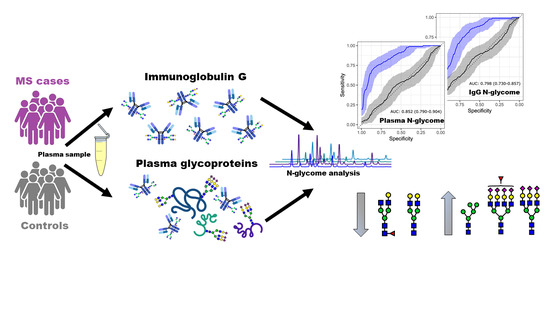
Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Ethical Statement
2.3. N-Glycome Analysis
2.3.1. IgG Isolation from Human Plasma
2.3.2. N-Glycan Release from IgG and Total Plasma Proteins
2.3.3. Fluorescent Labelling and HILIC-SPE Purification of Released N-Glycans
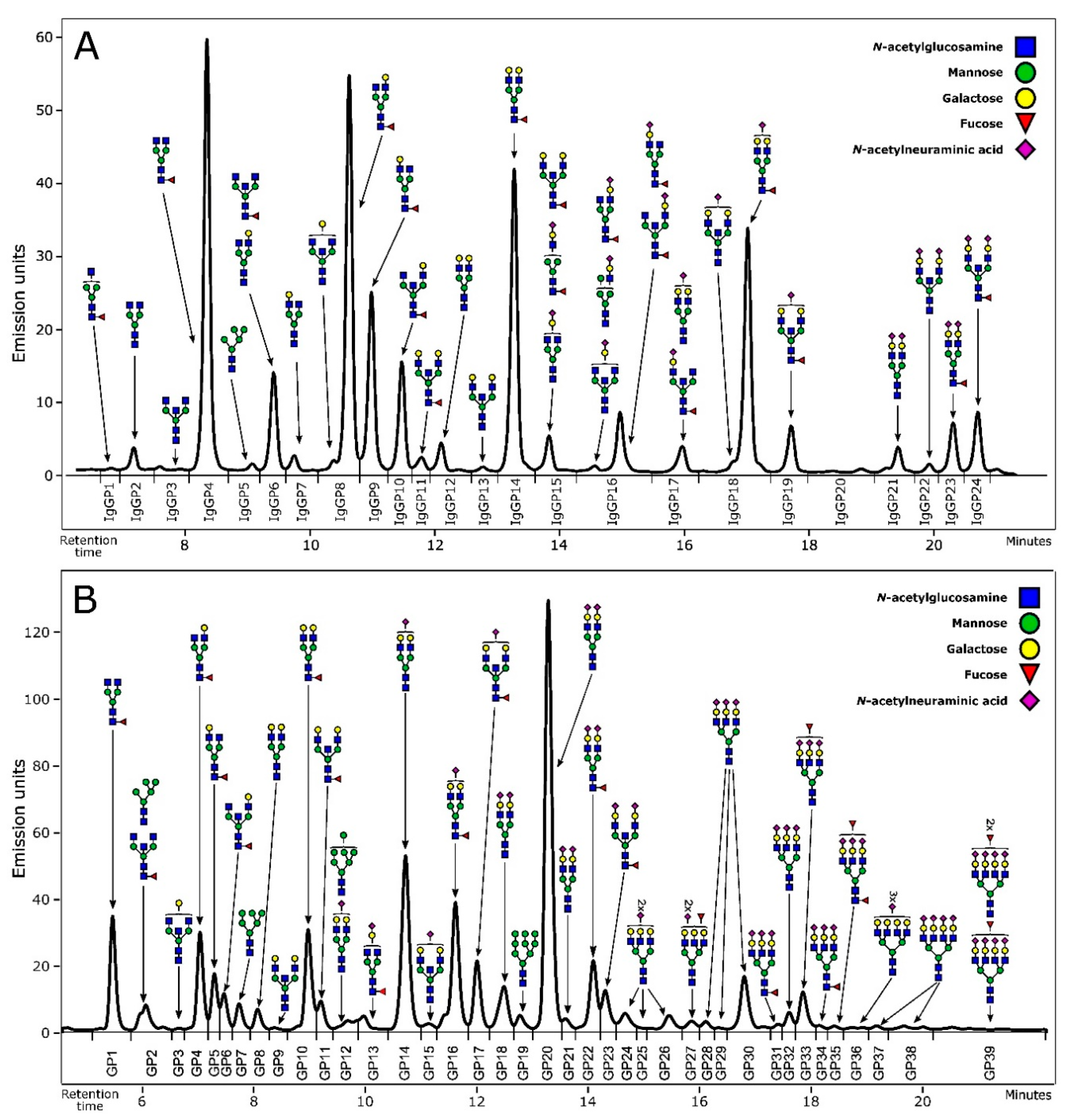
2.3.4. N-Glycan Profiling and Structure Assignment
2.4. Statistical Analysis
3. Results
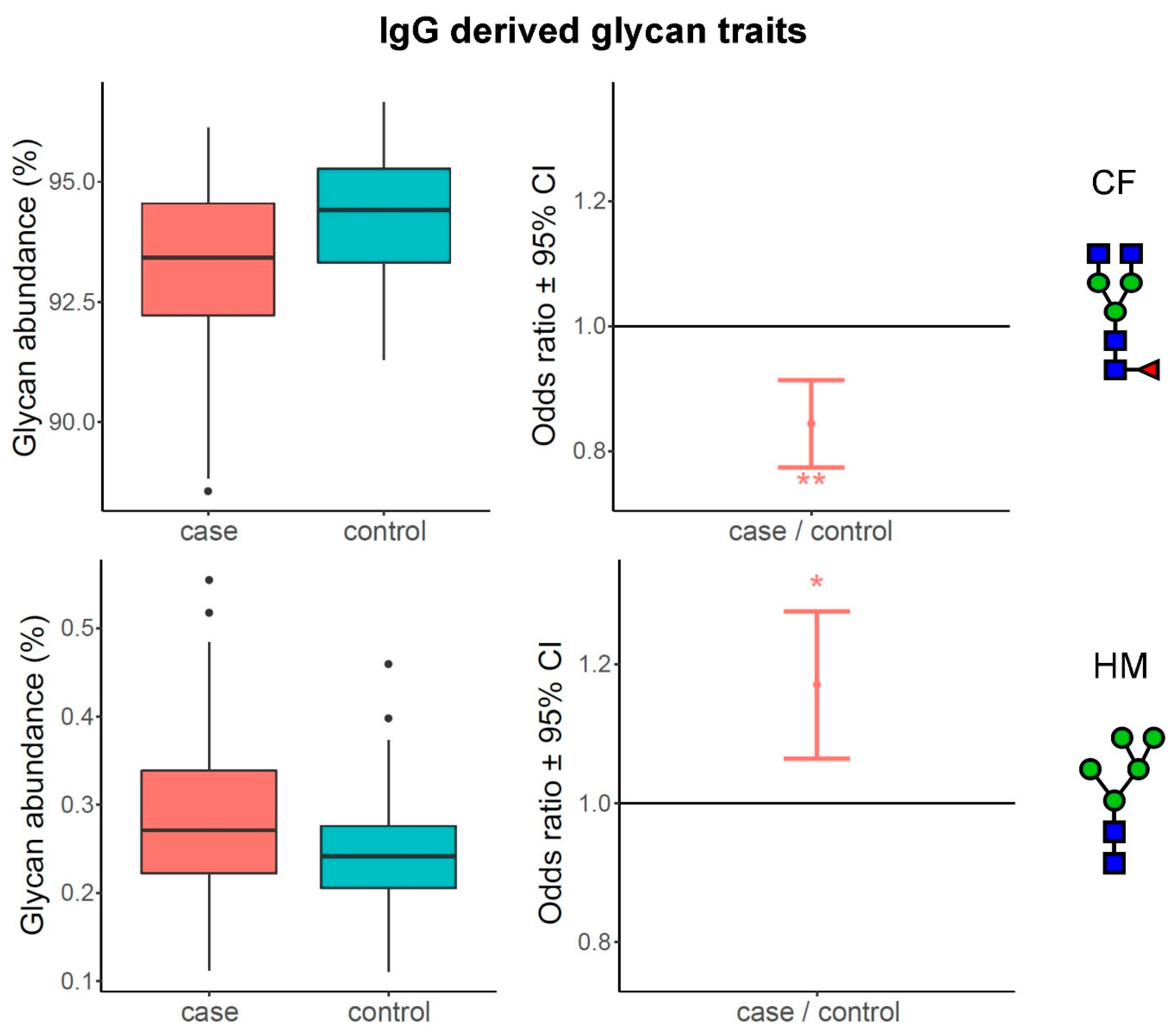
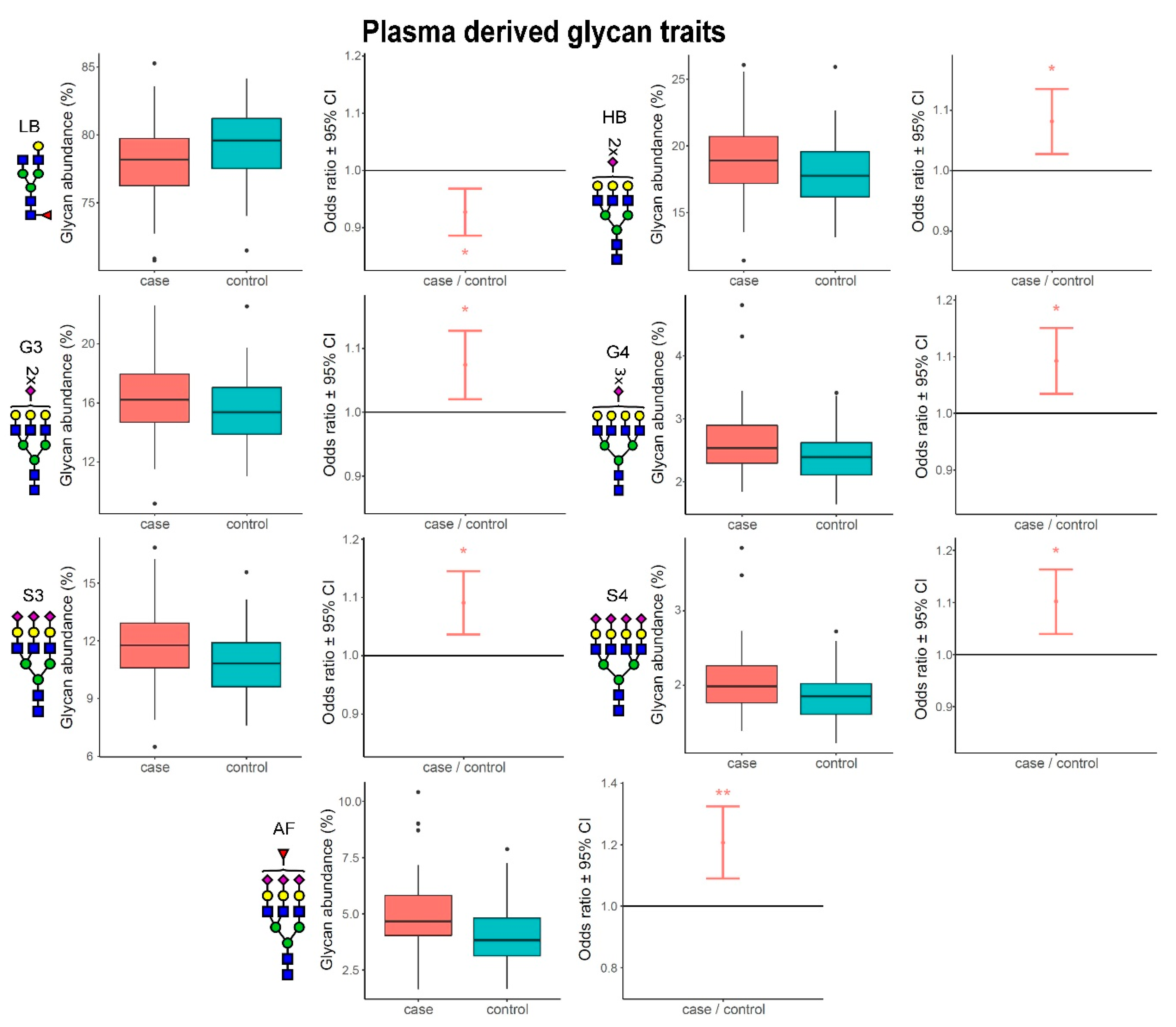
3.1. Alterations of N-Glycome Composition in Multiple Sclerosis
3.2. Complexity of Glycans Associated with Multiple Sclerosis
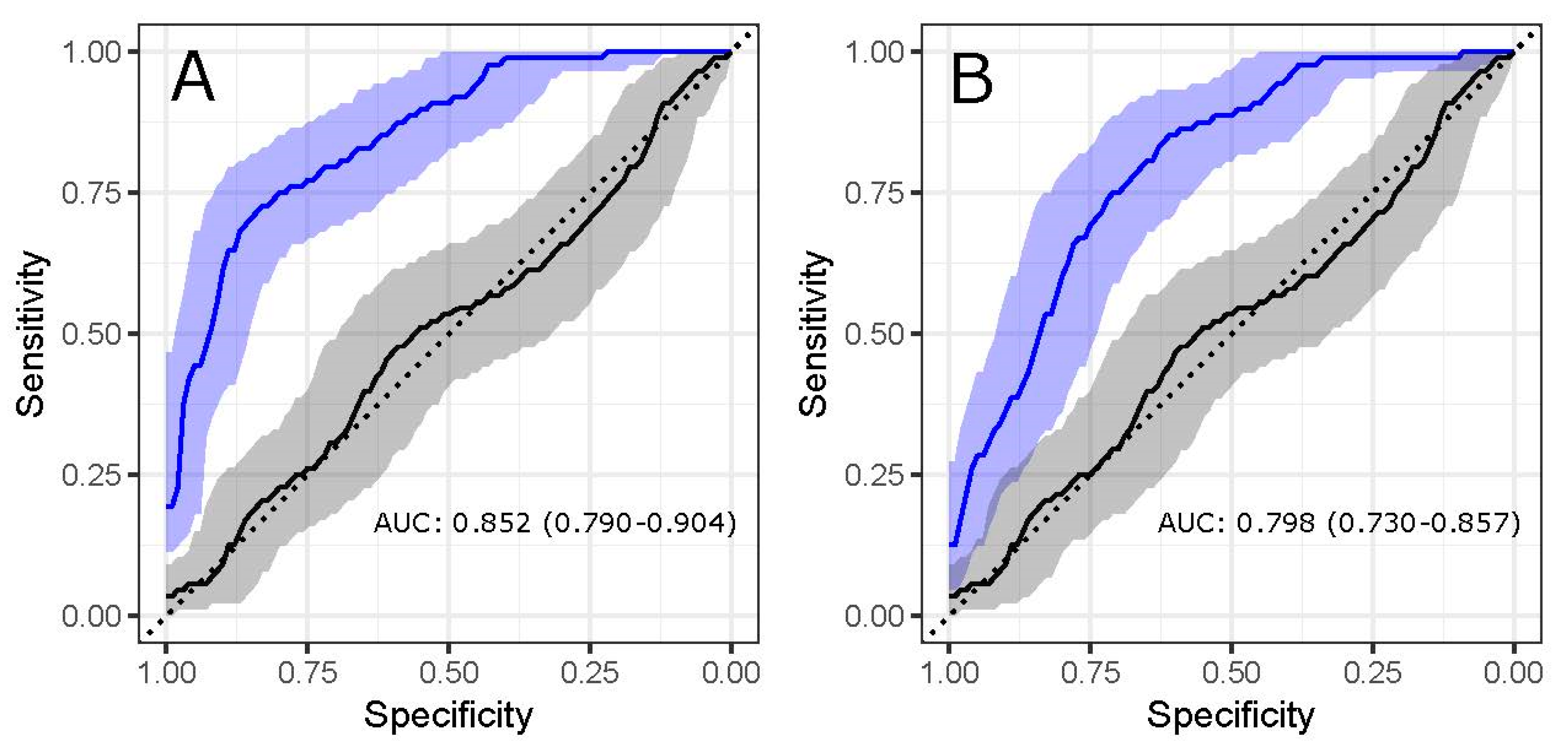
3.3. Glycans Show Value in Disease Status Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Dunn, S.E.; Steinman, L. The Gender Gap in Multiple Sclerosis: Intersection of Science and Society. JAMA Neurol. 2013, 70, 634. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.; Rumrill, P.D. Multiple sclerosis: Etiology, symptoms, incidence and prevalence, and implications for community living and employment. Work 2015, 52, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Cierny, D.; Lehotsky, J.; Hanysova, S.; Michalik, J.; Kantorova, E.; Sivak, S.; Kurca, E.; Dobrota, D.; Jesenska, L. The age at onset in Multiple Sclerosis is associated with patient’s prognosis. Bratisl. Med. J. 2017, 118, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.M.; Wilde, K.; Wilson, J.F.; Yong, K.K.; Counsell, C.E. A new prevalence study of multiple sclerosis in Orkney, Shetland and Aberdeen city. J. Neurol. Neurosurg. Psychiatry 2012, 83, 719–724. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- McWhirter, R.E.; McQuillan, R.; Visser, E.; Counsell, C.; Wilson, J.F. Genome-wide homozygosity and multiple sclerosis in Orkney and Shetland Islanders. Eur. J. Hum. Genet. 2012, 20, 198–202. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.A.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; Freeman, C.; Hunt, S.E.; et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium (IMSGC); Beecham, A.H.; Patsopoulos, N.A.; Xifara, D.K.; Davis, M.F.; Kemppinen, A.; Cotsapas, C.; Shah, T.S.; Spencer, C.; Booth, D.; et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 2013, 45, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Grigorian, A.; Mkhikian, H.; Li, C.F.; Newton, B.L.; Zhou, R.W.; Demetriou, M. Pathogenesis of multiple sclerosis via environmental and genetic dysregulation of N-glycosylation. Semin. Immunopathol. 2012, 34, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Schwab, I.; Lux, A.; Nimmerjahn, F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin. Immunopathol. 2012, 34, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Sakae, Y.; Satoh, T.; Yagi, H.; Yanaka, S.; Yamaguchi, T.; Isoda, Y.; Iida, S.; Okamoto, Y.; Kato, K. Conformational effects of N -glycan core fucosylation of immunoglobulin G Fc region on its interaction with Fcγ receptor IIIa. Sci. Rep. 2017, 7, 13780. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef]
