Methrotexate Treatment Inmunomodulates Abnormal Cytokine Expression by T CD4 Lymphocytes Present in DMARD-Naïve Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Results
2.1. Patient Demographic Characteristics
2.2. New-Onset DMARD-Naïve RA Patients Show An Expansion of CD4+IL-17A+ and CD4+IFNγ+ T-Lymphocytes
2.3. Different Patterns of Distribution Of IL-17A, IFNγ And IL-4 CD4+ T-Lymphocytes Are Observed in MTX Responder and Nonresponder Ra Patients
3. Discussions
4. Materials and Methods
4.1. Inclusion and Exclusion Criteria
4.2. Study Protocol
4.3. Clinical Laboratory Assays
4.4. Isolation of Peripheral Blood Mononuclear Cells
4.5. In Vitro Culture
4.6. Surface and Intracellular Lymphocyte Staining
4.7. Cytokines Serum Levels
4.8. STATs Flow Cytometry
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| IFNg | Interferon gamma |
| IL-4 | Interleukin-4 |
| IL-17 | Interleukin-17a |
| RA | Rheumatoid arthritis |
| DMARDs | Disease-Modifying antirheumatic drugs |
| PBMC | Peripheral blood mononuclear cells |
| FITC | Fluorescein-Isothiocyanate |
| PE | Phycoerythrin |
| Apc-Alexa780 | Allophycocyanin-alexa-780 |
| Pe-Cy7 | Phycoerythrin-Cyanine Seven |
| mAb | Monoclonal antibodies |
| MTX | Methrotexate |
| Th | T helper |
| TN | T naïve |
| TCM | T central memory |
| TEM | T effector memory |
| TE | T effector |
| PMA | Phorbol-12-Myristate-13-Acetate |
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W.J. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J. Pathogenesis of rheumatoid arthritis. Med. Clin. N. Am. 1997, 81, 29–55. [Google Scholar] [CrossRef]
- Cope, A.P. T cells in rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, S1. [Google Scholar] [CrossRef]
- Okada, R.; Kondo, T.; Matsuki, F.; Takata, H.; Takiguchi, M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int. Immunol. 2008, 20, 1189–1199. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Sallusto, F.; Monticelli, S. The many faces of CD4 T cells: Roles in immunity and disease. Semin. Immunol. 2013, 25, 249–251. [Google Scholar] [CrossRef]
- Schmitt, N.; Ueno, H. Regulation of human helper T cell subset differentiation by cytokines. Curr. Opin. Immunol. 2015, 34, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.-L.; Miossec, P. The role of T cells in rheumatoid arthritis: New subsets and new targets. Curr. Opin. Rheumatol. 2007, 19, 284–288. [Google Scholar] [CrossRef]
- Lönnberg, T.; Chen, Z.; Lahesmaa, R. From a gene-centric to whole-proteome view of differentiation of T helper cell subsets. Brief. Funct. Genomics. 2013, 12, 471–482. [Google Scholar] [CrossRef]
- Vahedi, G.; Takahashi, H.; Nakayamada, S.; Sun, H.-W.; Sartorelli, V.; Kanno, Y.; O’Shea, J.J. STATs shape the active enhancer landscape of T cell populations. Cell 2012, 151, 981–993. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Jenkins, M.K. CD4+ memory T cell survival. Curr. Opin. Immunol. 2011, 23, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lanzavecchia, A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur. J. Immunol. 2009, 39, 2076–2082. [Google Scholar] [CrossRef]
- Monserrat, J.; Bohórquez, C.; Lahoz, A.M.G.; Movasat, A.; Pérez, A.; Ruíz, L.; Diaz, D.; Chara, L.; Sánchez, A.I.; Albarrán, F.; et al. The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament. Cells 2019, 8, 871. [Google Scholar] [CrossRef]
- Wehrens, E.J.; Prakken, B.J.; Wijk, F.V. T cells out of control—Impaired immune regulation in the inflamed joint. Nat. Rev. Rheumatol. 2012, 9, 34–42. [Google Scholar] [CrossRef]
- Alex, P.; Szodoray, P.; Knowlton, N.; Dozmorov, I.M.; Turner, M.; Frank, M.B.; Arthur, R.E.; Willis, L.; Flinn, D.; Hynd, R.F.; et al. Multiplex serum cytokine monitoring as a prognostic tool in rheumatoid arthritis. Clin. Exp. Rheumatol. 2007, 25, 584–592. [Google Scholar]
- Arroyo-Villa, I.; Bautista-Caro, M.-B.; Balsa, A.; Aguado-Acín, P.; Nuño, L.; Bonilla-Hernán, M.-G.; Puig-Kröger, A.; Martín-Mola, E.; Miranda-Carus, M.-E. Frequency of Th17 CD4+ T Cells in Early Rheumatoid Arthritis: A Marker of Anti-CCP Seropositivity. PLoS ONE 2012, 7, e42189. [Google Scholar] [CrossRef]
