The Biocontrol Root-Oomycete, Pythium Oligandrum, Triggers Grapevine Resistance and Shifts in the Transcriptome of the Trunk Pathogenic Fungus, Phaeomoniella Chlamydospora
Abstract
1. Introduction
2. Results
2.1. Evaluation of Grapevine Transcriptomic Responses
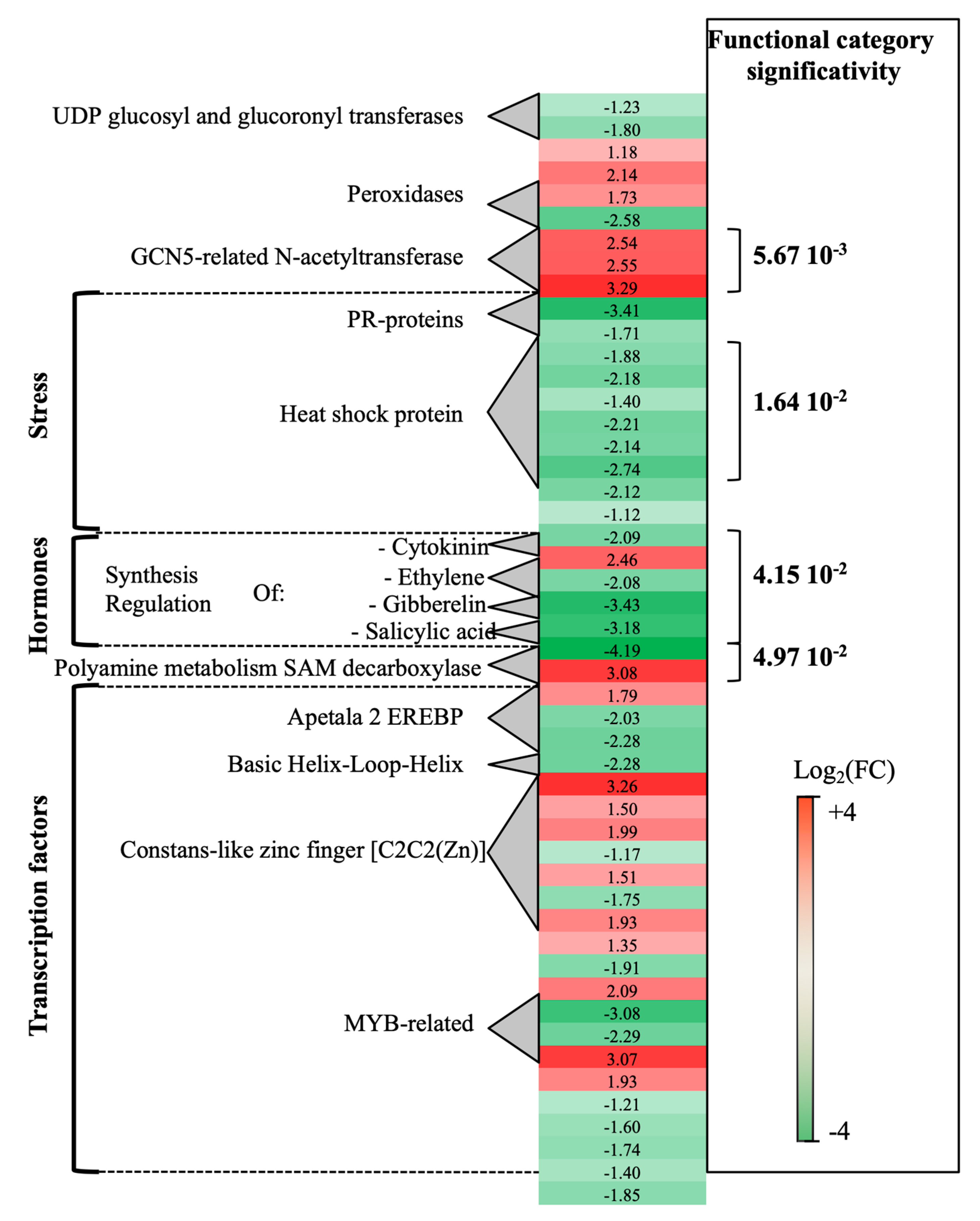
2.1.1. Grapevine Trunk Transcriptional Response to P. oligandrum Root Colonization
