Noncanonical Constitutive Androstane Receptor Signaling in Gene Regulation
Abstract
1. Introduction
2. Activation of CAR Nuclear Translocation
3. Canonical CAR Pathway
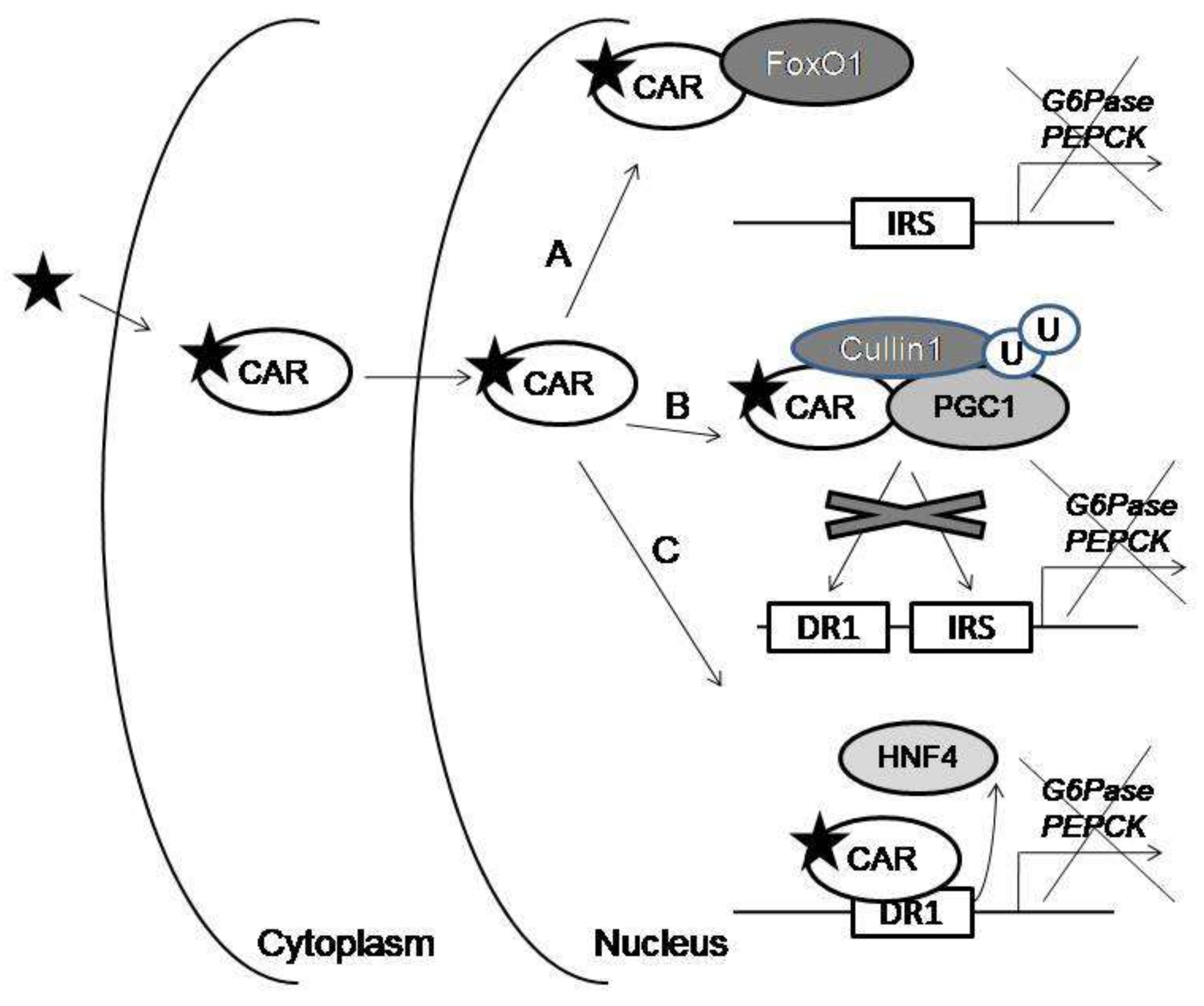
4. Noncanonical CAR Signaling in Gluconeogenic Gene Regulation
5. Noncanonical CAR Signaling in Hepatocyte Proliferation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, S.P.; Cidlowski, J.A.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br. J. Pharmacol. 2017, 174, S208–S224. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, S.; Yamazaki, Y.; Takizawa, D.; Negishi, M. New insights on the xenobiotic-sensing nuclear receptors in liver diseases--CAR and PXR--. Curr. Drug Metab. 2008, 9, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Buchman, C.D.; Chai, S.C.; Chen, T. A current structural perspective on PXR and CAR in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2018, 14, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Busby, S.A.; Wisecarver, S.; Vincent, J.; Griffin, P.R.; Fernandez, E.J. Helix 11 dynamics is critical for constitutive androstane receptor activity. Structure 2011, 19, 37–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, R.X.; Lambert, M.H.; Wisely, B.B.; Warren, E.N.; Weinert, E.E.; Waitt, G.M.; Williams, J.D.; Collins, J.L.; Moore, L.B.; Willson, T.M.; et al. A structural basis for constitutive activity in the human CAR/RXRalpha heterodimer. Mol. Cell. 2004, 16, 919–928. [Google Scholar] [CrossRef]
- Li, H.; Wang, H. Activation of xenobiotic receptors: Driving into the nucleus. Expert Opin. Drug Metab. Toxicol. 2010, 6, 409–426. [Google Scholar] [CrossRef]
- Molnár, F.; Küblbeck, J.; Jyrkkärinne, J.; Prantner, V.; Honkakoski, P. An update on the constitutive androstane receptor (CAR). Drug Metabol. Drug Interact. 2013, 28, 79–93. [Google Scholar] [CrossRef]
- Mutoh, S.; Sobhany, M.; Moore, R.; Perera, L.; Pedersen, L.; Sueyoshi, T.; Negishi, M. Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci. Signal. 2013, 6, ra31. [Google Scholar] [CrossRef]
- Honkakoski, P.; Zelko, I.; Sueyoshi, T.; Negishi, M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 1998, 18, 5652–5658. [Google Scholar] [CrossRef]
- Kawamoto, T.; Sueyoshi, T.; Zelko, I.; Moore, R.; Washburn, K.; Negishi, M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 1999, 19, 6318–6322. [Google Scholar] [CrossRef]
- Urquhart, B.L.; Tirona, R.G.; Kim, R.B. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: Implications for interindividual variability in response to drugs. J. Clin. Pharmacol. 2007, 47, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.Y.; Klaassen, C.D. RNA-Seq reveals common and unique PXR- and CAR-target gene signatures in the mouse liver transcriptome. Biochim. Biophys. Acta 2016, 1859, 1198–1217. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xie, W. A brief history of the discovery of PXR and CAR as xenobiotic receptors. Acta Pharm. Sin. B 2016, 6, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Wu, X.; Yang, X.; Zhang, Y.; Dai, Y.; Zhang, Y.; Wu, X.; Liu, Y.; Cui, X.; Jin, H.; et al. The Therapeutic Role of Xenobiotic Nuclear Receptors against Metabolic Syndrome. Curr. Drug Metab. 2019, 20, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Huang, H.; Hoffman, B.; Liebermann, D.A.; Ledda-Columbano, G.M.; Columbano, A.; Locker, J. Gadd45β is an inducible coactivator of transcription that facilitates rapid liver growth in mice. J. Clin. Investig. 2011, 121, 4491–4502. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.; Negishi, M. Phenobarbital-elicited activation of nuclear receptor CAR in induction of cytochrome P450 genes. Biochem. Biophys. Res. Commun. 2000, 277, 1–6. [Google Scholar] [CrossRef]
- Honkakoski, P.; Sueyoshi, T.; Negishi, M. Drug-activated nuclear receptors CAR and PXR. Ann. Med. 2003, 35, 172–182. [Google Scholar] [CrossRef]
- Carazo, A.; Dusek, J.; Holas, O.; Skoda, J.; Hyrsova, L.; Smutny, T.; Soukup, T.; Dosedel, M.; Pávek, P. Teriflunomide Is an Indirect Human Constitutive Androstane Receptor (CAR) Activator Interacting With Epidermal Growth Factor (EGF) Signaling. Front Pharmacol. 2018, 9, 993. [Google Scholar] [CrossRef]
- Mackowiak, B.; Wang, H. Mechanisms of xenobiotic receptor activation: Direct vs. indirect. Biochim. Biophys. Acta 2016, 1859, 1130–1140. [Google Scholar] [CrossRef]
- Zelko, I.; Sueyoshi, T.; Kawamoto, T.; Moore, R.; Negishi, M. The peptide near the C terminus regulates receptor CAR nuclear translocation induced by xenochemicals in mouse liver. Mol. Cell. Biol. 2001, 21, 2838–2846. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, K.; Kobayashi, K.; Moore, R.; Kawamoto, T.; Negish, I.M. Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett. 2003, 548, 17–20. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sueyoshi, T.; Inoue, K.; Moore, R.; Negishi, M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol. Pharmacol. 2003, 64, 1069–1075. [Google Scholar] [CrossRef]
- Sueyoshi, T.; Moore, R.; Sugatani, J.; Matsumura, Y.; Negishi, M. PPP1R16A, the membrane subunit of protein phosphatase 1beta, signals nuclear translocation of the nuclear receptor constitutive active/androstane receptor. Mol. Pharmacol. 2008, 73, 1113–1121. [Google Scholar] [CrossRef]
- Timsit, Y.E.; Negishi, M. CAR and PXR: The xenobiotic-sensing receptors. Steroids 2007, 72, 231–246. [Google Scholar] [CrossRef]
- Koike, C.; Moore, R.; Negishi, M. Localization of the nuclear receptor CAR at the cell membrane of mouse liver. FEBS Lett. 2005, 579, 6733–6736. [Google Scholar] [CrossRef]
- Mutoh, S.; Osabe, M.; Inoue, K.; Moore, R.; Pedersen, L.; Perera, L.; Rebolloso, Y.; Sueyoshi, T.; Negishi, M. Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR1I3). J. Biol. Chem. 2009, 284, 34785–34792. [Google Scholar] [CrossRef]
