The Effects of Asbestos Fibers on Human T Cells
Abstract
1. Introduction
2. Effect of Asbestos Fibers on Various T Lymphocytes
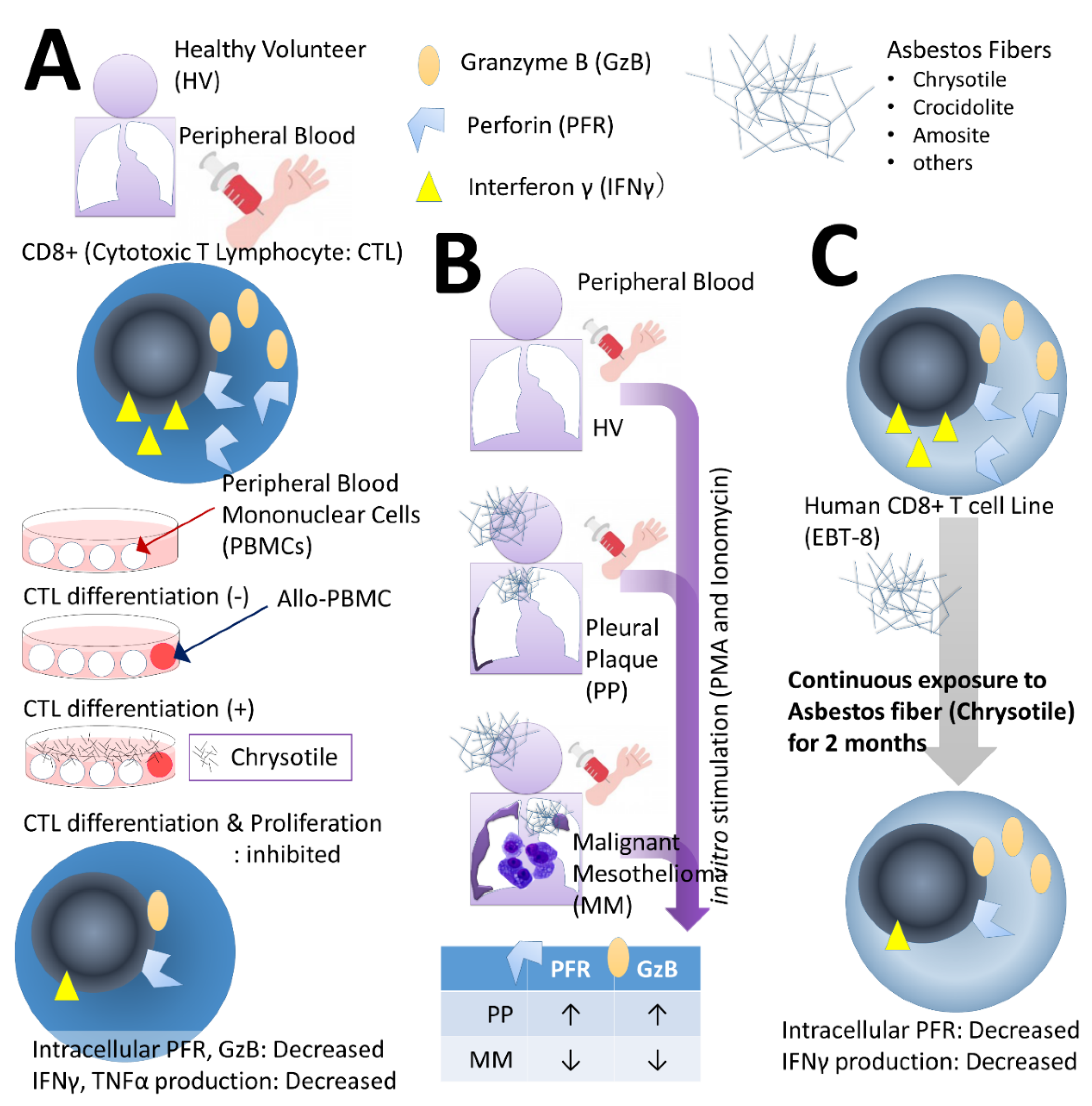
2.1. Effects on CTLs
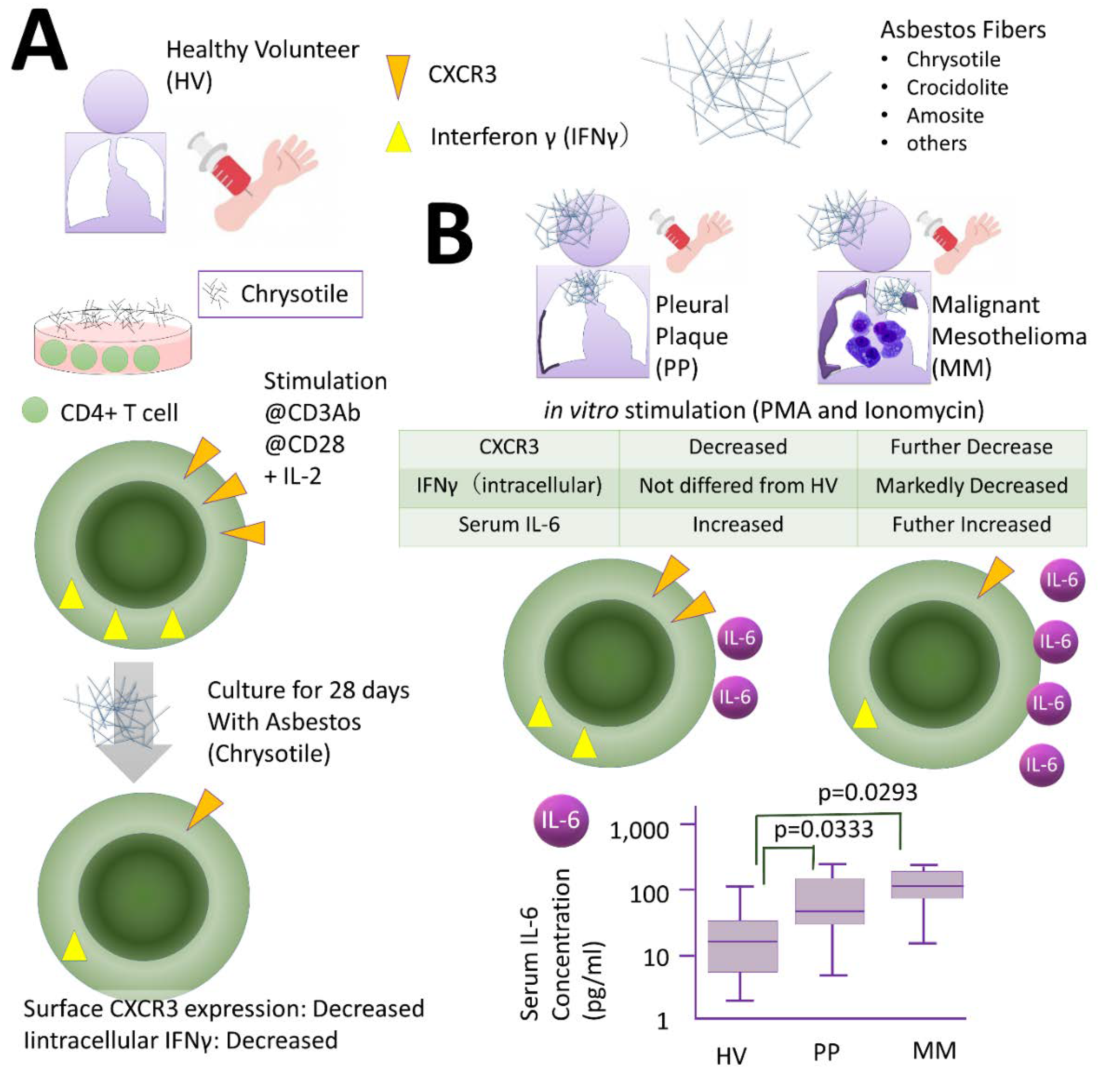
2.2. Effects of Th Cells
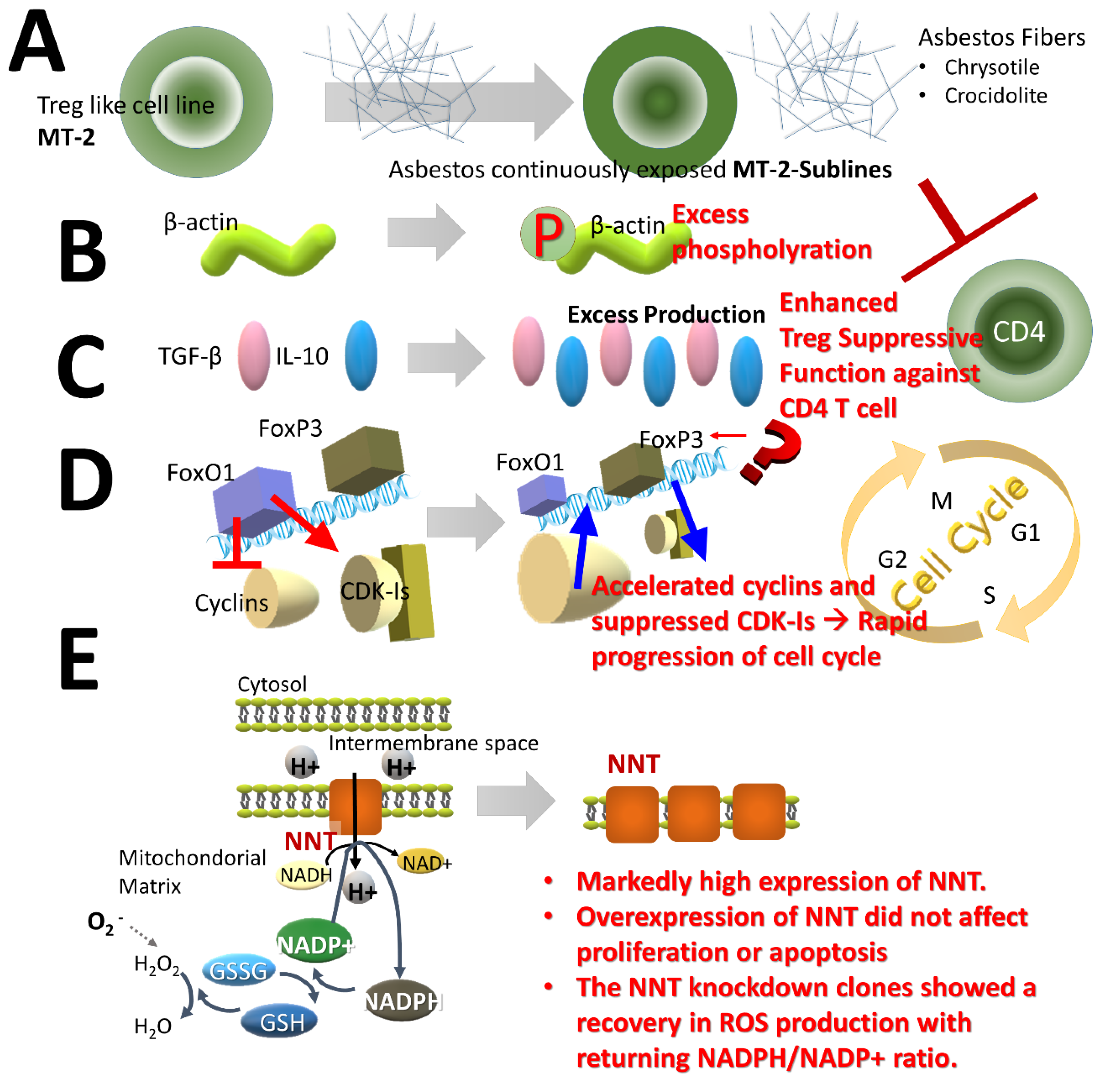
2.3. Effects of Treg Cells
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PP | pleural plaque |
| MM | malignant mesothelioma |
| ROS | reactive oxygen species |
| ANCA | anti-neutrophil cytoplasmic antibody |
| CTL | cytotoxic T Lymphocyte |
| Th | CD4+ helper T cell |
| Treg | regulatory T cell |
| HV | healthy volunteers |
| PBL | Peripheral blood lymphocyte |
| MLR | mixed lymphocyte reaction |
| PBMC | peripheral blood mononuclear cell |
| GzB | granzyme B |
| PFR | perforin |
| IFN | interferon |
| TNF | tumor necrosis factor |
| PMA | phorbol 12-myristate 13-acetate |
| IL | interleukin |
| CXCR | CXC chemokine receptor |
| TGF | transforming growth factor |
| HTLV | human T cell leukemia/lymphoma virus |
| FoxO1 | forkhead box protein O1 |
| CDK-Is | cyclin-dependent kinase-inhibitors |
| FoxP3 | forkhead box P3 |
| NNT | nicotinamide nucleotide transhydrogenase |
| NADPH | reduced nicotinamide adenine dinucleotide phosphate |
| NK | natural killer |
References
- Vallyathan, V.; Green, F.H. The role of analytical techniques in the diagnosis of asbestos-associated disease. Crit. Rev. Clin. Lab. Sci. 1985, 22, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Sporn, T.A. Mineralogy of asbestos. Recent Results Cancer Res. 2011, 189, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pigg, B.J. The uses of chrysotile. Ann. Occup. Hyg. 1994, 38, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.V.; Kahwa, I.A. Asbestos: Old foe in 21st century developing countries. Sci. Total Environ. 2003, 307, 1–9. [Google Scholar] [CrossRef]
- Lemen, R.A. Introduction: History of the use of asbestos. Med. Lav. 1997, 88, 288–292. [Google Scholar] [PubMed]
- Kamp, D.W. Asbestos-induced lung diseases: An update. Transl. Res. 2009, 153, 143–152. [Google Scholar] [CrossRef]