- Lauc, G.; Huffman, J.E.; Pučić, M.; Zgaga, L.; Adamczyk, B.; Mužinić, A.; Novokmet, M.; Polašek, O.; Gornik, O.; Krištić, J.; et al. Loci Associated with N-Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef]
- Baranzini, S.E.; Srinivasan, R.; Khankhanian, P.; Okuda, D.T.; Nelson, S.J.; Matthews, P.M.; Hauser, S.L.; Oksenberg, J.R.; Pelletier, D. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain 2010, 133, 2603–2611. [Google Scholar] [CrossRef]
- Knopf, J.; Magorivska, I.; Maler, J.M.; Spitzer, P.; Bilyy, R.; Biermann, M.H.C.; Hychka, K.; Bondt, A.; Wuhrer, M.; Toes, R.E.M.; et al. Low amounts of bisecting glycans characterize cerebrospinal fluid-borne IgG. J. Neuroimmunol. 2018, 320, 19–24. [Google Scholar] [CrossRef]
- Wuhrer, M.; Selman, M.H.J.; McDonnell, L.A.; Kümpfel, T.; Derfuss, T.; Khademi, M.; Olsson, T.; Hohlfeld, R.; Meinl, E.; Krumbholz, M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflamm. 2015, 12. [Google Scholar] [CrossRef]
- Housley, W.J.; Pitt, D.; Hafler, D.A. Biomarkers in multiple sclerosis. Clin. Immunol. 2015, 161, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Pavić, T.; Dilber, D.; Kifer, D.; Selak, N.; Keser, T.; Ljubičić, Đ.; Vukić Dugac, A.; Lauc, G.; Rumora, L.; Gornik, O. N-glycosylation patterns of plasma proteins and immunoglobulin G in chronic obstructive pulmonary disease. J. Transl. Med. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12. [Google Scholar] [CrossRef]