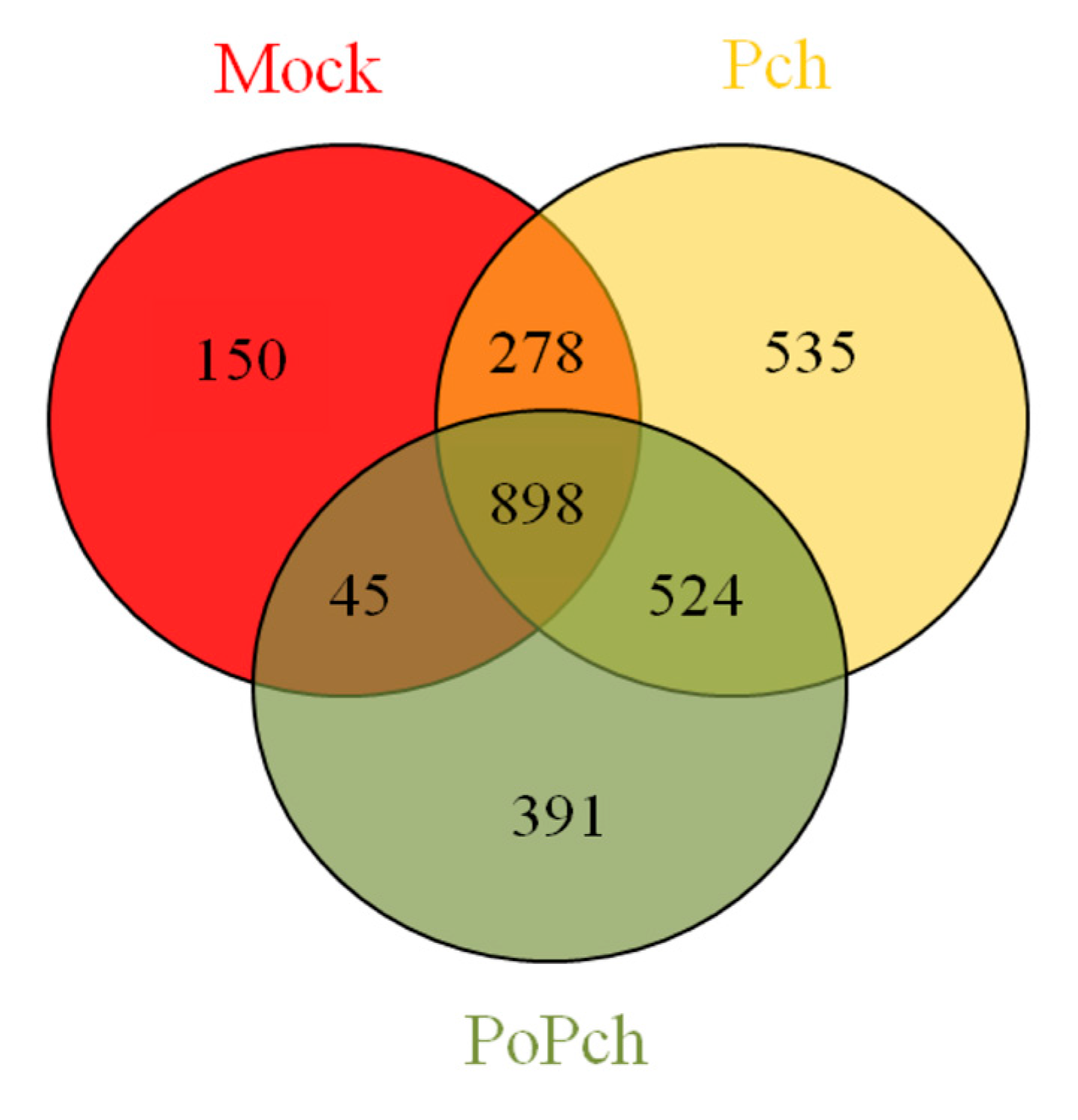
2.1.2. Transcriptomic Changes in Grapevine Wood Tissues Following P. chlamydospora or P. oligandrum + P. chlamydospora inoculations
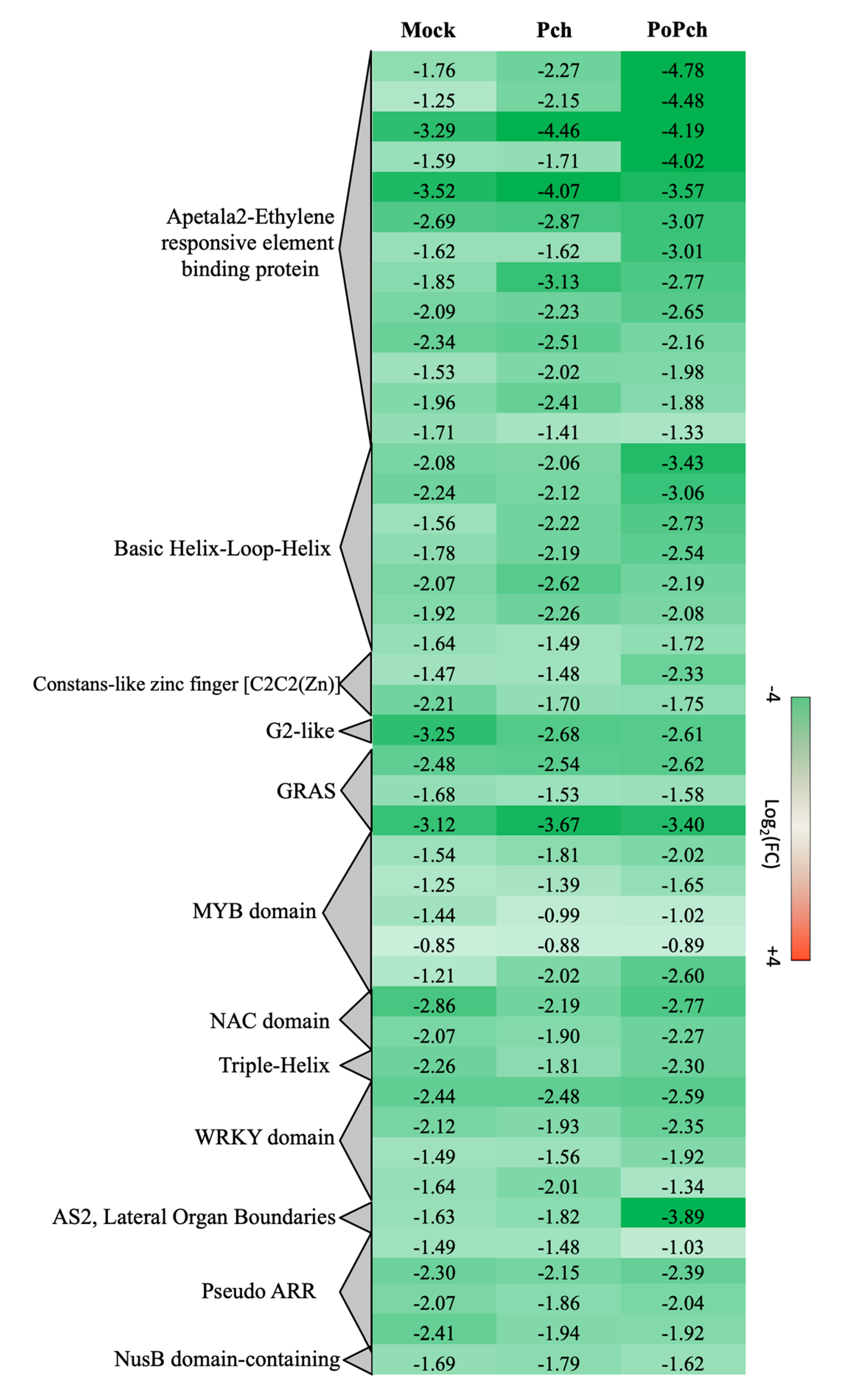
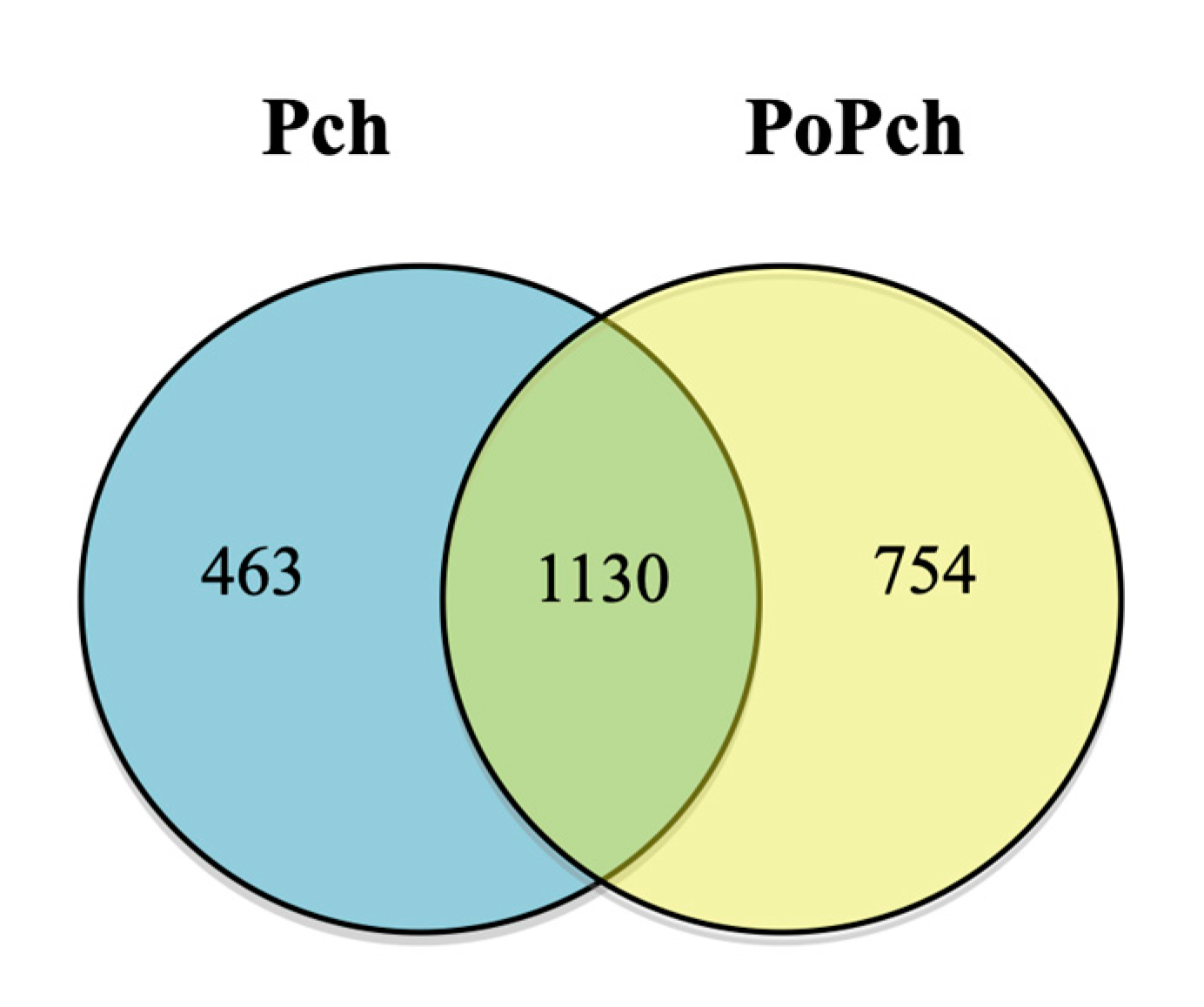
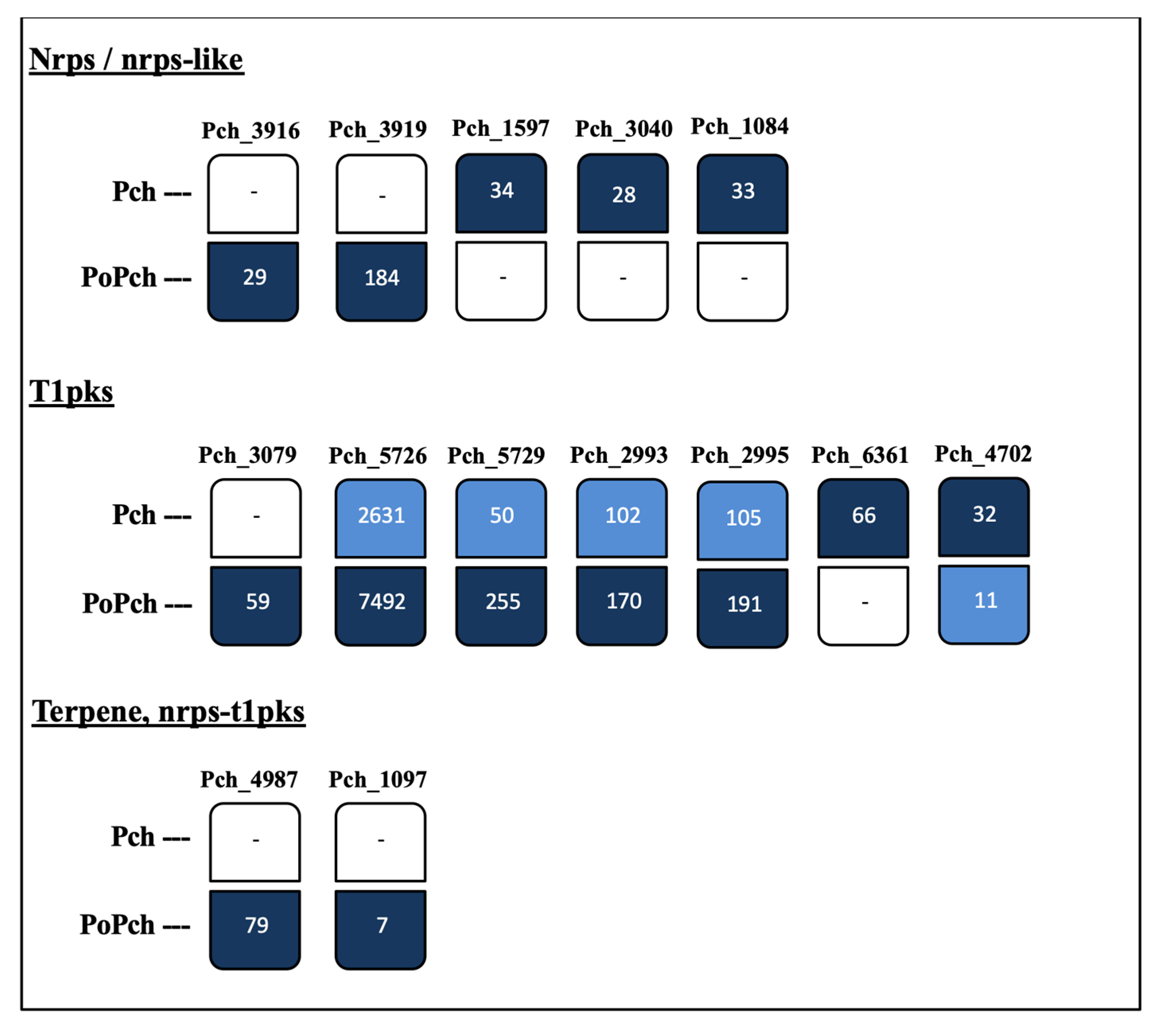
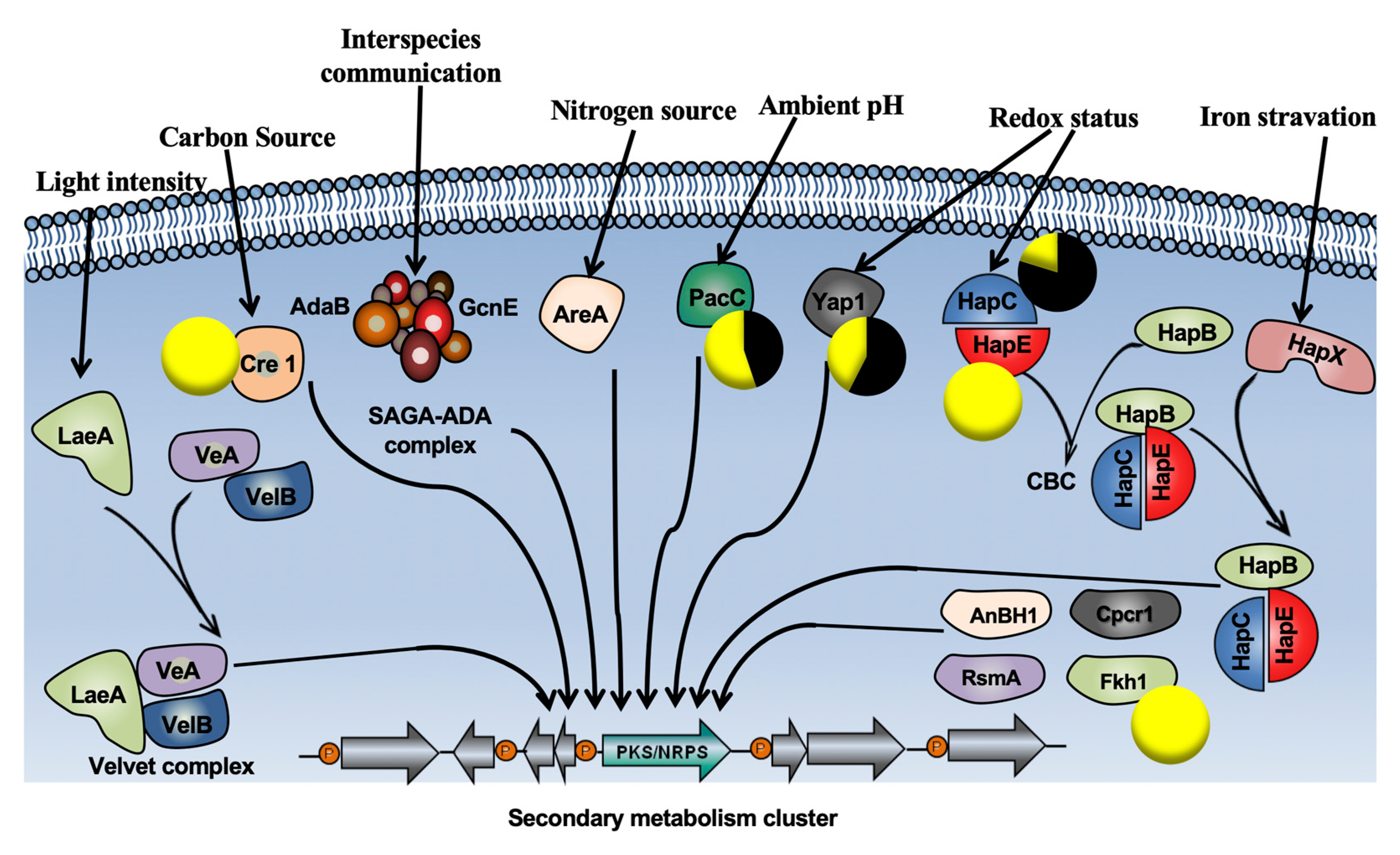
2.2. Evaluation of P. chlamydospora Transcriptomic Responses
3. Discussion