- Deane, K.D.; O’Donnell, C.I.; Hueber, W.; Majka, D.S.; Lazar, A.A.; Derber, L.A.; Gilliland, M.W.R.; Edison, J.; Norris, J.M.; Robinson, W.H.; et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010, 62, 3161–3172. [Google Scholar] [CrossRef]
- Kokkonen, H.; Söderström, I.; Rocklöv, J.; Hallmans, G.; Lejon, K.; Rantapää-Dahlqvist, S.; Söderström, I.; Rocklöv, J. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kosmaczewska, A.; Swierkot, J.; Ciszak, L.; Szteblich, A.; Chrobak, A.; Karabon, L.; Partyka, A.; Szechinski, J.; Wiland, P.; Frydecka, I. Patients with the most advanced rheumatoid arthritis remain with Th1 systemic defects after TNF inhibitors treatment despite clinical improvement. Rheumatol. Int. 2013, 34, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Goodall, J.C.; Busch, R.; Gaston, H. Relationship of CD146 expression to secretion of interleukin (IL)-17, IL-22 and interferon-γ by CD4(+) T cells in patients with inflammatory arthritis. Clin. Exp. Immunol. 2015, 179, 378–391. [Google Scholar] [CrossRef]
- Adamson, A.S.; Collins, K.; Laurence, A.; O’Shea, J. The Current STATus of lymphocyte signaling: New roles for old players. Curr. Opin. Immunol. 2009, 21, 161–166. [Google Scholar] [CrossRef]
- Kuuliala, K.; Kuuliala, A.; Koivuniemi, R.; Oksanen, S.; Hämäläinen, M.; Moilanen, E.; Kautiainen, H.; Leirisalo-Repo, M.; Repo, H. Constitutive STAT3 Phosphorylation in Circulating CD4+ T Lymphocytes Associates with Disease Activity and Treatment Response in Recent-Onset Rheumatoid Arthritis. PLoS ONE 2015, 10, e0137385. [Google Scholar] [CrossRef] [PubMed]
- Kuuliala, K.; Kuuliala, A.; Koivuniemi, R.; Kautiainen, H.; Repo, H.; Leirisalo-Repo, M. STAT6 and STAT1 Pathway Activation in Circulating Lymphocytes and Monocytes as Predictor of Treatment Response in Rheumatoid Arthritis. PLoS ONE 2016, 11, e0167975. [Google Scholar] [CrossRef]
- Ramiro, S.; Landewé, R.; Breedveld, F.C.; Buch, M.; Burmester, G.; Dougados, M.; Emery, P.; Gaujoux-Viala, C.; Gossec, L.; Nam, J.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann. Rheum. Dis. 2013, 73, 492–509. [Google Scholar] [CrossRef]
- Firestein, G.S. Immunologic Mechanisms in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Rheumatol. 2005, 11, S39–S44. [Google Scholar] [CrossRef]
- Fekete, A.; Soos, L.; Szekanecz, Z.; Szabo, Z.; Szodoray, P.; Barath, S.; Lakos, G. Disturbances in B- and T-cell homeostasis in rheumatoid arthritis: Suggested relationships with antigen-driven immune responses. J. Autoimmun. 2007, 29, 154–163. [Google Scholar] [CrossRef]
- Kohem, C.L.; Brezinschek, R.I.; Wisbey, H.; Tortorella, C.; Lipsky, P.E.; Oppenheimer-Marks, N. Enrichment of differentiated CD45RBdim. Arthritis Rheum. 1996, 39, 844–854. [Google Scholar] [CrossRef]
- Neidhart, M.; Pataki, F.; Schönbächler, J.; Brühlmann, P. Flow cytometric characterisation of the “false naive” (CD45RA+, CD45R0-, CD29 bright+) peripheral blood T-lymphocytes in health and in rheumatoid arthritis. Rheumatol. Int. 1996, 16, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Fehr, K.; Pataki, F.; Michel, B.A. The levels of memory (CD45RA?, RO+) CD4+ and CD8+ peripheral blood T-lymphocytes correlate with IgM rheumatoid factors in rheumatoid arthritis. Rheumatol. Int. 1996, 15, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, F.; Morgan, A.W.; Bingham, S.J.; Quinn, M.; Buch, M.; Verburg, R.J.; Henwood, J.; Douglas, S.H.; Masurel, A.; Conaghan, P.G.; et al. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood 2002, 100, 4550–4556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotake, S.; Nanke, Y.; Yago, T.; Kawamoto, M.; Kobashigawa, T.; Yamanaka, H. Ratio of Circulating IFNγ + “Th17 Cells” in Memory Th Cells Is Inversely Correlated with the Titer of Anti-CCP Antibodies in Early-Onset Rheumatoid Arthritis Patients Based on Flow Cytometry Methods of the Human Immunology Project. BioMed Res. Int. 2016, 2016, 9694289. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Falciani, F.; Curnow, S.J.; Ross, E.J.; Lee, C.-Y.; Akbar, A.N.; Lord, J.; Gordon, C.; Buckley, C.D.; Salmon, M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 2005, 7, R784–R795. [Google Scholar] [CrossRef]
