BMP-2/β-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model
Abstract
1. Introduction
2. Results
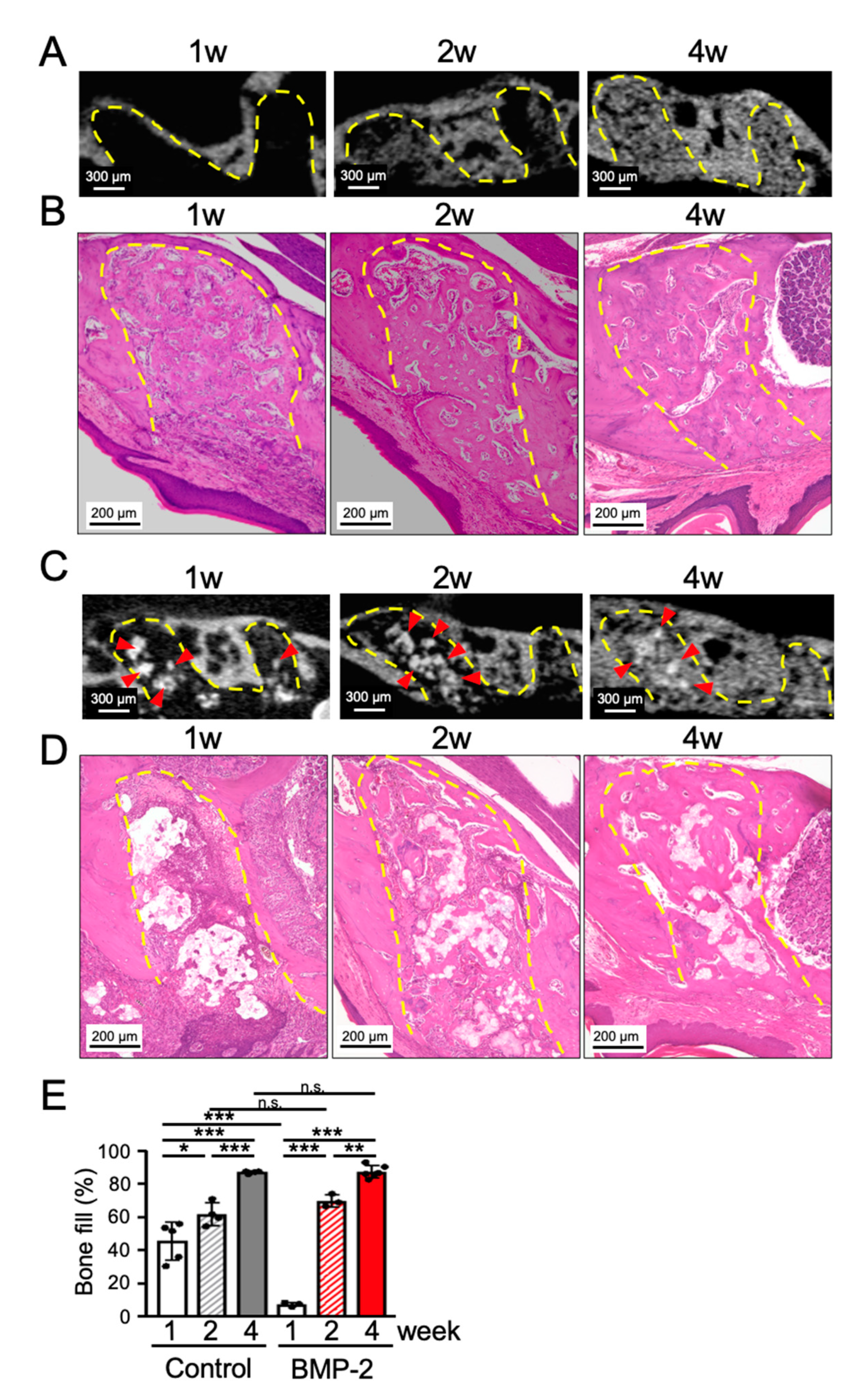
2.1. Transplantation of BMP-2/β-TCP Could Not Accelerate the Bone Regeneration in the Tooth Extraction Socket during the Normal Wound Healing Process
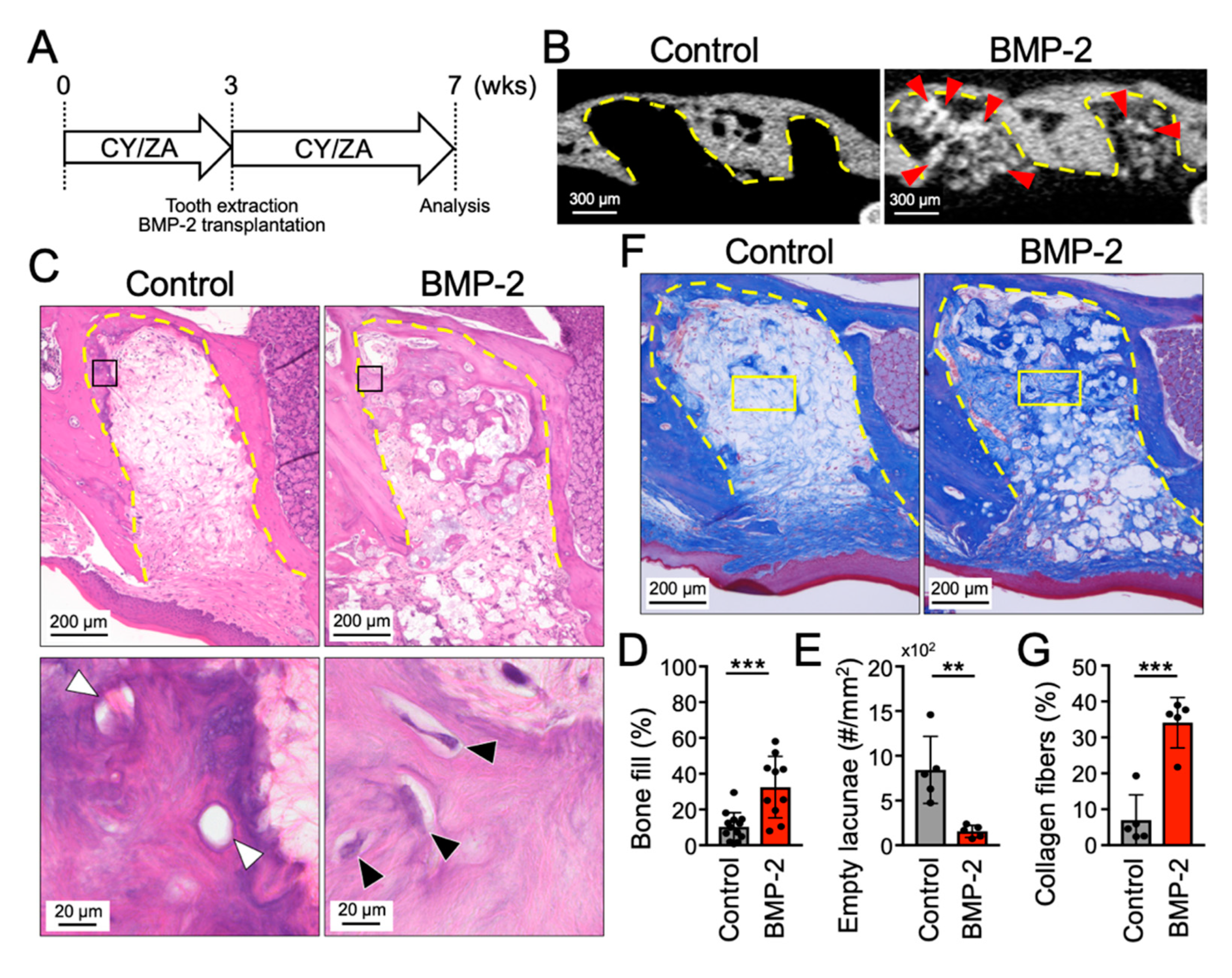
2.2. Transplantation of BMP-2/β-TCP Partially Induced Bone Formation in the Tooth Extraction Socket in a Mouse Model of MRONJ Prevention
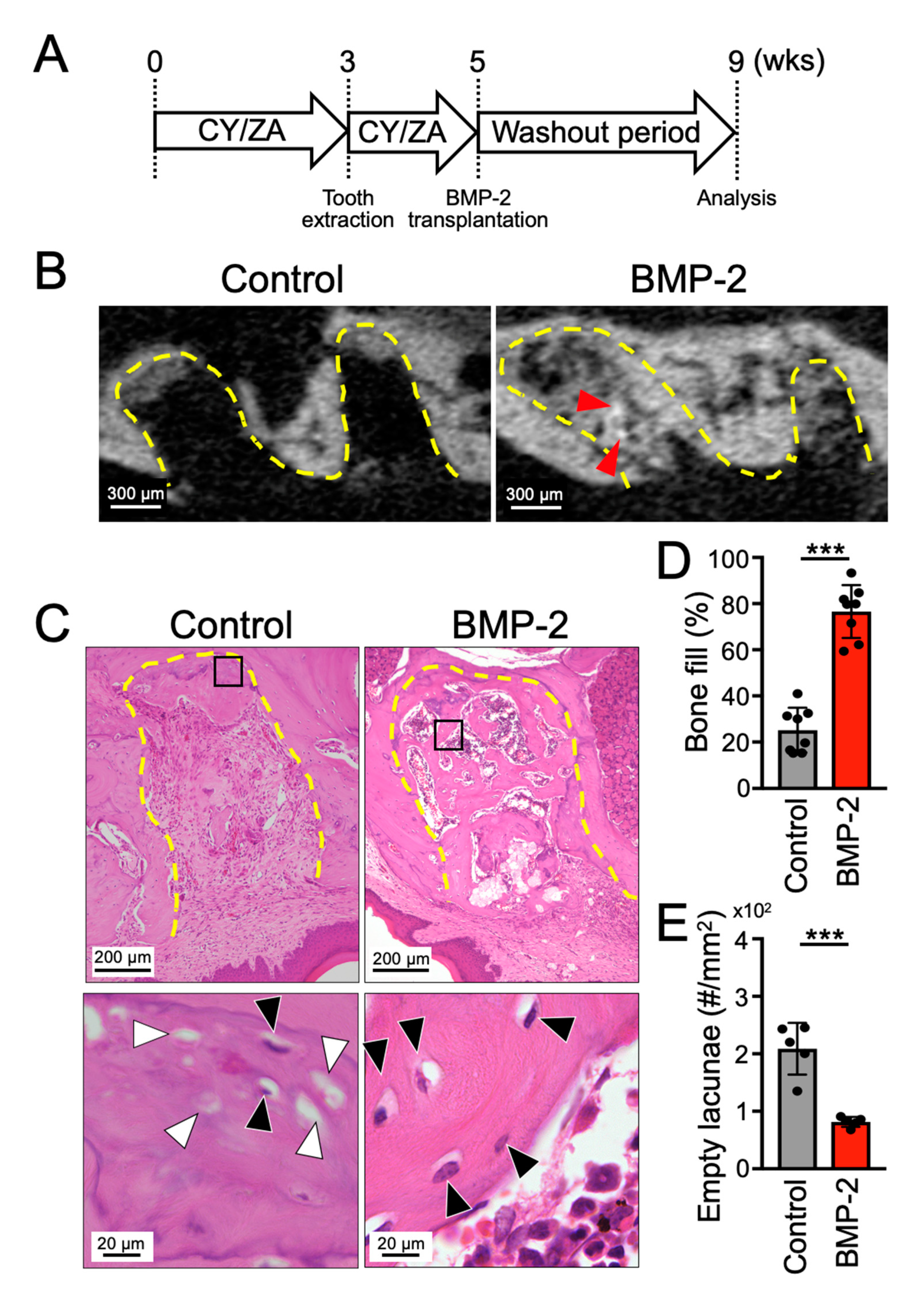
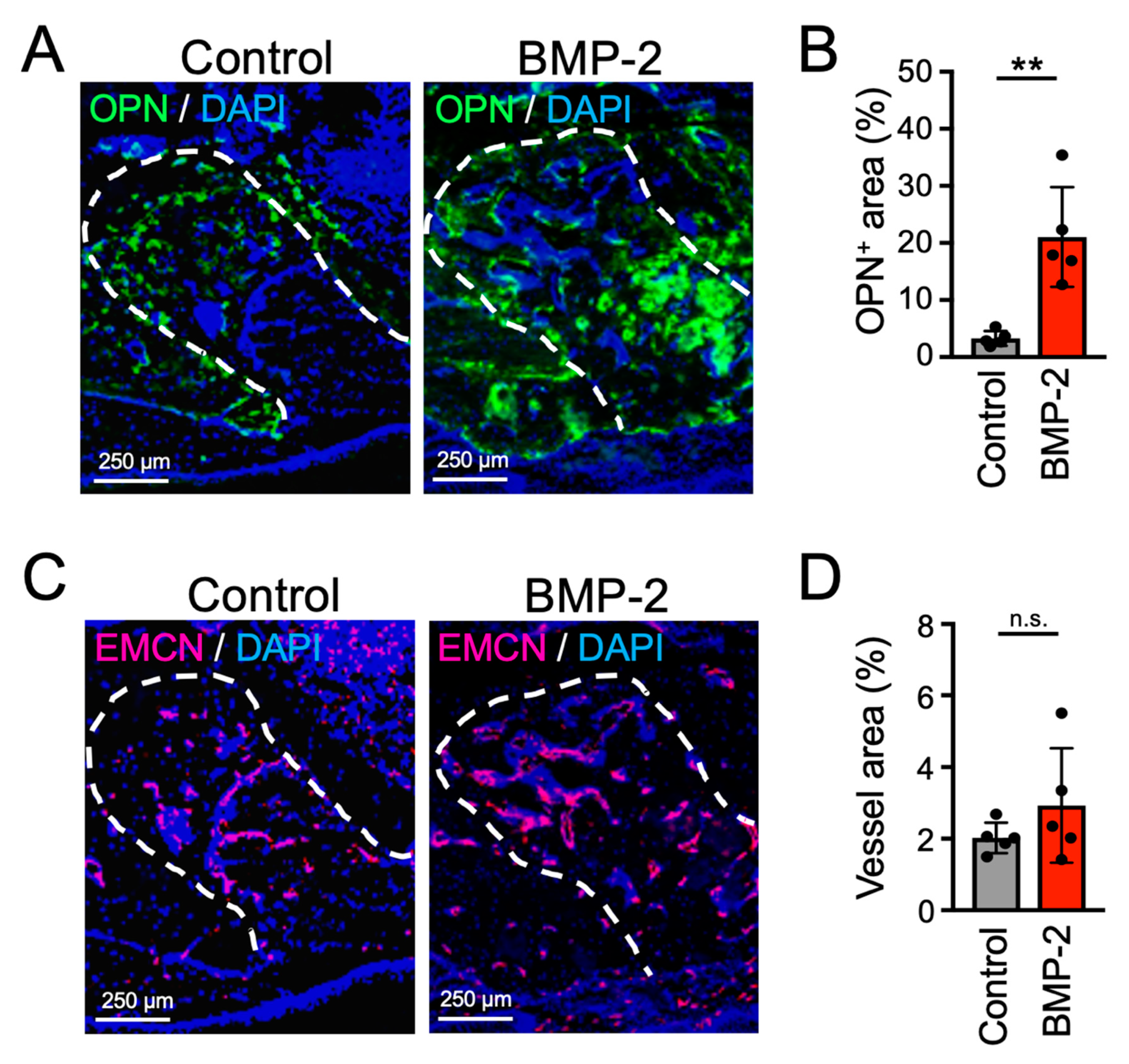
2.3. Transplantation of BMP-2/β-TCP Strongly Induced Bone Formation in the Tooth Extraction Socket in a Mouse Model of MRONJ Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Model
4.3. Micro Computed Tomography (Micro-CT) Analysis
4.4. Histological Analysis
5. Statistic Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hortobagyi, G.N.; Theriault, R.L.; Porter, L.; Blayney, D.; Lipton, A.; Sinoff, C.; Wheeler, H.; Simeone, J.F.; Seaman, J.; Knight, R.D. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N. Engl. J. Med. 1996, 335, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Lichtenstein, A.; Porter, L.; Dimopoulos, M.A.; Bordoni, R.; George, S.; Lipton, A.; Keller, A.; Ballester, O.; Kovacs, M.J.; et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N. Engl. J. Med. 1996, 334, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Hillner, B.E.; Ingle, J.N.; Chlebowski, R.T.; Gralow, J.; Yee, G.C.; Janjan, N.A.; Cauley, J.A.; Blumenstein, B.A.; Albain, K.S.; Lipton, A.; et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J. Clin. Oncol. 