with acute myeloid leukemia. Int. J. Clin. Exp. Pathol. 2017, 10, 550–555. [Google Scholar]
- Tian, C.; Zhang, L.; Li, X.; Zhang, Y.; Li, J.; Chen, L. Low miR-192 expression predicts poor prognosis in pediatric acute myeloid leukemia. Cancer Biomark. 2018, 22, 209–215. [Google Scholar]
- Li, B.; Ge, L.; Li, M.; Wang, L.; Li, Z. miR-448 suppresses proliferation and invasion by regulating IGF1R in colorectal cancer cells. Am. J. Transl. Res. 2016, 8, 3013–3022. [Google Scholar] [PubMed]
- Wang, C.; Xu, C.; Niu, R.; Hu, G.; Gu, Z.; Zhuang, Z. MiR-890 inhibits proliferation and invasion and induces apoptosis in triple-negative breast cancer cells by targeting CD147. BMC Cancer 2019, 19, 577. [Google Scholar]
- Li, S.M.; Zhao, Y.Q.; Hao, Y.L.; Liang, Y.Y. Upregulation of miR-504-3p is associated with favorable prognosis of acute myeloid leukemia and may serve as a tumor suppressor by targeting MTHFD2. Eur. Rev. Med. Pharm. Sci. 2019, 23, 1203–1213. [Google Scholar]
- Correia, N.C.; Melao, A.; Povoa, V.; Sarmento, L.; Gomez de Cedron, M.; Malumbres, M.; Enguita, F.J.; Barata, J.T. microRNAs regulate TAL1 expression in T-cell acute lymphoblastic leukemia. Oncotarget 2016, 7, 8268–8281. [Google Scholar]
- Lou, Q.; Liu, R.; Yang, X.; Li, W.; Huang, L.; Wei, L.; Tan, H.; Xiang, N.; Chan, K.; Chen, J.; et al. miR-448 targets IDO1 and regulates CD8(+) T cell response in human colon cancer. J. Immunother. Cancer 2019, 7, 210–214. [Google Scholar]
- Gao, H.Y.; Wang, W.; Luo, X.G.; Jiang, Y.F.; He, X.; Xu, P.; Chen, X.; Li, X.Y. Screening of prognostic risk microRNAs for acute myeloid leukemia. Hematology 2018, 23, 747–755. [Google Scholar]
- Sun, M.X.; An, Q.; Chen, L.M.; Guo, L. MIR-520f Regulated Itch Expression and Promoted Cell Proliferation in Human Melanoma. Cells. Dose Response 2020, 18, 1559325820918450. [Google Scholar]
- Zheng, H.Z.; Sun, Y.B.; Zhan, T.; Zeng, K.D.; Liu, C.Z.; Zhang, J.L. miR-9-5p prompts malignancies of acute myeloid leukemia cells mainly by targeting p27. Int. J. Clin. Exp. Med. 2018, 11, 2076–2083. [Google Scholar]
- Chen, L.; Hu, W.; Li, G.; Guo, Y.; Wan, Z.; Yu, J. Inhibition of miR-9-5p suppresses prostate cancer progress by targeting StarD13. Cell Mol. Biol. Lett. 2019, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Rui, K.; Tang, X.; Ma, J.; Wang, Y.; Tian, X.; Zhang, Y.; Xu, H.; Lu, L.; Wang, S. MicroRNA-9 Regulates the Differentiation and Function of Myeloid-Derived Suppressor Cells via Targeting Runx1. J. Immunol. 2015, 195, 1301–1311. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Y.; Song, X.; Song, X.; Niu, L.; Xie, L. Tumor-derived exosomal miRNA-320d as a biomarker for metastatic colorectal cancer. J. Clin. Lab. Anal. 2019, 33, e23004. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhou, Y.; Ji, X.; Liu, J.; Liu, D.; Yin, P.; Peng, Y.; Hao, M.; Zhang, L.; et al. Synergistic Effects of BMP9 and miR-548d-5p on Promoting Osteogenic Differentiation of Mesenchymal Stem Cells. Biomed. Res. Int. 2015, 2015, 309747. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Li, Y.; Zhao, G. Downregulation of PPARgamma by miR-548d-5p suppresses the adipogenic differentiation of human bone marrow mesenchymal stem cells and enhances their osteogenic potential. J. Transl Med. 2014, 12, 168. [Google Scholar] [CrossRef]
- Liu, X.; Li, H. Diagnostic Value of miR-34a in Bone Marrow Mononuclear Cells of Acute Myeloid Leukemia Patients. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, Y.; Lin, L.; Ma, X.; Chen, H. Identification of serum miR-34a as a potential biomarker in acute myeloid leukemia. Cancer Biomark. 2018, 22, 799–805. [Google Scholar] [CrossRef]
- Liu, L.; Ren, W.; Chen, K. MiR-34a Promotes Apoptosis and Inhibits Autophagy by Targeting HMGB1 in Acute Myeloid Leukemia Cells. Cell Physiol. Biochem. 2017, 41, 1981–1992. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Dong, K.; Lin, F.; Long, M.; Ouyang, Y.; Wei, J.; Chen, X.; Weng, Y.; He, T.; et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal. 2015, 27, 443–452. [Google Scholar] [CrossRef]
- Bi, L.; Zhou, B.; Li, H.; He, L.; Wang, C.; Wang, Z.; Zhu, L.; Chen, M.; Gao, S. A novel miR-375-HOXB3-CDCA3/DNMT3B regulatory circuitry contributes to leukemogenesis in acute myeloid leukemia. BMC Cancer 2018, 18, 182. [Google Scholar]
- Eldaly, M.N.; Metwally, F.M.; Shousha, W.G.; El-Saiid, A.S.; Ramadan, S.S. Clinical Potentials of miR-576-3p, miR-613, NDRG2 and YKL40 in Colorectal Cancer Patients. Asian Pac. J. Cancer Prev. 2020, 21, 1689–1695. [Google Scholar] [PubMed]
- Meng, F.M.; Meng, F.M.; Song, X.L. MiR-576-3p is a novel marker correlated with poor clinical outcome in bladder cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 973–977. [Google Scholar]
- Greenawalt, E.J.; Edmonds, M.D.; Jain, N.; Adams, C.M.; Mitra, R.; Eischen, C.M. Targeting of SGK1 by miR-576-3p Inhibits Lung Adenocarcinoma Migration and Invasion. Mol. Cancer Res. 2019, 17, 289–298. [Google Scholar] [PubMed]
- Hu, Q.; Liu, F.; Yan, T.; Wu, M.; Ye, M.; Shi, G.; Lv, S.; Zhu, X. MicroRNA5763p inhibits the migration and proangiogenic abilities of hypoxiatreated glioma cells through hypoxiainducible factor1alpha. Int. J. Mol. Med. 2019, 43, 2387–2397. [Google Scholar]
- He, J.; Mai, J.; Li, Y.; Chen, L.; Xu, H.; Zhu, X.; Pan, Q. miR-597 inhibits breast cancer cell proliferation, migration and invasion through FOSL2. Oncol Rep. 2017, 37, 2672–2678. [Google Scholar]