- Chara, L.; Sánchez-Atrio, A.; Pérez, A.; Cuende, E.; Albarrán, F.; Turrión, A.; Chevarria, J.; Barco, A.A.D.; Sánchez, M.A.; Monserrat, J.; et al. The number of circulating monocytes as biomarkers of the clinical response to methotrexate in untreated patients with rheumatoid arthritis. J. Transl. Med. 2015, 13, 2. [Google Scholar] [CrossRef]
- Rossol, M.; Kraus, S.; Pierer, M.; Baerwald, C.; Wagner, U. The CD14brightCD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012, 64, 671–677. [Google Scholar] [CrossRef]
- Cosmi, L.; De Palma, R.; Santarlasci, V.; Maggi, L.; Capone, M.; Frosali, F.; Rodolico, G.; Querci, V.; Abbate, G.; Angeli, R.; et al. Human interleukin 17–producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008, 205, 1903–1916. [Google Scholar] [CrossRef]
- Kotake, S.; Yago, T.; Kobashigawa, T.; Nanke, Y. The Plasticity of Th17 Cells in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Med. 2017, 6, 67. [Google Scholar] [CrossRef]
- Wessels, J.A.M.; Guchelaar, H.-J.; Huizinga, T.W.J. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatol. Oxf. 2007, 47, 249–255. [Google Scholar] [CrossRef]
- Möller, B.; Kukoc-Zivojnov, N.; Okamgba, S.; Kessler, U.; Puccetti, E.; Ottmann, O.G.; Kaltwasser, J.P.; Hoelzer, D.; Ruthardt, M. Folinic acid antagonizes methotrexate-induced differentiation of monocyte progenitors. Rheumatol. Int. 2002, 22, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.C.; Woollard, K.J.; Griffiths, H.R. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br. J. Pharmacol. 2003, 138, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.; Osen, W.; Debatin, K.-M. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin. Exp. Immunol. 2002, 128, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- González, V.M.; Stewart, A.; Ritter, P.L.; Lorig, K. Translation and validation of arthritis outcome measures into Spanish. Arthritis Rheum. 1995, 38, 1429–1446. [Google Scholar] [CrossRef]
- Gestel, A.M.V.; Prevoo, M.L.L.; Hof, M.A.V.; Rijswijk, M.H.V.; De Putte, L.B.A.V.D.; Riel, P.L.C.M.V. Development and validation of the european league against rheumatism response criteria for rheumatoid arthritis: Comparison with the preliminary american college of rheumatology and the world health organization/international league against rheumatism criteria. Arthritis Rheum. 1996, 39, 34–40. [Google Scholar] [CrossRef]
- Böyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 1968, 97, 77–89. [Google Scholar]
- Monserrat, J.; De Pablo, R.; Reyes, E.; Diaz, D.; Barcenilla, H.; Zapata, M.R.; De La Hera, A.; Prieto, A.; Alvarez-Mon, M. Clinical relevance of the severe abnormalities of the T cell compartment in septic shock patients. Crit. Care 2009, 13, R26. [Google Scholar] [CrossRef]
- Roederer, M. Compensation in Flow Cytometry. Curr. Protoc. Cytom. 2002, 22, 1–14. [Google Scholar] [CrossRef]
- Roederer, M.; Darzynkiewicz, Z.; Parks, D.R. Guidelines for the Presentation of Flow Cytometric Data. Methods Cell Biol. 2004, 75, 241–256. [Google Scholar] [CrossRef]
 ) and healthy controls (
) and healthy controls ( ). % (percentages) refers to total population of the indicated lymphocytes. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Panel 1. The first dot plots represent the selected gates and percentages of TN, TCM, TEM and TE CD4+ T-lymphocytes in two representative situations: a healthy control and RA patient at baseline before Methotrexate (MTX) treatment. Panel 2, 3 and 4. Dot plots represent the percentages of IL-17A, IFNγ and IL-4-producing CD4+ T cells in the presence and absence of PMA stimulation in the two representative cases described in panel 1. R1 and R2 represents the regions that include the TNaïve and TEM CD4+ lymphocyte subsets, respectively.