- Attanoos, R.L. Asbestos-Related Lung Disease. Surg. Pathol. Clin. 2010, 3, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Jamrozik, E.; de Klerk, N.; Musk, A.W. Asbestos-related disease. Intern. Med. J. 2011, 41, 372–380. [Google Scholar] [CrossRef]
- Nielsen, L.S.; Bælum, J.; Rasmussen, J.; Dahl, S.; Olsen, K.E.; Albin, M.; Hansen, N.C.; Sherson, D. Occupational asbestos exposure and lung cancer—A systematic review of the literature. Arch. Environ. Occup. Health 2014, 69, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.; Vehmas, T.; Oksa, P.; Rantanen, J.; Vainio, H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: Recommendations. Scand. J. Work Environ. Health 2015, 41, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S. Asbestos-related lung cancer and malignant mesothelioma of the pleura: Selected current issues. Semin. Respir. Crit. Care Med. 2015, 36, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, N.; Shinohara, Y.; Suzuki, Y. Mineral phases and some reexamined characteristics of the International Union against Cancer standard asbestos samples. Am. J. Ind. Med. 1996, 30, 515–528. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Lusvardi, G.; Zoboli, A.; Di Giuseppe, D.; Gualtieri, M.L. Biodurability and release of metals during the dissolution of chrysotile, crocidolite and fibrous erionite. Environ. Res. 2019, 171, 550–557. [Google Scholar] [CrossRef]
- Kamp, D.W.; Graceffa, P.; Pryor, W.A.; Weitzman, S.A. The role of free radicals in asbestos-induced diseases. Free Radic. Biol. Med. 1992, 12, 293–315. [Google Scholar] [CrossRef]
- Jaurand, M.C. Mechanisms of fiber-induced genotoxicity. Environ. Health Perspect. 1997, 105 (Suppl. 5), 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef]
- Upadhyay, D.; Kamp, D.W. Asbestos-induced pulmonary toxicity: Role of DNA damage and apoptosis. Exp. Biol. Med. 2003, 228, 650–659. [Google Scholar] [CrossRef]
- Tossavainen, A.; Karjalainen, A.; Karhunen, P.J. Retention of asbestos fibers in the human body. Environ. Health Perspect. 1994, 102 (Suppl. 5), 253–255. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Maed, M.; Lee, S.; Nishimura, Y.; Kumagai-Takei, N.; Hayashi, H.; Yamamoto, S.; HGatayama, T.; Kojima, Y.; Tabata, R.; et al. Asbestos-induced cellular and molecular alteration of immunocompetent cells and their relationship with chronic inflammation and carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 492608. [Google Scholar] [CrossRef]