- Gizaw, S.T.; Ohashi, T.; Tanaka, M.; Hinou, H.; Nishimura, S.-I. Glycoblotting method allows for rapid and efficient glycome profiling of human Alzheimer’s disease brain, serum and cerebrospinal fluid towards potential biomarker discovery. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1716–1727. [Google Scholar] [CrossRef]
- Chen, C.C.; Engelborghs, S.; Dewaele, S.; Le Bastard, N.; Martin, J.-J.; Vanhooren, V.; Libert, C.; De Deyn, P.P. Altered Serum Glycomics in Alzheimer Disease: A Potential Blood Biomarker? Rejuvenation Res. 2010, 13, 439–444. [Google Scholar] [CrossRef]
- Váradi, C.; Nehéz, K.; Hornyák, O.; Viskolcz, B.; Bones, J. Serum N-Glycosylation in Parkinson’s Disease: A Novel Approach for Potential Alterations. Molecules 2019, 24, 2220. [Google Scholar] [CrossRef]
- Sarrats, A.; Saldova, R.; Pla, E.; Fort, E.; Harvey, D.J.; Struwe, W.B.; de Llorens, R.; Rudd, P.M.; Peracaula, R. Glycosylation of liver acute-phase proteins in pancreatic cancer and chronic pancreatitis. Proteom. Clin. Appl. 2010, 4, 432–448. [Google Scholar] [CrossRef]
- Wright, R.D.; Cooper, D. Glycobiology of leukocyte trafficking in inflammation. Glycobiology 2014, 24, 1242–1251. [Google Scholar] [CrossRef]
- Steinman, M.D.L. Multiple Sclerosis: A Coordinated Immunological Attack against Myelin in the Central Nervous System. Cell 1996, 85, 299–302. [Google Scholar] [CrossRef]
