Characterization of Rat Cardiovascular System by Anacrotic/Dicrotic Notches in the Condition of Increase/Decrease of NO Bioavailability
Abstract
:1. Introduction
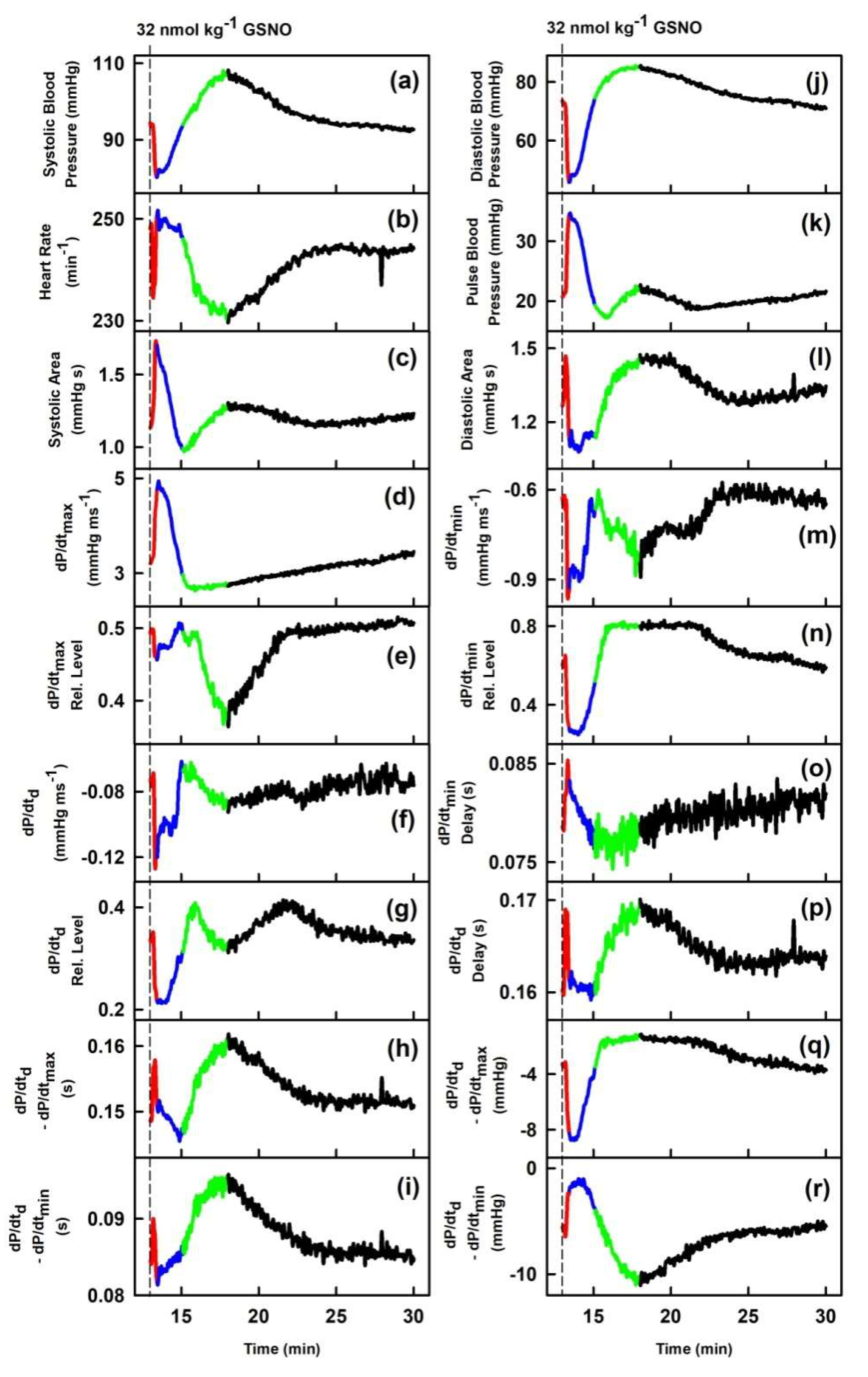
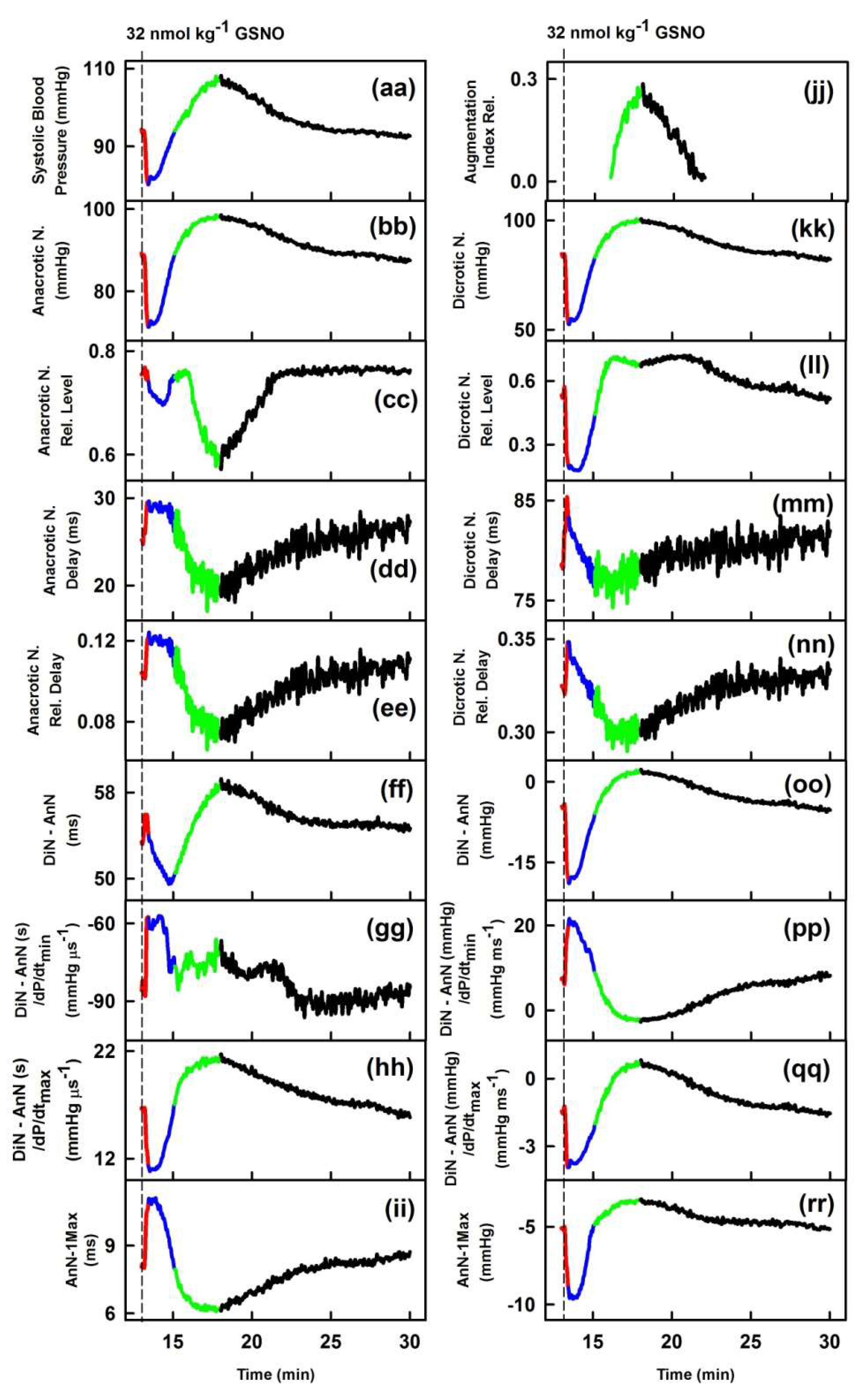
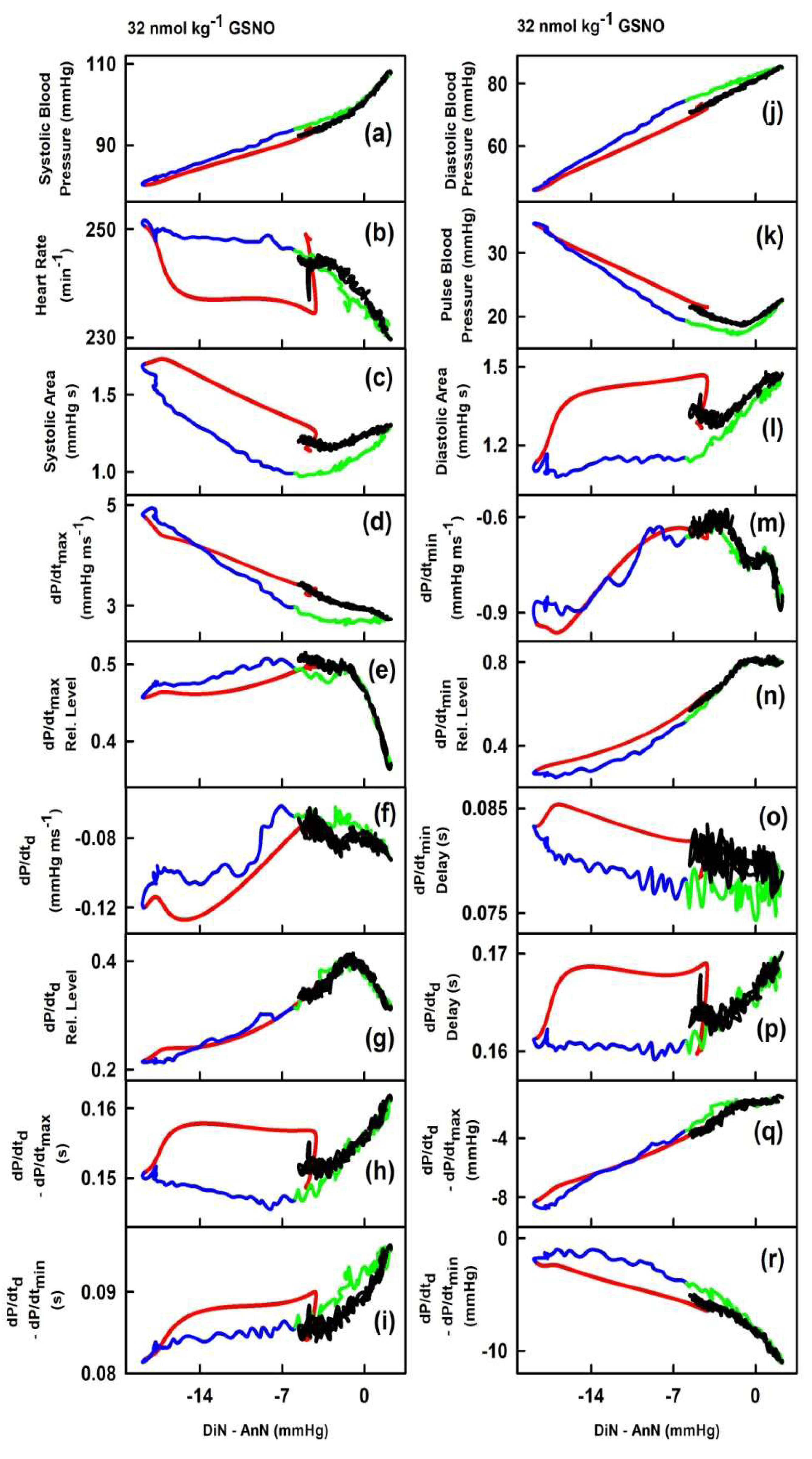
2. Results
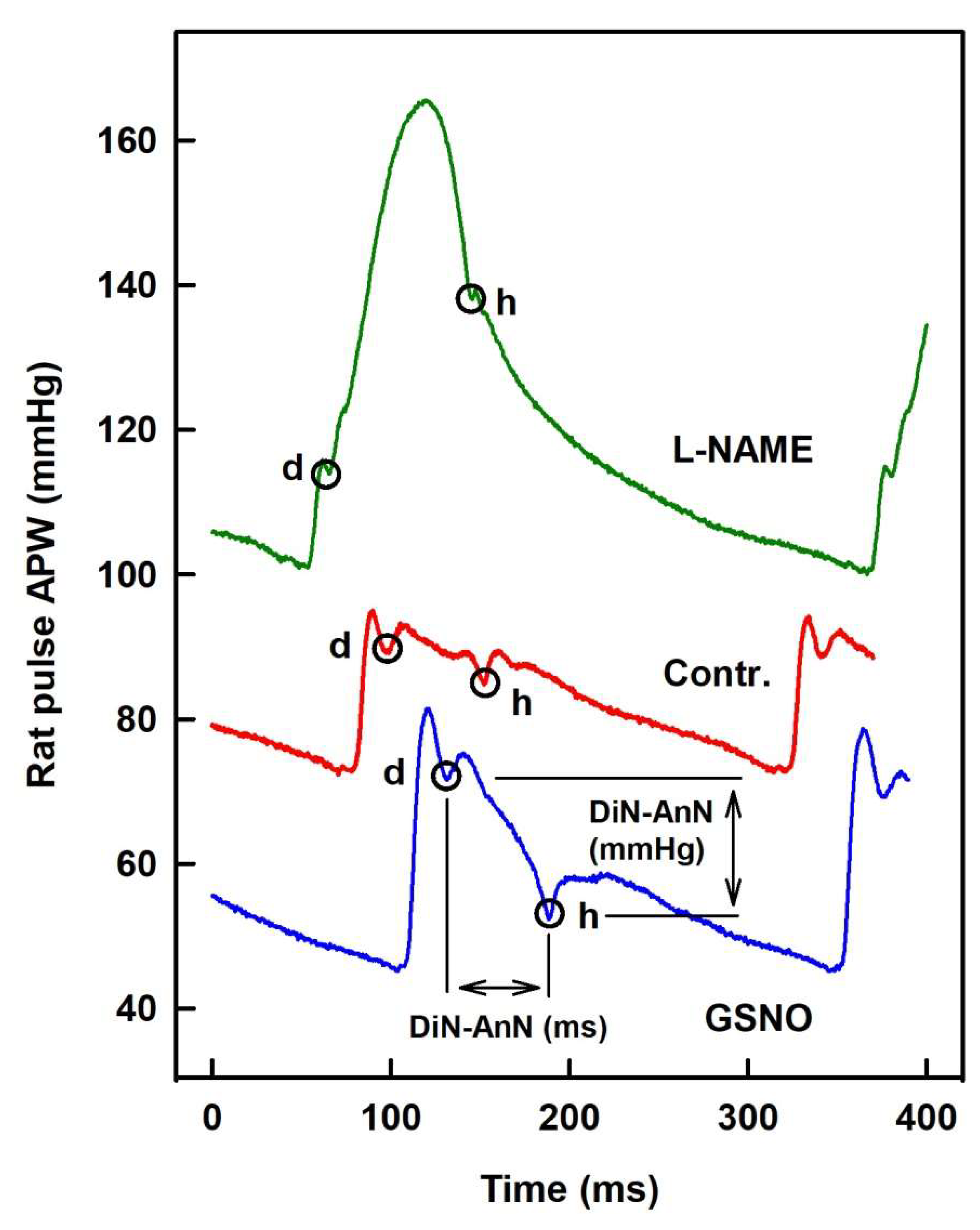
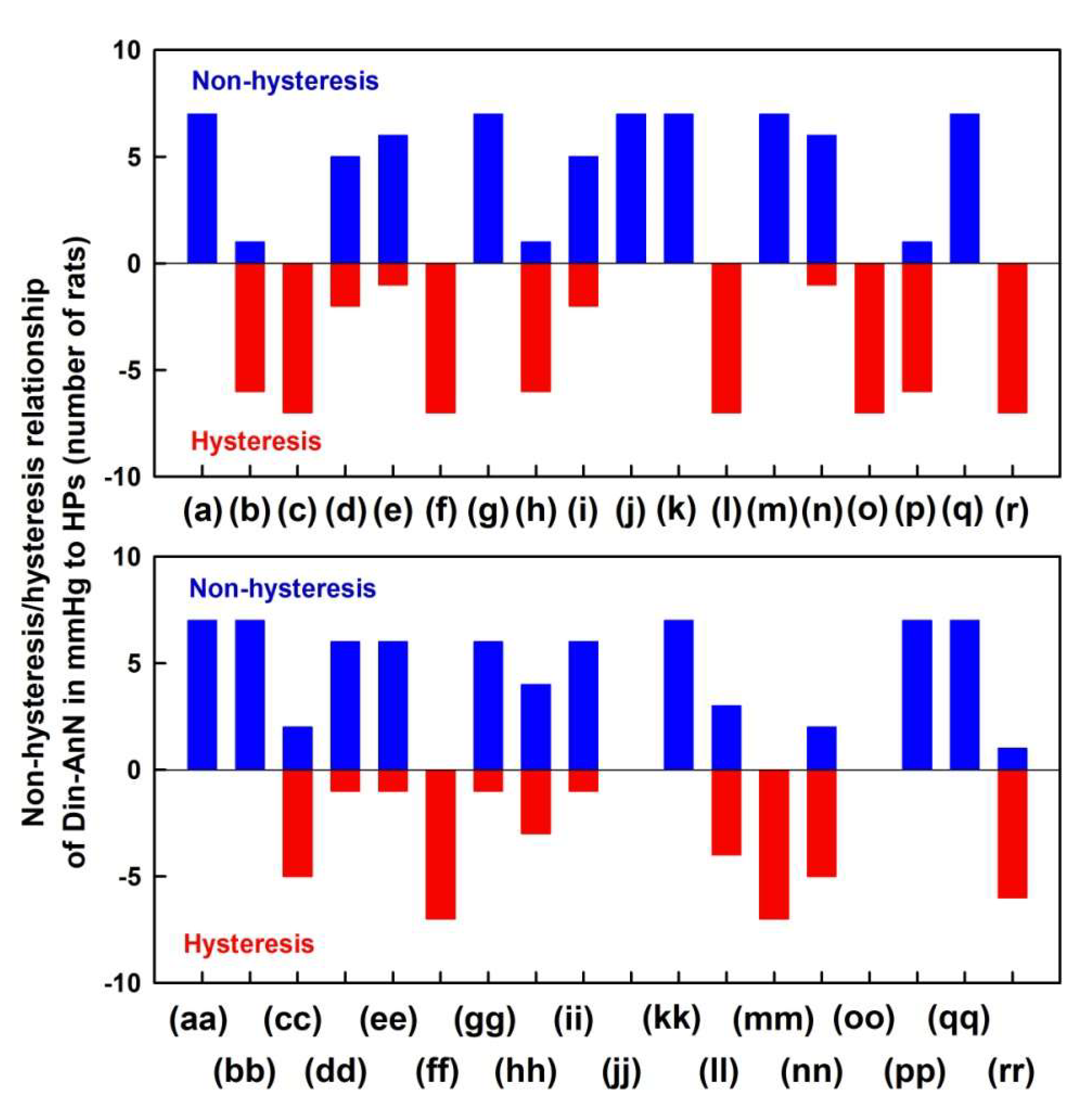
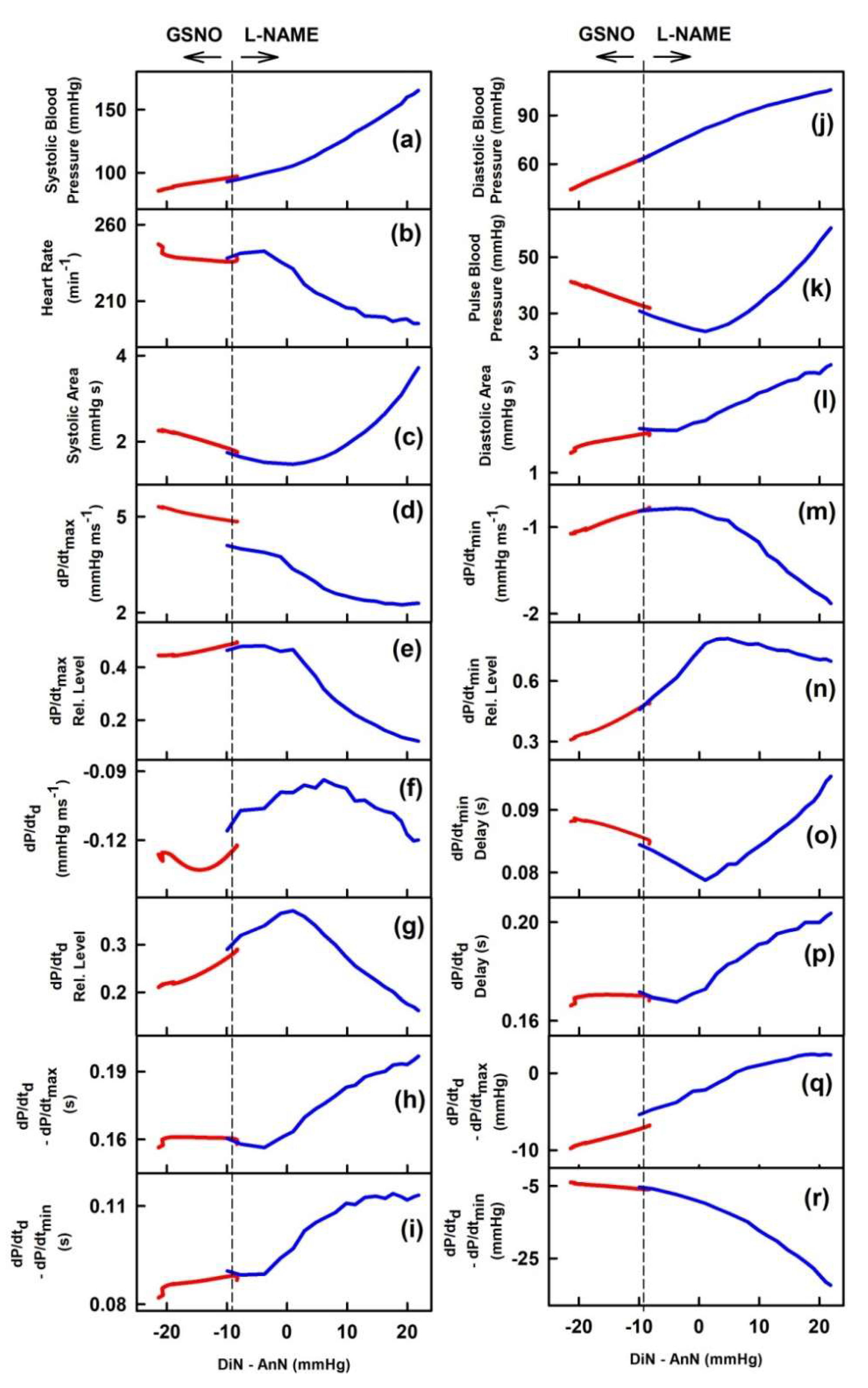
2.1. The Cross-Relationships of 34 HPs to the Difference between BP of Dicrotic and Anacrotic Notches
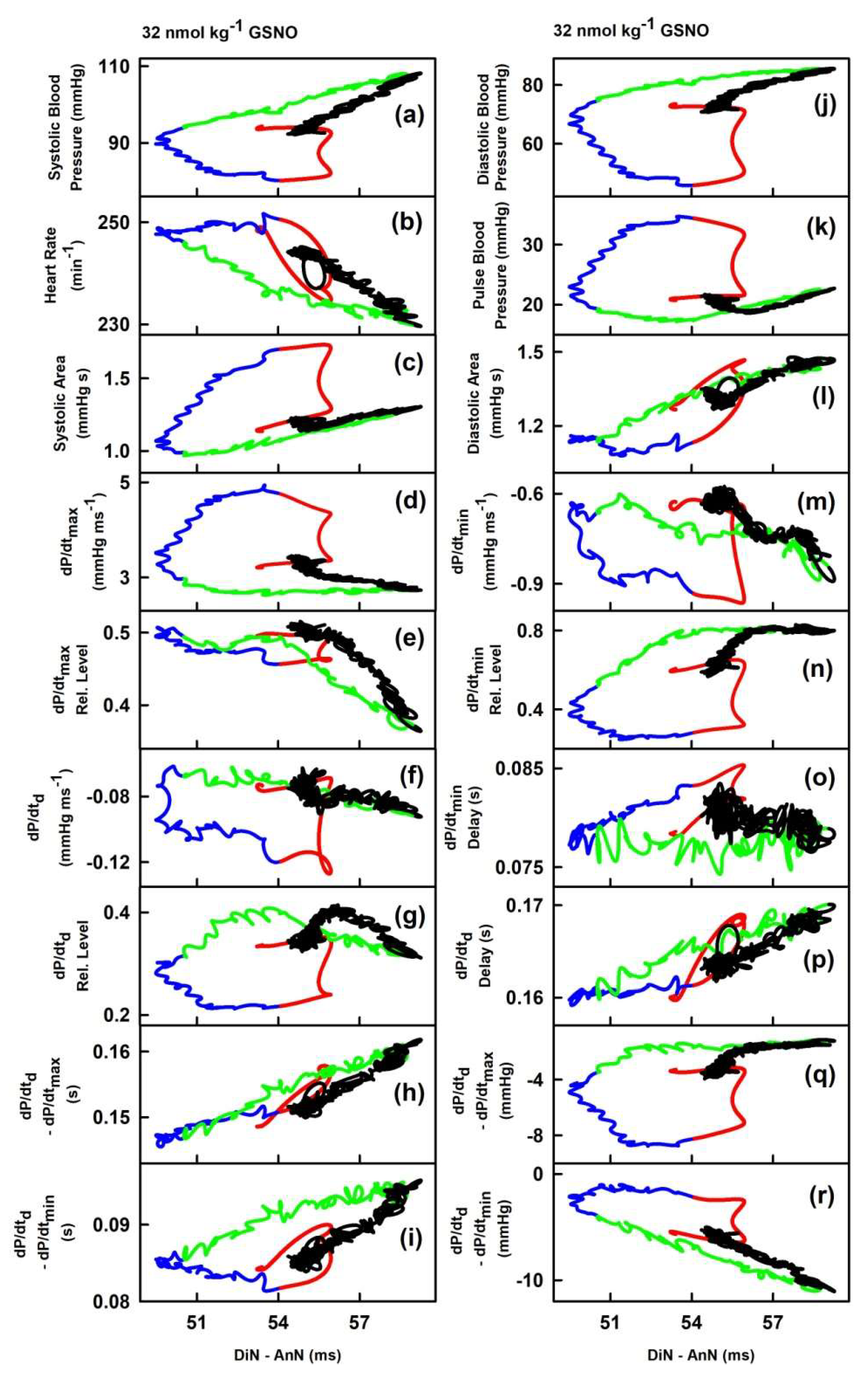
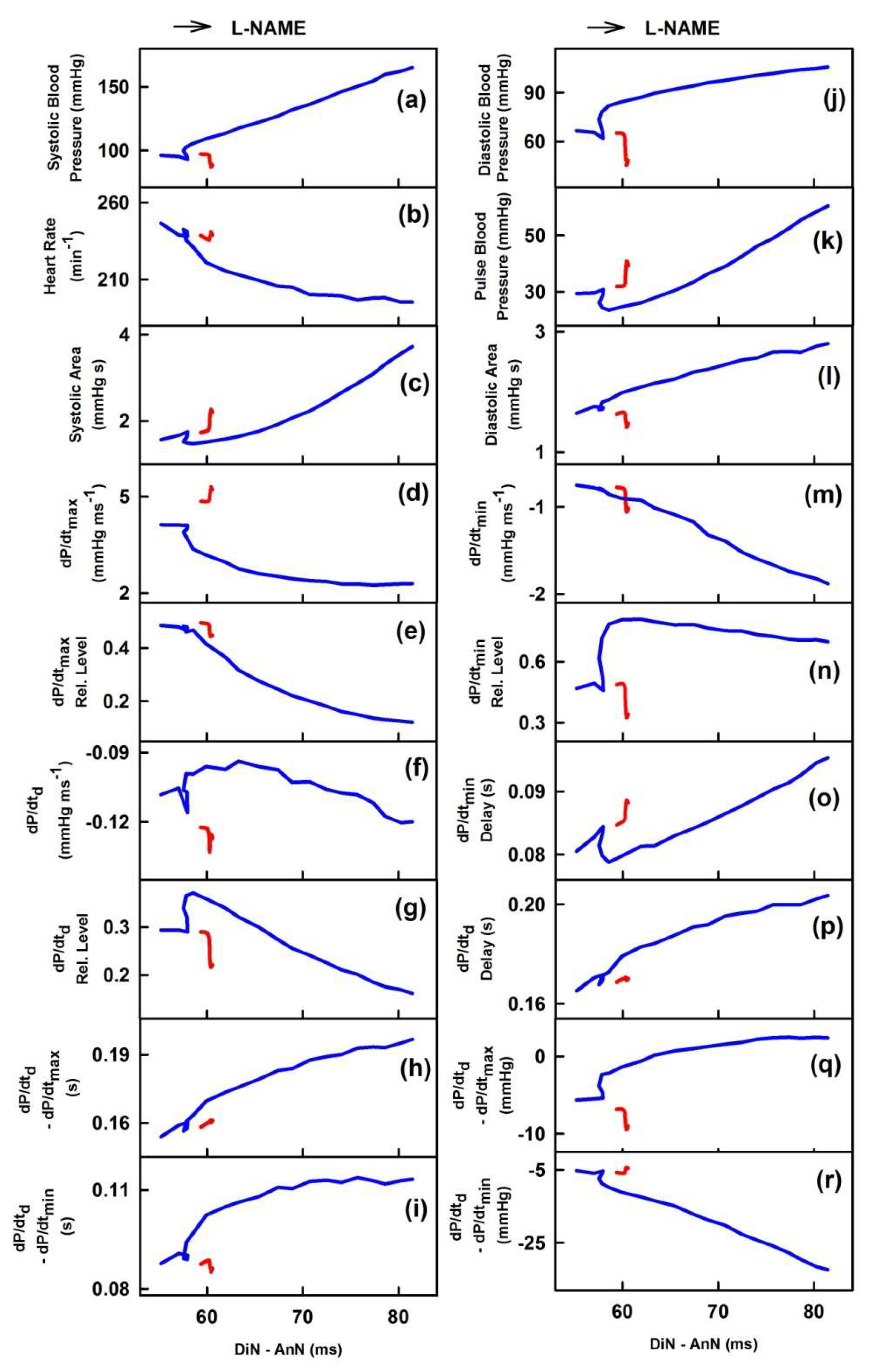
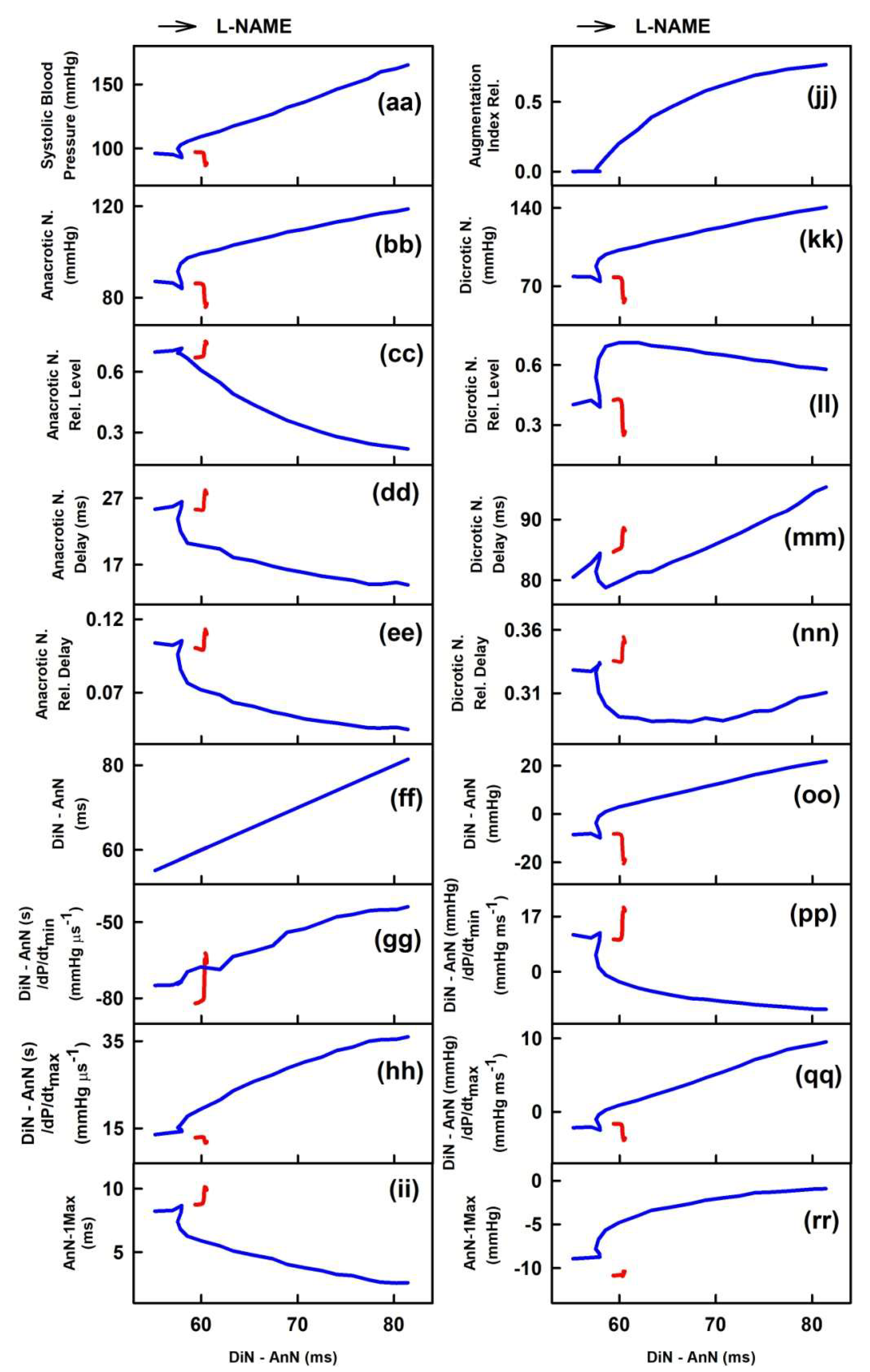
2.2. The Cross-Relationships of 34 HPs to the Time Interval between Dicrotic and Anacrotic Notches
2.3. Cross-Relathionships of DiN-AnN to 34 Rat HPs for Conditions of Increase/Decreased NO Bioavailability
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Animals, APW Measurement, and Data Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AnN | Anacrotic notch |
| APW | Arterial pulse waveform |
| BP | Blood pressure |
| DiN | Dicrotic notch |
| GSNO | S-nitrosoglutathione |
| HPs | Hemodynamic parameters |
| l-NAME | N(ω)-nitro-l-arginine methyl ester |
