Can miRNAs Be Considered as Diagnostic and Therapeutic Molecules in Ischemic Stroke Pathogenesis?—Current Status
Abstract
:1. Introduction
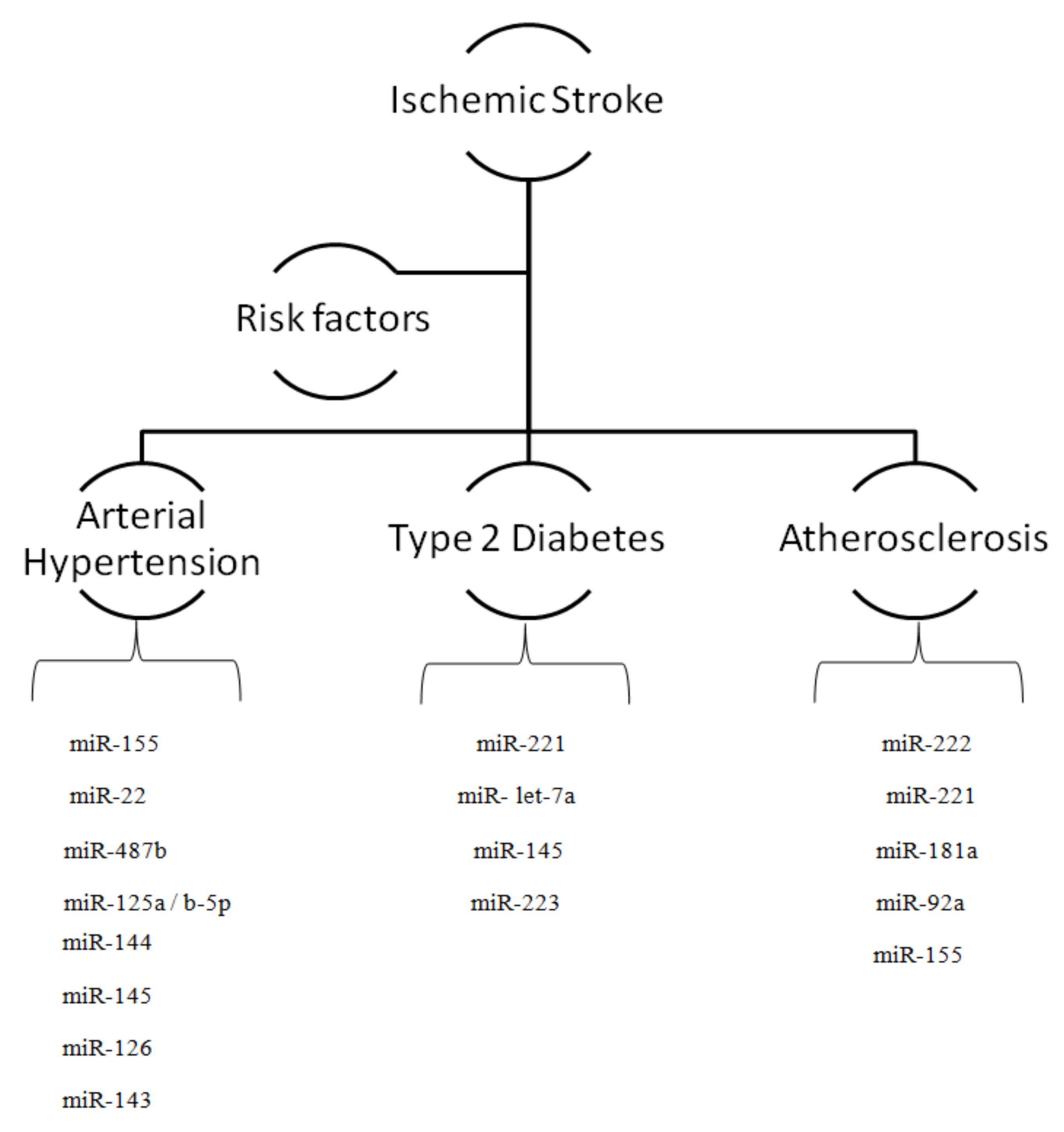
2. MicroRNAs and Risk Factors for Ischemic Stroke
2.1. MicroRNAs and Arterial Hypertension
2.2. MicroRNAs and Diabetes
2.3. MicroRNAs and Atherosclerosis
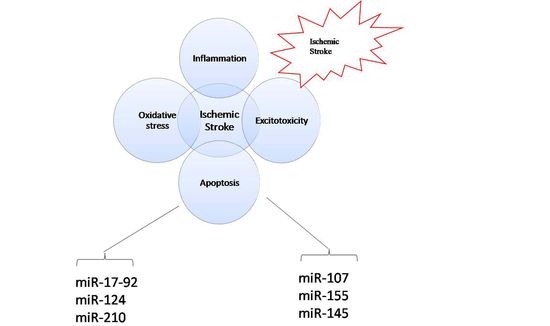
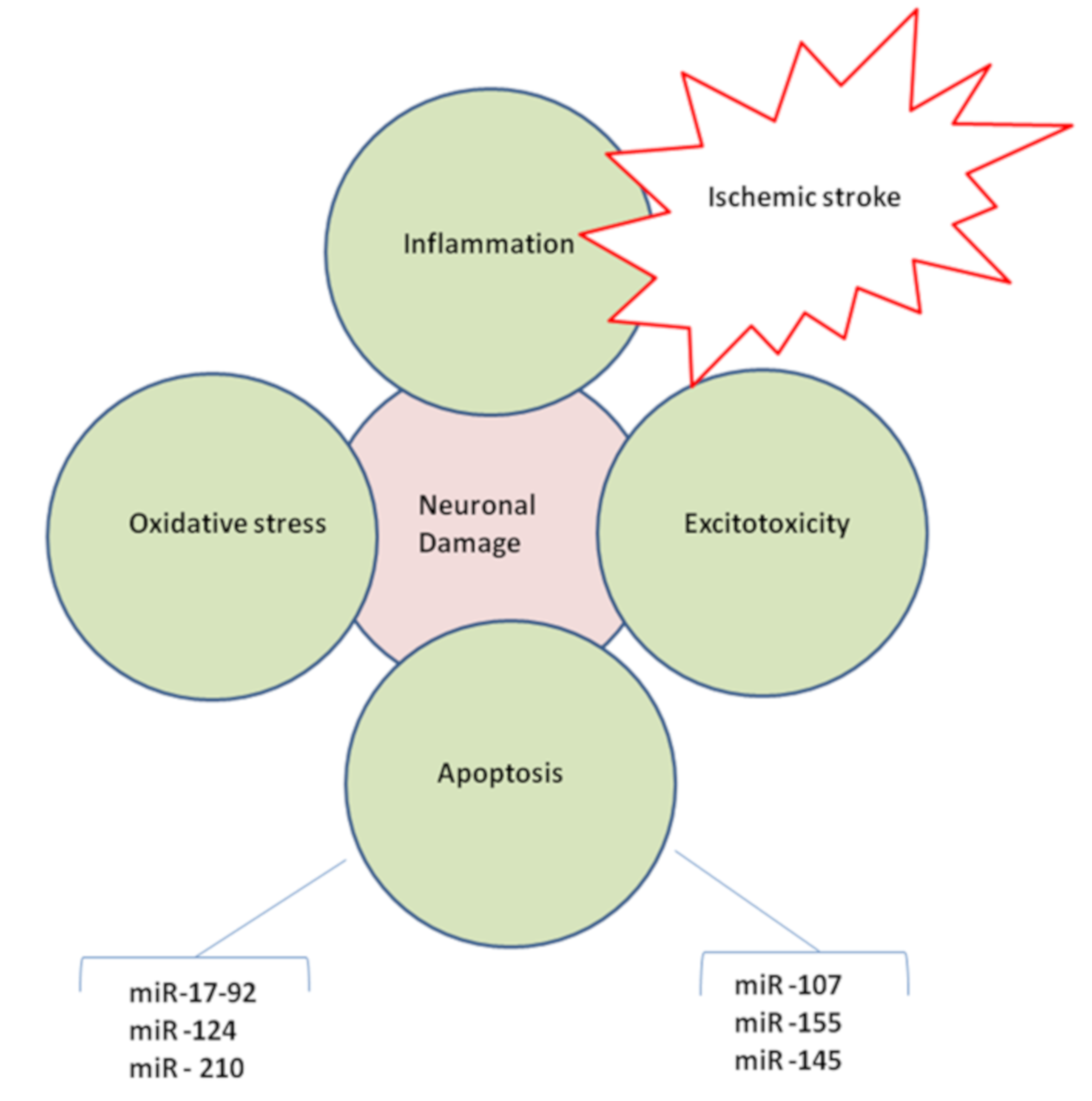
2.4. MicroRNAs in Brain Injury
2.5. MicroRNAs and Ischemic Excitotoxicity
2.6. MicroRNAs and Oxidative Stress
2.7. MicroRNAs and Alzheimer’s Disease
2.8. MicroRNAs and Inflammation
2.9. MicroRNAs, BBB Disruption, and Edema
2.10. MicroRNAs and Neuronal Death
2.11. MicroRNAs in Neurogenesis
2.12. MicroRNAs in Angiogenesis
3. MiRNA Profiling and Ischemic Stroke
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| (q:d)PCR | (quantitative, digital) PCR |
| AQP | aquaporin |
| Bcl | B-cell lymphoma |
| CAM | cellular adhesion molecule |
| CRP | C-reactive protein |
| ECM | extracellular matrix |
| HIF 1 | hypoxia-inducible factor 1 |
| ICAM-1 | intercellular adhesion molecule 1 |
| IGF-I | anti-apoptotic insulin receptor 1 |
| IGFR1 | insulin-like growth factor 1 |
| IS | ischemic stroke |
| LDL | low-density lipoprotein |
| MCAO | medial cerebral artery occlusion |
| Mcl-1 | myeloid cell leukemia 1 |
| miRNA | micro RNA |
| MMP | metalloproteinases |
| mRNA | messenger RNA |
| NF-κB | nuclear factor kappa B |
| NGS | Next Generation Sequencing |
| NMDA | N-Methyl-D-aspartic acid |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| PDGF | platelet-derived growth factor |
| ROS | reactive oxygen species |
| SHR | spontaneously hypertensive rat |
| SMC | smooth muscle cell |
| STAT3 | signal transducer and activator of transcription 2 |
| TNFα | tumor necrosis factor α |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| tPA | tissue plasminogen activator |
| PGC-1α | proliferator-activated receptor gamma coactivator 1-alpha |
| STAT-5 | signal transducer and activator of transcription 5 |
| FGF-2 | fibroblast growth factor 2 |
| PDGF | platelet-derived growth factor |
References
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, e121–e122. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Chopp, M. Function of neural stem cells in ischemic brain repair processes. J. Cereb. Blood Flow Metab. 2016, 36, 2034–2043. [Google Scholar] [CrossRef]
- Béjot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. La Presse Médicale 2016, 45, e391–e398. [Google Scholar]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Ekker, M.S.; Boot, E.M.; Singhal, A.B.; Tan, K.S.; Debette, S.; Tuladhar, A.M.; de Leeuw, F.-E. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018, 17, 790–801. [Google Scholar] [CrossRef]
- Davis, B.R.; Vogt, T.; Frost, P.H.; Burlando, A.; Cohen, J.; Wilson, A.; Brass, L.M.; Frishman, W.; Price, T.; Stamler, J. Risk factors for stroke and type of stroke in persons with isolated systolic hypertension. Stroke 1998, 29, 1333–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanizaki, Y.; Kiyohara, Y.; Kato, I.; Iwamoto, H.; Nakayama, K.; Shinohara, N.; Arima, H.; Tanaka, K.; Ibayashi, S.; Fujishima, M. Incidence and risk factors for subtypes of cerebral infarction in a general population: The Hisayama study. Stroke 2000, 31, 2616–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iso, H.; Rexrode, K.; Hennekens, C.H.; Manson, J.E. Application of computer tomography-oriented criteria for stroke subtype classification in a prospective study. Ann. Epidemiol. 2000, 10, 81–87. [Google Scholar] [CrossRef]
- Ohira, T.; Shahar, E.; Chambless, L.E.; Rosamond, W.D.; Mosley, T.H., Jr.; Folsom, A.R. Risk factors for ischemic stroke subtypes: The Atherosclerosis Risk in Communities study. Stroke 2006, 37, 2493–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, Z.N.; Hussein, M.Q.; Haji, G.F. Hypertension as a risk factor: Is it different in ischemic stroke and acute myocardial infarction comparative cross-sectional study? Int. J. Hypertens. 2011, 2011, 701029. [Google Scholar] [CrossRef] [Green Version]
- Petty, G.W.; Brown, R.D., Jr.; Whisnant, J.P.; Sicks, J.D.; O’Fallon, W.M.; Wiebers, D.O. Ischemic stroke subtypes: A population-based study of functional outcome, survival, and recurrence. Stroke 2000, 31, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, K.R.; Tamariz, J.; Desai, K.M.; Brand, F.J.; Liu, A.; Saul, I.; Bhattacharya, S.K.; Pileggi, A. Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke 2011, 42, 1404–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dela Peña, I.C.; Yoo, A.; Tajiri, N.; Acosta, S.A.; Ji, X.; Kaneko, Y.; Borlongan, C.V. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J. Cereb. Blood Flow Metab. 2015, 35, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Yemisci, M.; Caban, S.; Gursoy-Ozdemir, Y.; Lule, S.; Novoa-Carballal, R.; Riguera, R.; Fernandez-Megia, E.; Andrieux, K.; Couvreur, P.; Capan, Y. Systemically administered brain-targeted nanoparticles transport peptides across the blood—brain barrier and provide neuroprotection. J. Cereb. Blood Flow Metab. 2015, 35, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, T.V.; Manzanero, S.; Furtado, M.; Biggins, P.J.; Hsieh, Y.-H.; Gelderblom, M.; MacDonald, K.P.; Salimova, E.; Li, Y.-I.; Korn, O. An atypical role for the myeloid receptor Mincle in central nervous system injury. J. Cereb. Blood Flow Metab. 2017, 37, 2098–2111. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Nguyen, H.M.; Maezawa, I.; Grössinger, E.M.; Garing, A.L.; Köhler, R.; Jin, L.-W.; Wulff, H. The potassium channel KCa3. 1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 2016, 36, 2146–2161. [Google Scholar] [CrossRef] [Green Version]