- Trottier, E.; Belzil, A.; Stoltz, C.; Anderson, A. Localization of a phenobarbital-responsive element (PBRE) in the 5′-flanking region of the rat CYP2B2 gene. Gene 1995, 158, 263–268. [Google Scholar] [CrossRef]
- Honkakoski, P.; Negishi, M. Characterization of a phenobarbital-responsive enhancer module in mouse P450 Cyp2b10 gene. J. Biol. Chem. 1997, 272, 14943–14949. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, T.; Kawamoto, T.; Zelko, I.; Honkakoski, P.; Negishi, M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J. Biol. Chem. 1999, 274, 6043–6046. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, T.; Negishi, M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Faucette, S.; Sueyoshi, T.; Moore, R.; Ferguson, S.; Negishi, M.; LeCluyse, E.L. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J. Biol. Chem. 2003, 278, 14146–14152. [Google Scholar] [CrossRef]
- Muangmoonchai, R.; Smirlis, D.; Wong, S.C.; Edwards, M.; Phillips, I.R.; Shephard, E.A. Xenobiotic induction of cytochrome P450 2B1 (CYP2B1) is mediated by the orphan nuclear receptor constitutive androstane receptor (CAR) and requires steroid co-activator 1 (SRC-1) and the transcription factor Sp1. Biochem. J. 2001, 355, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Min, G.; Kemper, J.K.; Kemper, B. Glucocorticoid receptor-interacting protein 1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor in vivo. J. Biol. Chem. 2002, 277, 26356–26363. [Google Scholar] [CrossRef]
- Shiraki, T.; Sakai, N.; Kanaya, E.; Jingami, H. Activation of orphan nuclear constitutive androstane receptor requires subnuclear targeting by peroxisome proliferator-activated receptor gamma coactivator-1 alpha. A possible link between xenobiotic response and nutritional state. J. Biol. Chem. 2003, 278, 11344–11350. [Google Scholar] [CrossRef]
- Xia, J.; Kemper, B. Structural determinants of constitutive androstane receptor required for its glucocorticoid receptor interacting protein-1-mediated nuclear accumulation. J. Biol. Chem. 2005, 280, 7285–7293. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, J.; Kojima, H.; Ueda, A.; Kakizaki, S.; Yoshinari, K.; Gong, Q.H.; Owens, I.S.; Negishi, M.; Sueyoshi, T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology 2001, 33, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Lahtela, J.T.; Arranto, A.J.; Sotaniemi, E.A. Enzyme inducers improve insulin sensitivity in non-insulin-dependent diabetic subjects. Diabetes 1985, 34, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Hamadeh, H.K.; Webb, H.K.; Yamamoto, Y.; Sueyoshi, T.; Afshari, C.A.; Lehmann, J.M.; Negishi, M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002, 61, 1–6. [Google Scholar] [CrossRef]
- Dong, B.; Saha, P.K.; Huang, W.; Chen, W.; Abu-Elheiga, L.A.; Wakil, S.J.; Stevens, R.D.; Ilkayeva, O.; Newgard, C.B.; Chan, L.; et al. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc. Natl. Acad. Sci. USA 2009, 106, 18831–18836. [Google Scholar] [CrossRef]