2003, 21, 4042–4057. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, W.; Zhou, R.; Jia, S.; Tang, W.; Luo, Y.; Zhang, J. Bisphosphonates in the Treatment of Patients with Metastatic Breast, Lung, and Prostate Cancer: A Meta-Analysis. Medicine 2015, 94, e2014. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw-2014 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Khan, A.; Morrison, A.; Cheung, A.; Hashem, W.; Compston, J. Osteonecrosis of the jaw (ONJ): Diagnosis and management in 2015. Osteoporos. Int. 2016, 27, 853–859. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Sawase, T. Medication-related osteonecrosis of the jaw: A literature review. J. Oral Biosci. 2019, 61, 99–104. [Google Scholar] [CrossRef]
- Voss, P.J.; Poxleitner, P.; Schmelzeisen, R.; Stricker, A.; Semper-Hogg, W. Update MRONJ and perspectives of its treatment. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 232–235. [Google Scholar] [CrossRef]
- Miksad, R.A.; Lai, K.C.; Dodson, T.B.; Woo, S.B.; Treister, N.S.; Akinyemi, O.; Bihrle, M.; Maytal, G.; August, M.; Gazelle, G.S.; et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist 2011, 16, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.A.; Rosen, V.; Cordes, P.; Hewick, R.M.; Kriz, M.J.; Luxenberg, D.P.; Sibley, B.S.; Wozney, J.M. Purification and characterization of other distinct bone-inducing factors. Proc. Natl. Acad. Sci. USA 1988, 85, 9484–9488. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Heim, S.E.; Gornet, M.F.; Zdeblick, T.A. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J. Spinal Disord. Tech. 2003, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Sandhu, H.S.; Gornet, M.F.; Longley, M.C. Use of rhBMP-2 in combination with structural cortical allografts: Clinical and radiographic outcomes in anterior lumbar spinal surgery. J. Bone Jt. Surg. Am. 2005, 87, 1205–1212. [Google Scholar] [CrossRef]
- Fiorellini, J.P.; Howell, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef]
- Williams, J.T., Jr.; Ragland, P.S.; Clarke, S. Constrained components for the unstable hip following total hip arthroplasty: A literature review. Int. Orthop. 2007, 31, 273–277. [Google Scholar] [CrossRef]
- Wozney, J.M. Overview of bone morphogenetic proteins. Spine 2002, 27 (Suppl. 1), S2–S8. [Google Scholar] [CrossRef]
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; Kumegawa, M.; Hakeda, Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000, 27, 479–486. [Google Scholar] [CrossRef]
- Itoh, K.; Udagawa, N.; Katagiri, T.; Iemura, S.; Ueno, N.; Yasuda, H.; Higashio, K.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J.; et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 2001, 142, 3656–3662. [Google Scholar] [CrossRef]
- Pearson, H.B.; Mason, D.E.; Kegelman, C.D.; Zhao, L.; Dawahare, J.H.; Kacena, M.A.; Boerckel, J.D. Effects of Bone Morphogenetic Protein-2 on Neovascularization During Large Bone Defect Regeneration. Tissue Eng. Part A 2019, 25, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Brierly, G.I.; Ren, J.; Baldwin, J.; Saifzadeh, S.; Theodoropoulos, C.; Tsurkan, M.V.; Lynham, A.; Hsu, E.; Nikolarakos, D.; Werner, C.; et al. Investigation of Sustained BMP Delivery in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ) in a Rat Model. Macromol. Biosci. 2019, 19, e1900226. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, S.M.; Jung, J.M. Peroneus Longus activity according to various angles of a ramp during cross-ramp walking and one-legged standing. J. Back Musculoskelet. Rehabil. 2017, 30, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.S. Use of open bone-graft epiphysiodesis in the treatment of slipped capital femoral epiphysis. J. Pediatr. Orthop. 1998, 18, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Ogose, A.; Hotta, T.; Kawashima, H.; Kondo, N.; Gu, W.; Kamura, T.; Endo, N. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Honeycomb blocks composed of carbonate apatite, beta-tricalcium phosphate, and hydroxyapatite for bone regeneration: Effects of composition on biological responses. Mater. Today Bio 2019, 4, 100031. [Google Scholar] [CrossRef]
- Ono, M.; Sonoyama, W.; Yamamoto, K.; Oida, Y.; Akiyama, K.; Shinkawa, S.; Nakajima, R.; Pham, H.T.; Hara, E.S.; Kuboki, T. Efficient bone formation in a swine socket lift model using Escherichia coli-derived recombinant human bone morphogenetic protein-2 adsorbed in beta-tricalcium phosphate. Cells Tissues Organs 2014, 199, 249–255. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Transplantation of Noncultured Stromal Vascular Fraction Cells of Adipose Tissue Ameliorates Osteonecrosis of the Jaw-Like Lesions in Mice. J. Bone Miner. Res. 2018, 33, 154–166. [Google Scholar] [CrossRef]
- Koenig, B.B.; Cook, J.S.; Wolsing, D.H.; Ting, J.; Tiesman, J.P.; Correa, P.E.; Olson, C.A.; Pecquet, A.L.; Ventura, F.; Grant, R.A.; et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol. Cell. Biol. 1994, 14, 5961–5974. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yoo, H.Y.; Kim, G.T.; Lee, J.W.; Lee, Y.A.; Kim, D.Y.; Kwon, Y.D. Short-Term Teriparatide and Recombinant Human Bone Morphogenetic Protein-2 for Regenerative Approach to Medication-Related Osteonecrosis of the Jaw: A Preliminary Study. J. Bone Miner. Res. 2017, 32, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Jeppsson, C.; Aspenberg, P. BMP-2 can inhibit bone healing. Bone-chamber study in rabbits. Acta Orthop. Scand. 1996, 67, 589–592. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Ono, M.; Oida, Y.; Hara, E.S.; Komori, T.; Akiyama, K.; Nguyen, H.T.T.; Aung, K.T.; Pham, H.T.; Tosa, I.; et al. Bone Marrow Cells Inhibit BMP-2-Induced Osteoblast Activity in the Marrow Environment. J. Bone Miner. Res. 2019, 34, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Prevalence of bisphosphonate-related osteonecrosis of the jaw-like lesions is increased in a chemotherapeutic dose-dependent manner in mice. Bone 2018, 112, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Ono, M.; Hara, E.S.; Ueda, J.; Nguyen, H.T.T.; Nguyen, H.T.; Yonezawa, T.; Maeba, T.; Kimura-Ono, A.; Takarada, T.; et al. Type IV collagen alpha6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Sci. Rep. 2018, 8, 2612. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikai, A.; Ono, M.; Tosa, I.; Nguyen, H.T.T.; Hara, E.S.; Nosho, S.; Kimura-Ono, A.; Nawachi, K.; Takarada, T.; Kuboki, T.; et al. BMP-2/β-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model. Int. J. Mol. Sci. 2020, 21, 7028. https://doi.org/10.3390/ijms21197028
Mikai A, Ono M, Tosa I, Nguyen HTT, Hara ES, Nosho S, Kimura-Ono A, Nawachi K, Takarada T, Kuboki T, et al. BMP-2/β-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model. International Journal of Molecular Sciences. 2020; 21(19):7028. https://doi.org/10.3390/ijms21197028
Chicago/Turabian StyleMikai, Akihiro, Mitsuaki Ono, Ikue Tosa, Ha Thi Thu Nguyen, Emilio Satoshi Hara, Shuji Nosho, Aya Kimura-Ono, Kumiko Nawachi, Takeshi Takarada, Takuo Kuboki, and et al. 2020. "BMP-2/β-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model" International Journal of Molecular Sciences 21, no. 19: 7028. https://doi.org/10.3390/ijms21197028
APA StyleMikai, A., Ono, M., Tosa, I., Nguyen, H. T. T., Hara, E. S., Nosho, S., Kimura-Ono, A., Nawachi, K., Takarada, T., Kuboki, T., & Oohashi, T. (2020). BMP-2/β-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model. International Journal of Molecular Sciences, 21(19), 7028. https://doi.org/10.3390/ijms21197028