- Huria, T.; Beeraka, N.M.; Al-Ghamdi, B.; Fern, R. Premyelinated central axons express neurotoxic NMDA receptors: Relevance to early developing white-matter injury. J. Cereb. Blood Flow Metab. 2015, 35, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Nakka, V.P.; Prakash-babu, P.; Vemuganti, R. Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: Potential therapeutic targets for acute CNS injuries. Mol. Neurobiol. 2016, 53, 532–544. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Moore, K.J. MicroRNA regulation of atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [Green Version]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Jastrzebska-Kurkowska, I.; Czlonkowska, A.; Postula, M. MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke—A comprehensive review and bioinformatic analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, L.M.; Bernal, A.; San Martín, N.; Lorenzo, M.; Fernández-Veledo, S.; Gálvez, B.G. Metabolic rescue of obese adipose-derived stem cells by Lin28/Let7 pathway. Diabetes 2013, 62, 2368–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. miR-155 gene: A typical multifunctional microRNA. Biochim. Biophys. Acta 2009, 1792, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, X.; Dong, L.; Zhao, J.; Zhang, C.; Zhu, C. Acetylbritannilactone modulates microRNA-155-mediated inflammatory response in ischemic cerebral tissues. Mol. Med. 2015, 21, 197–209. [Google Scholar] [CrossRef]
- Edbauer, D.; Neilson, J.R.; Foster, K.A.; Wang, C.-F.; Seeburg, D.P.; Batterton, M.N.; Tada, T.; Dolan, B.M.; Sharp, P.A.; Sheng, M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 2010, 65, 373–384. [Google Scholar] [CrossRef] [Green Version]
- von Engelhardt, J.; Coserea, I.; Pawlak, V.; Fuchs, E.C.; Köhr, G.; Seeburg, P.H.; Monyer, H. Excitotoxicity in vitro by NR2A-and NR2B-containing NMDA receptors. Neuropharmacology 2007, 53, 10–17. [Google Scholar] [CrossRef]
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.; van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A key regulator of astrocyte-mediated inflammatory response. PLoS ONE 2012, 7, e44789. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, Y.; Yang, S.; Li, H.; Zhao, G.; Wang, F.; Yang, L.; Wang, D.W. MiR-320a contributes to atherogenesis by augmenting multiple risk factors and down-regulating SRF. J. Cell. Mol. Med. 2015, 19, 970–985. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Yang, J.; Xu, L.; Zhang, C. Cell-specific effects of miR-221/222 in vessels: Molecular mechanism and therapeutic application. J. Mol. Cell. Cardiol. 2012, 52, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Ji, B.; Wang, X.; Liu, J.; Zheng, Z.; Long, C.; Tang, Y.; Hu, S. Expression of microRNA-1 and microRNA-21 in different protocols of ischemic conditioning in an isolated rat heart model. Cardiology 2012, 122, 36–43. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Han, R.; Liu, H.; Sun, D.; Liu, X. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J. Clin. Neurosci. 2015, 22, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Sinnaeve, P.; Van der Schueren, B.; Mathieu, C.; Janssens, S.; Holvoet, P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J. Clin. Endocrinol. Metab. 2012, 97, E1213–E1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, J.; Ma, Y.; Tang, G.; Liu, Y.; Chen, X.; Zhang, Z.; Zeng, L.; Wang, Y.; Ouyang, Y.-B. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J. Cereb. Blood Flow Metab. 2015, 35, 1977–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepramaniam, S.; Ying, L.K.; Armugam, A.; Wintour, E.; Jeyaseelan, K. MicroRNA-130a represses transcriptional activity of aquaporin 4 M1 promoter. J. Biol. Chem. 2012, 287, 12006–12015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, G.; Liu, Y.; Liu, T.; Yan, W.; Liu, X.; Wang, Y.; Shi, J.; Jia, L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp. Brain Res. 2012, 216, 225–230. [Google Scholar] [CrossRef]
- Shi, H.; Sun, B.-l.; Zhang, J.; Lu, S.; Zhang, P.; Wang, H.; Yu, Q.; Stetler, R.A.; Vosler, P.S.; Chen, J. miR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2013, 12, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Kriegel, A.J.; Baker, M.A.; Liu, Y.; Liu, P.; Cowley, A.W., Jr.; Liang, M. Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension 2015, 66, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Yang, P.; Xiong, Q.; Song, X.; Yang, X.; Liu, L.; Yuan, W.; Rui, Y.-C. MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J. Hypertens. 2010, 28, 1646–1654. [Google Scholar] [CrossRef]