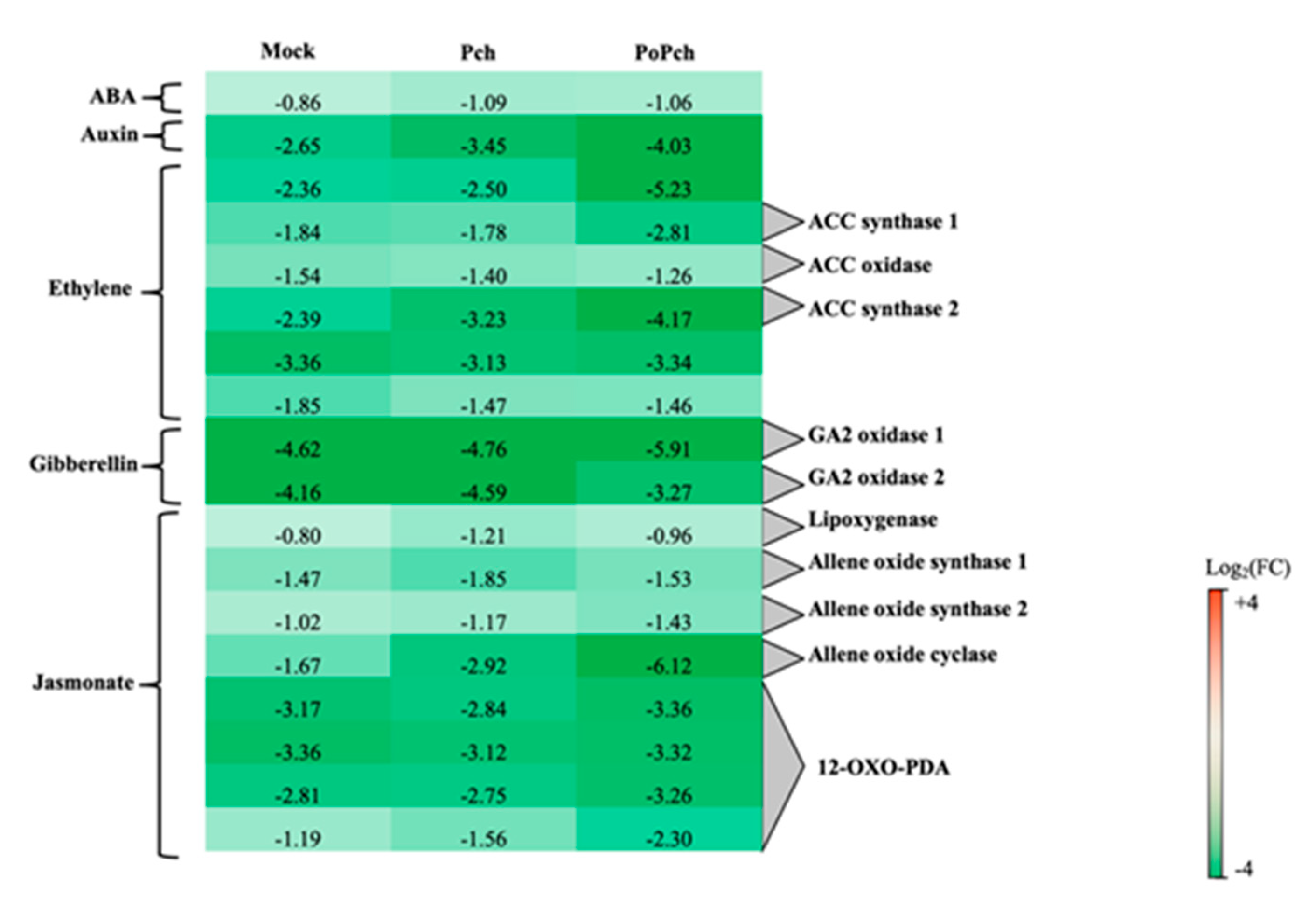
3.1. P. oligandrum Induced Genes Involved in the Jasmonic/Ethylene Signaling Pathways
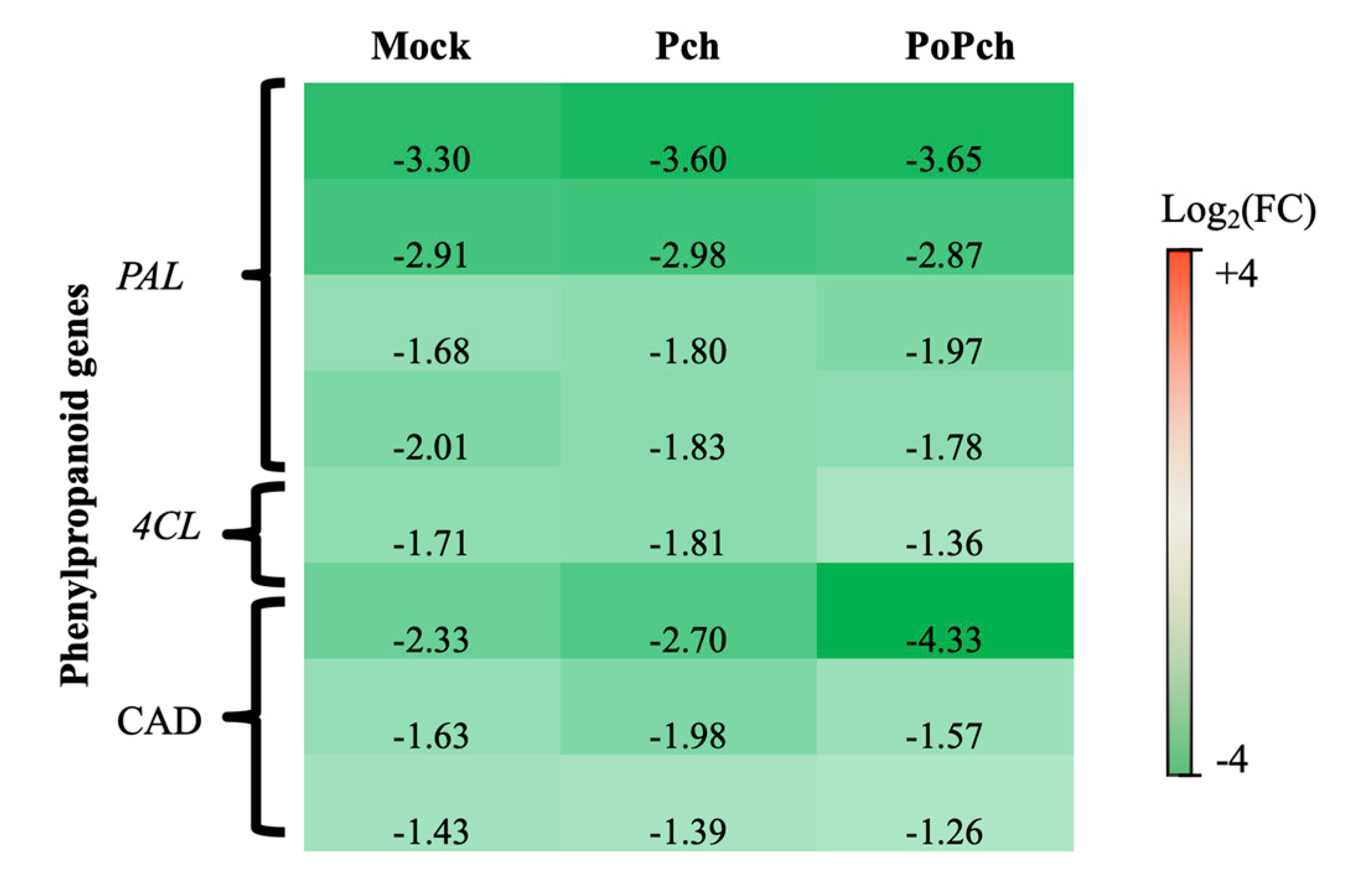
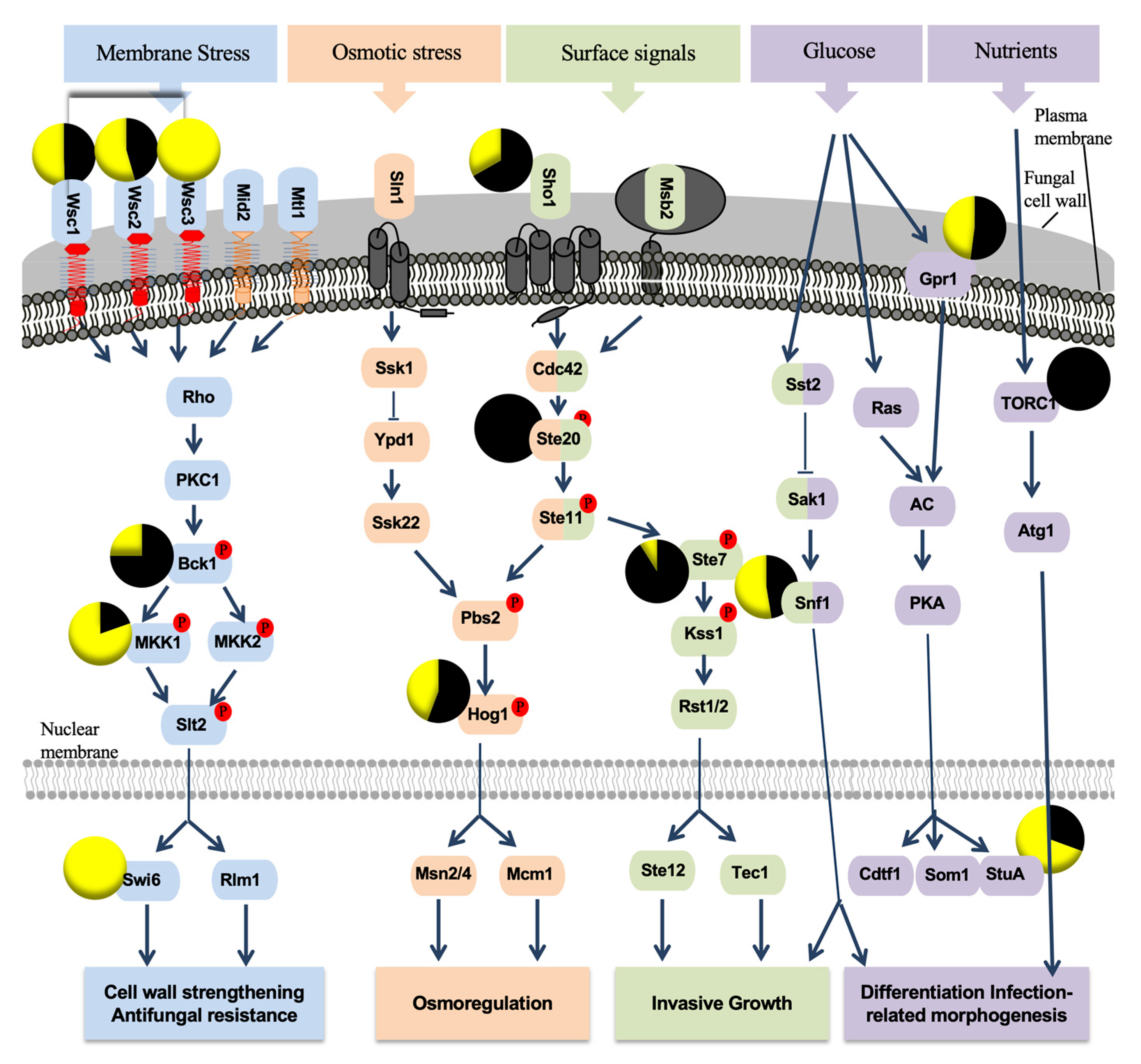
3.2. P. oligandrum Indirectly Modulates the Expression Level of P. chlamydospora Pathogencity Related-Genes
4. Methods
4.1. Plant and Microorganism Materials
4.2. RNA Extraction