- Li, S.; Liu, Z.; Fang, X.D.; Wang, X.Y.; Fei, B.Y. MicroRNA (miR)-597-5p Inhibits Colon Cancer Cell Migration and Invasion by Targeting FOS-Like Antigen 2 (FOSL2). Front. Oncol. 2019, 9, 495. [Google Scholar] [PubMed]
- Zhang, Q.; Hu, H.; Chen, S.Y.; Liu, C.J.; Hu, F.F.; Yu, J.; Wu, Y.; Guo, A.Y. Transcriptome and Regulatory Network Analyses of CD19-CAR-T Immunotherapy for B-ALL. Genom. Proteom. Bioinform. 2019, 17, 190–200. [Google Scholar]
- Xu, W.; Hua, Y.; Deng, F.; Wang, D.; Wu, Y.; Zhang, W.; Tang, J. MiR-145 in cancer therapy resistance and sensitivity: A comprehensive review. Cancer Sci. 2020, 111, 3122–3131. [Google Scholar] [CrossRef]
- Shi, Q.; Xing, G.; Qi, M.; Xing, Y. Lower Serum miR-145 Predicts Poor Prognosis in Patients with Acute Myeloid Leukemia. Clin. Lab. 2020, 66, 7754. [Google Scholar]
- Ishii, H.; Vodnala, S.K.; Achyut, B.R.; So, J.Y.; Hollander, M.C.; Greten, T.F.; Lal, A.; Yang, L. miR-130a and miR-145 reprogram Gr-1+CD11b+ myeloid cells and inhibit tumor metastasis through improved host immunity. Nat. Commun. 2018, 9, 2611. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Zhang, Y.; Wang, Z.; Ding, J.; Song, Y.; Zhao, W. Cisplatin-mediated down-regulation of miR-145 contributes to up-regulation of PD-L1 via the c-Myc transcription factor in cisplatin-resistant ovarian carcinoma cells. Clin. Exp. Immunol. 2020, 200, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.A.; Cummings, C.L.; Korb, B.; Boaglio, S.; Oehler, V.G. Deregulated KLF4 Expression in Myeloid Leukemias Alters Cell Proliferation and Differentiation through MicroRNA and Gene Targets. Mol. Cell. Boil. 2016, 36, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bugno, J.; Hu, C.; Yang, Y.; Herold, T.; Qi, J.; Chen, P.; Gurbuxani, S.; Arnovitz, S.; Strong, J.; et al. Eradication of acute myeloid leukemia with FLT3 ligand-targeted miR-150 nanoparticles. Cancer Res. 2016, 76, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Butrym, A.; Rybka, J.; Baczynska, D.; Tukiendorf, A.; Kuliczkowski, K.; Mazur, G. Low expression of microRNA-204 (miR-204) is associated with poor clinical outcome of acute myeloid leukemia (AML) patients. J. Exp. Clin. Cancer Res. 2015, 34, 68. [Google Scholar] [CrossRef]
- Xue, F.; Che, H. The long non-coding RNA LOC285758 promotes invasion of acute myeloid leukemia cells by down-regulating miR-204-5p. FEBS Open Bio. 2020, 10, 734–743. [Google Scholar] [PubMed]
- Xu, X.; Zhang, F.; Chen, X.; Ying, Q. MicroRNA-518b functions as a tumor suppressor in glioblastoma by targeting PDGFRB. Mol. Med. Rep. 2017, 16, 5326–5332. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.S.; Ryu, H.S.; Kim, N.; Kim, J.; Lee, E.; Moon, H.; Kim, K.H.; Jin, M.-S.; Kwon, N.H.; Kim, S.; et al. Tumor suppressor microRNA-204-5p regulates growth, metastasis, and immune microenvironment remodeling in breast cancer. Cancer Res. 2019, 79, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, S.; Zhang, L.; Zhang, J.; Cai, H.; Zhu, J.; Huang, C.; Wang, J. miR-518b is down-regulated, and involved in cell proliferation and invasion by targeting Rap1b in esophageal squamous cell carcinoma. FEBS Lett. 2012, 586, 3508–3521. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, A.; Brown, M.D.; Finelli, A.; Jewett, M.A.; Diamandis, E.P.; Yousef, G.M. Prognostic urinary miRNAs for the assessment of small renal masses. Clin. Biochem. 2020, 75, 15–22. [Google Scholar]
- Xia, W.; Gong, D.; Qin, X.; Cai, Z. MicroRNA-671-3p suppresses proliferation and invasion of breast cancer cells by targeting DEPTOR. Nan Fang Yi Ke Da Xue Xue Bao 2020, 40, 42–48. [Google Scholar] [PubMed]
- Yao, Y.; Zhou, Y.; Fu, X. miR-671-3p is downregulated in non-small cell lung cancer and inhibits cancer progression by directly targeting CCND2. Mol. Med. Rep. 2019, 19, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Bi, H.; Zhang, Q.; Zhang, S.; Zhou, L.; Zhu, X.; Zhou, J. Upregulated microRNA-671-3p promotes tumor progression by suppressing forkhead box P2 expression in non-small-cell lung cancer. Mol. Med. Rep. 2019, 20, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-F.; You, C.-Y.; Chen, Y.-S.; Jiang, H.; Zheng, X.; Tang, W.-W.; Wang, X.-Y.; Xu, H.-Y.; Geng, F. MicroRNA-671-3p promotes proliferation and migration of glioma cells via targeting CKAP4. OncoTargets Ther. 2018, 11, 6217–6226. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Xuan, C.; Chen, Q.; Xiang, Q.; Wang, J.; Liu, Y.; Luo, L.; Zhao, S.; Deng, Y.; et al. Identifying the key genes and microRNAs in prostate cancer bone metastasis by bioinformatics analysis. FEBS Open Bio. 2020, 10, 674–688. [Google Scholar] [CrossRef]
- Hu, Q.L.; Xu, Z.P.; Lan, Y.F.; Li, B. miR-636 represses cell survival by targeting CDK6/Bcl-2 in cervical cancer. Kaohsiung J. Med. Sci. 2020, 36, 328–335. [Google Scholar] [CrossRef]
- Erdogan, B.; Facey, C.; Qualtieri, J.; Tedesco, J.; Rinker, E.; Isett, R.B.; Tobias, J.; Baldwin, D.A.; Thompson, J.E.; Carroll, M.; et al. Diagnostic microRNAs in myelodysplastic syndrome. Exp. Hematol. 2011, 39, 915–926. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Song, X.; Niu, L.; Tang, Y.; Song, X.; Xie, L. Circulating Exosomal miR-150-5p and miR-99b-5p as Diagnostic Biomarkers for Colorectal Cancer. Front. Oncol. 2019, 9, 1129. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Yang, Y.; Luo, M.; Zhang, M.; Wang, X.; Liu, L.; Hou, N.; Guo, Q.; Song, T.; et al. MiR-99b-5p and miR-203a-3p Function as Tumor Suppressors by Targeting IGF-1R in Gastric Cancer. Sci. Rep. 2018, 8, 10119. [Google Scholar] [CrossRef]