- Friese, R.S.; Altshuler, A.E.; Zhang, K.; Miramontes-Gonzalez, J.P.; Hightower, C.M.; Jirout, M.L.; Salem, R.M.; Gayen, J.R.; Mahapatra, N.R.; Biswas, N. MicroRNA-22 and promoter motif polymorphisms at the Chga locus in genetic hypertension: Functional and therapeutic implications for gene expression and the pathogenesis of hypertension. Hum. Mol. Genet. 2013, 22, 3624–3640. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.-Y.; Li, P.; Yang, Y.-B.; Liu, M.-L. Xuezhikang, extract of red yeast rice, improved abnormal hemorheology, suppressed caveolin-1 and increased eNOS expression in atherosclerotic rats. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Nossent, A.Y.; Eskildsen, T.V.; Andersen, L.B.; Bie, P.; Brønnum, H.; Schneider, M.; Andersen, D.C.; Welten, S.M.; Jeppesen, P.L.; Hamming, J.F. The 14q32 microRNA-487b targets the antiapoptotic insulin receptor substrate 1 in hypertension-induced remodeling of the aorta. Ann. Surg. 2013, 258, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Chen, Y.; Lei, M. Biomarkers associated with ischemic stroke in diabetes mellitus patients. Cardiovasc. Toxicol. 2016, 16, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ye, X.; Chopp, M.; Venkat, P.; Zacharek, A.; Yan, T.; Ning, R.; Yu, P.; Cui, G.; Chen, J. miR-145 regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem Cells Transl. Med. 2016, 5, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, J.E. ASK1 modulates the expression of microRNA Let7A in microglia under high glucose in vitro condition. Front. Cell. Neurosci. 2015, 9, 198. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Han, W.; Xiao, B.; Li, N.; Zhu, D.; Gao, P. Differential expression of microRNAs in the aorta of spontaneously hypertensive rats. Sheng Li Xue Bao Acta Physiol. Sin. 2008, 60, 553–560. [Google Scholar]
- Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Strapazzon, G.; Ragazzo, F.; Bratti, P.; Fabricio, A.S.; Squarcina, E.; Gion, M.; Palatini, P. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am. J. Hypertens. 2011, 24, 241–246. [Google Scholar] [CrossRef]
- Sun, H.-X.; Zeng, D.-Y.; Li, R.-T.; Pang, R.-P.; Yang, H.; Hu, Y.-L.; Zhang, Q.; Jiang, Y.; Huang, L.-Y.; Tang, Y.-B. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 2012, 60, 1407–1414. [Google Scholar] [CrossRef] [Green Version]
- DeCicco, D.; Zhu, H.; Brureau, A.; Schwaber, J.S.; Vadigepalli, R. MicroRNA network changes in the brain stem underlie the development of hypertension. Physiol. Genom. 2015, 47, 388–399. [Google Scholar] [CrossRef]
- Yong, M.; Kaste, M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke 2008, 39, 2749–2755. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Cui, X.; Zacharek, A.; Cui, Y.; Roberts, C.; Chopp, M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke 2011, 42, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Air, E.L.; Kissela, B.M. Diabetes, the metabolic syndrome, and ischemic stroke: Epidemiology and possible mechanisms. Diabetes Care 2007, 30, 3131–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, E.; Fuchs, P.F.; Heldin, J.; Barkefors, I.; Bondjers, C.; Genové, G.; Arrondel, C.; Gerwins, P.; Kurschat, C.; Schermer, B. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009, 1, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Venkat, P.; Zacharek, A.; Chopp, M. Neurorestorative therapy for stroke. Front. Hum. Neurosci. 2014, 8, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyamasundar, S.; Jadhav, S.P.; Bay, B.H.; Tay, S.S.W.; Kumar, S.D.; Rangasamy, D.; Dheen, S.T. Analysis of epigenetic factors in mouse embryonic neural stem cells exposed to hyperglycemia. PLoS ONE 2013, 8, e65945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menet, R.; Bernard, M.; ElAli, A. Hyperlipidemia in stroke pathobiology and therapy: Insights and perspectives. Front. Physiol. 2018, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Hansson, G.K.; Robertson, A.-K.L.; Söderberg-Nauclér, C. Inflammation and atherosclerosis. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 297–329. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Gimbrone, M.A. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991, 251, 788–791. [Google Scholar] [CrossRef] [Green Version]
- Siess, W. Platelet interaction with bioactive lipids formed by mild oxidation of low-density lipoprotein. Pathophysiol. Haemost. Thromb. 2006, 35, 292–304. [Google Scholar] [CrossRef] [Green Version]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [Green Version]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.-H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Gong, Y.; Yuan, J.; Zhang, W.; Zhao, G.; Li, H.; Sun, A.; Zou, Y.; Ge, J. microRNA-181a represses ox-LDL-stimulated inflammatory response in dendritic cell by targeting c-Fos. J. Lipid Res. 2012, 53, 2355–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Chen, S.; Liu, X.; Lin, L.; Huang, X.; Guo, Z.; Liu, J.; Wang, Y.; Yuan, W. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 2011, 215, 286–293. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, M.; Corbalán-Campos, J.; Heyll, K.; Weber, C.; Schober, A. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 796–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.-F.; Yang, L.-X.; Guo, R.-W.; Liu, H.; Shi, Y.-K.; Wang, H.; Ye, J.-S.; Yang, Z.-H.; Liang, X. miR-155 inhibits oxidized low-density lipoprotein-induced apoptosis of RAW264. 7 cells. Mol. Cell. Biochem. 2013, 382, 253–261. [Google Scholar] [CrossRef]
- Li, X.; Kong, D.; Chen, H.; Liu, S.; Hu, H.; Wu, T.; Wang, J.; Chen, W.; Ning, Y.; Li, Y. miR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci. Rep. 2016, 6, 21789. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.Y.; Fan, W.D.; Fang, R.; Wu, G.F. Regulation of microRNA-155 in endothelial inflammation by targeting nuclear factor (NF)-κB P65. J. Cell. Biochem. 2014, 115, 1928–1936. [Google Scholar] [CrossRef]
- Huang, R.-S.; Hu, G.-Q.; Lin, B.; Lin, Z.-Y.; Sun, C.-C. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J. Investig. Med. 2010, 58, 961–967. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Liang, X.; Zhu, G. MicroRNA-155 promotes atherosclerosis inflammation via targeting SOCS1. Cell. Physiol. Biochem. 2015, 36, 1371–1381. [Google Scholar] [CrossRef]