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Balaan, M.R.; Weber, S.L.; Banks, D.E. Clinical aspects of coal workers’ pneumoconiosis and silicosis. Occup. Med. 1993, 8, 19–34. [Google Scholar]
- Castranova, V.; Vallyathan, V. Silicosis and coal workers’ pneumoconiosis. Environ. Health Perspect. 2000, 108 (Suppl. 4), 675–684. [Google Scholar] [CrossRef] [PubMed]
- Uber, C.L.; McReynolds, R.A. Immunotoxicology of silica. Crit. Rev. Toxicol. 1982, 10, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Goldsmith, D.F. Silica exposure and autoimmune diseases. Am. J. Ind. Med. 1995, 28, 603–608. [Google Scholar] [CrossRef]
- Haustein, U.F.; Anderegg, U. Silica induced scleroderma—Clinical and experimental aspects. J. Rheumatol. 1998, 25, 1917–1926. [Google Scholar] [PubMed]
- Sluis-Cremer, G.K.; Hessel, P.A.; Nizdo, E.H.; Churchill, A.R.; Zeiss, E.A. Silica, silicosis, and progressive systemic sclerosis. Br. J. Ind. Med. 1985, 42, 838–843. [Google Scholar] [CrossRef]
- Cojocaru, M.; Niculescu, T.; Spătaru, E. Antineutrophil cytoplasm antibodies in patients with silicosis. Rom. J. Intern. Med. 1996, 34, 233–237. [Google Scholar]
- Mulloy, K.B. Silica exposure and systemic vasculitis. Environ. Health Perspect. 2003, 111, 1933–1938. [Google Scholar] [CrossRef][Green Version]
- Caplan, A. Certain unusual radiological appearances in the chest of coal-miners suffering from rheumatoid arthritis. Thorax 1953, 8, 29–37. [Google Scholar] [CrossRef]
- Caplan, A. Rheumatoid disease and pneumoconiosis (Caplan’s syndrome). Proc. R. Soc. Med. 1959, 52, 1111–1113. [Google Scholar] [CrossRef]
- Kumagai-Takei, N.; Maeda, M.; Chen, Y.; Matsuzaki, H.; Lee, S.; Nishimura, Y.; Hiratsuka, J.; Otsuki, T. Asbestos induces reduction of tumor immunity. Clin. Dev. Immunol. 2011, 2011, 481439. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Matsuzaki, H.; Lee, S.; Kumagai-Takei, N.; Yamamoto, S.; Hatayama, T.; Yoshitome, K.; Nishimura, Y. Environmental factors and human health: Fibrous and particulate substance-induced immunological disorders and construction of a health-promoting living environment. Environ. Health Prev. Med. 2016, 21, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kumagai-Takei, N.; Nishimura, Y.; Maeda, M.; Hayashi, H.; Matsuzaki, H.; Lee, S.; Hiratsuka, J.; Otsuki, T. Effect of asbestos exposure on differentiation of cytotoxic T lymphocytes in mixed lymphocyte reaction of human peripheral blood mononuclear cells. Am. J. Respir. Cell Mol. Biol. 2013, 49, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kumagai-Takei, N.; Nishimura, Y.; Matsuzaki, H.; Lee, S.; Yoshitome, K.; Hayashi, H.; Otsuki, T. The Suppressed Induction of Human Mature Cytotoxic T Lymphocytes Caused by Asbestos Is Not due to Interleukin-2 Insufficiency. J. Immunol. Res. 2016, 2016, 7484872. [Google Scholar] [CrossRef]
- Maeda, M.; Yamamoto, S.; Chen, Y.; Kumagai-Takei, N.; Hayashi, H.; Matsuzaki, H.; Lee, S.; Hatayama, T.; Miyahara, N.; Katoh, M.; et al. Resistance to asbestos-induced apoptosis with continuous exposure to crocidolite on a human T cell. Sci. Total Environ. 2012, 429, 174–182. [Google Scholar] [CrossRef]
- Kumagai-Takei, N.; Nishimura, Y.; Maeda, M.; Hayashi, H.; Matsuzaki, H.; Lee, S.; Kishimoto, T.; Fukuoka, K.; Nakano, T.; Otsuki, T. Functional properties of CD8(+) lymphocytes in patients with pleural plaque and malignant mesothelioma. J. Immunol. Res. 2014, 2014, 670140. [Google Scholar] [CrossRef]
- Furuya, S.; Chimed-Ochir, O.; Takahashi, K.; David, A.; Takala, J. Global Asbestos Disaster. Int. J. Environ. Res. Public Health 2018, 15, 1000. [Google Scholar] [CrossRef]
- Asada, H.; Okada, N.; Hashimoto, K.; Yamanishi, K.; Sairenji, T.; Hirota, S.; Nomura, S.; Kitamura, Y.; Yoshikawa, K. Establishment and characterization of the T-cell line, EBT-8 latently infected with Epstein-Barr virus from large granular lymphocyte leukemia. Leukemia 1994, 8, 1415–1423. [Google Scholar]
- Kumagai-Takei, N.; Nishimura, Y.; Matsuzaki, H.; Lee, S.; Yoshitome, K.; Otsuki, T. Decrease in Intracellular Perforin Levels and IFN-γ Production in Human CD8(+) T Cell Line following Long-Term Exposure to Asbestos Fibers. J. Immunol. Res. 2018, 2018, 4391731. [Google Scholar] [CrossRef]