| NO | Nitric oxide |
References
- Forte, M.; Conti, V.; Damato, A.; Ambrosio, M.; Puca, A.A.; Sciarretta, S.; Frati, G.; Vecchione, C.; Carrizzo, A. Targeting Nitric Oxide with Natural Derived Compounds as a Therapeutic Strategy in Vascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7364138. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.A.; Dirsch, V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide Biol. Chem. 2009, 21, 77–91. [Google Scholar] [CrossRef]
- Barnett, S.D.; Buxton, I.L.O. The role of S-nitrosoglutathione reductase (GSNOR) in human disease and therapy. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 340–354. [Google Scholar] [CrossRef]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Torregrossa, A.C.; Parthasarathy, D.K.; Bryan, N.S. Natural product nitric oxide chemistry: New activity of old medicines. Evid. Based Complementary Altern. Med. eCAM 2012, 2012, 873210. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C.; Marletta, M.A. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 2012, 16, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-nitrosoglutathione. Biochim. Biophys. Acta 2013, 1830, 3173–3181. [Google Scholar] [CrossRef] [Green Version]
- Kopincová, J.; Púzserová, A.; Bernátová, I. l-NAME in the cardiovascular system-nitric oxide synthase activator? Pharmacol. Rep. 2012, 64, 511–520. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; O’Rourke, M. Genesis of the normal and abnormal arterial pulse. Curr. Probl. Cardiol. 2000, 25, 303–367. [Google Scholar] [CrossRef]
- Stoner, L.; Young, J.M.; Fryer, S. Assessments of arterial stiffness and endothelial function using pulse wave analysis. Int. J. Vasc. Med. 2012, 2012, 903107. [Google Scholar] [CrossRef] [Green Version]
- Lekakis, J.P.; Zakopoulos, N.A.; Protogerou, A.D.; Papaioannou, T.G.; Kotsis, V.T.; Pitiriga, V.; Tsitsirikos, M.D.; Stamatelopoulos, K.S.; Papamichael, C.M.; Mavrikakis, M.E. Arterial stiffness assessed by pulse wave analysis in essential hypertension: Relation to 24-h blood pressure profile. Int. J. Cardiol. 2005, 102, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A.P.; Butlin, M.; Walsh, A. Arterial blood pressure measurement and pulse wave analysis--their role in enhancing cardiovascular assessment. Physiol. Meas. 2010, 31, R1–R47. [Google Scholar] [CrossRef] [PubMed]