- Gao, J.; He, J.; Zhai, Y.; Wada, T.; Xie, W. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J. Biol. Chem. 2009, 284, 25984–25992. [Google Scholar] [CrossRef]
- Masuyama, H.; Hiramatsu, Y. Treatment with a constitutive androstane receptor ligand ameliorates the signs of preeclampsia in high-fat diet-induced obese pregnant mice. Mol. Cell. Endocrinol. 2012, 348, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Daitoku, H.; Hatta, M.; Tanaka, K.; Fukamizu, A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. USA 2003, 100, 11285–11290. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Koike, C.; Negishi, M.; Yamamoto, Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 2004, 24, 7931–7940. [Google Scholar] [CrossRef] [PubMed]
- Kazantseva, Y.A.; Yarushkin, A.A.; Pustylnyak, V.O. CAR-mediated repression of Foxo1 transcriptional activity regulates the cell cycle inhibitor p21 in mouse livers. Toxicology 2014, 321, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Puigserver, P.; Chen, G.; Donovan, J.; Wu, Z.; Rhee, J.; Adelmant, G.; Stafford, J.; Kahn, C.R.; Granner, D.K.; et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001, 413, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef]
- Gao, J.; Yan, J.; Xu, M.; Ren, S.; Xie, W. CAR Suppresses Hepatic Gluconeogenesis by Facilitating the Ubiquitination and Degradation of PGC1α. Mol. Endocrinol. 2015, 29, 1558–1570. [Google Scholar] [CrossRef]
- Odom, D.T.; Zizlsperger, N.; Gordon, D.B.; Bell, G.W.; Rinaldi, N.J.; Murray, H.L.; Volkert, T.L.; Schreiber, J.; Rolfe, P.A.; Gifford, D.K.; et al. Control of pancreas and liver gene expression by HNF transcription factors. Science 2004, 303, 1378–1381. [Google Scholar] [CrossRef]
- Miao, J.; Fang, S.; Bae, Y.; Kemper, J.K. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J. Biol. Chem. 2006, 281, 14537–14546. [Google Scholar] [CrossRef]
- Kachaylo, E.M.; Yarushkin, A.A.; Pustylnyak, V.O. Constitutive androstane receptor activation by 2,4,6-triphenyldioxane-1,3 suppresses the expression of the gluconeogenic genes. Eur. J. Pharmacol. 2012, 679, 139–143. [Google Scholar] [CrossRef]
- Yarushkin, A.A.; Kachaylo, E.M.; Pustylnyak, V.O. The constitutive androstane receptor activator 4-[(4R,6R)-4,6-diphenyl-1,3-dioxan-2-yl]-N,N-dimethylaniline inhibits the gluconeogenic genes PEPCK and G6Pase through the suppression of HNF4α and FOXO1 transcriptional activity. Br. J. Pharmacol. 2013, 168, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Lukowicz, C.; Ellero-Simatos, S.; Régnier, M.; Oliviero, F.; Lasserre, F.; Polizzi, A.; Montagner, A.; Smati, S.; Boudou, F.; Lenfant, F.; et al. Dimorphic metabolic and endocrine disorders in mice lacking the constitutive androstane receptor. Sci. Rep. 2019, 9, 20169. [Google Scholar] [CrossRef] [PubMed]