). % (percentages) refers to total population of the indicated lymphocytes. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Panel 1. The first dot plots represent the selected gates and percentages of TN, TCM, TEM and TE CD4+ T-lymphocytes in two representative situations: a healthy control and RA patient at baseline before Methotrexate (MTX) treatment. Panel 2, 3 and 4. Dot plots represent the percentages of IL-17A, IFNγ and IL-4-producing CD4+ T cells in the presence and absence of PMA stimulation in the two representative cases described in panel 1. R1 and R2 represents the regions that include the TNaïve and TEM CD4+ lymphocyte subsets, respectively.
 ) and healthy controls (
) and healthy controls ( ). % (percentages) refers to total population of the indicated lymphocytes. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Panel 1. The first dot plots represent the selected gates and percentages of TN, TCM, TEM and TE CD4+ T-lymphocytes in two representative situations: a healthy control and RA patient at baseline before Methotrexate (MTX) treatment. Panel 2, 3 and 4. Dot plots represent the percentages of IL-17A, IFNγ and IL-4-producing CD4+ T cells in the presence and absence of PMA stimulation in the two representative cases described in panel 1. R1 and R2 represents the regions that include the TNaïve and TEM CD4+ lymphocyte subsets, respectively.
). % (percentages) refers to total population of the indicated lymphocytes. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Panel 1. The first dot plots represent the selected gates and percentages of TN, TCM, TEM and TE CD4+ T-lymphocytes in two representative situations: a healthy control and RA patient at baseline before Methotrexate (MTX) treatment. Panel 2, 3 and 4. Dot plots represent the percentages of IL-17A, IFNγ and IL-4-producing CD4+ T cells in the presence and absence of PMA stimulation in the two representative cases described in panel 1. R1 and R2 represents the regions that include the TNaïve and TEM CD4+ lymphocyte subsets, respectively.
 ) DMARD-naïve RA patients and (
) DMARD-naïve RA patients and ( ) healthy controls. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Dot plots represent the percentages of IFNγ+IL-17A+ double-positive expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a healthy control and a RA patient at baseline before MTX treatment.
) healthy controls. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Dot plots represent the percentages of IFNγ+IL-17A+ double-positive expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a healthy control and a RA patient at baseline before MTX treatment.
 ) DMARD-naïve RA patients and (
) DMARD-naïve RA patients and ( ) healthy controls. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Dot plots represent the percentages of IFNγ+IL-17A+ double-positive expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a healthy control and a RA patient at baseline before MTX treatment.
) healthy controls. All values are expressed as the mean cell numbers ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Dot plots represent the percentages of IFNγ+IL-17A+ double-positive expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a healthy control and a RA patient at baseline before MTX treatment.
 ) DMARD-naïve RA patients and (
) DMARD-naïve RA patients and ( ) healthy controls. All values are expressed as the mean MFI or percentage ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Histograms represent the mean fluorescence intensity (MFI) of STAT-1, STAT-3 and STAT-6 total protein stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls, in two representative cases: a healthy control and a RA patient at baseline before MTX treatment. (c) Histograms represent the percentages of STAT-1, STAT-3 and STAT-6 phosphorylation stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls in two representative cases: a healthy control and an RA patient at baseline before MTX treatment.
) healthy controls. All values are expressed as the mean MFI or percentage ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Histograms represent the mean fluorescence intensity (MFI) of STAT-1, STAT-3 and STAT-6 total protein stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls, in two representative cases: a healthy control and a RA patient at baseline before MTX treatment. (c) Histograms represent the percentages of STAT-1, STAT-3 and STAT-6 phosphorylation stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls in two representative cases: a healthy control and an RA patient at baseline before MTX treatment.