- Maeda, M.; Nishimura, Y.; Hayashi, H.; Kumagai, N.; Chen, Y.; Murakami, S.; Miura, Y.; Hiratsuka, J.; Kishimoto, T.; Otsuki, T. Decreased CXCR3 expression in CD4+ T cells exposed to asbestos or derived from asbestos-exposed patients. Am. J. Respir. Cell Mol. Biol. 2011, 45, 795–803. [Google Scholar] [CrossRef]
- Miura, Y.; Nishimura, Y.; Katsuyama, H.; Maeda, M.; Hayashi, H.; Dong, M.; Hyodoh, F.; Tomita, M.; Matsuo, Y.; Uesaka, A.; et al. Involvement of IL-10 and Bcl-2 in resistance against an asbestos-induced apoptosis of T cells. Apoptosis 2006, 11, 1825–1835. [Google Scholar] [CrossRef]
- Hyodoh, F.; Takata-Tomokuni, A.; Miura, Y.; Sakaguchi, H.; Hatayama, T.; Hatada, S.; Katsuyama, H.; Matsuo, Y.; Otsuki, T. Inhibitory effects of anti-oxidants on apoptosis of a human polyclonal T-cell line, MT-2, induced by an asbestos, chrysotile-A. Scand. J. Immunol. 2005, 61, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Chen, Y.; Kumagai-Takei, N.; Hayashi, H.; Matsuzaki, H.; Lee, S.; Hiratsuka, J.; Nishimura, Y.; Kimura, Y.; Otsuki, T. Alteration of cytoskeletal molecules in a human T cell line caused by continuous exposure to chrysotile asbestos. Immunobiology 2013, 218, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Borelli, V.; Trevisan, E.; Francesca, V.; Zabucchi, G. The Secretory Response of Rat Peritoneal Mast Cells on Exposure to Mineral Fibers. Int. J. Environ. Res. Public Health 2018, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Toyokuni, S. Differences and similarities between carbon nanotubes and asbestos fibers during mesothelial carcinogenesis: Shedding light on fiber entry mechanism. Cancer Sci. 2012, 103, 1378–1390. [Google Scholar] [CrossRef]
- Maeda, M.; Chen, Y.; Hayashi, H.; Kumagai-Takei, N.; Matsuzaki, H.; Lee, S.; Nishimura, Y.; Otsuki, T. Chronic exposure to asbestos enhances TGF-β1 production in the human adult T cell leukemia virus-immortalized T cell line MT-2. Int. J. Oncol. 2014, 45, 2522–2532. [Google Scholar] [CrossRef]