- Xue, Y.; Wei, Z.; Ding, H.; Wang, Q.; Zhou, Z.; Zheng, S.; Zhang, Y.; Hou, D.; Liu, Y.; Zen, K. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis 2015, 241, 671–681. [Google Scholar] [CrossRef]
- Mackenzie, N.; Staines, K.; Zhu, D.; Genever, P.; Macrae, V. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem. Funct. 2014, 32, 209–216. [Google Scholar] [CrossRef] [PubMed]
- White, B.C.; Sullivan, J.M.; DeGracia, D.J.; O’Neil, B.J.; Neumar, R.W.; Grossman, L.I.; Rafols, J.A.; Krause, G.S. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J. Neurol. Sci. 2000, 179, 1–33. [Google Scholar] [CrossRef]
- Liu, F.J.; Lim, K.Y.; Kaur, P.; Sepramaniam, S.; Armugam, A.; Wong, P.T.H.; Jeyaseelan, K. microRNAs involved in regulating spontaneous recovery in embolic stroke model. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaseelan, K.; Lim, K.Y.; Armugam, A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008, 39, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Martinez, B.; Peplow, P.V. Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury. Neural Regen. Res. 2016, 11, 1375. [Google Scholar] [CrossRef]
- Yu, P.; Chen, W. Advances in the diagnosis of exosomal miRNAs in ischemic stroke. Neuropsychiatr. Dis. Treat. 2019, 15, 2339. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, D.-B.; Li, R.-Y.; Zhou, X.; Yu, D.-J.; Lan, X.-Y.; Li, J.-P.; Liu, J.-L. Diagnosis of hyperacute and acute ischaemic stroke: The potential utility of exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cerebrovasc. Dis. 2018, 45, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-B.; Liu, J.-L.; Wang, W.; Li, R.-Y.; Yu, D.-J.; Lan, X.-Y.; Li, J.-P. Plasma exosomal miR-422a and miR-125b-2-3p serve as biomarkers for ischemic stroke. Curr. Neurovascular Res. 2017, 14, 330–337. [Google Scholar] [CrossRef]
- Castillo, J.; Loza, M.I.; Mirelman, D.; Brea, J.; Blanco, M.; Sobrino, T.; Campos, F. A novel mechanism of neuroprotection: Blood glutamate grabber. J. Cereb. Blood Flow Metab. 2016, 36, 292–301. [Google Scholar] [CrossRef]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef] [Green Version]
- Dharap, A.; Bowen, K.; Place, R.; Li, L.-C.; Vemuganti, R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J. Cereb. Blood Flow Metab. 2009, 29, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Huang, J.; Chen, X.; Gu, X.; Wang, Y.; Zeng, L.; Yang, G.-Y. Increase of circulating miR-223 and insulin-like growth factor-1 is associated with the pathogenesis of acute ischemic stroke in patients. BMC Neurol. 2014, 14, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, T.-H.; Hwang, H.-M.; Chen, J.-J.; Wu, T.; Li, A.H.; Wang, H.-L. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol. Dis. 2005, 18, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-B.; Zhang, Z.; Li, T.-B.; Lou, Z.; Li, S.-Y.; Yang, H.; Yang, J.; Luo, X.-J.; Peng, J. Up-regulation of brain-enriched miR-107 promotes excitatory neurotoxicity through down-regulation of glutamate transporter-1 expression following ischaemic stroke. Clin. Sci. 2014, 127, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Demir, M.; Guldiken, S.; Turgut, N.; Turgut, B.; Tugrul, A. Oxidative stress and total antioxidant capacity in diabetic and nondiabetic acute ischemic stroke patients. Clin. Appl. Thromb. Hemost. 2009, 15, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.E.; Karatas, H.; Liu, Y.; Yalcin, A.; Montaner, J.; Lo, E.H.; Van Leyen, K. STAT-dependent upregulation of 12/15-lipoxygenase contributes to neuronal injury after stroke. J. Cereb. Blood Flow Metab. 2015, 35, 2043–2051. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-D.; Yang, D.-I.; Lin, T.-K.; Shaw, F.-Z.; Liou, C.-W.; Chuang, Y.-C. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 7199–7215. [Google Scholar] [CrossRef] [Green Version]
- Tao, Z.; Zhao, H.; Wang, R.; Liu, P.; Yan, F.; Zhang, C.; Ji, X.; Luo, Y. Neuroprotective effect of microRNA-99a against focal cerebral ischemia–reperfusion injury in mice. J. Neurol. Sci. 2015, 355, 113–119. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Tan, X.; Liu, B.; Zhang, Y.; Li, C. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J. Neurochem. 2015, 134, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Li, L.; Liu, B.; Zhang, Y.; Chen, Q.; Li, C. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS ONE 2014, 9, e102342. [Google Scholar] [CrossRef]
- Chan, S.Y.; Loscalzo, J. MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle 2010, 9, 1072–1083. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yu, J.-T.; Wang, H.-F.; Meng, X.-F.; Tan, C.-C.; Wang, J.; Wang, C.; Tan, L. Association between stroke and Alzheimer’s disease: Systematic review and meta-analysis. J. Alzheimer’s Dis. 2015, 43, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Mellinger, J.L.; Pencina, K.M.; Massaro, J.M.; Hoffmann, U.; Seshadri, S.; Fox, C.S.; O’Donnell, C.J.; Speliotes, E.K. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J. Hepatol. 2015, 63, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Salinas, J.; Lin, H.; Aparico, H.J.; Huan, T.; Liu, C.; Rong, J.; Beiser, A.; Himali, J.J.; Freedman, J.E.; Larson, M.G. Whole blood microRNA expression associated with stroke: Results from the Framingham Heart Study. PLoS ONE 2019, 14, e0219261. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, M.; Reddy, P.H. Peripheral biomarkers of stroke: Focus on circulatory microRNAs. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Armugam, A.; Sepramaniam, S.; Lim, K.Y.; Setyowati, K.D.; Wang, C.W.; Jeyaseelan, K. Expression profile of MicroRNAs in young stroke patients. PLoS ONE 2009, 4, e7689. [Google Scholar] [CrossRef] [Green Version]