 ) DMARD-naïve RA patients and (
) DMARD-naïve RA patients and ( ) healthy controls. All values are expressed as the mean MFI or percentage ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Histograms represent the mean fluorescence intensity (MFI) of STAT-1, STAT-3 and STAT-6 total protein stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls, in two representative cases: a healthy control and a RA patient at baseline before MTX treatment. (c) Histograms represent the percentages of STAT-1, STAT-3 and STAT-6 phosphorylation stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls in two representative cases: a healthy control and an RA patient at baseline before MTX treatment.
) healthy controls. All values are expressed as the mean MFI or percentage ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls. (b) Histograms represent the mean fluorescence intensity (MFI) of STAT-1, STAT-3 and STAT-6 total protein stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls, in two representative cases: a healthy control and a RA patient at baseline before MTX treatment. (c) Histograms represent the percentages of STAT-1, STAT-3 and STAT-6 phosphorylation stimulated with IFNγ, IL-6 and IL-4, respectively, and their negative controls in two representative cases: a healthy control and an RA patient at baseline before MTX treatment.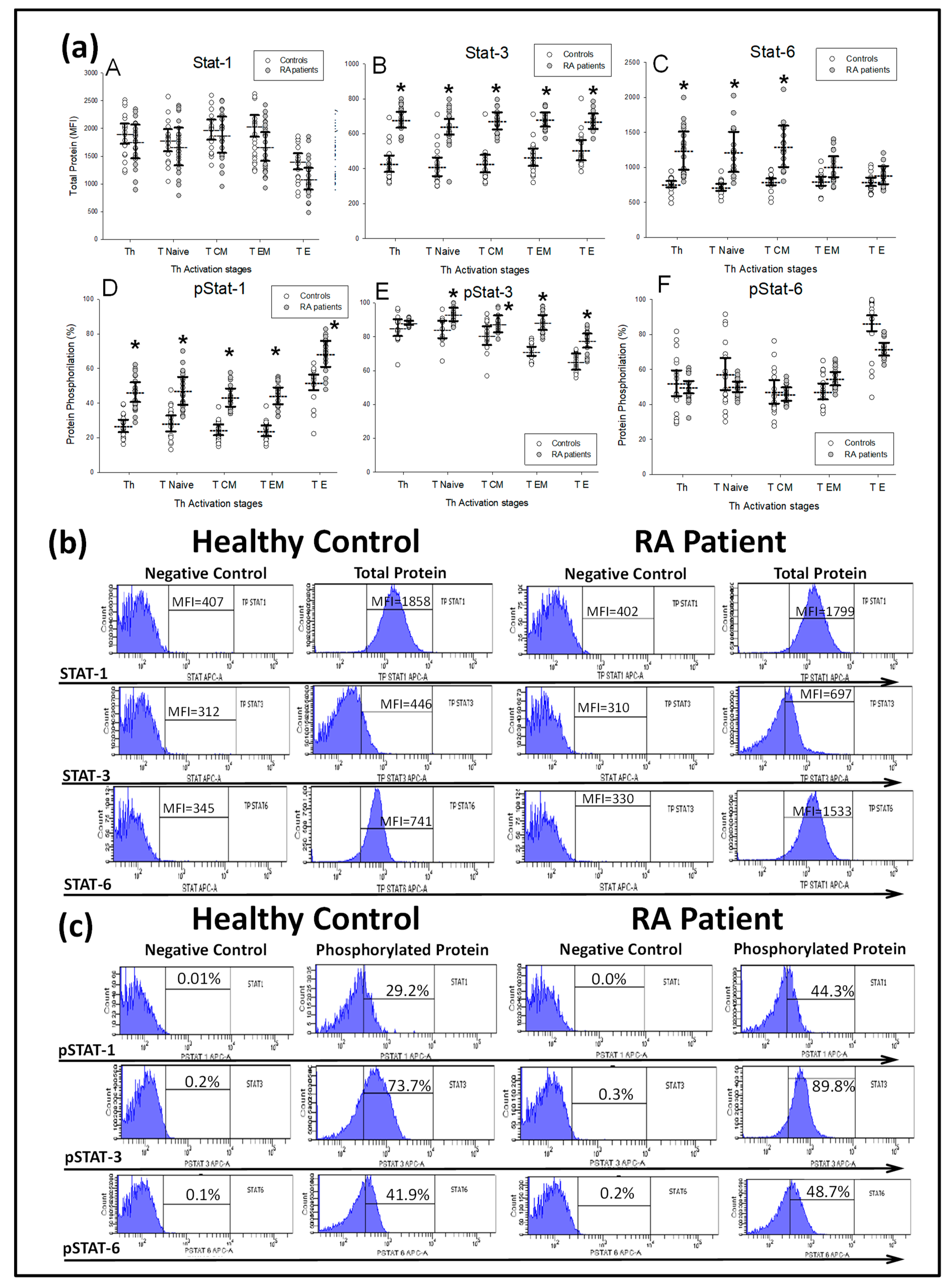
 ) RA patients and (
) RA patients and ( ) as healthy controls (panels A, B and C). All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls.