- Ying, C.; Maeda, M.; Nishimura, Y.; Kumagai-Takei, N.; Hayashi, H.; Matsuzaki, H.; Lee, S.; Yoshitome, K.; Yamamoto, S.; Hatayama, T.; et al. Enhancement of regulatory T cell-like suppressive function in MT-2 by long-term and low-dose exposure to asbestos. Toxicology 2015, 338, 86–94. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Lee, S.; Maeda, M.; Kumagai-Takei, N.; Nishimura, Y.; Otsuki, T. FoxO1 regulates apoptosis induced by asbestos in the MT-2 human T-cell line. J. Immunotoxicol. 2016, 13, 620–627. [Google Scholar] [CrossRef]
- Lee, S.; Matsuzaki, H.; Maeda, M.; Yamamoto, S.; Kumagai-Takei, N.; Hatayama, T.; Ikeda, M.; Yoshitome, K.; Nishimura, Y.; Otsuki, T. Accelerated cell cycle progression of human regulatory T cell-like cell line caused by continuous exposure to asbestos fibers. Int. J. Oncol. 2017, 50, 66–74. [Google Scholar] [CrossRef]
- Maeda, M.; Matsuzaki, H.; Yamamoto, S.; Lee, S.; Kumagai-Takei, N.; Yoshitome, K.; Min, Y.; Sada, N.; Nishimura, Y.; Otsuki, T. Aberrant expression of FoxP3 in a human T cell line possessing regulatory T cell-like function and exposed continuously to asbestos fibers. Oncol. Rep. 2018, 40, 748–758. [Google Scholar] [CrossRef]
- Van den Heuvel, L.; Smeitink, J. The oxidative phosphorylation (OXPHOS) system: Nuclear genes and human genetic diseases. Bioessays 2001, 23, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.J.; van den Heuvel, L.P.; Smeitink, J.A. Genetic defects in the oxidative phosphorylation (OXPHOS) system. Expert Rev. Mol. Diagn. 2004, 4, 143–156. [Google Scholar] [CrossRef]
- Yamamoto, S.; Lee, S.; Matsuzaki, H.; Kumagai-Takei, N.; Yoshitome, K.; Sada, N.; Shimizu, Y.; Ito, T.; Nishimura, Y.; Otsuki, T. Enhanced expression of nicotinamide nucleotide transhydrogenase (NNT) and its role in a human T cell line continuously exposed to asbestos. Environ. Int. 2020, 138, 105654. [Google Scholar] [CrossRef]
- Nishimura, Y.; Miura, Y.; Maeda, M.; Kumagai, N.; Murakami, S.; Hayashi, H.; Fukuoka, K.; Nakano, T.; Otsuki, T. Impairment in cytotoxicity and expression of NK cell-activating receptors on human NK cells following exposure to asbestos fibers. Int. J. Immunopathol. Pharmacol. 2009, 22, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Maeda, M.; Kumagai, N.; Hayashi, H.; Miura, Y.; Otsuki, T. Decrease in phosphorylation of ERK following decreased expression of NK cell-activating receptors in human NK cell line exposed to asbestos. Int. J. Immunopathol. Pharmacol. 2009, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Pfau, J.C.; Serve, K.M.; Noonan, C.W. Autoimmunity and asbestos exposure. Autoimmune Dis. 2014, 2014, 782045. [Google Scholar] [CrossRef]