- Liu, C.-J.; Huang, F.-Z.; Yang, J.-H.; Liu, C.-P.; Mao, X.-H.; Yi, W.-M.; Shen, X.-B.; Peng, C.; Chen, M.-F.; Jiang, B.; et al. The role of miR-99b in mediating hepatocellular carcinoma invasion and migration. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 2273–2281. [Google Scholar]
- Chen, S.; Chen, Y.; Zhu, Z.; Tan, H.; Lu, J.; Qin, P.; Xu, L. Identification of the key genes and microRNAs in adult acute myeloid leukemia with FLT3 mutation by bioinformatics analysis. Int. J. Med Sci. 2020, 17, 1269–1280. [Google Scholar] [PubMed]
- Ma, X.; Jin, L.; Lei, X.; Tong, J.; Wang, R. MicroRNA-363-3p inhibits cell proliferation and induces apoptosis in retinoblastoma cells via the Akt/mTOR signaling pathway by targeting PIK3CA. Oncol. Rep. 2020, 43, 1365–1374. [Google Scholar] [PubMed]
- Zhang, L.; Wang, L.; Lu, N.; Wang, J.; Yan, R.; Yan, H.; Zhang, J.; Zhang, M. Micro RNA-363 inhibits esophageal squamous cell carcinoma progression by directly targeting sperm-associated antigen 5. J. Int. Med Res. 2020, 48. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Gao, Y.; Ma, X.; He, W.; Zhang, Y.; Zhang, F.; Fan, Y.; Gu, L.; Li, P.; et al. miR-363 suppresses the proliferation, migration and invasion of clear cell renal cell carcinoma by downregulating S1PR1. Cancer Cell Int. 2020, 20, 227. [Google Scholar] [PubMed]
- Zhang, R.; Li, Y.; Dong, X.; Peng, L.; Nie, X. MiR-363 sensitizes cisplatin-induced apoptosis targeting in Mcl-1 in breast cancer. Med Oncol. 2014, 31, 347. [Google Scholar] [PubMed]
- Zhang, H.; Zhang, N.; Wang, R.; Shao, T.; Feng, Y.; Yao, Y.; Wu, Q.; Zhu, S.; Cao, J.; Zhang, H.; et al. High expression of miR-363 predicts poor prognosis and guides treatment selection in acute myeloid leukemia. J. Transl. Med. 2019, 17, 106. [Google Scholar]
- Smallwood, D.T.; Apollonio, B.; Willimott, S.; Lezina, L.; Alharthi, A.; Ambrose, A.R.; De Rossi, G.; Ramsay, A.G.; Wagner, S.D. Extracellular vesicles released by CD40/IL-4-stimulated CLL cells confer altered functional properties to CD4+ T cells. Blood 2016, 128, 542–552. [Google Scholar]
- Huber, V.; Vallacchi, V.; Fleming, V.; Hu, X.; Cova, A.; Dugo, M.; Shahaj, E.; Sulsenti, R.; Vergani, E.; Filipazzi, P.; et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J. Clin. Investig. 2018, 128, 5505–5516. [Google Scholar]
- Liu, J.; Wang, D.; Long, Z.; Liu, J.; Li, W. CircRNA8924 Promotes Cervical Cancer Cell Proliferation, Migration and Invasion by Competitively Binding to MiR-518d-5p /519-5p Family and Modulating the Expression of CBX8. Cell Physiol. Biochem. 2018, 48, 173–184. [Google Scholar]
- Huang, J.; Cao, D.; Sha, J.; Zhu, X.; Han, S. DLL3 is regulated by LIN28B and miR-518d-5p and regulates cell proliferation, migration and chemotherapy response in advanced small cell lung cancer. Biochem. Biophys. Res. Commun. 2019, 514, 853–860. [Google Scholar]
- Organista-Nava, J.; Gómez-Gómez, Y.; Illades-Aguiar, B.; Alarcón-Romero, L.D.C.; Saavedra-Herrera, M.V.; Rivera-Ramírez, A.B.; Garzón-Barrientos, V.H.; Leyva-Vázquez, M.A. High miR-24 expression is associated with risk of relapse and poor survival in acute leukemia. Oncol. Rep. 2015, 33, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhang, H.; Cai, T.-T.; Liu, Y.-N.; Ni, J.-J.; He, J.; Peng, J.-Y.; Chen, Q.-Y.; Mo, H.-Y.; Cui, J.; et al. Exosomal miR-24-3p impedes T-cell function by targetingFGF11and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016, 240, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Fayyad-Kazan, H.; Hamade, E.; Rouas, R.; Najar, M.; Fayyad-Kazan, M.; El Zein, N.; ElDirani, R.; Hussein, N.; Fakhry, M.; Al-Akoum, C.; et al. Downregulation of microRNA-24 and -181 parallels the upregulation of IFN-gamma secreted by activated human CD4 lymphocytes. Hum. Immunol. 2014, 75, 677–685. [Google Scholar] [CrossRef]
- Lovat, F.; Nigita, G.; Distefano, R.; Nakamura, T.; Gasparini, P.; Tomasello, L.; Fadda, P.; Ibrahimova, N.; Catricala, S.; Palamarchuk, A.; et al. Combined loss of function of two different loci of miR-15/16 drives the pathogenesis of acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2020, 117, 12332–12340. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Lu, F.; Han, X.-Y.; Ji, M.; Zhou, Y.; Zhang, A.-M.; Wang, H.-C.; Ma, D.-X.; Ji, C.-Y. MiR-424 and miR-27a increase TRAIL sensitivity of acute myeloid leukemia by targeting PLAG1. Oncotarget 2016, 7, 25276–25290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, L.-J.; Sun, G.-K.; Zhang, T.-J.; Wu, D.-H.; Zhou, J.-D.; Ma, B.-B.; Xu, Z.-J.; Wen, X.-M.; Chen, Q.; Yao, D.-M.; et al. Down-regulation of miR-29c is a prognostic biomarker in acute myeloid leukemia and can reduce the sensitivity of leukemic cells to decitabine. Cancer Cell Int. 2019, 19, 177. [Google Scholar] [CrossRef]