) as healthy controls (panels A, B and C). All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls.
 ) RA patients and (
) RA patients and ( ) as healthy controls (panels A, B and C). All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls.
) as healthy controls (panels A, B and C). All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for RA patients vs. healthy controls.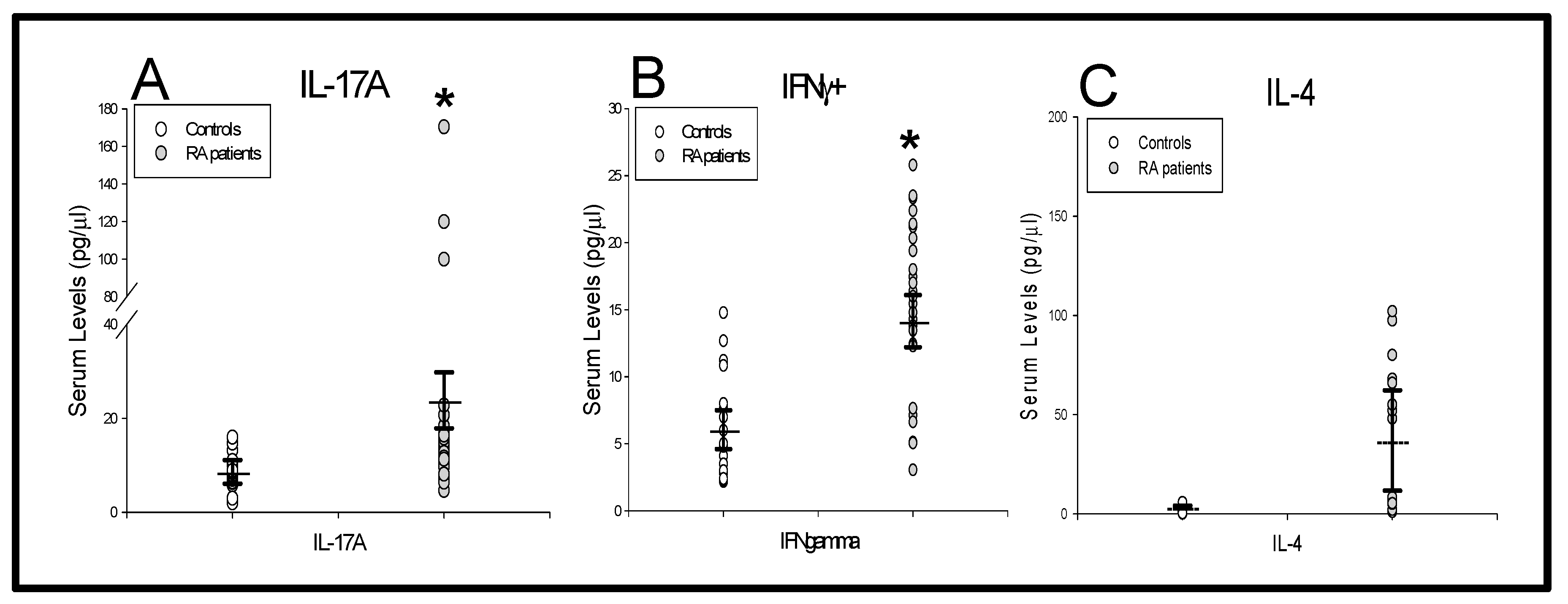
 ) responder and (
) responder and ( ) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls (
) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls ( ). All values are expressed as the mean cell numbers (nº/μL) ± S.E.M. *, p < 0.05 for responder or nonresponder RA patients vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline. (d) Dot plots represent the percentages of IFNγ+, IL-17A+ and IL-4+ expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a nonresponder and a responder RA patient at baseline before MTX treatment.
). All values are expressed as the mean cell numbers (nº/μL) ± S.E.M. *, p < 0.05 for responder or nonresponder RA patients vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline. (d) Dot plots represent the percentages of IFNγ+, IL-17A+ and IL-4+ expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a nonresponder and a responder RA patient at baseline before MTX treatment.