- Elovaara, I.; Ukkonen, M.; Leppäkynnäs, M.; Lehtimäki, T.; Luomala, M.; Peltola, J.; Dastidar, P. Adhesion Molecules in Multiple Sclerosis: Relation to Subtypes of Disease and Methylprednisolone Therapy. Arch. Neurol. 2000, 57, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Dore-Duffy, P.; Newman, W.; Balabanov, R.; Lisak, R.P.; Mainolfi, E.; Rothlein, R.; Peterson, M. Circulating, soluble adhesion proteins in cerebrospinal fluid and serum of patients with multiple sclerosis: Correlation with clinical activity. Ann. Neurol. 1995, 37, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Diamond, B. General Features of Autoimmune Disease. In The Autoimmune Diseases, 5th ed.; Rose, N.R., Mackay, I.R., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 19–37. ISBN 978-0-12-384929-8. [Google Scholar]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Wormald, M.R.; Rudd, P.M.; Fischer, P.B.; Dwek, R.A.; Sim, R.B. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1995, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.Y.; Vaida, F.; Augst, R.M.; Yu, D.Y.; Singh, K.K. Mannose Binding Lectin Mediated Complement Pathway in Multiple Sclerosis. J. Neuroimmunol. 2011, 239, 98–100. [Google Scholar] [CrossRef][Green Version]
- Watkins, L.M.; Neal, J.W.; Loveless, S.; Michailidou, I.; Ramaglia, V.; Rees, M.I.; Reynolds, R.; Robertson, N.P.; Morgan, B.P.; Howell, O.W. Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J. Neuroinflamm. 2016, 13. [Google Scholar] [CrossRef]
- Olesen, M.N.; Soelberg, K.; Debrabant, B.; Nilsson, A.C.; Lillevang, S.T.; Grauslund, J.; Brandslund, I.; Madsen, J.S.; Paul, F.; Smith, T.J.; et al. Cerebrospinal fluid biomarkers for predicting development of multiple sclerosis in acute optic neuritis: A population-based prospective cohort study. J. Neuroinflamm. 2019, 16. [Google Scholar] [CrossRef]