- Lim, E.L.; Trinh, D.L.; Ries, R.E.; Wang, J.; Gerbing, R.B.; Ma, Y.; Topham, J.; Hughes, M.; Pleasance, E.; Mungall, A.J.; et al. MicroRNA Expression-Based Model Indicates Event-Free Survival in Pediatric Acute Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-X.; You, L.-S.; Liu, H.; Mao, L.-P.; Ye, X.; Qian, W.-B. Apoptosis of acute myeloid leukemia HL-60 cells induced by CDK inhibitor SNS-032 and its molecular mechanisms. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015, 44, 174–178. [Google Scholar] [PubMed]
- Xu, P.; Zhou, D.; Yan, G.; Ouyang, J.; Chen, B. Correlation of miR-181a and three HOXA genes as useful biomarkers in acute myeloid leukemia. Int. J. Lab. Hematol. 2020, 42, 16–22. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, M.-X.; Luo, Z.-Y. Expression of miR-181a in Acute Myeloid Leukaemia and Its Effect on Cell Proliferation. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016, 24, 985–989. [Google Scholar]
- Liu, X.; Liao, W.; Peng, H.; Luo, X.; Luo, Z.; Jiang, H.; Xu, L. miR-181a promotes G1/S transition and cell proliferation in pediatric acute myeloid leukemia by targeting ATM. J. Cancer Res. Clin. Oncol. 2016, 142, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.-Y.; Feng, Y.; Pang, Y.; Zhou, X.-H.; Xu, B.; Yan, M.-X. MiR-181a Promotes Proliferation of Human Acute Myeloid Leukemia Cells by Targeting ATM. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016, 24, 347–351. [Google Scholar] [PubMed]
- Guo, Q.; Luan, J.; Li, N.; Zhang, Z.; Zhu, X.; Zhao, L.; Wei, R.; Sun, L.; Shi, Y.; Yin, X.; et al. MicroRNA-181 as a prognostic biomarker for survival in acute myeloid leukemia: A meta-analysis. Oncogene 2017, 8, 89130–89141. [Google Scholar] [CrossRef] [PubMed]
- Nanbakhsh, A.; Visentin, G.; Olive, D.; Janji, B.; Mussard, E.; Dessen, P.; Meurice, G.; Zhang, Y.; Louache, F.; Bourhis, J.-H.; et al. miR-181a modulates acute myeloid leukemia susceptibility to natural killer cells. OncoImmunology 2015, 4, e996475. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Kohanbash, G.; Lotze, M.T. MicroRNAs in immune regulation—Opportunities for cancer immunotherapy. Int. J. Biochem. Cell Boil. 2010, 42, 1256–1261. [Google Scholar] [CrossRef]
- Papapetrou, E.P.; Kovalovsky, D.; Beloeil, L.; Sant’Angelo, D.; Sadelain, M. Harnessing endogenous miR-181a to segregate transgenic antigen receptor expression in developing versus post-thymic T cells in murine hematopoietic chimeras. J. Clin. Investig. 2009, 119, 157–168. [Google Scholar] [CrossRef]
- Kagoya, Y.; Nakatsugawa, M.; Saso, K.; Guo, T.; Anczurowski, M.; Wang, C.-H.; Butler, M.O.; Arrowsmith, C.H.; Hirano, N. DOT1L inhibition attenuates graft-versus-host disease by allogeneic T cells in adoptive immunotherapy models. Nat. Commun. 2018, 9, 1915. [Google Scholar] [CrossRef]
- Achberger, S.; Aldrich, W.; Tubbs, R.; Crabb, J.W.; Singh, A.D.; Triozzi, P.L. Circulating immune cell and microRNA in patients with uveal melanoma developing metastatic disease. Mol. Immunol. 2014, 58, 182–186. [Google Scholar] [CrossRef]
- Vignard, V.; Labbé, M.; Marec, N.; André-Grégoire, G.; Jouand, N.; Fonteneau, J.-F.; Labarrière, N.; Fradin, D. MicroRNAs in Tumor Exosomes Drive Immune Escape in Melanoma. Cancer Immunol. Res. 2020, 8, 255–267. [Google Scholar] [CrossRef]
- Su, R.; Lin, H.-S.; Zhang, X.-H.; Yin, X.-L.; Ning, H.-M.; Liu, B.; Zhai, P.-F.; Gong, J.-N.; Shen, C.; Song, L.; et al. MiR-181 family: Regulators of myeloid differentiation and acute myeloid leukemia as well as potential therapeutic targets. Oncogene 2014, 34, 3226–3239. [Google Scholar] [CrossRef]
- Lim, S.P.; Ioannou, N.; Ramsay, A.G.; Darling, D.; Gäken, J.; Mufti, G.J. miR-181c -BRK1 axis plays a key role in actin cytoskeleton-dependent T cell function. J. Leukoc. Boil. 2018, 103, 855–866. [Google Scholar]
- Le Dieu, R.; Taussig, D.C.; Ramsay, A.G.; Mitter, R.; Miraki-Moud, F.; Fatah, R.; Lee, A.M.; Lister, T.A.; Gribben, J.G. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood 2009, 114, 3909–3916. [Google Scholar] [PubMed]
- Zhang, Z.; Xue, Z.; Liu, Y.; Liu, H.; Guo, X.; Li, Y.; Yang, H.; Zhang, L.; Da, Y.; Yao, Z.; et al. MicroRNA-181c promotes Th17 cell differentiation and mediates experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2018, 70, 305–314. [Google Scholar]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tang, X.; Shi, X.; Su, L. miR-532-5p promotes breast cancer proliferation and migration by targeting RERG. Exp. Ther. Med. 2019, 19, 400–408. [Google Scholar]
- Zhang, J.; Zhou, W.; Liu, Y.; Liu, T.; Li, C.; Wang, L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5’UTR of RUNX3. Oncol. Lett. 2018, 15, 7215–7220. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Liu, Z.; Zhang, X.; Jia, J. miRNA-532-5p functions as an oncogenic microRNA in human gastric cancer by directly targeting RUNX3. J. Cell. Mol. Med. 2016, 20, 95–103. [Google Scholar]
- Lin, X.; Ling, Q.; Lv, Y.; Ye, W.; Huang, J.; Guo, Q.; Wang, J.; Li, Z.; Jin, J. Plasma exosome-derived microRNA-532 as a novel predictor for acute myeloid leukemia. Cancer Biomarkers 2020, 28, 151–158. [Google Scholar]
- Ibrahim, S.; Szóstek-Mioduchowska, A.; Skarzynski, D.J. Expression profiling of selected miRNAs in equine endometrium in response to LPS challenge in vitro: A new understanding of the inflammatory immune response. Veter. Immunol. Immunopathol. 2019, 209, 37–44. [Google Scholar] [CrossRef]
- Shomali, N.; Mansoori, B.; Mohammadi, A.; Shirafkan, N.; Ghasabi, M.; Baradaran, B. MiR-146a functions as a small silent player in gastric cancer. Biomed. Pharmacother. 2017, 96, 238–245. [Google Scholar] [CrossRef]