- Ledda-Columbano, G.M.; Pibiri, M.; Concas, D.; Molotzu, F.; Simbula, G.; Cossu, C.; Columbano, A. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis 2003, 24, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Columbano, A.; Simbula, M.; Pibiri, M.; Perra, A.; Pisanu, A.; Uccheddu, A.; Ledda-Columbano, G.M. Potential utility of xenobiotic mitogens in the context of liver regeneration in the elderly and living-related transplantation. Lab. Investig. 2008, 88, 408–415. [Google Scholar] [CrossRef]
- Kazantseva, Y.A.; Pustylnyak, Y.A.; Pustylnyak, V.O. Role of Nuclear Constitutive Androstane Receptor in Regulation of Hepatocyte Proliferation and Hepatocarcinogenesis. Biochemistry (Mosc) 2016, 81, 338–347. [Google Scholar] [CrossRef]
- Costa, R.H.; Kalinichenko, V.V.; Tan, Y.; Wang, I.C. The CAR nuclear receptor and hepatocyte proliferation. Hepatology 2005, 42, 1004–1008. [Google Scholar] [CrossRef]
- Tschuor, C.; Kachaylo, E.; Limani, P.; Raptis, D.A.; Linecker, M.; Tian, Y.; Herrmann, U.; Grabliauskaite, K.; Weber, A.; Columbano, A.; et al. Constitutive androstane receptor (Car)-driven regeneration protects liver from failure following tissue loss. J. Hepatol. 2016, 65, 66–74. [Google Scholar] [CrossRef]
- Blanco-Bose, W.E.; Murphy, M.J.; Ehninger, A.; Offner, S.; Dubey, C.; Huang, W.; Moore, D.D.; Trumpp, A. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology 2008, 48, 1302–1311. [Google Scholar] [CrossRef]
- Shizu, R.; Shindo, S.; Yoshida, T.; Numazawa, S. MicroRNA-122 down-regulation is involved in phenobarbital-mediated activation of the constitutive androstane receptor. PLoS ONE 2012, 7, e41291. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Nakao, K.; Miyaaki, H.; Ichikawa, T. Antitumor function of microRNA-122 against hepatocellular carcinoma. J. Gastroenterol. 2014, 49, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hsu, S.H.; Wang, X.; Kutay, H.; Bid, H.K.; Yu, J.; Ganju, R.K.; Jacob, S.T.; Yuneva, M.; Ghoshal, K. Reciprocal regulation of microRNA-122 and c-Myc in hepatocellular cancer: Role of E2F1 and transcription factor dimerization partner 2. Hepatology 2014, 59, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, J.H.; Xiao, Z.D.; Zhang, Q.Q.; Chen, Y.Q.; Zhou, H.; Qu, L.H. Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology 2010, 52, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Xi, Y.; Zhu, W.N.; Zeng, C.; Zhang, Z.Q.; Guo, Z.C.; Hao, D.L.; Liu, G.; Feng, L.; Chen, H.Z.; et al. Positive regulation of hepatic miR-122 expression by HNF4α. J. Hepatol. 2011, 55, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Kazantseva, Y.A.; Yarushkin, A.A.; Mostovich, L.A.; Pustylnyak, Y.A.; Pustylnyak, V.O. Xenosensor CAR mediates down-regulation of miR-122 and up-regulation of miR-122 targets in the liver. Toxicol. Appl. Pharmacol. 2015, 288, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yarushkin, A.A.; Mazin, M.E.; Pustylnyak, Y.A.; Prokopyeva, E.A.; Pustylnyak, V.O. Promotion of liver growth by CAR is accompanied by Akt pathway activation and FoxM1-Nedd4-mediated repression of PTEN. Arch. Biochem. Biophys. 2019, 672, 108065. [Google Scholar] [CrossRef]