| Case | Control | p-Value | |
|---|---|---|---|
| Participants, N | 83 | 88 | |
| Sex, Nfemale (%) | 58 (70) | 62 (70) | 1.000 |
| Age, median (min-max) | 52 (29–77) | 50 (28–77) | 0.830 |
| Type of multiple sclerosis, N (%) | |||
| Relapsing remitting | 31 (37) | ||
| Secondary progressive with relapses | 25 (30) | ||
| Secondary progressive without relapses | 15 (18) | ||
| Primary progressive | 12 (14) | ||
| Expanded Disability Status Scale, median (IQR) | |||
| Relapsing remitting | 2.0 (1.0–4.5) | ||
| Secondary progressive with relapses | 6.0 (5.0–8.0) | ||
| Secondary progressive without relapses | 6.5 (6.0–7.5) | ||
| Primary progressive | 6.0 (6.0–7.5) | ||
| Sample | Glycan Peak | Glycan Structure | Odds Ratio | 95% CI | p-Value | Adjusted p-Value |
|---|---|---|---|---|---|---|
| IgG | IgGP5 | M5 | 1.17 | 1.06–1.28 | 7.48 × 10−4 | 1.48 × 10−2 |
| IgG | IgGP9 | FA2[3]G1 | 0.92 | 0.88–0.96 | 6.38 × 10−4 | 1.48 × 10−2 |
| IgG | IgGP17 | FA2[3]BG1S1, A2G2S1 | 1.16 | 1.05–1.27 | 1.95 × 10−3 | 3.21 × 10−2 |
| IgG | IgGP21 | A2G2S2 | 1.24 | 1.09–1.38 | 3.78 × 10−4 | 1.48 × 10−2 |
| Sample | Glycan Peak | Glycan Structure | Odds Ratio | 95% CI | p-Value | Adjusted p-Value |
| plasma | GP4 | FA2[6]G1 | 0.90 | 0.84–0.97 | 4.19 × 10−3 | 3.57 × 10−2 |
| plasma | GP5 | FA2[3]G1 | 0.86 | 0.79–0.94 | 1.37 × 10−3 | 1.87 × 10−2 |
| plasma | GP10 | FA2G2 | 0.88 | 0.82–0.95 | 1.04 × 10−3 | 1.86 × 10−2 |