- Emming, S.; Chirichella, M.; Monticelli, S. MicroRNAs as modulators of T cell functions in cancer. Cancer Lett. 2018, 430, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.D.; Kageyama, R.; Shehata, H.M.; Fassett, M.S.; Mar, D.J.; Wigton, E.J.; Johansson, K.; Litterman, A.J.; Odorizzi, P.; Simeonov, D.; et al. miR-15/16 Restrain Memory T Cell Differentiation, Cell Cycle, and Survival. Cell Rep. 2019, 28, 2169–2181.e4. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Garden, O.A.; Lang, F.; Cobb, B.S. MicroRNA-15b/16 Enhances the Induction of Regulatory T Cells by Regulating the Expression of Rictor and mTOR. J. Immunol. 2015, 195, 5667–5677. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J. Cell. Mol. Med. 2020. [Google Scholar] [CrossRef]
- Fan, X.; Wang, J.; Qin, T.; Zhang, Y.; Liu, W.; Jiang, K.; Huang, D. Exosome miR-27a-3p secreted from adipocytes targets ICOS to promote antitumor immunity in lung adenocarcinoma. Thorac. Cancer 2020, 11, 1453–1464. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, H.; Yu, J.; Ren, X. The role of miRNA-29 family in cancer. Eur. J. Cell Boil. 2013, 92, 123–128. [Google Scholar] [CrossRef]
- Schmitt, M.; Margue, C.; Behrmann, I.; Kreis, S. MiRNA-29: A microRNA Family with Tumor-Suppressing and Immune-Modulating Properties. Curr. Mol. Med. 2013, 13, 572–585. [Google Scholar] [CrossRef]
- Chandiran, K.; Lawlor, R.; Pannuti, A.; Perez, G.G.; Srinivasan, J.; Golde, T.E.; Miele, L.; Osborne, B.A.; Minter, L.M. Notch1 primes CD4 T cells for T helper type I differentiation through its early effects on miR-29. Mol. Immunol. 2018, 99, 191–198. [Google Scholar] [CrossRef]
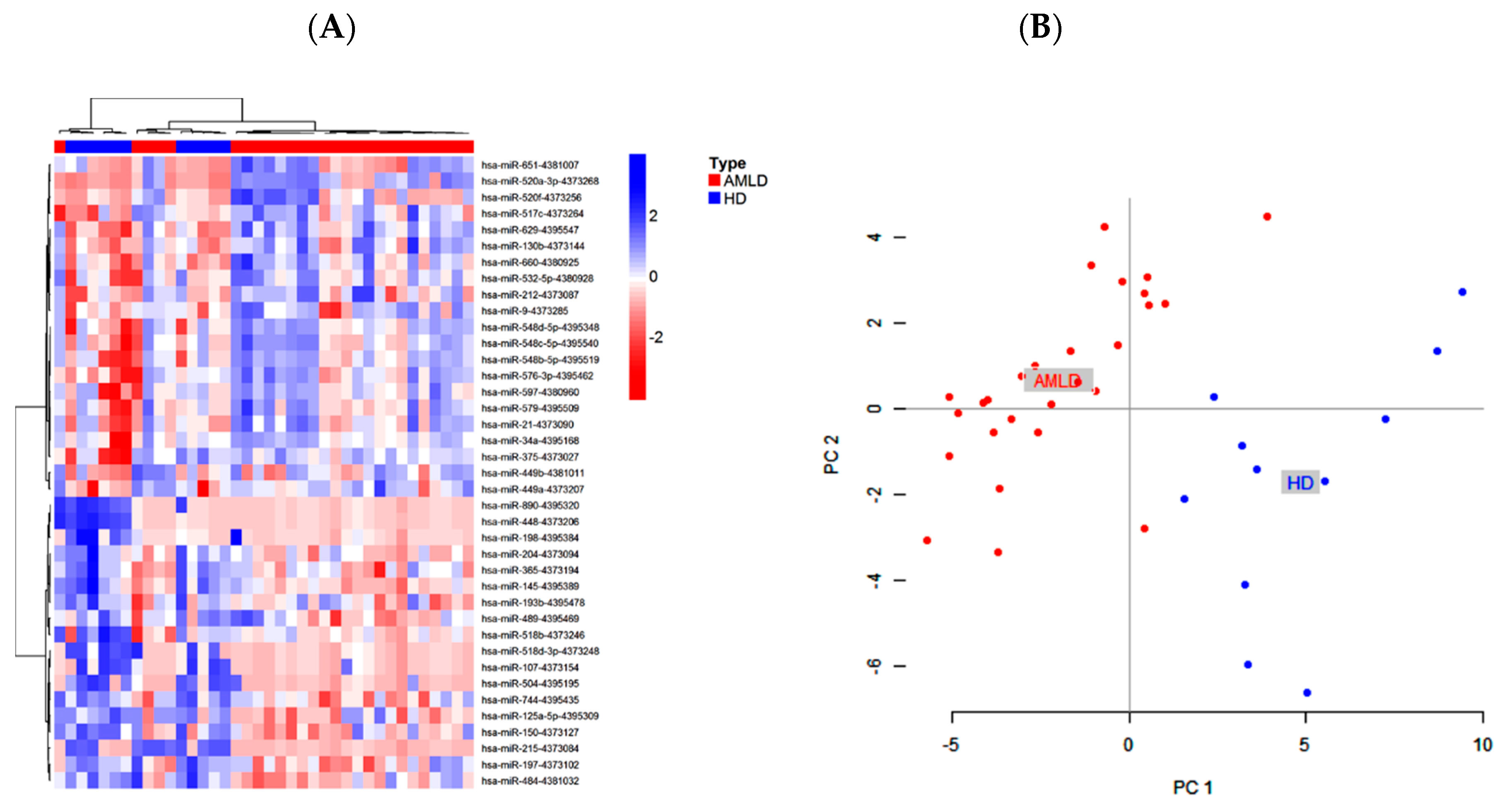

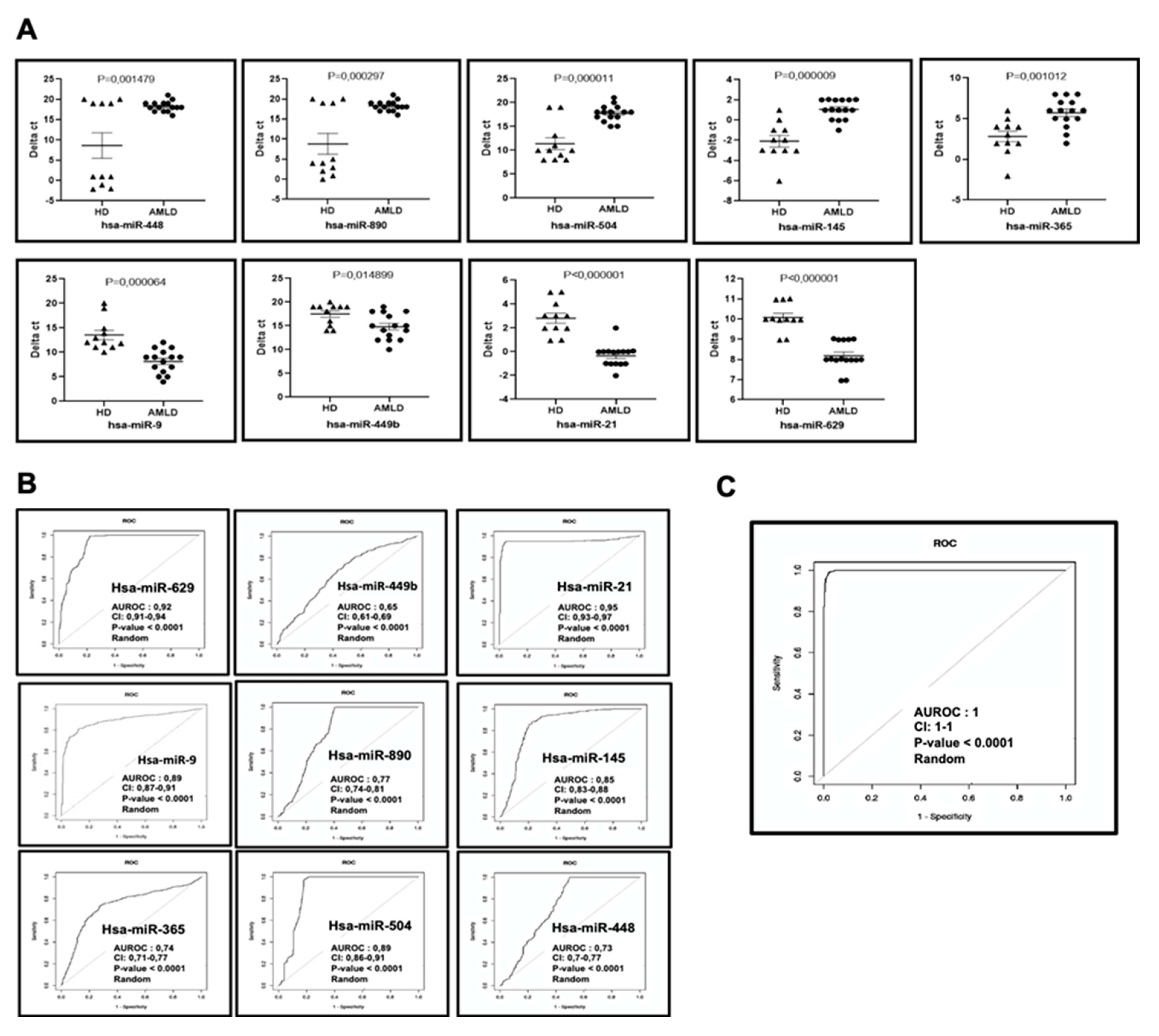

| Up regulated miRNAs in AMLD vs. HD | ||
| MiR Connotation | pValue | FC AMLD1 vs. HD |
| hsa-miR-520a-3p | 0.0000004 | 103.59 |
| hsa-miR-548b-5p | 0.0000187 | 15.35 |
| hsa-miR-651 | 0.0007066 | 13.01 |
| hsa-miR-449b | 0.0029965 | 7.84 |
| hsa-miR-520f | 0.0012403 | 7.19 |
| hsa-miR-330-5p | 0.0476803 | 6.55 |
| hsa-miR-34c-5p | 0.0300460 | 5.10 |
| hsa-miR-9 | 0.0030388 | 15.45 |
| hsa-miR-34a | 0.0008391 | 14.22 |
| hsa-miR-548d-5p | 0.0000390 | 13.09 |
| hsa-miR-655 | 0.0061917 | 8.55 |
| hsa-miR-548c-5p | 0.0002937 | 7.99 |
| hsa-miR-135a | 0.0225966 | 6.95 |
| hsa-miR-502-5p | 0.0028352 | 6.68 |
| hsa-miR-576-3p | 0.0008681 | 5.99 |
| hsa-miR-449a | 0.0003304 | 5.97 |
| hsa-miR-518f | 0.0015237 | 5.88 |
| hsa-miR-21 | 0.0000118 | 5.61 |
| hsa-miR-629 | 0.0000000 | 5.60 |
| hsa-miR-195 | 0.0000050 | 5.32 |
| hsa-miR-517c | 0.0011033 | 5.02 |
| hsa-miR-597 | 0.0026663 | 4.94 |
| hsa-miR-199b-5p | 0.0421635 | 4.85 |
| hsa-miR-548d-3p | 0.0203888 | 4.76 |
| hsa-miR-181a | 0.0039392 | 4.76 |
| hsa-miR-570 | 0.0180477 | 4.75 |
| hsa-miR-660 | 0.0000101 | 4.56 |
| hsa-miR-202 | 0.0100613 | 4.24 |
| hsa-miR-24 | 0.0225184 | 4.24 |
| hsa-miR-375 | 0.0016871 | 4.18 |
| hsa-miR-130b | 0.0000115 | 3.81 |
| hsa-miR-361-5p | 0.0020377 | 3.65 |
| hsa-miR-451 | 0.0019980 | 3.58 |
| hsa-miR-181c | 0.0131902 | 3.49 |
| hsa-miR-579 | 0.0002417 | 3.49 |
| hsa-miR-362-3p | 0.0106066 | 3.22 |
| hsa-miR-25 | 0.0005764 | 3.15 |
| hsa-miR-29c | 0.0095014 | 3.00 |
| hsa-miR-511 | 0.0103669 | 2.90 |
| hsa-miR-146b-3p | 0.0096730 | 2.88 |
| hsa-miR-532-5p | 0.0014276 | 2.70 |
| hsa-miR-106a | 0.0012818 | 2.62 |
| hsa-miR-874 | 0.0089434 | 2.61 |
| hsa-miR-212 | 0.0012351 | 2.50 |
| hsa-miR-598 | 0.0046231 | 2.48 |
| hsa-miR-221 | 0.0144840 | 2.47 |
| hsa-miR-18a | 0.0052162 | 2.46 |
| hsa-miR-27a | 0.0160879 | 2.45 |
| hsa-miR-590-5p | 0.0015733 | 2.40 |
| hsa-miR-20b | 0.0471303 | 2.36 |
| hsa-miR-101 | 0.0162702 | 2.23 |
| hsa-miR-146a | 0.0174218 | 2.22 |
| hsa-miR-142-3p | 0.0246946 | 2.21 |
| hsa-miR-146b-5p | 0.0305051 | 2.14 |
| hsa-miR-93 | 0.0175670 | 1.97 |
| hsa-miR-140-5p | 0.0362266 | 1.96 |
| hsa-miR-18b | 0.0224577 | 1.90 |
| hsa-miR-423-5p | 0.0088564 | 1.87 |
| hsa-miR-92a | 0.0443479 | 1.71 |
| hsa-miR-210 | 0.0429423 | 1.68 |
| hsa-miR-483-5p | 0.0423541 | 1.62 |
| Downregulated miRNAs in AMLD vs. HD | ||
| MiR Connotation | p Value | FC AMLD1 vs. HD |
| hsa-miR-326 | 0.00663326 | 0.103 |
| hsa-miR-198 | 0.00282623 | 0.078 |
| hsa-miR-518d-3p | 0.00001285 | 0.018 |
| hsa-miR-107 | 0.00000130 | 0.012 |
| hsa-miR-215 | 0.00082673 | 0.009 |
| hsa-miR-504 | 0.00000004 | 0.006 |
| hsa-miR-890 | 0.00000995 | 0.001 |
| hsa-miR-448 | 0.00007498 | 0.001 |
| hsa-miR-532-3p | 0.05245365 | 0.616 |
| hsa-miR-342-3p | 0.04884326 | 0.602 |
| hsa-miR-200c | 0.04994680 | 0.547 |
| hsa-miR-491-5p | 0.01187570 | 0.543 |
| hsa-miR-28-3p | 0.00718598 | 0.531 |
| hsa-miR-99b | 0.00686813 | 0.444 |
| hsa-miR-574-3p | 0.01232231 | 0.432 |
| hsa-miR-744 | 0.00082444 | 0.358 |
| hsa-miR-190 | 0.05420973 | 0.353 |
| hsa-miR-150 | 0.00317263 | 0.322 |
| hsa-miR-197 | 0.00005892 | 0.270 |
| hsa-miR-125a-5p | 0.00614751 | 0.262 |
| hsa-miR-203 | 0.02014882 | 0.258 |
| hsa-miR-891a | 0.02627127 | 0.248 |
| hsa-miR-485-3p | 0.05481495 | 0.247 |
| hsa-miR-489 | 0.00938728 | 0.245 |
| hsa-miR-204 | 0.00027158 | 0.207 |
| hsa-miR-193b | 0.00487572 | 0.203 |
| hsa-miR-184 | 0.01694239 | 0.203 |
| hsa-miR-484 | 0.00012474 | 0.173 |
| hsa-miR-618 | 0.03435969 | 0.152 |
| hsa-miR-134 | 0.01390311 | 0.145 |
| hsa-miR-433 | 0.04689247 | 0.135 |