4.3. Microarray Analyses, Data Processing and Deposition
4.4. RNAseq Analyses
4.5. Reverse Transcriptase Quantitative PCR Experiments
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Kaplan, J.; Travadon, R.; Cooper, M.; Hillis, V.; Lubell, M.; Baumgartner, K. Identifying economic hurdles to early adoption of preventative practices: The case of trunk diseases in California winegrape vineyards. Wine Econ. Policy 2016, 5, 127–141. [Google Scholar] [CrossRef]
- Calzarano, F.; Marco, S.D.; Cesari, A. Benefit of fungicide treatment after trunk renewal of vines with different types of esca necrosis. Phytopathol. Mediterr. 2004, 43, 10. [Google Scholar]
- Lorrain, B.; Ky, I.; Pasquier, G.; Jourdes, M.; Dubrana, L.G.; Gény, L.; Rey, P.; Donèche, B.; Teissedre, P.-L. Effect of Esca disease on the phenolic and sensory attributes of Cabernet Sauvignon grapes, musts and wines: Esca effect on grapes and wines composition. Aust. J. Grape Wine Res. 2012, 18, 64–72. [Google Scholar] [CrossRef]
- Les Maladies du Bois de la Vigne—Réunion du Groupe de Travail Maladies du Bois. Available online: https://www.maladie-du-bois-vigne.fr/Zoom-sur/Reunion-du-Groupe-de-travail-Maladies-du-bois (accessed on 30 June 2020).
