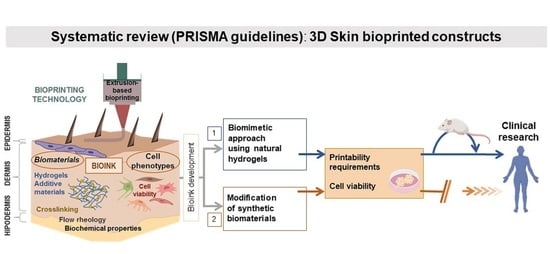
Overview of Current Advances in Extrusion Bioprinting for Skin Applications
Abstract
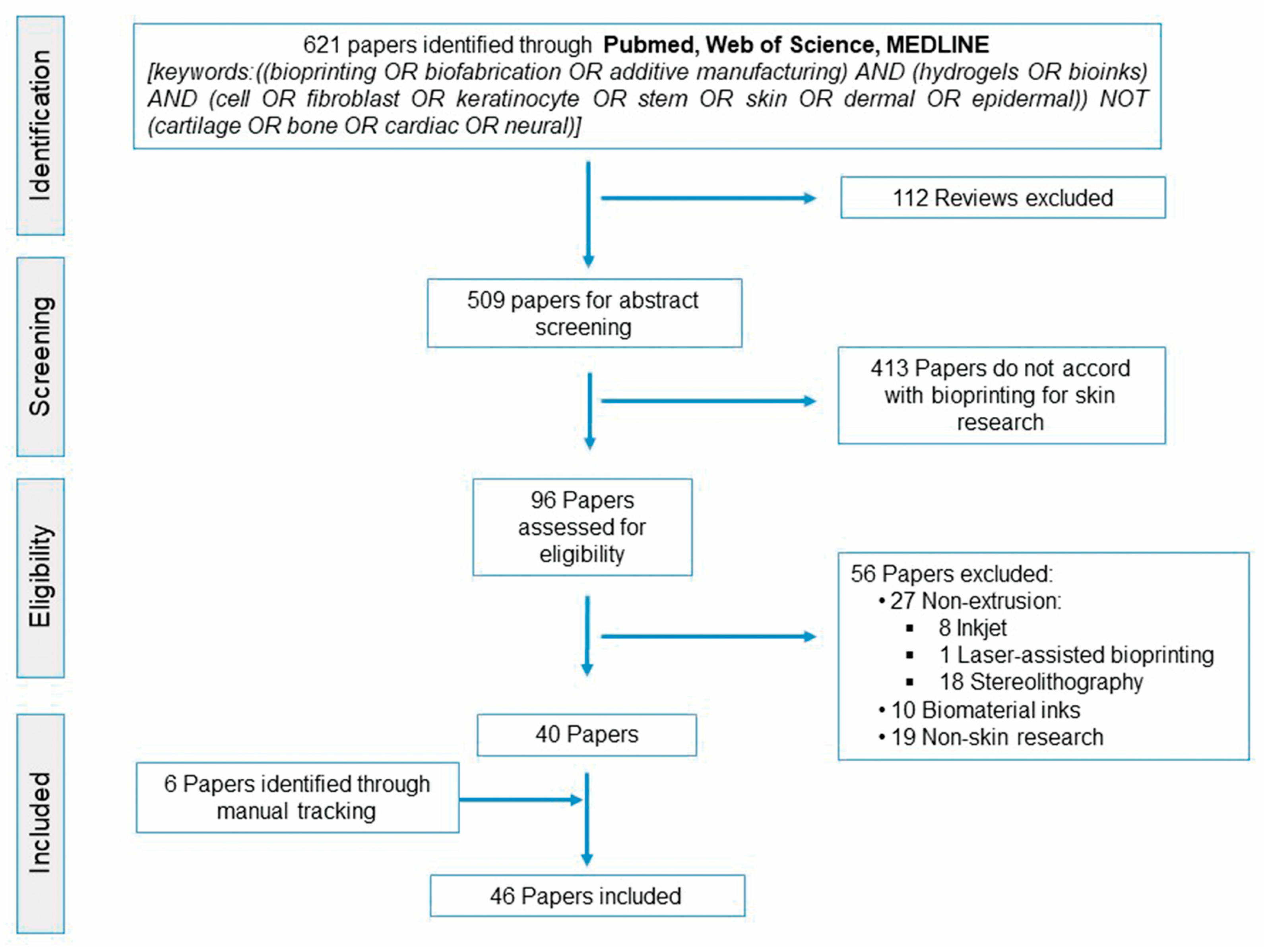
1. Introduction
2. Skin Biology and Relevant Aspects for Bioprinting
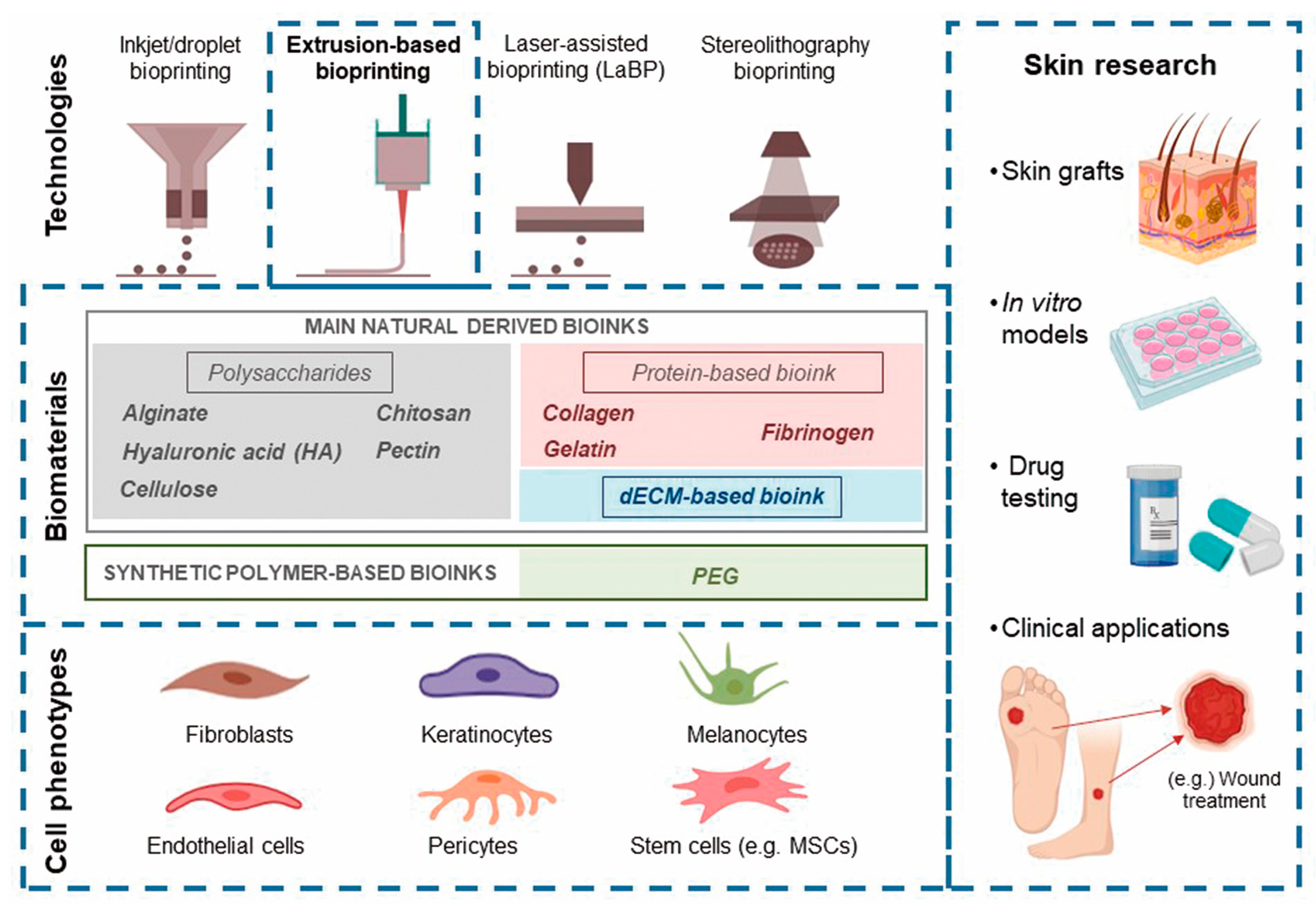
3. Overview of Current Research on Extrusion-Based Skin Bioprinting
3.1. Cells Applied in Skin Bioprinting
3.2. Stem Cell Sources
4. Cell-Laden Bioinks
4.1. Fibrinogen-Based Bioinks
4.2. dECM-Based Bioinks
4.3. Collagen-Based Bioinks
4.4. Alginate-Based Bioinks
4.5. Gelatin-Based Bioinks
4.6. Other Hybrid Bioinks
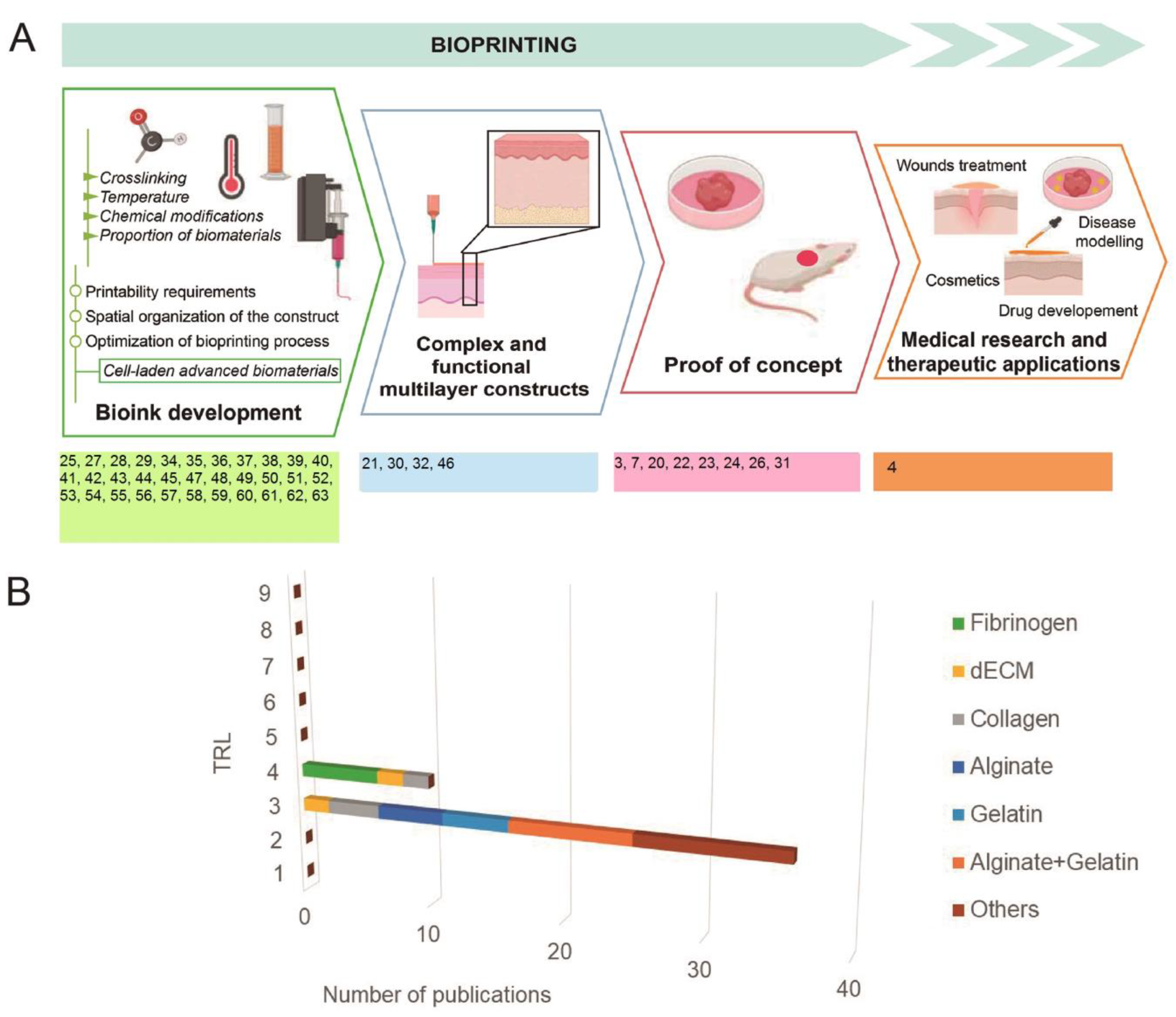
5. Skin Bioprinting, from Bench to Society, Technology Readiness Pathway
6. Limitations and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Michael, S.; Bharti, K.; Ferrer, M.; Song, M.J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020, 12, 035002. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Ooka, M.; Zhang, L.; Song, M.J.; Huang, R.; Kleinstreuer, N.C.; Simeonov, A.; Xia, M.; Ferrer, M. Two-Dimensional Cellular and Three-Dimensional Bio-Printed Skin Models to Screen Topical-Use Compounds for Irritation Potential. Front. Bioeng. Biotechnol. 2020, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P.W. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef]
- Dussoyer, M.; Courtial, E.J.; Albouy, M.; Thepot, A.; Dos Santos, M.; Marquette, C.A. Mechanical Properties of 3D Bioprinted Dermis: Characterization and Improvement; Science Repository OÜ: Lewes, DE, USA, 2019. [Google Scholar]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Randall, M.J.; Jüngel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front. Bioeng. Biotechnol. 2018, 6, 154. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. A contemporary view of platelet-rich plasma therapies: Moving toward refined clinical protocols and precise indications. Regen. Med. 2018, 13, 717–728. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.K.L.; Fernandes, B.L.; de Souza, M.A. Autologous Matrix of Platelet-Rich Fibrin in Wound Care Settings: A Systematic Review of Randomized Clinical Trials. J. Funct. Biomater. 2020, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Andia, I.; Aragón-Sánchez, J.; Herrera-Ramos, E.; Iruzubieta Barragán, F.J.; Serrano-Aguilar, P. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis: Platelet-rich plasma for diabetic foot ulcers. Wound Repair Regen. 2019, 27, 170–182. [Google Scholar] [CrossRef]
- San Sebastian, K.M.; Lobato, I.; Hernández, I.; Burgos-Alonso, N.; Gomez-Fernandez, M.C.; López, J.L.; Rodríguez, B.; March, A.G.; Grandes, G.; Andia, I. Efficacy and safety of autologous platelet rich plasma for the treatment of vascular ulcers in primary care: Phase III study. BMC Fam. Pract. 2014, 15, 211. [Google Scholar] [CrossRef]
- Perez-Zabala, E.; Basterretxea, A.; Larrazabal, A.; Perez-del-Pecho, K.; Rubio-Azpeitia, E.; Andia, I. Biological approach for the management of non-healing diabetic foot ulcers. J. Tissue Viability 2016, 25, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Platelet-rich plasma: Underlying biology and clinical correlates. Regen. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Derr, K.; Zou, J.; Luo, K.; Song, M.J.; Sittampalam, G.S.; Zhou, C.; Michael, S.; Ferrer, M.; Derr, P. Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function. Tissue Eng. Part C Methods 2019, 25, 334–343. [Google Scholar] [CrossRef]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab. Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef]
- Seol, Y.-J.; Lee, H.; Copus, J.S.; Kang, H.-W.; Cho, D.-W.; Atala, A.; Lee, S.J.; Yoo, J.J. 3D bioprinted biomask for facial skin reconstruction. Bioprinting 2018, 10, e00028. [Google Scholar] [CrossRef] [PubMed]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef] [PubMed]