| hsa-miR-145 | 0.00000042 | 0.120 |
| hsa-miR-149 | 0.02704296 | 0.119 |
| hsa-miR-328 | 0.00004261 | 0.105 |
| hsa-miR-654-5p | 0.03086797 | 0.079 |
| hsa-miR-365 | 0.00000686 | 0.066 |
| hsa-miR-518b | 0.00169071 | 0.063 |
| MiRNAs Connotation | p Value AMLD1 | FC AMLD1 | p Value AMLD2 | FC AMLD2 | p Value AMLD Total | FC. AMLD Total |
|---|---|---|---|---|---|---|
| hsa-miR-520a-3p | 4.08 × 10−7 | 103.58 | 0.016 | 3.724 | 9.22 × 10−5 | 31.587 |
| hsa-miR-9 | 0.003 | 15.451 | 3.20 × 10−5 | 41.678 | 0.0002 | 22.022 |
| hsa-miR-548b-5p | 1.87 × 10−5 | 15.354 | 0.22 | 2.003 | 0.0026 | 7.418 |
| hsa-miR-34a | 0.0008 | 14.221 | 0.006 | 13.443 | 6.19 × 10−5 | 13.938 |
| hsa-miR-548d-5p | 3.89 × 10−5 | 13.091 | 0.021 | 5.268 | 7.85 × 10−5 | 9.458 |
| hsa-miR-651 | 0.0007 | 13.011 | 0.004 | 7.129 | 0.0006 | 10.496 |
| hsa-miR-548c-5p | 0.0002 | 7.993 | 0.150 | 1.961 | 0.0031 | 4.839 |
| hsa-miR-449b | 0.0029 | 7.843 | 0.007 | 6.301 | 0.0019 | 7.253 |
| hsa-miR-520f | 0.0012 | 7.190 | 0.001 | 3.562 | 0.0011 | 5.594 |
| hsa-miR-502-5p | 0.0028 | 6.676 | 0.132 | 2.898 | 0.0109 | 4.955 |
| hsa-miR-576-3p | 0.0008 | 5.989 | 2.11 × 10−5 | 8.364 | 2.68 × 10−5 | 6.748 |
| hsa-miR-449a | 0.0003 | 5.972 | 0.078 | 2.814 | 0.0021 | 4.565 |
| hsa-miR-21 | 1.17 × 10−5 | 5.611 | 1.76 × 10−7 | 8.913 | 8.07 × 10−8 | 6.619 |
| hsa-miR-629 | 2.98 × 10−8 | 5.600 | 2.23 × 10−7 | 3.693 | 3.41 × 10−9 | 4.826 |
| hsa-miR-195 | 5.006 × 10−6 | 5.315 | 0.20 | 1.546 | 0.002 | 3.419 |
| hsa-miR-517c | 0.0011 | 5.019 | 0.35 | 1.264 | 0.020 | 3.068 |
| hsa-miR-597 | 0.0026 | 4.941 | 0.0008 | 8.215 | 0.0001 | 5.925 |
| hsa-miR-181a | 0.0039 | 4.760 | 0.0161 | 2.755 | 0.0044 | 3.916 |
| hsa-miR-660 | 1.00 × 10−5 | 4.558 | 0.0590 | 1.536 | 0.0003 | 3.091 |
| hsa-miR-375 | 0.001 | 4.177 | 0.0009 | 9.544 | 0.0001 | 5.611 |
| hsa-miR-130b | 1.14 × 10−5 | 3.815 | 0.1794 | 1.766 | 0.0077 | 2.897 |
| hsa-miR-361-5p | 0.002 | 3.651 | 0.0168 | 1.920 | 0.0025 | 2.902 |
| hsa-miR-451 | 0.0019 | 3.584 | 0.0128 | 2.659 | 0.0016 | 3.221 |
| hsa-miR-579 | 0.0002 | 3.487 | 0.0290 | 1.927 | 0.0006 | 2.821 |
| hsa-miR-25 | 0.0005 | 3.154 | 5.15 × 10−5 | 4.060 | 2.71 × 10−5 | 3.451 |
| hsa-miR-106a | 0.0012 | 2.616 | 0.07 | 1.558 | 0.0050 | 2.174 |
| hsa-miR-212 | 0.0012 | 2.501 | 0.49 | 1.003 | 0.0251 | 1.805 |
| hsa-miR-598 | 0.0046 | 2.482 | 0.24 | 1.346 | 0.0280 | 1.994 |
| hsa-miR-18a | 0.0052 | 2.459 | 0.0008 | 3.101 | 0.0011 | 2.671 |
| hsa-miR-744 | 0.0008 | 0.358 | 3.03 × 10−5 | 0.201 | 8.80 × 10−5 | 0.291 |
| hsa-miR-150 | 0.0031 | 0.322 | 1.10 × 10−5 | 0.116 | 0.0001 | 0.223 |
| hsa-miR-197 | 5.8910−5 | 0.270 | 0.19 | 0.734 | 0.0041 | 0.386 |
| hsa-miR-204 | 0.0002 | 0.207 | 1.39 × 10−5 | 0.113 | 8.73 × 10−6 | 0.167 |
| hsa-miR-193b | 0.0048 | 0.203 | 9.67 × 10−7 | 0.052 | 0.0001 | 0.125 |
| hsa-miR-484 | 0.0001 | 0.173 | 0.11 | 0.552 | 0.0023 | 0.262 |
| hsa-miR-145 | 4.21 × 10−7 | 0.120 | 4.47 × 10−6 | 0.112 | 3.11 × 10−9 | 0.117 |
| hsa-miR-328 | 4.26 × 10−5 | 0.105 | 0.42 | 1.135 | 0.014 | 0.245 |
| hsa-miR-198 | 0.0028 | 0.078 | 0.22 | 0.409 | 0.013 | 0.141 |
| hsa-miR-365 | 6.86 × 10−6 | 0.066 | 0.0005 | 0.132 | 3.58 × 10−6 | 0.084 |
| hsa-miR-518b | 0.001 | 0.063 | 4.28 × 10−5 | 0.007 | 5.05 × 10−5 | 0.028 |
| hsa-miR-518d-3p | 1.28 × 10−5 | 0.018 | 0.37 | 0.656 | 0.005 | 0.066 |
| hsa-miR-107 | 1.30 × 10−6 | 0.012 | 0.11 | 0.174 | 0.0004 | 0.031 |
| hsa-miR-215 | 0.0008 | 0.009 | 0.06 | 0.083 | 0.0024 | 0.019 |
| hsa-miR-504 | 3.50 × 10−8 | 0.006 | 5.70 × 10−6 | 0.012 | 1.70 × 10−10 | 0.008 |
| hsa-miR-890 | 9.94 × 10−6 | 0.001 | 0.0001 | 0.001 | 9.74 × 10−8 | 0.001 |
| hsa-miR-448 | 7.49 × 10−5 | 0.001 | 0.0007 | 0.001 | 1.63 × 10−6 | 0.001 |
| A | ||
| KEGG ID and Term | Count | p value |
| hsa05206: MicroRNAs in cancer | 60 | 6.05 × 10−11 |
| hsa05200: Pathways in cancer | 62 | 1.51 × 10−10 |
| hsa04014: Ras signaling pathway | 48 | 2.29 × 10−5 |
| hsa05221:Acute myeloid leukemia | 45 | 3.98 × 10−5 |
| hsa04068: FoxO signaling pathway | 52 | 0.000106 |
| hsa04151: PI3K-Akt signaling pathway | 54 | 0.000182 |
| hsa04012: ErbB signaling pathway | 46 | 0.00181 |
| hsa05202: Transcriptional misregulation in cancer | 49 | 0.003887 |
| hsa04066: HIF-1 signaling pathway | 47 | 0.003892 |
| hsa05231: Choline metabolism in cancer | 42 | 0.00457 |
| hsa04520: Adherens junction | 50 | 0.004866 |
| hsa04210: Apoptosis | 35 | 0.005405 |
| hsa04620: Toll-like receptor signaling pathway | 37 | 0.005751 |
| hsa04010: MAPK signaling pathway | 52 | 0.009669 |
| hsa04672: Intestinal immune network for IgA production | 16 | 0.010748 |
| hsa04064: NF-kappa B signaling pathway | 37 | 0.012295 |
| hsa04150: mTOR signaling pathway | 30 | 0.014025 |
| hsa04650: Natural killer cell mediated cytotoxicity | 34 | 0.014803 |
| hsa04062: Chemokine signaling pathway | 48 | 0.01481 |
| hsa04550: Signaling pathways regulating pluripotency of stem cells | 45 | 0.016837 |
| hsa04660: T cell receptor signaling pathway | 32 | 0.031392 |
| hsa04115: p53 signaling pathway | 37 | 0.045474 |
| hsa04330: Notch signaling pathway | 26 | 0.047169 |
| hsa04110: Cell cycle | 44 | 0.049139 |
| B | ||
| Gene Ontology: GO ID and Term | Count | p value |
| Biological Process | ||
| GO:0010604~positive regulation of macromolecule metabolic process | 70 | 2.52384 × 10−16 |