- Compant, S.; Brader, G.; Muzammil, S.; Sessitsch, A.; Lebrihi, A.; Mathieu, F. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl 2013, 58, 435–455. [Google Scholar] [CrossRef]
- Mounier, E.; Boulisset, F.; Elbaz, N.; Dubournet, P.; Pajot, E. Esquive® WP limits development of grapevine wood diseases and reductions in the productive potential of land. In Proceedings of the 5th Conférence Internationale sur les Méthodes Alternatives de Protection des Plantes, Nouceau Sièle, Lille, France, 11–13 March 2015; Association Française de Protection des Plantes (AFPP): Alfortville, France, 2015; pp. 251–261. [Google Scholar]
- Blundell, R.; Lynch, M.; Haden, T.; Arreguin, M.; Gallagher, T.; Eskalen, A. Final Report: Evaluation of Vintec (Trichoderma atroviride_SC1) as Pruning Wound Protectants Against Selected Fungi Associated with Grapevine Trunk Diseases; Department of Plant Pathology, University of California: Davis, CA, USA, 2020. [Google Scholar]
- Gerbore, J.; Vallance, J.; Yacoub, A.; Delmotte, F.; Grizard, D.; Regnault-Roger, C.; Rey, P. Characterization of Pythium oligandrum populations that colonize the rhizosphere of vines from the Bordeaux region. FEMS Microbiol. Ecol. 2014, 90, 153–167. [Google Scholar] [CrossRef]
- Benhamou, N.; le Floch, G.; Vallance, J.; Gerbore, J.; Grizard, D.; Rey, P. Pythium oligandrum: An example of opportunistic success. Microbiology 2012, 158, 2679–2694. [Google Scholar] [CrossRef]
- Gerbore, J.; Benhamou, N.; Vallance, J.; Le Floch, G.; Grizard, D.; Regnault-Roger, C.; Rey, P. Biological control of plant pathogens: Advantages and limitations seen through the case study of Pythium oligandrum. Environ. Sci. Pollut. Res. 2014, 21, 4847–4860. [Google Scholar] [CrossRef]
- Mohamed, N.; Lherminier, J.; Farmer, M.-J.; Fromentin, J.; Béno, N.; Houot, V.; Milat, M.-L.; Blein, J.-P. Defense responses in grapevine leaves against Botrytis cinerea induced by application of a Pythium oligandrum strain or its elicitin, oligandrin, to roots. Phytopathology 2007, 97, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Laveau, C.; Letouze, A.; Louvet, G.; Bastien, S.; Guérin-Dubrana, L. Differential aggressiveness of fungi implicated in esca and associated diseases of grapevine in France. Phytopathol. Mediterr. 2009, 48, 15. [Google Scholar]
- Daraignes, L.; Gerbore, J.; Yacoub, A.; Dubois, L.; Romand, C.; Zekri, O.; Roudet, J.; Chambon, P.; Fermaud, M. Efficacy of P. oligandrum affected by its association with bacterial BCAs and rootstock effect in controlling grapevine trunk diseases. Biol. Control 2018, 119, 59–67. [Google Scholar] [CrossRef]
- Yacoub, A.; Gerbore, J.; Magnin, N.; Chambon, P.; Dufour, M.-C.; Corio-Costet, M.-F.; Guyoneaud, R.; Rey, P. Ability of Pythium oligandrum strains to protect Vitis vinifera L., by inducing plant resistance against Phaeomoniella chlamydospora, a pathogen involved in Esca, a grapevine trunk disease. Biol. Control 2016, 92, 7–16. [Google Scholar] [CrossRef]
- Antonielli, L.; Compant, S.; Strauss, J.; Sessitsch, A.; Berger, H. Draft genome sequence of Phaeomoniella chlamydospora strain RR-HG1, a grapevine trunk disease (esca)-related member of the ascomycota. Genome Announc. 2014, 2, e00098-14. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Rolshausen, P.; Cantu, D. Draft genome sequence of the Ascomycete Phaeoacremonium aleophilum strain UCR-PA7, a causal agent of the esca disease complex in grapevines. Genome Announc. 2013, 1, e00390-13. [Google Scholar] [CrossRef]
- Robert-Siegwald, G.; Vallet, J.; Abou-Mansour, E.; Xu, J.; Rey, P.; Bertsch, C.; Rego, C.; Larignon, P.; Fontaine, F.; Lebrun, M.-H. Draft genome sequence of Diplodia seriata F98.1, a fungal species involved in grapevine trunk diseases. Genome Announc. 2017, 5, e00061-17. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Turrà, D.; Segorbe, D.; Di Pietro, A. Protein kinases in plant-pathogenic fungi: Conserved regulators of infection. Annu. Rev. Phytopathol. 2014, 52, 267–288. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Yacoub, A.; Gerbore, J.; Magnin, N.; Haidar, R.; Compant, S.; Rey, P. Transcriptional analysis of the interaction between the oomycete biocontrol agent, Pythium oligandrum, and the roots of Vitis vinifera L. Biol. Control 2018, 120, 26–35. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine pathogenic microorganisms: Understanding infection strategies and host response scenarios. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- De Bona, G.S.; Adrian, M.; Negrel, J.; Chiltz, A.; Klinguer, A.; Poinssot, B.; Héloir, M.-C.; Angelini, E.; Vincenzi, S.; Bertazzon, N. Dual mode of action of grape cane extracts against Botrytis cinerea. J. Agric. Food Chem. 2019, 67, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Esmaeel, Q.; Jacquard, C.; Clément, C.; Sanchez, L.; Ait Barka, E. Genome sequencing and traits analysis of Burkholderia strains reveal a promising biocontrol effect against grey mould disease in grapevine (Vitis vinifera L.). World J. Microbiol. Biotechnol. 2019, 35, 40. [Google Scholar] [CrossRef] [PubMed]
- Haidar, R.; Roudet, J.; Bonnard, O.; Dufour, M.C.; Corio-Costet, M.F.; Fert, M.; Gautier, T.; Deschamps, A.; Fermaud, M. Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiol. Res. 2016, 192, 172–184. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, J.; Li, X.; Xing, Q.; Chethana, K.W.T.; Zhao, W. Transcriptional response of grapevine to infection with the fungal pathogen Lasiodiplodia theobromae. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Hase, S.; Shimizu, A.; Nakaho, K.; Takenaka, S.; Takahashi, H. Induction of transient ethylene and reduction in severity of tomato bacterial wilt by Pythium oligandrum. Plant Pathol. 2006, 55, 537–543. [Google Scholar] [CrossRef]
- Hase, S.; Takahashi, S.; Takenaka, S.; Nakaho, K.; Arie, T.; Seo, S.; Ohashi, Y.; Takahashi, H. Involvement of jasmonic acid signalling in bacterial wilt disease resistance induced by biocontrol agent Pythium oligandrum in tomato. Plant Pathol. 2008, 57, 870–876. [Google Scholar] [CrossRef]