 ) responder and (
) responder and ( ) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls (
) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls ( ). All values are expressed as the mean cell numbers (nº/μL) ± S.E.M. *, p < 0.05 for responder or nonresponder RA patients vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline. (d) Dot plots represent the percentages of IFNγ+, IL-17A+ and IL-4+ expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a nonresponder and a responder RA patient at baseline before MTX treatment.
). All values are expressed as the mean cell numbers (nº/μL) ± S.E.M. *, p < 0.05 for responder or nonresponder RA patients vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline. (d) Dot plots represent the percentages of IFNγ+, IL-17A+ and IL-4+ expression by CD4+ T-lymphocytes after in vitro PMA stimulation in two representative situations: a nonresponder and a responder RA patient at baseline before MTX treatment.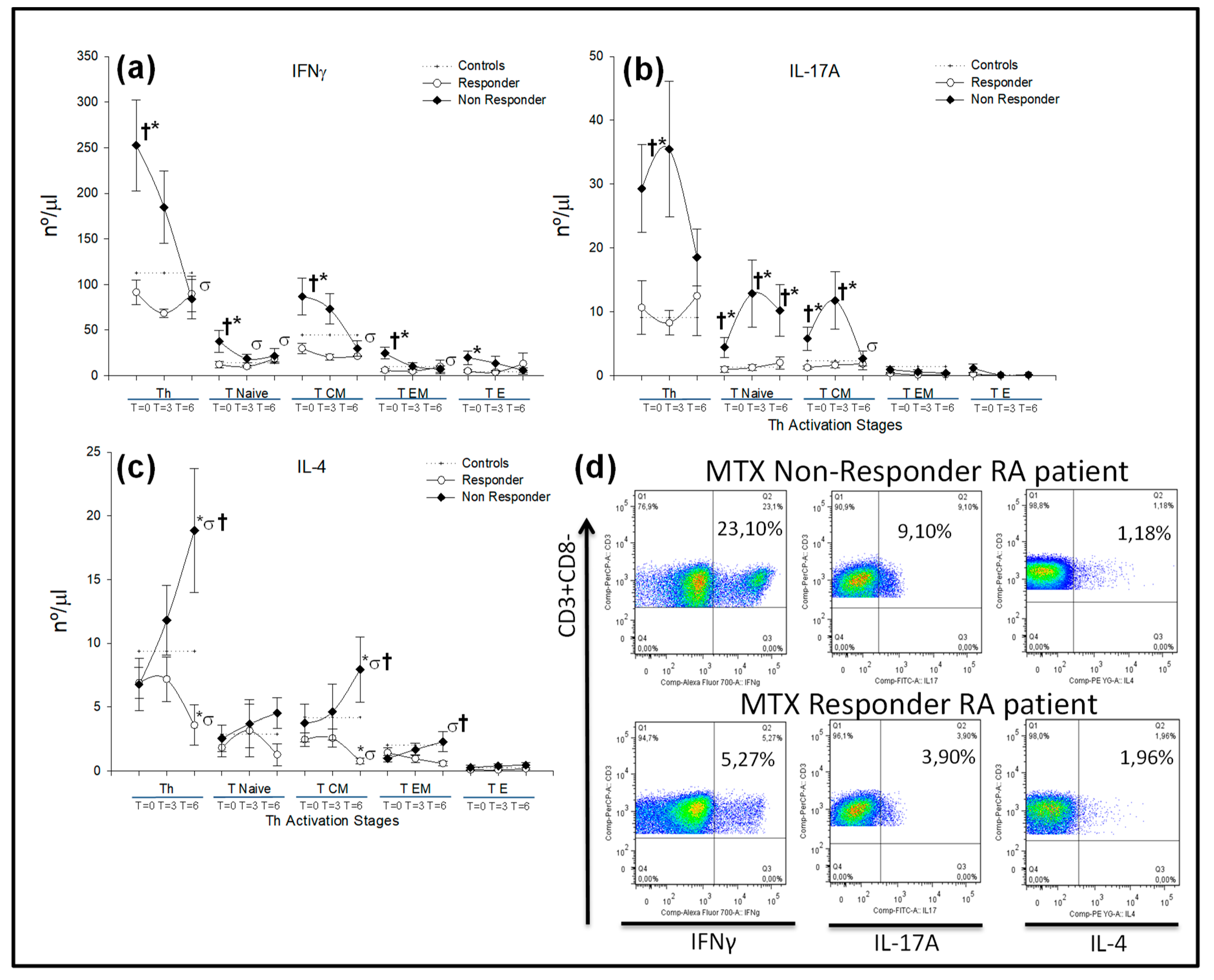
 ) responder and (
) responder and ( ) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls (
) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls ( ). All values are expressed as the mean cell numbers ± S.E.M. †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline.