| GO:0010628~positive regulation of gene expression | 68 | 1.05757 × 10−12 |
| GO:0042127~regulation of cell proliferation | 69 | 1.97068 × 10−11 |
| GO:0008219~cell death | 68 | 3.48089 × 10−10 |
| GO:0006915~apoptotic process | 66 | 4.40894 × 10−10 |
| GO:0002682~regulation of immune system process | 65 | 6.91088 × 10-10 |
| GO:0045595~regulation of cell differentiation | 68 | 1.57355 × 10−9 |
| GO:0060548~negative regulation of cell death | 62 | 1.0149 × 10−8 |
| GO:0008284~positive regulation of cell proliferation | 62 | 1.04 × 10−8 |
| GO:0097190~apoptotic signaling pathway | 54 | 1.20513 × 10−8 |
| GO:0043410~positive regulation of MAPK cascade | 56 | 2.1753 × 10−6 |
| GO:0050776~regulation of immune response | 57 | 4.45138 × 10−6 |
| GO:0070489~T cell aggregation | 57 | 6.58436 × 10−6 |
| GO:0051726~regulation of cell cycle | 58 | 8.80856 × 10−6 |
| GO:0043408~regulation of MAPK cascade | 60 | 1.07104 × 10−5 |
| GO:0045596~negative regulation of cell differentiation | 65 | 1.11453 × 10−5 |
| GO:0097191~extrinsic apoptotic signaling pathway | 47 | 1.20729 × 10−5 |
| GO:0002819~regulation of adaptive immune response | 33 | 9.06477 × 10−5 |
| GO:0007219~Notch signaling pathway | 43 | 9.88879 × 10−5 |
| GO:0046651~lymphocyte proliferation | 45 | 0.000115119 |
| GO:0006955~immune response | 62 | 0.000124958 |
| GO:0002683~negative regulation of immune system process | 45 | 0.000299385 |
| GO:0001959~regulation of cytokine-mediated signaling pathway | 37 | 0.00094784 |
| GO:0030099~myeloid cell differentiation | 54 | 0.001058782 |
| GO:0097194~execution phase of apoptosis | 31 | 0.00363619 |
| GO:0070227~lymphocyte apoptotic process | 32 | 0.008369403 |
| GO:0097300~programmed necrotic cell death | 13 | 0.011048278 |
| Molecular Function | ||
| GO:0019899~enzyme binding | 67 | 2.15784 × 10−7 |
| GO:0000981~RNA polymerase II transcription factor activity. SSB | 65 | 1.2604 × 10−5 |
| GO:0044212~transcription regulatory region DNA binding | 64 | 3.84654 × 10−5 |
| GO:0000975~regulatory region DNA binding | 64 | 5.11725 × 10−5 |
| GO:0044877~macromolecular complex binding | 62 | 5.44329 × 10−5 |
| GO:0019900~kinase binding | 62 | 6.77415 × 10−5 |
| GO:0008134~transcription factor binding | 64 | 0.000131465 |
| GO:0003690~double-stranded DNA binding | 62 | 0.000142272 |
| GO:0003682~chromatin binding | 52 | 0.00091488 |
| GO:0016301~kinase activity | 61 | 0.001966828 |
| GO:0001077~transcriptional activator activity. RNA polymerase II SSB | 49 | 0.002467546 |
| GO:0050839~cell adhesion molecule binding | 45 | 0.007658872 |
| GO:0001085~RNA polymerase II transcription factor binding | 40 | 0.01087498 |
| GO:0005126~cytokine receptor binding | 49 | 0.010986866 |
| GO:0071837~HMG box domain binding | 16 | 0.012673444 |
| GO:0019838~growth factor binding | 42 | 0.021399477 |
| GO:0042826~histone deacetylase binding | 40 | 0.02268476 |
| GO:0008327~methyl-CpG binding | 15 | 0.039968845 |
| GO:0005125~cytokine activity | 37 | 0.04028971 |
| GO:0070851~growth factor receptor binding | 38 | 0.046274794 |
| Cellular Component | ||
| GO:0000785~chromatin | 55 | 0.000142352 |
| GO:0000790~nuclear chromatin | 51 | 0.000199439 |
| GO:0005829~cytosol | 68 | 0.001232442 |
| GO:0044454~nuclear chromosome part | 52 | 0.002342868 |
| GO:0000792~heterochromatin | 25 | 0.002647328 |
| GO:0000791~euchromatin | 20 | 0.003993973 |
| GO:0005578~proteinaceous extracellular matrix | 42 | 0.004102668 |
| GO:0044427~chromosomal part | 56 | 0.00606402 |
| GO:0005794~Golgi apparatus | 50 | 0.006148438 |
| GO:0005741~mitochondrial outer membrane | 32 | 0.010046195 |
| GO:0005576~extracellular region | 61 | 0.010189067 |
| GO:0005667~transcription factor complex | 47 | 0.017135475 |
| GO:0016604~nuclear body | 42 | 0.017270393 |
| GO:0019867~outer membrane | 32 | 0.018716537 |
| GO:0098857~membrane microdomain | 51 | 0.020024305 |
| GO:0045121~membrane raft | 40 | 0.024624821 |
| GO:0005739~mitochondrion | 58 | 0.027721756 |
| GO:0005912~adherens junction | 51 | 0.02781721 |
| GO:0070161~anchoring junction | 36 | 0.028521889 |
| MiRNAs Connotation | pvalue FAV vs. ADV | FC FAV vs. ADV |
|---|---|---|
| hsa-miR-886-3p | 0.003362062 | 0.242595592 |
| hsa-miR-671-3p | 0.003550766 | 0.533445656 |
| hsa-miR-187 | 0.00401137 | 0.071572526 |
| hsa-miR-886-5p | 0.007422418 | 0.36174907 |
| hsa-miR-99b | 0.011423156 | 0.405734354 |
| hsa-miR-337-5p | 0.016909231 | 0.147489611 |
| hsa-miR-501-5p | 0.019700757 | 0.515368965 |
| hsa-miR-125a-5p | 0.019760174 | 0.252095185 |
| hsa-miR-532-3p | 0.023040754 | 0.49492166 |
| hsa-miR-636 | 0.024538272 | 0.436038555 |
| hsa-miR-196b | 0.02857687 | 0.210572395 |
| hsa-miR-363 | 0.030540457 | 0.459860525 |
| hsa-miR-152 | 0.037124343 | 0.44325062 |
| hsa-miR-125a-3p | 0.045392856 | 0.277844263 |
| hsa-miR-616 | 0.046918254 | 0.557903991 |
| hsa-miR-184 | 0.04693333 | 0.148403457 |
| hsa-miR-328 | 0.046990145 | 0.315492217 |
| hsa-miR-490-3p | 0.049822819 | 0.075649011 |
| hsa-miR-222 | 0.049940917 | 3.023869496 |
| MIRNAs Connotation | p Value | FC AMLD vs. AMLPT |
|---|---|---|
| hsa-miR-10b | 0.01421971 | 47.195 |
| hsa-miR-22 | 0.02777678 | 30.223 |
| has-miR-155 | 2.1736 × 10−6 | 15.886 |
| hsa-miR-29c | 7.6596 × 10−7 | 11.930 |
| hsa-miR-181a | 0.00018836 | 11.307 |
| hsa-miR-181c | 0.00060928 | 10.557 |
| hsa-miR-31 | 0.04346473 | 9.674 |
| hsa-miR-193a-3p | 0.0017743 | 9.137 |