- Won, J.-Y.; Lee, M.-H.; Kim, M.-J.; Min, K.-H.; Ahn, G.; Han, J.-S.; Jin, S.; Yun, W.-S.; Shim, J.-H. A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink. Artif. Cells Nanomed. Biotechnol. 2019, 47, 644–649. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.; Min, K.-H.; Kim, C.; Lee, J.-S.; Kang, D.; Won, J.-Y.; Cho, D.-W.; Kim, J.-Y.; Jin, S.; Yun, W.-S.; et al. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci. Rep. 2017, 7, 8624. [Google Scholar] [CrossRef]
- Kajave, N.S.; Schmitt, T.; Nguyen, T.-U.; Kishore, V. Dual crosslinking strategy to generate mechanically viable cell-laden printable constructs using methacrylated collagen bioinks. Mater. Sci. Eng. C 2020, 107, 110290. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef]
- Attalla, R.; Puersten, E.; Jain, N.; Selvaganapathy, P.R. 3D bioprinting of heterogeneous bi- and tri-layered hollow channels within gel scaffolds using scalable multi-axial microfluidic extrusion nozzle. Biofabrication 2018, 11, 015012. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, T.L.; Zhang, H.B.; Yin, R.X.; Yang, S.M.; Wei, J.; Zhang, W.J. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 2018, 13, 035008. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.-S.; Gao, G.; Cho, D.-W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef]
- Crook, J.M.; Tomaskovic-Crook, E. Bioprinting 3D Human Induced Pluripotent Stem Cell Constructs for Multilineage Tissue Engineering and Modeling. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2140, pp. 251–258. ISBN 978-1-07-160519-6. [Google Scholar]
- Motealleh, A.; Dorri, P.; Schäfer, A.H.; Kehr, N.S. 3D bioprinting of triphasic nanocomposite hydrogels and scaffolds for cell adhesion and migration. Biofabrication 2019, 11, 035022. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.W.; Mota, C.; ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol–Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, L.; Lavrentieva, A.; Pepelanova, I.; Bahnemann, J.; Geier, D.; Becker, T.; Scheper, T.; Beutel, S. Development and Application of an Additively Manufactured Calcium Chloride Nebulizer for Alginate 3D-Bioprinting Purposes. J. Funct. Biomater. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Laude, A.; Yeong, W.Y. Investigation of cell viability and morphology in 3D bio-printed alginate constructs with tunable stiffness: 3D BIO-PRINTED ALGINATE CONSTRUCTS WITH TUNABLE STIFFNESS. J. Biomed. Mater. Res. A 2017, 105, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Dubbin, K.; Hori, Y.; Lewis, K.K.; Heilshorn, S.C. Dual-Stage Crosslinking of a Gel-Phase Bioink Improves Cell Viability and Homogeneity for 3D Bioprinting. Adv. Healthc. Mater. 2016, 5, 2488–2492. [Google Scholar] [CrossRef]
- Tigner, T.J.; Rajput, S.; Gaharwar, A.K.; Alge, D.L. Comparison of Photo Cross Linkable Gelatin Derivatives and Initiators for Three-Dimensional Extrusion Bioprinting. Biomacromolecules 2020, 21, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2017, 29, 1604983. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium Alginate/Gelatine Hydrogels for Direct Bioprinting—The Effect of Composition Selection and Applied Solvents on the Bioink Properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef] [PubMed]
- Compaan, A.M.; Song, K.; Huang, Y. Gellan Fluid Gel as a Versatile Support Bath Material for Fluid Extrusion Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shen, H.; Zhi, Y.; Si, J.; Shi, J.; Guo, L.; Shen, S.G. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf. B Biointerfaces 2019, 181, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning Alginate-Gelatin Bioink Properties by Varying Solvent and Their Impact on Stem Cell Behavior. Sci. Rep. 2018, 8, 8020. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, X.; Khademhosseini, A.; Zhang, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 2018, 10, 024102. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef]