- Žikić, D. A mathematical model of pressure and flow waveforms in the aortic root. Eur. Biophys. J. 2017, 46, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nier, B.A.; Harrington, L.S.; Carrier, M.J.; Weinberg, P.D. Evidence for a specific influence of the nitrergic pathway on the peripheral pulse waveform in rabbits. Exp. Physiol. 2008, 93, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Rafati, M.; Havaee, E.; Moladoust, H.; Sehhati, M. Appraisal of different ultrasonography indices in patients with carotid artery atherosclerosis. EXCLI J. 2017, 16, 727–741. [Google Scholar] [CrossRef]
- Hao, Y.; Cheng, F.; Pham, M.; Rein, H.; Patel, D.; Fang, Y.; Feng, Y.; Yan, J.; Song, X.; Yan, H.; et al. A Noninvasive, Economical, and Instant-Result Method to Diagnose and Monitor Type 2 Diabetes Using Pulse Wave: Case-Control Study. JMIR mHealth uHealth 2019, 7, e11959. [Google Scholar] [CrossRef] [Green Version]
- Marais, L.; Pernot, M.; Khettab, H.; Tanter, M.; Messas, E.; Zidi, M.; Laurent, S.; Boutouyrie, P. Arterial Stiffness Assessment by Shear Wave Elastography and Ultrafast Pulse Wave Imaging: Comparison with Reference Techniques in Normotensives and Hypertensives. Ultrasound Med. Biol. 2019, 45, 758–772. [Google Scholar] [CrossRef]