- Guedes, J.R.; Custódia, C.M.; Silva, R.J.; de Almeida, L.P.; Pedroso de Lima, M.C.; Cardoso, A.L. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum. Mol. Genet. 2014, 23, 6286–6301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Lee, J.E. miR-155 is involved in Alzheimer’s disease by regulating T lymphocyte function. Front. Aging Neurosci. 2015, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, D.; Huang, H.-Z.; Wang, Z.-H.; Hou, T.-Y.; Yang, X.; Pang, P.; Wei, N.; Zhou, Y.-F.; Dupras, M.-J. A novel microRNA-124/PTPN1 signal pathway mediates synaptic and memory deficits in Alzheimer’s disease. Biol. Psychiatry 2018, 83, 395–405. [Google Scholar] [CrossRef]
- Hu, Y.-K.; Wang, X.; Li, L.; Du, Y.-H.; Ye, H.-T.; Li, C.-Y. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor 1. Neurosci. Bull. 2013, 29, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, F. Micro RNAs in Alzheimer’s disease: Diagnostic markers or therapeutic agents? Front. Pharmacol. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Tomás, M.; Alvarez-López, M.J.; Sanchez-Roige, S.; Lalanza, J.F.; Bayod, S.; Sanfeliu, C.; Pallàs, M.; Escorihuela, R.M.; Kaliman, P. Epigenetic alterations in hippocampus of SAMP8 senescent mice and modulation by voluntary physical exercise. Front. Aging Neurosci. 2014, 6, 51. [Google Scholar] [PubMed]
- Ma, X.; Liu, L.; Meng, J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci. Lett. 2017, 661, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cerutti, C.; Lopez-Ramirez, M.A.; Pryce, G.; King-Robson, J.; Simpson, J.E.; Van Der Pol, S.M.; Hirst, M.C.; De Vries, H.E.; Sharrack, B. Brain endothelial miR-146a negatively modulates T-cell adhesion through repressing multiple targets to inhibit NF-κB activation. J. Cereb. Blood Flow Metab. 2015, 35, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrak, R.E.; Griffin, W.S.T. Glia and their cytokines in progression of neurodegeneration. Neurobiol. Aging 2005, 26, 349–354. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Luna, J.M.; Moon, Y.P.; Liu, K.M.; Spitalnik, S.; Paik, M.C.; Cheung, K.; Sacco, R.L.; Elkind, M.S. High-sensitivity C-reactive protein and interleukin-6–dominant inflammation and ischemic stroke risk: The Northern Manhattan Study. Stroke 2014, 45, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrarese, C.; Mascarucci, P.; Zoia, C.; Cavarretta, R.; Frigo, M.; Begni, B.; Sarinella, F.; Frattola, L.; De Simoni, M.G. Increased cytokine release from peripheral blood cells after acute stroke. J. Cereb. Blood Flow Metab. 1999, 19, 1004–1009. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, G.; Granger, D.N. Cell adhesion molecules and ischemic stroke. Neurol. Res. 2008, 30, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Yenari, M.A. Cellular targets of brain inflammation in stroke. Curr. Opin. Investig. Drugs (Lond. Engl. 2000) 2003, 4, 522–529. [Google Scholar]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappaB in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chopp, M.; Zhang, Z.; Jiang, N.; Powers, C. The expression of P-and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998, 785, 207–214. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Molecular insights and therapeutic targets for blood–brain barrier disruption in ischemic stroke: Critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol. Dis. 2010, 38, 376–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Wang, J.; Gao, L.; Wang, R.; Liu, X.; Gao, Z.; Tao, Z.; Xu, C.; Song, J.; Ji, X. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke 2013, 44, 1706–1713. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Wang, X.; Chen, S.; Liu, H.; Wang, Y.; Xu, X.; Cheng, J.; Jia, J.; Zhen, X. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav. Immun. 2015, 49, 75–85. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. In From Innate Immunity to Immunological Memory; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 311, pp. 17–58. [Google Scholar]
- Zador, Z.; Stiver, S.; Wang, V.; Manley, G.T. Role of aquaporin-4 in cerebral edema and stroke. In Aquaporin; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 190, pp. 159–170. [Google Scholar]
- Li, M.; Liu, J.; Bi, Y.; Chen, J.; Zhao, L. Potential medications or compounds acting on toll-like receptors in cerebral ischemia. Curr. Neuropharmacol. 2018, 16, 160–175. [Google Scholar] [CrossRef]
- Yao, L.; Kan, E.M.; Lu, J.; Hao, A.; Dheen, S.T.; Kaur, C.; Ling, E.-A. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: Role of TLR4 in hypoxic microglia. J. Neuroinflamm. 2013, 10, 785. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Y.J.; Wu, X.Y.; Hong, Z.; Wei, W.S. Micro RNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J. Neurochem. 2015, 132, 713–723. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Guedes, J.R.; Pereira de Almeida, L.; Pedroso de Lima, M.C. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 2012, 135, 73–88. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.-Y.; Li, Y.-J.; Hong, Z.; Wei, W.-S. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J. Neuroinflamm. 2012, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Tahamtan, A.; Teymoori-Rad, M.; Nakstad, B.; Salimi, V. Anti-inflammatory microRNAs and their potential for inflammatory diseases treatment. Front. Immunol. 2018, 9, 1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Kumar, M.; Aich, J.; Hariharan, M.; Brahmachari, S.K.; Agrawal, A.; Ghosh, B. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc. Natl. Acad. Sci. USA 2009, 106, 5761–5766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S. TLR signaling. In From Innate Immunity to Immunological Memory; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 311, pp. 1–16. [Google Scholar]