- Montheil, T.; Maumus, M.; Valot, L.; Lebrun, A.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. Inorganic Sol–Gel Polymerization for Hydrogel Bioprinting. ACS Omega 2020, 5, 2640–2647. [Google Scholar] [CrossRef]
- Zidarič, T.; Milojević, M.; Gradišnik, L.; Stana Kleinschek, K.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Gómez-Florit, M.; Hamilton, A.G.; Detamore, M.S.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Human platelet lysate-based nanocomposite bioink for bioprinting hierarchical fibrillar structures. Biofabrication 2019, 12, 015012. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.M.; Sercombe, T.B.; Liew, L.J.; Dilley, R.J.; Doyle, B.J. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Leong, K.F.; Li, L. 3D Bioprinting of Highly Thixotropic Alginate/Methylcellulose Hydrogel with Strong Interface Bonding. ACS Appl. Mater. Interfaces 2017, 9, 20086–20097. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.; Dorati, R.; Scocozza, F.; Mariotti, C.; Chiesa, E.; Bruni, G.; Genta, I.; Auricchio, F.; Conti, M.; Conti, B. Preliminary investigation on a new natural based poly(gamma-glutamic acid)/Chitosan bioink. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2718–2732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, X.; Li, L.; Chen, Z.-N.; Gao, G.; Yao, R.; Sun, W. 3D printing human induced pluripotent stem cells with novel hydroxypropyl chitin bioink: Scalable expansion and uniform aggregation. Biofabrication 2018, 10, 044101. [Google Scholar] [CrossRef]
- Wang, L.L.; Highley, C.B.; Yeh, Y.-C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single- and double-network hydrogels containing dynamic covalent crosslinks: 3D EXTRUSION BIOPRINTING. J. Biomed. Mater. Res. A 2018, 106, 865–875. [Google Scholar] [CrossRef]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Mater. Horiz. 2018, 5, 1100–1111. [Google Scholar] [CrossRef]
- Rutz, A.L.; Gargus, E.S.; Hyland, K.E.; Lewis, P.L.; Setty, A.; Burghardt, W.R.; Shah, R.N. Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties. Acta Biomater. 2019, 99, 121–132. [Google Scholar] [CrossRef]
- Xin, S.; Chimene, D.; Garza, J.E.; Gaharwar, A.K.; Alge, D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019, 7, 1179–1187. [Google Scholar] [CrossRef]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Invest. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Nguyen Thi Phuong, T.; Tien, N.L.B.; Tran, D.K.; Minh, L.B.; Thanh, V.V.; Gia Anh, P.; Pham, V.H.; Thi Nga, V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J. Clin. Med. 2019, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Meng, M.; Wu, J.; Zhang, J.; Hou, Z.; Gao, H.; Xu, H.; Liu, B.; Tang, W.; Jiang, L.; et al. Compared to the amniotic membrane, Wharton’s jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration. Stem Cell Res. Ther. 2017, 8, 72. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. STEM CELLS Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Orive, G.; Andia, I. Delivering growth factors for therapeutics. Trends Pharmacol. Sci. 2008, 29, 37–41. [Google Scholar] [CrossRef]
- Quílez, C.; de Aranda Izuzquiza, G.; García, M.; López, V.; Montero, A.; Valencia, L.; Velasco, D. Bioprinting for Skin. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; pp. 217–228. ISBN 978-1-07-160520-2. [Google Scholar]
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef]