- Diegel, R.; Black, B.; Pfau, J.C.; McNew, T.; Noonan, C.; Flores, R. Case series: Rheumatological manifestations attributed to exposure to Libby Asbestiform Amphiboles. J. Toxicol. Environ. Health A 2018, 81, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Franklin, P.; de Klerk, N.; Creaney, J.; Brims, F.; Musk, B.; Pfau, J. Autoimmune antibodies and asbestos exposure: Evidence from Wittenoom, Western Australia. Am. J. Ind. Med. 2018, 61, 615–620. [Google Scholar] [CrossRef]
- Gerard, J.; Chu, G.J.; van Zandwijk, N.; Rasko, J.E.J. The Immune Microenvironment in Mesothelioma: Mechanisms of Resistance to Immunotherapy. Front. Oncol. 2019, 9, 1366. [Google Scholar] [CrossRef]
- François Huaux, F. Emerging Role of Immunosuppression in Diseases Induced by Micro- and Nano-Particles: Time to Revisit the Exclusive Inflammatory Scenario. Front. Immunol. 2018, 9, 2364. [Google Scholar] [CrossRef]
- Lee, S.; Hayashi, H.; Mastuzaki, H.; Kumagai-Takei, N.; Otsuki, T. Silicosis and autoimmunity. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Matsuzaki, H.; Kumagai-Takei, N.; Yoshitome, K.; Maeda, M.; Chen, Y.; Kusaka, M.; Urakami, K.; Hayashi, H.; Fujimoto, W.; et al. Silica exposure and altered regulation of autoimmunity. Environ. Health Prev. Med. 2014, 19, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Nishimura, Y.; Kumagai, N.; Hayashi, H.; Hatayama, T.; Katoh, M.; Miyahara, N.; Yamamoto, S.; Hirastuka, J.; Otsuki, T. Dysregulation of the immune system caused by silica and asbestos. J. Immunotoxicol. 2010, 7, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Comar, M.; Zanotta, N.; Zanconati, F.; Cortale, M.; Bonotti, A.; Cristaudo, A.; Bovenzi, M. Chemokines involved in the early inflammatory response and in pro-tumoral activity in asbestos-exposed workers from an Italian coastal area with territorial clusters of pleural malignant mesothelioma. Lung Cancer 2016, 94, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Nishimura, Y.; Hayashi, H.; Kumagai, N.; Chen, Y.; Murakami, S.; Miura, Y.; Hiratsuka, J.; Kishimoto, T.; Otsuki, T. Reduction of CXCR3 in an in vitro model of continuous asbestos exposure on a human T-cell line, MT-2. Am. J. Respir. Cell Mol. Biol. 2011, 45, 470–479. [Google Scholar] [CrossRef]
- Suffee, N.; Richard, B.; Hlawaty, H.; Oudar, O.; Charnaux, N.; Sutton, A. Angiogenic properties of the chemokine RANTES/CCL5. Biochem. Soc. Trans. 2011, 39, 1649–1653. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Schmitt, M.M. Platelets and their chemokines in atherosclerosis-clinical applications. Front. Physiol. 2014, 5, 294. [Google Scholar] [CrossRef]
- Okada, A.; Yaguchi, T.; Kanno, T.; Gotoh, A.; Nakano, T.; Nishizaki, T. PDGF-D/PDGF-ββ receptor-regulated chemotaxis of malignant mesothelioma cells. Cell Physiol. Biochem. 2012, 9, 241–250. [Google Scholar] [CrossRef]
- Batra, H.; Antony, V.B. Pleural mesothelial cells in pleural and lung diseases. J. Thorac. Dis. 2015, 7, 964–980. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumagai-Takei, N.; Lee, S.; Srinivas, B.; Shimizu, Y.; Sada, N.; Yoshitome, K.; Ito, T.; Nishimura, Y.; Otsuki, T. The Effects of Asbestos Fibers on Human T Cells. Int. J. Mol. Sci. 2020, 21, 6987. https://doi.org/10.3390/ijms21196987
Kumagai-Takei N, Lee S, Srinivas B, Shimizu Y, Sada N, Yoshitome K, Ito T, Nishimura Y, Otsuki T. The Effects of Asbestos Fibers on Human T Cells. International Journal of Molecular Sciences. 2020; 21(19):6987. https://doi.org/10.3390/ijms21196987
Chicago/Turabian StyleKumagai-Takei, Naoko, Suni Lee, Bandaru Srinivas, Yurika Shimizu, Nagisa Sada, Kei Yoshitome, Tatsuo Ito, Yasumitsu Nishimura, and Takemi Otsuki. 2020. "The Effects of Asbestos Fibers on Human T Cells" International Journal of Molecular Sciences 21, no. 19: 6987. https://doi.org/10.3390/ijms21196987
APA StyleKumagai-Takei, N., Lee, S., Srinivas, B., Shimizu, Y., Sada, N., Yoshitome, K., Ito, T., Nishimura, Y., & Otsuki, T. (2020). The Effects of Asbestos Fibers on Human T Cells. International Journal of Molecular Sciences, 21(19), 6987. https://doi.org/10.3390/ijms21196987