| hsa-miR-542-5p | 0.00255845 | 9.027 |
| hsa-miR-24 | 0.0202524 | 8.248 |
| hsa-miR-517a | 0.00148494 | 7.944 |
| hsa-miR-205 | 0.03307049 | 7.550 |
| hsa-miR-146b-5p | 2.0118 10−7 | 7.488 |
| hsa-miR-32 | 0.01163137 | 7.056 |
| hsa-miR-150 | 0.00052394 | 6.297 |
| hsa-miR-196b | 0.01001577 | 6.294 |
| hsa-miR-29a | 0.00499311 | 6.213 |
| hsa-miR-95 | 0.00012723 | 5.918 |
| hsa-miR-140-3p | 2.4156 × 10−6 | 5.603 |
| hsa-miR-503 | 0.03701134 | 5.591 |
| hsa-miR-616 | 0.00153298 | 5.563 |
| hsa-miR-345 | 1.9647 × 10−5 | 5.303 |
| hsa-miR-324-3p | 0.00632634 | 5.086 |
| hsa-miR-627 | 0.00796799 | 5.039 |
| hsa-miR-523 | 0.04255625 | 4.853 |
| hsa-miR-574-3p | 0.05035657 | 4.795 |
| hsa-miR-362-3p | 0.01189798 | 4.470 |
| hsa-miR-339-3p | 0.00211171 | 4.439 |
| hsa-miR-518f | 0.01914946 | 4.261 |
| hsa-miR-548b-5p | 0.03672825 | 4.166 |
| hsa-miR-652 | 0.00221981 | 3.952 |
| hsa-miR-489 | 0.01868762 | 3.762 |
| hsa-miR-708 | 0.04542188 | 3.758 |
| hsa-miR-362-5p | 0.00066885 | 3.745 |
| hsa-miR-324-5p | 0.0004552 | 3.737 |
| hsa-miR-146b-3p | 0.00350397 | 3.544 |
| hsa-miR-212 | 9.932810−5 | 3.541 |
| hsa-miR-106b | 0.00022376 | 3.533 |
| hsa-miR-628-5p | 0.00724292 | 3.350 |
| hsa-miR-138 | 0.00521557 | 3.343 |
| hsa-miR-342-3p | 0.00134169 | 3.336 |
| hsa-miR-146a | 0.0132347 | 3.315 |
| hsa-miR-140-5p | 0.0013407 | 3.143 |
| hsa-miR-374b | 0.00172676 | 2.978 |
| hsa-miR-548c-5p | 0.04039887 | 2.967 |
| hsa-miR-361-5p | 0.0093724 | 2.963 |
| hsa-miR-200c | 0.00275003 | 2.961 |
| hsa-miR-340 | 0.03578044 | 2.952 |
| hsa-miR-200b | 1.0495 × 10−5 | 2.810 |
| hsa-miR-126 | 0.00102753 | 2.638 |
| hsa-miR-744 | 0.01146915 | 2.633 |
| hsa-miR-502-5p | 0.02889317 | 2.510 |
| hsa-miR-590-5p | 0.00094189 | 2.509 |
| hsa-miR-28-5p | 0.01105795 | 2.491 |
| hsa-miR-142-5p | 0.00951069 | 2.371 |
| hsa-let-7g | 0.00883628 | 2.368 |
| hsa-miR-374a | 0.01189576 | 2.338 |
| hsa-miR-199b-5p | 0.04657583 | 2.248 |
| hsa-miR-532-5p | 0.01865158 | 2.236 |
| hsa-miR-27a | 0.02344286 | 2.230 |
| hsa-miR-186 | 0.01390867 | 2.225 |
| hsa-miR-15b | 0.01605429 | 2.115 |
| hsa-miR-195 | 0.02292656 | 2.105 |
| hsa-miR-26a | 0.02280752 | 2.101 |
| hsa-miR-106a | 0.00925626 | 2.099 |
| hsa-miR-26b | 0.03471509 | 1.985 |
| hsa-miR-30c | 0.03123522 | 1.869 |
| hsa-miR-30b | 0.04525501 | 1.765 |
| hsa-miR-491-5p | 0.0305936 | 1.652 |
| hsa-miR-375 | 0.03634904 | 0.393 |
| hsa-miR-99b | 0.00549437 | 0.391 |
| hsa-miR-365 | 0.04837696 | 0.373 |
| hsa-miR-92a | 0.00974346 | 0.371 |
| hsa-miR-671-3p | 2.371 × 10−5 | 0.312 |
| hsa-miR-450a | 0.04665015 | 0.232 |
| hsa-miR-484 | 0.00424698 | 0.187 |
| hsa-miR-885-5p | 0.00758973 | 0.185 |
| hsa-miR-190 | 0.00698937 | 0.154 |
| hsa-miR-654-3p | 0.01975562 | 0.123 |
| hsa-miR-328 | 0.01366178 | 0.121 |
| hsa-miR-211 | 0.02452068 | 0.099 |
| hsa-miR-296-5p | 0.00035013 | 0.078 |
| hsa-miR-486-5p | 4.0707 × 10−5 | 0.075 |
| hsa-miR-490-3p | 0.02091499 | 0.055 |
| hsa-miR-485-3p | 0.00378225 | 0.039 |
| hsa-miR-23a | 0.01913452 | 0.015 |
| hsa-miR-518d-3p | 0.00055143 | 0.008 |
| hsa-miR-326 | 8.7126 × 10−7 | 0.003 |
| Clinical Details of AML Patients (42) | Number |
|---|---|
| Sex and age | |
| Males | 20 |
| Females | 22 |
| Age < 55y | 17 |
| Age ≥ 55y | 25 |
| Classification of AML Patients | |
| Cytogenetic abnormalities | |
| Normal Karyotype | 10 |
| Chromosome 9 deletion | 4 |
| Inversion (16) MYH11-CBFB | 4 |
| Translocation (t8;21) | 4 |
| Translocation (t15;17) | 6 |
| Translocation (X:21) (p11;q22) | 2 |
| trisomy 8 | 1 |
| complex Karyotype | 3 |
| WHO 2016 System | |
| AML with recurrent genetic abnormalities (t8;21) | 4 |
| AML with recurrent genetic abnormalities (inv 16) | 4 |
| AML with recurrent genetic abnormalities (t15;17) | 6 |
| Acute monoblastic/monocytic leukemia | 2 |
| Pure erythroid leukemia | 2 |
| AML not otherwise specified (NOS) | 20 |
| AML with myelodysplasia-related changes | 6 |
| ELN 2017 Genetic Risk Stratification | |
| Favorable | 14 |
| Intermediate | 7 |
| Adverse | 16 |
| unkown | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa Agha, D.; Rouas, R.; Najar, M.; Bouhtit, F.; Naamane, N.; Fayyad-Kazan, H.; Bron, D.; Meuleman, N.; Lewalle, P.; Merimi, M. Identification of Acute Myeloid Leukemia Bone Marrow Circulating MicroRNAs. Int. J. Mol. Sci. 2020, 21, 7065. https://doi.org/10.3390/ijms21197065
Moussa Agha D, Rouas R, Najar M, Bouhtit F, Naamane N, Fayyad-Kazan H, Bron D, Meuleman N, Lewalle P, Merimi M. Identification of Acute Myeloid Leukemia Bone Marrow Circulating MicroRNAs. International Journal of Molecular Sciences. 2020; 21(19):7065. https://doi.org/10.3390/ijms21197065
Chicago/Turabian StyleMoussa Agha, Douâa, Redouane Rouas, Mehdi Najar, Fatima Bouhtit, Najib Naamane, Hussein Fayyad-Kazan, Dominique Bron, Nathalie Meuleman, Philippe Lewalle, and Makram Merimi. 2020. "Identification of Acute Myeloid Leukemia Bone Marrow Circulating MicroRNAs" International Journal of Molecular Sciences 21, no. 19: 7065. https://doi.org/10.3390/ijms21197065
APA StyleMoussa Agha, D., Rouas, R., Najar, M., Bouhtit, F., Naamane, N., Fayyad-Kazan, H., Bron, D., Meuleman, N., Lewalle, P., & Merimi, M. (2020). Identification of Acute Myeloid Leukemia Bone Marrow Circulating MicroRNAs. International Journal of Molecular Sciences, 21(19), 7065. https://doi.org/10.3390/ijms21197065