- Klein, L.W.; Shahrrava, A. The Incisura. Cardiol. Rev. 2019, 27, 274–278. [Google Scholar] [CrossRef]
- Munir, S.; Guilcher, A.; Kamalesh, T.; Clapp, B.; Redwood, S.; Marber, M.; Chowienczyk, P. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension 2008, 51, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Klocke, R.; Cockcroft, J.R.; Taylor, G.J.; Hall, I.R.; Blake, D.R. Arterial stiffness and central blood pressure, as determined by pulse wave analysis, in rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Takazawa, K.; Kobayashi, H.; Shindo, N.; Tanaka, N.; Yamashina, A. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens Res. 2007, 30, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Misak, A.; Kurakova, L.; Berenyiova, A.; Tomasova, L.; Grman, M.; Cacanyiova, S.; Ondrias, K. Patterns and Direct/Indirect Signaling Pathways in Cardiovascular System in the Condition of Transient Increase of NO. BioMed Res. Int. 2020, 2020, 6578213. [Google Scholar] [CrossRef] [PubMed]
- Kurakova, L.; Misak, A.; Tomasova, L.; Cacanyiova, S.; Berenyiova, A.; Ondriasova, E.; Balis, P.; Grman, M.; Ondrias, K. Mathematical relationships of patterns of 35 rat haemodynamic parameters for conditions of hypertension resulting from decreased nitric oxide bioavailability. Exp. Physiol. 2020, 105, 312–334. [Google Scholar] [CrossRef]
- Politi, M.T.; Ghigo, A.; Fernández, J.M.; Khelifa, I.; Gaudric, J.; Fullana, J.M.; Lagrée, P.Y. The dicrotic notch analyzed by a numerical model. Comput. Biol. Med. 2016, 72, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yang, L.; Wen, W.; Zhang, S.; Hao, D.; Khalid, S.G.; Zheng, D. Quantification of radial arterial pulse characteristics change during exercise and recovery. J. Physiol. Sci. 2018, 68, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Yang, L.; Liu, C.; Cui, J.; Li, Y.; Yang, X.; Zhang, S.; Zheng, D. Athletic differences in the characteristics of the photoplethysmographic pulse shape: Effect of maximal oxygen uptake and maximal muscular voluntary contraction. BioMed Res. Int. 2015, 2015, 752570. [Google Scholar] [CrossRef] [PubMed]
- Kristek, F.; Grman, M.; Ondrias, K. In Vivo Measurement of H(2)S, Polysulfides, and “SSNO(-) Mix”-Mediated Vasoactive Responses and Evaluation of Ten Hemodynamic Parameters from Rat Arterial Pulse Waveform. Methods Mol. Biol. 2019, 2007, 109–124. [Google Scholar] [CrossRef]