- Michinaga, S.; Koyama, Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int. J. Mol. Sci. 2015, 16, 9949–9975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukeirat, M.; Sarkar, S.N.; Hu, H.; Quintana, D.D.; Simpkins, J.W.; Ren, X. MiR-34a regulates blood–brain barrier permeability and mitochondrial function by targeting cytochrome c. J. Cereb. Blood Flow Metab. 2016, 36, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Suárez, Y.; Sessa, W.C. MicroRNAs as novel regulators of angiogenesis. Circ. Res. 2009, 104, 442–454. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; He, Q.-W.; Li, Q.; Chen, X.-L.; Baral, S.; Jin, H.-J.; Zhu, Y.-Y.; Li, M.; Xia, Y.-P.; Mao, L. MicroRNA-150 regulates blood–brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J. 2016, 30, 2097–2107. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-R.; Tsuji, K.; Lee, S.-R.; Lo, E.H. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J. Neurosci. 2004, 24, 671–678. [Google Scholar] [CrossRef]
- Deng, X.; Zhong, Y.; Gu, L.; Shen, W.; Guo, J. MiR-21 involve in ERK-mediated upregulation of MMP9 in the rat hippocampus following cerebral ischemia. Brain Res. Bull. 2013, 94, 56–62. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef]
- Vella, J.; Zammit, C.; Di Giovanni, G.; Muscat, R.; Valentino, M. The central role of aquaporins in the pathophysiology of ischemic stroke. Front. Cell. Neurosci. 2015, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Huang, C.; Ding, H.; Dong, J.; Gao, Z.; Yang, X.; Tang, Y.; Dong, Q. Aquaporin-4 and cerebrovascular diseases. Int. J. Mol. Sci. 2016, 17, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, J.-C.; Yamauchi, H.; Fujioka, M.; Endres, M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J. Cereb. Blood Flow Metab. 2014, 34, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.-B.; Giffard, R.G. MicroRNAs affect BCL-2 family proteins in the setting of cerebral ischemia. Neurochem. Int. 2014, 77, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, K.-J.; Deng, Z.; Huang, H.; Hamblin, M.; Xie, C.; Zhang, J.; Chen, Y.E. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol. Dis. 2010, 38, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.-B.; Lu, Y.; Yue, S.; Xu, L.-J.; Xiong, X.-X.; White, R.E.; Sun, X.; Giffard, R.G. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol. Dis. 2012, 45, 555–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.-M.; Xu, L.; Giffard, R.G. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J. Cereb. Blood Flow Metab. 2013, 33, 1976–1982. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.-B.; Lu, Y.; Yue, S.; Giffard, R.G. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 2012, 12, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Buller, B.; Liu, X.; Wang, X.; Zhang, R.L.; Zhang, L.; Hozeska-Solgot, A.; Chopp, M.; Zhang, Z.G. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010, 277, 4299–4307. [Google Scholar] [CrossRef] [Green Version]
- Khodanovich, M.; Nemirovich-Danchenko, N.M. New neurons in the post-ischemic and injured brain: Migrating or resident? Front. Neurosci. 2019, 13, 588. [Google Scholar]
- Liu, X.S.; Chopp, M.; Wang, X.L.; Zhang, L.; Hozeska-Solgot, A.; Tang, T.; Kassis, H.; Zhang, R.L.; Chen, C.; Xu, J. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J. Biol. Chem. 2013, 288, 12478–12488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaloy, C.; Liu, L.; Lee, J.-A.; Su, H.; Shen, F.; Yang, G.-Y.; Young, W.L.; Ivey, K.N.; Gao, F.-B. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell 2010, 6, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.S.; Chopp, M.; Zhang, R.L.; Tao, T.; Wang, X.L.; Kassis, H.; Hozeska-Solgot, A.; Zhang, L.; Chen, C.; Zhang, Z.G. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS ONE 2011, 6, e23461. [Google Scholar] [CrossRef] [Green Version]
- Androutsellis-Theotokis, A.; Leker, R.R.; Soldner, F.; Hoeppner, D.J.; Ravin, R.; Poser, S.W.; Rueger, M.A.; Bae, S.-K.; Kittappa, R.; McKay, R.D. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006, 442, 823–826. [Google Scholar] [CrossRef]
- Wang, X.; Mao, X.; Xie, L.; Greenberg, D.A.; Jin, K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J. Cereb. Blood Flow Metab. 2009, 29, 1644–1654. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; He, X.; Wang, Y.; Tang, Y.; Zheng, C.; Cai, H.; Liu, J.; Fu, Y.; Yang, G. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 2014, 21, 37–43. [Google Scholar] [CrossRef]