- Moreno-Arotzena, O.; Meier, J.; del Amo, C.; García-Aznar, J. Characterization of Fibrin and Collagen Gels for Engineering Wound Healing Models. Materials 2015, 8, 1636–1651. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, C.; Borau, C.; Movilla, N.; Asín, J.; García-Aznar, J.M. Quantifying 3D chemotaxis in microfluidic-based chips with step gradients of collagen hydrogel concentrations. Integr. Biol. 2017, 9, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, C.; Perez-Valle, A.; Perez-Zabala, E.; Perez-del-Pecho, K.; Larrazabal, A.; Basterretxea, A.; Bully, P.; Andia, I. Wound Dressing Selection Is Critical to Enhance Platelet-Rich Fibrin Activities in Wound Care. Int. J. Mol. Sci. 2020, 21, 624. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Enhanced cell functions on graphene oxide incorporated 3D printed polycaprolactone scaffolds. Mater. Sci. Eng. C 2019, 102, 1–11. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.-S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthc. Mater. 2015, 4, 1359–1368. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Medronho, B.; Antunes, F.E.; Fernández-García, M.P.; Ventura, J.; Araújo, J.P.; Romano, A.; Lindman, B. Unusual extraction and characterization of nanocrystalline cellulose from cellulose derivatives. J. Mol. Liq. 2015, 210, 106–112. [Google Scholar] [CrossRef]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y.W. Signaling Properties of Hyaluronan Receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Baier, C.; Baader, S.L.; Jankowski, J.; Gieselmann, V.; Schilling, K.; Rauch, U.; Kappler, J. Hyaluronan is organized into fiber-like structures along migratory pathways in the developing mouse cerebellum. Matrix Biol. 2007, 26, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player: Hyaluronan in wound healing. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, B.; Zhang, C.; Wysk, R.A.; Chen, Y.-W. Bioprinting: An assessment based on manufacturing readiness levels. Crit. Rev. Biotechnol. 2017, 37, 333–354. [Google Scholar] [CrossRef] [PubMed]
- MedicalCountermeasures. Available online: https://www.medicalcountermeasures.gov/trl/integrated-trls/ (accessed on 31 August 2020).
- Naveau, A.; Smirani, R.; Catros, S.; de Oliveira, H.; Fricain, J.-C.; Devillard, R. A Bibliometric Study to Assess Bioprinting Evolution. Appl. Sci. 2017, 7, 1331. [Google Scholar] [CrossRef]
- Costa, P.F. Translating Biofabrication to the Market. Trends Biotechnol. 2019, 37, 1032–1036. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-Electrospinning and Its Application for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, B.; Xu, F. Recent Advances in 4D Bioprinting. Biotechnol. J. 2020, 15, 1900086. [Google Scholar] [CrossRef]
- Wu, Y.; Ravnic, D.J.; Ozbolat, I.T. Intraoperative Bioprinting: Repairing Tissues and Organs in a Surgical Setting. Trends Biotechnol. 2020, 38, 594–605. [Google Scholar] [CrossRef]
| Fibrinogen | ||||||
| Author Reference | Biomaterial | Cell Phenotypes (Source, Density (cell/mL)) | Rheology | Bioprinting Conditions | Post-Printing Processing | Bioprinted Construct Application/Evaluation |
| Jorgensen A.M. 2020 [20] | Fibrinogen (30 mg/mL), Glycerol (100 µL/mL), Gelatin (35 mg/mL), HA (3 mg/mL), Aprotinin (40 µg/mL) | Epidermis: (ratio 9:1) hKCs, hMCs. Dermis: hFBs, FDPCs, hDMECs. Hypodermis: pre-adipocytes. Total cell concentration: 20 × 106 | NO | Extrusion: Pneumatic; N. extrusors: Three; Nozzle Ø: 500 μm metal; Pressure: 60~90 kPa | Thrombin (20 IU/mL, 60 min, RT) | Proof-of-concept validation of full-thickness bioprinted skin constructs for wound closure. Testing and evaluation of printed skin grafts in mice. Construct evaluation: -SEM: analysis of the structure and morphology of the construct. Histology: H&E, Masson’s trichrome, and picrosirius red. Immunostaining: Lamin A + C, Pan-cytokeratin, Mel5, CD146, adiponectin, vimentin, ZO-1, keratin71 |