- Yarushkin, A.A.; Mazin, M.E.; Yunusova, A.Y.; Korchagina, K.V.; Pustylnyak, Y.A.; Prokopyeva, E.A.; Pustylnyak, V.O. CAR-mediated repression of Cdkn1a(p21) is accompanied by the Akt activation. Biochem. Biophys. Res. Commun. 2018, 504, 361–366. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Moore, R.; Goldsworthy, T.L.; Negishi, M.; Maronpot, R.R. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004, 64, 7197–7200. [Google Scholar] [CrossRef]
- Dong, B.; Lee, J.S.; Park, Y.Y.; Yang, F.; Xu, G.; Huang, W.; Finegold, M.J.; Moore, D.D. Activating CAR and β-catenin induces uncontrolled liver growth and tumorigenesis. Nat. Commun. 2015, 6, 5944. [Google Scholar] [CrossRef]
- Sidaway, J.E.; Orton, T.C.; Kalaitzi, K.; Jones, H.B.; Foster, A.; Lake, B.G. Analysis of β-catenin gene mutations and gene expression in liver tumours of C57BL/10J mice produced by chronic administration of sodium phenobarbital. Toxicology 2020, 430, 152343. [Google Scholar] [CrossRef]
- Yarushkin, A.A.; Mazin, M.E.; Pustylnyak, Y.A.; Prokopyeva, E.A.; Pustylnyak, V.O. Activation of the Akt pathway by a constitutive androstane receptor agonist results in β-catenin activation. Eur. J. Pharmacol. 2020, 879, 173135. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, K. Role of Nuclear Receptors PXR and CAR in Xenobiotic-Induced Hepatocyte Proliferation and Chemical Carcinogenesis. Biol. Pharm. Bull. 2019, 42, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Shizu, R.; Yoshinari, K. Nuclear receptor CAR-mediated liver cancer and its species differences. Expert Opin. Drug Metab. Toxicol. 2020, 16, 343–351. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pustylnyak, Y.A.; Gulyaeva, L.F.; Pustylnyak, V.O. Noncanonical Constitutive Androstane Receptor Signaling in Gene Regulation. Int. J. Mol. Sci. 2020, 21, 6735. https://doi.org/10.3390/ijms21186735
Pustylnyak YA, Gulyaeva LF, Pustylnyak VO. Noncanonical Constitutive Androstane Receptor Signaling in Gene Regulation. International Journal of Molecular Sciences. 2020; 21(18):6735. https://doi.org/10.3390/ijms21186735
Chicago/Turabian StylePustylnyak, Yuliya A., Lyudmila F. Gulyaeva, and Vladimir O. Pustylnyak. 2020. "Noncanonical Constitutive Androstane Receptor Signaling in Gene Regulation" International Journal of Molecular Sciences 21, no. 18: 6735. https://doi.org/10.3390/ijms21186735
APA StylePustylnyak, Y. A., Gulyaeva, L. F., & Pustylnyak, V. O. (2020). Noncanonical Constitutive Androstane Receptor Signaling in Gene Regulation. International Journal of Molecular Sciences, 21(18), 6735. https://doi.org/10.3390/ijms21186735