- Zeng, L.L.; He, X.S.; Liu, J.R.; Zheng, C.B.; Wang, Y.T.; Yang, G.Y. Lentivirus-mediated overexpression of microRNA-210 improves long-term outcomes after focal cerebral ischemia in mice. CNS Neurosci. Ther. 2016, 22, 961–969. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Akamatsu, Y.; Lee, C.C.; Stetler, R.A.; Lawton, M.T.; Yang, G.-Y. Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Prog. Neurobiol. 2014, 115, 138–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morancho, A.; Ma, F.; Barceló, V.; Giralt, D.; Montaner, J.; Rosell, A. Impaired vascular remodeling after endothelial progenitor cell transplantation in MMP9-deficient mice suffering cortical cerebral ischemia. J. Cereb. Blood Flow Metab. 2015, 35, 1547–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, K.-J.; Hamblin, M.; Eugene Chen, Y. Angiogenesis-regulating microRNAs and ischemic stroke. Curr. Vasc. Pharmacol. 2015, 13, 352–365. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-S.; Su, J.-L.; Cha, S.-T.; Tarn, W.-Y.; Wang, M.-Y.; Hsu, H.-C.; Lin, M.-T.; Chu, C.-Y.; Hua, K.-T.; Chen, C.-N. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J. Clin. Investig. 2011, 121, 3442–3445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Lai, T.-C.; Jan, Y.-H.; Lin, F.-M.; Wang, W.-C.; Xiao, H.; Wang, Y.-T.; Sun, W.; Cui, X.; Li, Y.-S. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J. Clin. Investig. 2013, 123, 1057–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Mao, L.; Gao, Y.; Baral, S.; Zhou, Y.; Hu, B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci. Rep. 2015, 5, 13316. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.-L.; Guo, F.; Liu, F.; Gao, F.-L.; Zhang, P.-Q.; Niu, X.; Guo, S.-C.; Yin, J.-H.; Wang, Y.; Deng, Z.-F. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol. Cell. Biochem. 2012, 370, 45–51. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Doehring, M.; Bretschneider, E.; Zechariah, A.; Kaltwasser, B.; Müller, B.; Koch, J.C.; Bähr, M.; Hermann, D.M.; Michel, U. MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol. 2013, 126, 251–265. [Google Scholar] [CrossRef]
- Caballero-Garrido, E.; Pena-Philippides, J.C.; Lordkipanidze, T.; Bragin, D.; Yang, Y.; Erhardt, E.B.; Roitbak, T. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J. Neurosci. 2015, 35, 12446–12464. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Gilyazova, I.R.; Klimentova, E.A.; Bulygin, K.V.; Izmailov, A.A.; Bermisheva, M.A.; Galimova, E.F.; Safiullin, R.I.; Galimov, S.N.; Pavlov, V.N.; Khusnutdinova, E.K. MicroRNA-200 family expression analysis in metastatic clear cell renal cell carcinoma patients. Cancer Gene Ther. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, S.; Chen, X.; Li, S.; Chen, W. Bioinformatic analysis of potential microRNAs in ischemic stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 1753–1759. [Google Scholar] [CrossRef]
- Pabinger, S.; Rödiger, S.; Kriegner, A.; Vierlinger, K.; Weinhäusel, A. A survey of tools for the analysis of quantitative PCR (qPCR) data. Biomol. Detect. Quantif. 2014, 1, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Pabinger, S.; Dander, A.; Fischer, M.; Snajder, R.; Sperk, M.; Efremova, M.; Krabichler, B.; Speicher, M.R.; Zschocke, J.; Trajanoski, Z. A survey of tools for variant analysis of next-generation genome sequencing data. Brief. Bioinform. 2014, 15, 256–278. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Gao, Y.; Wu, G.; Lei, X.; Zhang, Y.; Pan, W.; Yu, H. Bioinformatics analysis of microarray data to reveal the pathogenesis of brain ischemia. Mol. Med. Rep. 2018, 18, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, L.; Batte, K.E.; Trgovcich, J.; Wisler, J.; Marsh, C.B.; Piper, M. Methodological challenges in utilizing mi RNA s as circulating biomarkers. J. Cell. Mol. Med. 2014, 18, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Coenen-Stass, A.M.; Magen, I.; Brooks, T.; Ben-Dov, I.Z.; Greensmith, L.; Hornstein, E.; Fratta, P. Evaluation of methodologies for microRNA biomarker detection by next generation sequencing. RNA Biol. 2018, 15, 1133–1145. [Google Scholar]
- Eminaga, S.; Christodoulou, D.C.; Vigneault, F.; Church, G.M.; Seidman, J.G. Quantification of microRNA Expression with Next-Generation Sequencing. Curr. Protoc. Mol. Biol. 2013, 103. [Google Scholar] [CrossRef]
- Cheng, C.-A.; Lin, Y.-C.; Chiu, H.-W. Prediction of the Prognosis of Ischemic Stroke Patients After Intravenous Thrombolysis Using Artificial Neural Networks. Stud. Health Technol. Inform. 2014, 202, 115–118. [Google Scholar] [PubMed]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE Guidelines: Minimum Information for Publication of quantitative Real-Time PCR experiments. Clin Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Huggett, J.F.; Foy, C.A.; Benes, V.; Emslie, K.; Garson, J.A.; Haynes, R.; Hellemans, J.; Kubista, M.; Mueller, R.D.; Nolan, T. The Digital MIQE Guidelines: Minimum Information for Publication of Quantitative Digital PCR Experiments. Clin. Chem. 2013, 59, 892–902. [Google Scholar] [CrossRef]
- Huntley, R.P.; Kramarz, B.; Sawford, T.; Umrao, Z.; Kalea, A.; Acquaah, V.; Martin, M.J.; Mayr, M.; Lovering, R.C. Expanding the horizons of microRNA bioinformatics. RNA 2018, 24, 1005–1017. [Google Scholar] [CrossRef]
- Dichgans, M.; Pulit, S.L.; Rosand, J. Stroke genetics: Discovery, biology, and clinical applications. Lancet Neurol. 2019, 18, 587–599. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Zheng, D.; Sun, Y.; Wang, S.; Yan, Y. Bioinformatics analysis of the regulatory lncRNA-miRNA-mRNA network and drug prediction in patients with hypertrophic cardiomyopathy. Mol. Med. Rep. 2019, 20, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Zhang, D.; Lv, L.; Shi, W.; Song, Z.; Yi, B.; Lai, B.; Chen, Q.; Yang, S.; Hua, P. Bioinformatic gene analysis for potential biomarkers and therapeutic targets of atrial fibrillation-related stroke. J. Transl. Med. 2019, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yin, P.; Li, G.; Zhong, D. Genome-wide integration study of circulating miRNAs and peripheral whole-blood mRNAs of male acute ischemic stroke patients. Neuroscience 2018, 380, 27–37. [Google Scholar] [CrossRef]