| Liu X. 2020 [4] | Fibrinogen (2.5 mg/mL), NovoGel component 2 (60 mg/mL) | Epidermis: hNHKs (2 × 105 cell/cm2)—manually seeded; Dermis: hNFBs (8 × 106), hiPSC derived endothelial cells (7 × 106), placental microvascular hPCs (0.7 × 106) | NO | Nozzle Ø: 250 µm | Thrombin (1 U/mL, 24 h) | Bioprinting of vascularized full-thickness skin tissue equivalent of atopic dermatitis model for preclinical studies. Construct evaluation: Trans-epidermal electrical resistance measurement. Histology: H&E. Immunostaining: human collagen IV, laminin 5, integrin β, filaggrin, KRT10, loricrin, E-cadherin, CD-31, phalloidin, desmoglein, claudin-1. Cytokine measurement: ICAM, VCAM, VEGF-A, VEGF-C, VEGF-D |
| Derr K. 2018 [21] | Basal layer: Laminin/Entactin (1.61 mg/mL in DMEM); Dermis: Fibrinogen (7.7 mg/mL), Gelatin (0.045 mg/mL), Collagen I (4 mg/mL), Elastin (0.55% v/v) | Epidermis: hNKCs (6.15 × 106); Dermis: hNFBs (2 × 106) | NO | Epidermis: Extrusion: pneumatic; Basal layer: Extrusion: jetting; Dermis: Extrusion: plunger; N. Extrusors: three | Thrombin (5 U/mL, 1.5 h, RT) | Fabrication of morphologically and physiologically relevant skin substitutes. Construct evaluation: Histology: H&E. Immunostaining: collagen I, collagen VII, Ki67, cytokeratin 15, ZO-1, claudin 1, e-cadherin, phalloidin, filaggrin. OCT imaging. Permeability. Barrier function |
| Hakimi N. 2018 [22] | Epidermis: Fibrinogen (2.5%), HA (0.25%); Dermis: Fibrinogen (1.25%), HA (0.25%), Collagen I (2.5 mg/mL), Alginate (1%) | Epidermis: hKCs (1.5 × 106); Dermis: hFBs (4 × 105) | YES | Speed: 0.3–1.6 cm2/s | Thermal gelation, 30 min, CaCl2 (10 mM), Thrombin (50 IU) | Development of handheld printer for in situ bioprinting. Proof-of-concept in mice and porcine wound model. Construct evaluation: SEM for surface microstructure. Histology: H&E. Immunostaining: phalloidin, F-actin, keratin 14, keratin 10, α-SMA |
| Seol Y.J. 2018 [23] | Fibrinogen (20 mg/mL), Gelatin (30 mg/mL), HA (3 mg/mL), Glycerol (10% v/v) | Epidermis: hKCs (1 × 107); Dermis: hFBs (5 × 106) | NO | Extrusion: Pneumatic; Nozzle Ø: Teflon 300 µm; Pressure: 60 kPa | Thrombin (20 U/mL) | Bioengineered skin substitute combined with a wound dressing layer for facial wounds; Construct evaluation: Wound contraction measure in vivo. Histology: H&E |
| Cubo N. 2017 [24] | Dermis: Fibrinogen (2.3 mg/mL); Tranexamic acid, CaCl2 (0.1%) | Epidermis: hKCs (6 × 106); Dermis: hFBs (1.75 × 104) | NO | Extruders: two; Flow: 12 mL/min | Dermis: 37 °C, 30 min | Functional human bi-layered skin tested in immunodeficient mice model; Construct evaluation: Histology: H&E. Immunostaining: vimentin, keratin 5, keratin 10, filagrin, collagen VII, SMA |
| Decellularized Extracellular Matrix (dECM) | ||||||
| Reference | Biomaterial | Cell Phenotypes (Source, Density (cell/mL)) | Rheology | Bioprinting Conditions | Post-Printing Processing | Bioprinted Construct Application/Evaluation |
| Kim B.S. 2019 [7] | Dermis: s-dECM (1.5%), Fibrinogen (10 mg/mL), NaCl (1.1%), Aprotinin (5 µg/mL); Vasculature: Gelatin (10%), Glycerol (10%), Thrombin (100 U/mL); Hypodermis: a-dECM (2%), Fibrinogen (10 mg/mL), NaCl (1.1%), Aprotinin (5 µg/mL) | Epidermis: hKCs (5 × 106); Dermis: hFBs (5 × 105); Vasculature: HUVECs (1 × 107); Hypodermis: Pre-adipocytes (1 × 106) | NO | Hypodermis, Dermis and Vasculature: Extrusion; Epidermis: Inkjet | Dermis and hypodermis: (I) Sprayed thrombin (100 U/mL), (II) 30 °C, 10 min, III) 37 °C, 30 min | Development of a novel printing platform for a full-thickness skin model using dECM with a vascular channel. Construct evaluation: Histology: H&E, Masson’s trichrome., Immunostaining: CD31, keratin 10, filaggrin, laminin, collagen type I, fibronectin, BODIPY, p63, keratin 19, Ki67. Permeability of vascular channel |