- King, A.J.; Pfeffer, J.M.; Pfeffer, M.A.; Brenner, B.M. Systemic hemodynamic effects of endothelin in rats. Am. J. Physiol. 1990, 258, H787–H792. [Google Scholar] [CrossRef]
- Gardiner, S.M.; March, J.E.; Kemp, P.A.; Bennett, T. Bolus injection of human UII in conscious rats evokes a biphasic haemodynamic response. Br. J. Pharmacol. 2004, 143, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Charles, C.J.; Rademaker, M.T.; Richards, A.M. Apelin-13 induces a biphasic haemodynamic response and hormonal activation in normal conscious sheep. J. Endocrinol. 2006, 189, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.C.; Guo, Y.J.; Yu, F.Y.; Chen, L.L.; Li, W.Y.; Ji, E.S. Bosentan ameliorates hypertension in rats exposed to chronic intermittent hypoxia through inhibiting renal sympathetic nerve activity. Sheng Li Xue Bao 2018, 70, 354–360. [Google Scholar] [PubMed]
- Schmidt, R.; Weidner, C.; Schmelz, M. Time course of acetylcholine-induced activation of sympathetic efferents matches axon reflex sweating in humans. J. Peripher Nerv. Syst. 2011, 16, 30–36. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasova, L.; Misak, A.; Kurakova, L.; Grman, M.; Ondrias, K. Characterization of Rat Cardiovascular System by Anacrotic/Dicrotic Notches in the Condition of Increase/Decrease of NO Bioavailability. Int. J. Mol. Sci. 2020, 21, 6685. https://doi.org/10.3390/ijms21186685
Tomasova L, Misak A, Kurakova L, Grman M, Ondrias K. Characterization of Rat Cardiovascular System by Anacrotic/Dicrotic Notches in the Condition of Increase/Decrease of NO Bioavailability. International Journal of Molecular Sciences. 2020; 21(18):6685. https://doi.org/10.3390/ijms21186685
Chicago/Turabian StyleTomasova, Lenka, Anton Misak, Lucia Kurakova, Marian Grman, and Karol Ondrias. 2020. "Characterization of Rat Cardiovascular System by Anacrotic/Dicrotic Notches in the Condition of Increase/Decrease of NO Bioavailability" International Journal of Molecular Sciences 21, no. 18: 6685. https://doi.org/10.3390/ijms21186685
APA StyleTomasova, L., Misak, A., Kurakova, L., Grman, M., & Ondrias, K. (2020). Characterization of Rat Cardiovascular System by Anacrotic/Dicrotic Notches in the Condition of Increase/Decrease of NO Bioavailability. International Journal of Molecular Sciences, 21(18), 6685. https://doi.org/10.3390/ijms21186685