- Ouyang, Z.; Li, X.; Huang, L.; Hong, Y.; Zhang, Y.; Zhang, H.; Li, D.; Song, F. Elicitin-like proteins Oli-D1 and Oli-D2 from Pythium oligandrum trigger hypersensitive response in Nicotiana benthamiana and induce resistance against Botrytis cinerea in tomato: Oligandrins induce resistance in tomato. Mol. Plant Pathol. 2015, 16, 238–250. [Google Scholar] [CrossRef]
- Wang, A.; Lou, B.; Xu, T.; Lin, C. Defense responses in tomato fruit induced by oligandrin against Botrytis cinerea. Afr. J. Biotechnol. 2011, 10, 4596–4601. [Google Scholar]
- Gutterson, N.; Reuber, T.L. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr. Opin. Plant Biol. 2004, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Pré, M.; Atallah, M.; Champion, A.; De Vos, M.; Pieterse, C.M.J.; Memelink, J. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 2008, 147, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: I. Characterization of secondary metabolites in culture media and host responses to the pathogens in calli. Physiol. Mol. Plant Pathol. 2006, 69, 209–223. [Google Scholar] [CrossRef]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: II. Characterization of biomolecules in xylem sap and leaves of healthy and diseased vines. Physiol. Mol. Plant Pathol. 2006, 69, 195–208. [Google Scholar] [CrossRef]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: III. Enzymes produced by the pathogens and their role in fungus-to-plant or in fungus-to-fungus interactions. Physiol. Mol. Plant Pathol. 2006, 69, 182–194. [Google Scholar] [CrossRef]
- Morales-Cruz, A.; Amrine, K.C.H.; Blanco-Ulate, B.; Lawrence, D.P.; Travadon, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Distinctive expansion of gene families associated with plant cell wall degradation, secondary metabolism, and nutrient uptake in the genomes of grapevine trunk pathogens. BMC Genom. 2015, 16. [Google Scholar] [CrossRef]
- Luini, E.; Fleurat-Lessard, P.; Rousseau, L.; Roblin, G.; Berjeaud, J.-M. Inhibitory effects of polypeptides secreted by the grapevine pathogens Phaeomoniella chlamydospora and Phaeoacremonium aleophilum on plant cell activities. Physiol. Mol. Plant Pathol. 2010, 74, 403–411. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Wang, C.; Xu, J.-R. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genom. 2013, 14, 274. [Google Scholar] [CrossRef]
- Sista Kameshwar, A.K.; Qin, W. Comparative study of genome-wide plant biomass-degrading CAZymes in white rot, brown rot and soft rot fungi. Mycology 2018, 9, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Yip, V.L.; Withers, S.G. Breakdown of oligosaccharides by the process of elimination. Curr. Opin. Chem. Biol. 2006, 10, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, G.; Rey, P.; Déniel, F.; Benhamou, N.; Picard, K.; Tirilly, Y. Enhancement of development and induction of resistance in tomato plants by the antagonist, Pythium oligandrum. Agronomie 2003, 23, 455–460. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 10, R80. [Google Scholar] [CrossRef]
- Kauffmann, A.; Gentleman, R.; Huber, W. arrayQualityMetrics—A bioconductor package for quality assessment of microarray data. Bioinformatics 2009, 25, 415–416. [Google Scholar] [CrossRef]
- Carvalho, B.S.; Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R., Ed.; Statistics for Biology and Health; Springer Science + Business Media: New York, NY, USA, 2005; ISBN 978-0-387-25146-2. [Google Scholar]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A Free, Open-Source System for Microarray Data Management and Analysis. BioTechniques 2003, 34, 374–378. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef]
- Dufour, M.C.; Lambert, C.; Bouscaut, J.; Mérillon, J.M.; Corio-Costet, M.F. Benzothiadiazole-primed defence responses and enhanced differential expression of defence genes in Vitis vinifera infected with biotrophic pathogens Erysiphe necator and Plasmopara viticola: Elicitation and grapevine responses to mildews. Plant Pathol. 2013, 62, 370–382. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
| Families | Number of Genes Predicted in the Genome | Number of Genes Detected in Samples | |
|---|---|---|---|
| Pch | PoPch | ||
| Glycoside hydrolases (GH) | 345 (72) | 60 (17) | 70 (9) |
| Glycosyl transferases (GT) | 268 (17) | 34 (3) | 69 (7) |
| Carbohydrate-binding module (CBM) | 141 (26) | 27 (6) | 37 (4) |
| Carbohydrate esterases (CE) | 107 (12) | 13 (1) | 10 (3) |
| Auxiliary Activities (AA) | 56 (14) | 9 (0) | 7 (2) |
| Polysaccharide Lyases (PL) | 4 (1) | 2 (0) | 1 (1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yacoub, A.; Magnin, N.; Gerbore, J.; Haidar, R.; Bruez, E.; Compant, S.; Guyoneaud, R.; Rey, P. The Biocontrol Root-Oomycete, Pythium Oligandrum, Triggers Grapevine Resistance and Shifts in the Transcriptome of the Trunk Pathogenic Fungus, Phaeomoniella Chlamydospora. Int. J. Mol. Sci. 2020, 21, 6876. https://doi.org/10.3390/ijms21186876
Yacoub A, Magnin N, Gerbore J, Haidar R, Bruez E, Compant S, Guyoneaud R, Rey P. The Biocontrol Root-Oomycete, Pythium Oligandrum, Triggers Grapevine Resistance and Shifts in the Transcriptome of the Trunk Pathogenic Fungus, Phaeomoniella Chlamydospora. International Journal of Molecular Sciences. 2020; 21(18):6876. https://doi.org/10.3390/ijms21186876
Chicago/Turabian StyleYacoub, Amira, Noel Magnin, Jonathan Gerbore, Rana Haidar, Emilie Bruez, Stéphane Compant, Rémy Guyoneaud, and Patrice Rey. 2020. "The Biocontrol Root-Oomycete, Pythium Oligandrum, Triggers Grapevine Resistance and Shifts in the Transcriptome of the Trunk Pathogenic Fungus, Phaeomoniella Chlamydospora" International Journal of Molecular Sciences 21, no. 18: 6876. https://doi.org/10.3390/ijms21186876
APA StyleYacoub, A., Magnin, N., Gerbore, J., Haidar, R., Bruez, E., Compant, S., Guyoneaud, R., & Rey, P. (2020). The Biocontrol Root-Oomycete, Pythium Oligandrum, Triggers Grapevine Resistance and Shifts in the Transcriptome of the Trunk Pathogenic Fungus, Phaeomoniella Chlamydospora. International Journal of Molecular Sciences, 21(18), 6876. https://doi.org/10.3390/ijms21186876