| Won J.Y. 2019 [25] | dECM (2–3%) | hFBs (1.5 × 106 cells) | YES | Nozzle Ø: 500 µm | 37 °C, 30 min | Promotion of skin regeneration as well as the survival and proliferation of skin-derived cells by the application of dECM cell-laden bioink to form skin substitutes. Construct evaluation: Microarrays for gene expression of ECM, skin development and morphology |
| Kim B.S. 2018 [26] | Dermis and Hypodermis: Porcine s-dECM, Acetic acid (0.5 M), Pepsin | Epidermis: hKCs (6 × 106); Dermis: hFBs (5 × 105), EPCs (2.5 × 106); Hypodermis: hASCs (2.5 × 106) | YES | Epidermis: Inkjet; Nozzle Ø: Epidermis: 120 µm, Dermis and Hypodermis: 600 µm | 37 °C, 30 min | Fabrication of human full-skin pre-vascularized equivalent using dECM by printing different layers. Construct evaluation: Transepithelial electrical resistance, -Water permeability of the construct. SEM for bioink microstructure. Histology: H&E, Masson’s trichrome, Alcian blue. Immunostaining: keratin 10, involucrin, collagen type-I, fibronectin, decorin, laminin. Gene expression: collagen type-I, fibronectin, decorin, collagen type-III, vimentin, keratinocyte growth factor. In vivo wound healing. In vivo construct histology: H&E, re-epithelialization. In vivo construct immunostaining: CD31, cytokeratin. In vivo blood flow measurement |
| Ahn G. 2017 [27] | s-dECM (2.5%) Acidic pepsin | mFBs | YES | Extrusion: pneumatic; Nozzle Ø: 250 µm, Pressure: 60 kPa, Speed: 125 mm/min | During printing: Heating the nozzle and bed at 37 °C | Development of printing strategy of cell-laden dECM constructs by inducing simultaneous gelation. Construct evaluation: SEM to measure pore size. Immunostaining: F-actin |
| Collagen | ||||||
| Reference | Biomaterial | Cell Phenotypes (Source, Density (cell/mL)) | Rheology | Bioprinting Conditions | Post-Printing Processing | Bioprinted Construct Application/Evaluation |
| Baltazar T. 2020 [3] | Epidermis: KGM, Skin differentiation medium; Dermis: Collagen I (3.5 mg/mL), FBS (5%), pH reconstruction buffer (1X- 290 µL), HAM-F12 medium (290 µL) | Epidermis: hKCs (1 × 106); Dermis: hFBs (7 × 105), hECs (7 × 105), hPCs (3.5 × 105) | NO | Extrusion: Pneumatic; Epidermis: Nozzle Ø: 100 µm; Pressure: 35 kPa for 54 s; Dermis: Nozzle Ø: 150 µm, Temp: 4 ºC, Pressure: 50 kPa for 205 s | 37 °C | Fabrication of 3D bioprinted bilayered skin grafts. Construct evaluation: Histology: H&E. Immunostaining: filaggrin, cytokeratin 14, cytokeratin 10, collagen type-IV, Ki67, laminin 5, CD31. Endothelial network stability. In vivo graft histology: H&E, vascularization. In vivo graft immunostaining: cytokeratin 14, cytokeratin 10, Lectin I, GSL-B4, laminin 5, CD31, F4/80, involucrin. In vivo vascularization by perfusion |
| Kajave N.S. 2020 [28] | CMA (3 mg/mL), VA-086 photoinitiator (1%) | hMSCs (1 × 105) | YES | Nozzle Ø: 210 µm, Flow: 5 mm/s | (I) UV (365 nm/17 mW/cm2, 1 min); (II) Genipin (0.5 mM or 1 mM, 1 h, 37 °C) | Development of stable and printable CMA hydrogels with dual crosslinking process |
| Osidak E.O. 2019 [29] | Collagen ViscollTM (collagen I, 20, 30, and 40 mg/mL) neutralized in acetic acid (20 mM) | mFBs (0.5 × 106) | YES | Nozzle Ø: 250 µm, Temp: 15 °C, Flow: 5 mm/min | Printing bed at 37 °C for instant gelification | Adaptation of commercial Viscoll collagen to a bioink for 3D bioprinting of cell-laden constructs |