| plasma | GP27 | A3F1G3S2 | 1.18 | 1.06–1.31 | 1.67 × 10−3 | 1.93 × 10−2 |
| plasma | GP32 | A3G3S3 | 1.13 | 1.04–1.21 | 1.42 × 10−3 | 1.87 × 10−2 |
| plasma | GP33 | A3F1G3S3 | 1.20 | 1.08–1.33 | 6.12 × 10−4 | 1.66 × 10−2 |
| plasma | GP35 | FA3F1G3S3, A4F1G4S3 | 1.23 | 1.11–1.34 | 1.94 × 10−5 | 1.57 × 10−3 |
| plasma | GP38 | A4G4S4, A4F1G4S4 | 1.08 | 1.02–1.14 | 4.14 × 10−3 | 3.57 × 10−2 |
| plasma | GP39 | A4F1G4S4, A4F2G4S4 | 1.18 | 1.08–1.27 | 1.50 × 10−4 | 6.78 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvetko, A.; Kifer, D.; Gornik, O.; Klarić, L.; Visser, E.; Lauc, G.; Wilson, J.F.; Štambuk, T. Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential. Biomedicines 2020, 8, 410. https://doi.org/10.3390/biomedicines8100410
Cvetko A, Kifer D, Gornik O, Klarić L, Visser E, Lauc G, Wilson JF, Štambuk T. Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential. Biomedicines. 2020; 8(10):410. https://doi.org/10.3390/biomedicines8100410
Chicago/Turabian StyleCvetko, Ana, Domagoj Kifer, Olga Gornik, Lucija Klarić, Elizabeth Visser, Gordan Lauc, James F. Wilson, and Tamara Štambuk. 2020. "Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential" Biomedicines 8, no. 10: 410. https://doi.org/10.3390/biomedicines8100410
APA StyleCvetko, A., Kifer, D., Gornik, O., Klarić, L., Visser, E., Lauc, G., Wilson, J. F., & Štambuk, T. (2020). Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential. Biomedicines, 8(10), 410. https://doi.org/10.3390/biomedicines8100410