- Mick, E.; Shah, R.; Tanriverdi, K.; Murthy, V.; Gerstein, M.; Rozowsky, J.; Kitchen, R.; Larson, M.G.; Levy, D.; Freedman, J.E. Stroke and circulating extracellular RNAs. Stroke 2017, 48, 828–834. [Google Scholar] [CrossRef]
- Backes, C.; Meese, E.; Keller, A. Specific miRNA disease biomarkers in blood, serum and plasma: Challenges and prospects. Mol. Diagn. Ther. 2016, 20, 509–518. [Google Scholar] [CrossRef]
- Limkakeng, A.T., Jr.; Monte, A.A.; Kabrhel, C.; Puskarich, M.; Heitsch, L.; Tsalik, E.L.; Shapiro, N.I. Systematic molecular phenotyping: A path toward precision emergency medicine? Acad. Emerg. Med. 2016, 23, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Nan, F.; Yang, P.; Meng, Q.; Xie, Y.; Zhang, D.; Muhammad, K. Gan-based semi-supervised learning approach for clinical decision support in health-IoT platform. IEEE Access 2019, 7, 8048–8057. [Google Scholar] [CrossRef]
- Vijayan, M.; Kumar, S.; Yin, X.; Zafer, D.; Chanana, V.; Cengiz, P.; Reddy, P.H. Identification of novel circulatory microRNA signatures linked to patients with ischemic stroke. Hum. Mol. Genet. 2018, 27, 2318–2329. [Google Scholar] [CrossRef] [Green Version]
| miRNA | Target Gene | Process | Effects Produced by Modulating mRNA Role |
|---|---|---|---|
| miR-126 | VCAM1 [22] | Atherosclerosis | Reduced neutrophil infiltration |
| miR-155 | PU.1 [23] | Arterial hypertension | Reduced monocyte maturation |
| miR-155 | SOCS1 [24] | Inflammation | Suppression of inflammation |
| miR-125b | NR2A [25] | Excitotoxicity | Decreased NMDA activation |
| miR-125b | p53 [26] | Neuronal death | Reduced neuronal death |
| miR-146a | IRAK-1, IL-6, IL-8 [27] | Inflammation | Reduced inflammation |
| miR-145 | KLP4, KLP5 [28] | Atherosclerosis | Promotes SMC growth |
| Let-7a | Casp3 [22] | Apoptosis | Reduced apoptosis |
| miR-221 | KIT [29] | Type 2 Diabetes | Endothelial dysfunction |
| miR-221 | KIP1 [29] | Atherosclerosis | Promotes SMC growth |
| miR-222 | KIP2 [29] | Atherosclerosis | Promotes SMC growth |
| miR-223 | NR2A [30] | Excitotoxicity | Increased NMDA activation |
| miR-424 | NRF2 [31] | Inflammation | Reduced inflammation |
| miR-181a miR-25 | Bim (BCL2L11) [32] | Apoptosis | Reduced apoptosis |
| miR-29b, miR-130a | AQP4 [33,34] | Edema | Reduces edema formation |
| miR-29b, miR-15 | Bcl-2 [35,36] | Apoptosis | Reduced apoptosis |
| miRNA | Target | mRNA Activity | Effects Produced by Modulating mRNA Role |
|---|---|---|---|
| miR-424 | NRF2 [31] | Increase | Suppression of inflammation |
| miR-let- 7c-5p | Casp3 [115] | Decrease | Suppression of inflammation |
| miR-124 | C/EBP-α-PU.1 [125] | Decrease | Suppression of inflammation |
| miR-155 | SOCS1, MyD88 [24] | Decrease | Suppression of inflammation |
| miR-106a | IL-10 [27] | - | Suppression of inflammation |
| miR-146a | IL-6, IL-1β [27] | Decrease | Suppression of inflammation |
| miR-9 | MMP-9, MMP-13 [115] | Decrease | Suppression of inflammation |
| miR-219 | MPP9 [31] | Decrease | Suppression of inflammation |
| miR-181c | TLR4 [120] | Increase | Suppression of inflammation |
| miR-181a | IL1 [122] | Increase | Suppression of inflammation |
| Risk Factors | mRNA | Target | Source |
|---|---|---|---|
| Arterial Hypertension | miR-155 | PU.1 | [23] |
| miR-22 | CHGA | [39] | |
| miR-487b | IGF-I | [41] | |
| miR-125a/b-5p | ET-1 | [38] | |
| Diabetes | miR-221 | KIT | [29] |
| miR-let-7a | ASK-1 | [22] | |
| miR-145 | ABCA1 | [43] | |
| miR-223 | P2Y | [42] | |
| miR-144 | IRS-1 | [42] | |
| Atherosclerosis | miR-222 | KIP2 | [29] |
| miR-221 | KIP1 | [29] | |
| miR-145 | KLP4, KLP5 | [28] | |
| miR-126 | VCAM1 | [22] | |
| miR-143 | ABCA1 | [28] | |
| miR-92a | Kruppel factor | [28] | |
| miR-155 | ETS1, AT1r | [63] | |
| miR-181a | c-Fos | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulygin, K.V.; Beeraka, N.M.; Saitgareeva, A.R.; Nikolenko, V.N.; Gareev, I.; Beylerli, O.; Akhmadeeva, L.R.; Mikhaleva, L.M.; Torres Solis, L.F.; Solís Herrera, A.; et al. Can miRNAs Be Considered as Diagnostic and Therapeutic Molecules in Ischemic Stroke Pathogenesis?—Current Status. Int. J. Mol. Sci. 2020, 21, 6728. https://doi.org/10.3390/ijms21186728
Bulygin KV, Beeraka NM, Saitgareeva AR, Nikolenko VN, Gareev I, Beylerli O, Akhmadeeva LR, Mikhaleva LM, Torres Solis LF, Solís Herrera A, et al. Can miRNAs Be Considered as Diagnostic and Therapeutic Molecules in Ischemic Stroke Pathogenesis?—Current Status. International Journal of Molecular Sciences. 2020; 21(18):6728. https://doi.org/10.3390/ijms21186728
Chicago/Turabian StyleBulygin, Kirill V., Narasimha M. Beeraka, Aigul R. Saitgareeva, Vladimir N. Nikolenko, Ilgiz Gareev, Ozal Beylerli, Leila R. Akhmadeeva, Liudmila M. Mikhaleva, Luis Fernando Torres Solis, Arturo Solís Herrera, and et al. 2020. "Can miRNAs Be Considered as Diagnostic and Therapeutic Molecules in Ischemic Stroke Pathogenesis?—Current Status" International Journal of Molecular Sciences 21, no. 18: 6728. https://doi.org/10.3390/ijms21186728
APA StyleBulygin, K. V., Beeraka, N. M., Saitgareeva, A. R., Nikolenko, V. N., Gareev, I., Beylerli, O., Akhmadeeva, L. R., Mikhaleva, L. M., Torres Solis, L. F., Solís Herrera, A., Avila-Rodriguez, M. F., Somasundaram, S. G., Kirkland, C. E., & Aliev, G. (2020). Can miRNAs Be Considered as Diagnostic and Therapeutic Molecules in Ischemic Stroke Pathogenesis?—Current Status. International Journal of Molecular Sciences, 21(18), 6728. https://doi.org/10.3390/ijms21186728