| Attalla R. 2018 [30] | CaCl2 (100 mM); Alginate (0.5%), Collagen (2.5 mg/mL) or Alginate (1%), Fibrinogen (25 mg/mL) | HUVEC + RFP; mFBs + GFP; Cell concentration: 2 × 106 | YES | N. extrusors: three. (I) Nozzle Ø: 260 µm; (II) Nozzle Ø: 630 µm; (III) Nozzle Ø: 830 µm. Flow: 1–6 mL/min; Speed: 1–16 m/min | Fibrinogen bioink: Thrombin (250 U/mL, 30 min) | Fabrication of complex heterogeneous bi- and tri-layered hollow channels within multi-layered scaffolds using multi-axial nozzle. Construct evaluation: Cell distribution in the hollow channels |
| Shi Y. 2018 [31] | Collagen I-rat (8%), GelMA (5%), Tyrosinase (300 U/mL), I2959 photoinitiator (0.1%) | hMCs (3 × 104), hKCs (1 × 106), hFBs (1 × 106) | YES | Nozzle Ø: 200 µm, Temp: 17 °C, Pressure: 0.8–1.2 bar, Speed: 7–10 mm/s | UV (365 nm, 40 s) | Development of skin substitutes with GelMA bioink doped with tyrosinase enhancing the wound closure in vivo (rats) and prevention of scar formation. Construct evaluation: SEM: cell morphology. Histology: H&E. Wound closure measurement |
| Kim B.S. 2017 [32] | Dermis: Collagen I-porcine skin (2%) | Epidermis: hKCs (1 × 106); Dermis: hFBs (2 × 105) | NO | Epidermis: Inkjet; Dermis: Extrusion: pneumatic. Pressure: 5–200 kPa | 37 °C, for at least 30 min | Development of a hybrid and versatile 3D direct cell-printing system for human skin model biofabrication. Construct evaluation: Histology: H&E. Immunostaining: collagen type I, keratin 10, involucrin. Epidermis thickness |
| Alginate | ||||||
| Reference | Biomaterial | Cell Phenotypes (Source, Density) | Rheology | Bioprinting Conditions | Post-Printing Processing | Bioprinted Construct Application/Evaluation |
| Crook J.M. 2020 [33] | Alginate (5% w/v), Carboxymethyl chitosan (5% w/v), Agarose (1.5% w/v) | iPSCs (20–40 × 106 cells) | NO | Needle Ø: 19 G, 1 mL syringe, Pressure: 0.3 bar, Speed: 9 mm/s, Temp: 15 °C | CaCl2 (2% w/v, 10 min, RT) | Immunophenotyping (OCT4, SSEA4, TRA-1-‘60, TRA-1-81), Cell viability |
| Motealleh A. 2019 [34] | Alginate and Nanocomposites (DXPPMO-L-Asp-Alg and DXPPMO-D-Asp-Alg) | hDFs and mFBs (10,000 cells) | NO | Not reported | CaCl2 (22.5 M, 10 min) | 3D bioprinted triphasic chiral nanocomposite hydrogels to study the effect of the addition of nanocomposites and the chirality of enantiomers in cell activities. Cell morphology, adhesion, and migration |
| Ooi H.W. 2018 [35] | Alginate (2%), 5-Norbornene-2-methylamine and RGD Peptide Sequence (CGGGRGDS); photoinitiator and PEG linker | MFBs, ATDC5 Chondrocytes | YES | Metal needle Ø: 25 G Speed: 10 mm/s, Pressure: 30 kPa | UV (365 nm, 10 mW/cm2, 60 s) | Development of bioink with modified alginate, allowing its printability with low alginate concentration and high cell viability |
| Raddatz L. 2018 [36] | Alginate (0.5, 1, 2, 3 and 4% w/v) | hASCs and mFBs-GFP (5 × 106 cells/mL) | YES | Nozzle Ø: 0.256 mm Temp. platform: 37 °C Temp. syringes: RT, Pressure: 90.3 mPa | CaCl2 (500 mM, nebulized) | Development of a calcium chloride nebulizer to reduce the negative impact of high concentrations of CaCl2 on cell-laden bioinks |
| Shi P. 2017 [37] | Alginate (2%, 5%, and 10%) | mFBs (5 × 106 cells/mL) | YES | Nozzle Ø: 27 G | CaCl2 (100 mM, 5 min) | Analysis of the effect of hydrogel stiffness on cell activities of fibroblast in bioprinted cell-laden alginate hydrogels |
| Dubbin K. 2016 [38] | Alginate (2%), P1 peptide (2 mg) and C7 protein polymer (10%) | mFBs and hASCs (10 × 106 cells/mL) | YES | Blunt-tipped nozzle Ø: 32 G, Pressure: 10 psi, Speed: 4 mm/s | CaCl2 (10 mM, 10 min) | Study of the effect of two crosslinking processes in two component bioink to ensure high cell viability |
| Gelatin | ||||||
| Reference | Biomaterial | Cell Phenotypes (Source, Density) | Rheology | Bioprinting Conditions | Post-Printing Processing | Bioprinted Construct Application/Evaluation |