). All values are expressed as the mean cell numbers ± S.E.M. †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline.
 ) responder and (
) responder and ( ) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls (
) nonresponder RA patients. The dotted line represents the mean value recorded in healthy controls ( ). All values are expressed as the mean cell numbers ± S.E.M. †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline.
). All values are expressed as the mean cell numbers ± S.E.M. †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 6 months of follow-up time vs. baseline.
 ) nonresponder, (
) nonresponder, ( ) responder RA patients and (
) responder RA patients and ( ) healthy controls. All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for responders or nonresponders vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 3 or 6 months of follow-up time vs. baseline.
) healthy controls. All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for responders or nonresponders vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 3 or 6 months of follow-up time vs. baseline.
 ) nonresponder, (
) nonresponder, ( ) responder RA patients and (
) responder RA patients and ( ) healthy controls. All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for responders or nonresponders vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 3 or 6 months of follow-up time vs. baseline.
) healthy controls. All values are expressed as the mean serum levels ± S.E.M. *, p < 0.05 for responders or nonresponders vs. healthy controls; †, p < 0.05 for responders vs. nonresponders, σ p < 0.05 for 3 or 6 months of follow-up time vs. baseline.
| Healthy Controls (n = 29) | Eventual Responders (n = 31) | Eventual Non-Responders (n = 16) | ||
|---|---|---|---|---|
| Variables | (mean ± SD) | (mean ± SD) | (mean ± SD) | p-value |
| Age (years) | 48.70 ± 12.01 | 51.60 ± 10.01 | 52.02 ± 9.48 | 0.823 |
| Gender (women) | 72.10% | 74.19% | 75.00% | 0.902 |
| CRP (mg/L) | - | 16.12 ± 6.39 | 16.57 ± 5.33 | 0.942 |
| Rheumatoid factor (+) Prevalence (+) | - | 225.34 ± 88.46 87.09% | 233 ± 91.18 87.50% | 0.907 0.841 |
| Anti-CCP (IU/mL) Prevalence (+) | - | 435.72 ± 358.15 77.41% | 431.08 ± 276.10 81.25% | 0.965 0.809 |
| DAS28 | - | 3.62 ± 0.49 | 3.69 ± 0.46 | 0.689 |
| Erosions (+) | - | 27.01% | 27.53% | 0.759 |
| HAQ | - | 0.76 ± 0.56 | 0.78 ± 0.79 | 0.844 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat Sanz, J.; Bohórquez, C.; Gómez, A.M.; Movasat, A.; Pérez, A.; Ruíz, L.; Diaz, D.; Sánchez, A.I.; Albarrán, F.; Sanz, I.; et al. Methrotexate Treatment Inmunomodulates Abnormal Cytokine Expression by T CD4 Lymphocytes Present in DMARD-Naïve Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2020, 21, 6847. https://doi.org/10.3390/ijms21186847
Monserrat Sanz J, Bohórquez C, Gómez AM, Movasat A, Pérez A, Ruíz L, Diaz D, Sánchez AI, Albarrán F, Sanz I, et al. Methrotexate Treatment Inmunomodulates Abnormal Cytokine Expression by T CD4 Lymphocytes Present in DMARD-Naïve Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2020; 21(18):6847. https://doi.org/10.3390/ijms21186847
Chicago/Turabian StyleMonserrat Sanz, Jorge, Cristina Bohórquez, Ana Maria Gómez, Atusa Movasat, Ana Pérez, Lucía Ruíz, David Diaz, Ana Isabel Sánchez, Fernando Albarrán, Ignacio Sanz, and et al. 2020. "Methrotexate Treatment Inmunomodulates Abnormal Cytokine Expression by T CD4 Lymphocytes Present in DMARD-Naïve Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 21, no. 18: 6847. https://doi.org/10.3390/ijms21186847
APA StyleMonserrat Sanz, J., Bohórquez, C., Gómez, A. M., Movasat, A., Pérez, A., Ruíz, L., Diaz, D., Sánchez, A. I., Albarrán, F., Sanz, I., & Álvarez-Mon, M. (2020). Methrotexate Treatment Inmunomodulates Abnormal Cytokine Expression by T CD4 Lymphocytes Present in DMARD-Naïve Rheumatoid Arthritis Patients. International Journal of Molecular Sciences, 21(18), 6847. https://doi.org/10.3390/ijms21186847