| Tigner T.J. 2020 [39] | GelNB (10% w/v), GelMA (10% w/v), LAP or I2959 (4.46 mM) | mFBs (2 × 106 cells/mL) | YES | Needle Ø: 18 G; 5, 10 and 15 mm/s printing speed; 2–4 mm/s extrusion speed | Continuous exposure to UV (365 nm), Intensity: 5 mW/cm2 (LAP); 20 mW/cm2 (I2959) | Comparative analysis of photocrosslinkable gelatin derivatives (GelNB vs. GelMA) combined with different photoinitiators (LAP vs. I2959) |
| Pepelanova I. 2018 [40] | GelMA (5% w/v) and AlgHEMA (0, 1, 3% w/v) or SiNPs (0, 1, 2% w/v) | HASCs (1.5 × 106 cells/mL) | YES | Needle Ø: 0.40 mm, Pressure: 2.8–3.8 psi, Temp: 30 °C or 37 °C, Speed: 260 mm/min | UV (365 nm, 1.2 J/cm2, 25 °C) | Improvement of extrusion bioprinting by adding biocompatibles additives to increase the hydrogel viscosity (SiNPs and the novel AlgHEMA). Hydrogel brings a cell-promoting microenvironment for hADSCs |
| Liu W. 2017 [41] | GelMA (3%, 4%, 5%) and Photoinitiator (0.5%) | HUVECs (4 × 106 cells/mL) | YES | Cone-shaped nozzles and straight nozzle, Ø: 27 G; Temp: 21 °C; Speed: 400 mm/min 100 µL/min feeding rate | UV (3.95 W/cm2, 30 s) | Development of GelMA constructs that support cell viability, survival, and spreading |
| Ouyang L. 2017 [42] | MeHA (2.5 wt %), NorHA (2 wt %), GelMA (5 wt %), PEGDA (5 wt %), I2959 or LAP photoinitiator (0.05 wt %) | mFBs (2.5 × 106 cells/mL) | NO | Coaxial system: Core needle Ø: 23/24 G; shell needle Ø: 18 G; Flow rate: 0.4 mL/h | In-situ crosslinking UV (10–15 mW/cm2) or visible light | Development of a extrusion technology to print simple or complex filaments (core/shell) using a general strategy for photocrosslinkable hydrogels |
| Rutz A.L. 2015 [43] | Gelatin type A (5% w/v), Fibrinogen (3% w/v), TGFß (5% w/v), 4-arm PEG amine (20% w/v) and GelMA (10% w/v) | HDFs, HUVECs, hMSCs | YES | Nozzle Ø: 200 μm Pressure: 1–2.5 bar Speed: 5 mm/s (1–2 h of bioink incubation prior to printing at 37 °C) | (I) UV (365 nm, 15–20 mW/cm2, 10 min); (II) Thrombin (10 U/mL) and CaCl2 (40 mM) for 30 min | Development of versatile and cell-compatible bioink printing method for creating soft, printable gels from a variety of synthetic and natural polymers |
| Alginate + Gelatin | ||||||
| Reference | Biomaterial | Cell phenotypes (Source, Density) | Rheology | Bioprinting Conditions | Post-Printing Processing | Bioprinted Construct Application/Evaluation |
| Bociaga D. 2019 [44] | Alginate (5% w/v) gelatin (3–4% w/v) | hECs | YES | Flat-tip needle Ø: 430 µm, length: 16 mm) Temp: 34 °C, 37 °C, 40 °C. Thickness: 0.35 mm | CaCl2 (2%) | Control of mechanical properties, cell survival after extrusion, and degradation rate of hydrogels prepared in water vs. [DMEM + 10% FBS] |
| Compaan A.M. 2019 [45] | (I) Gelatin (5–10% w/v) and Alginate (2% w/v); (II) Gellan (0.5%), Gelatin (4%) and CaCl2·2H2O (0.1% w/v) (various gellan fluid bath formulations) | mFBs (5 × 106 cells/mL) | YES | Gellan bath enabled extrusion bioprinting; Stainless steel tips Ø: 23 G, Speed: 2.5–10 mm/s; Thickness: 0.1–0.15 mm | Enzyme-mediated covalent crosslinking: TG (37 °C, 45 min); Alginate structures: CaCl2, 2 h PEGDA structures: UV, 15 min | Analysis of the versatility and advantages of using gellan gum-based fluid gel formulations as a support bath material for the bioprinting of 3D hydrogels and the addition of TG for the gelation of native gelatin. Analysis of postprinting stability with different crosslinking protocols. Living fibroblasts spread and multiply, cell extension and cell–cell contacts better with bioink II (Gellan) |
| Liu P. 2019 [46] | Alginate (2 wt %), Gelatin (15 wt %) | hAECs, WJMSCs 1 × 106 cells/mL | YES | Pressure: 0.2 Mpa, Nozzle Ø: 0.33 μm, Speed: 7 mm/s, Temp: 30 °C | During printing: Instantaneous gelation at 4 °C; After printing: CaCl2 bath (2 wt %, 30 min, RT) | Cell phenotypes, gene expression microarrays: differentially expressed genes hAECs vs. hWJMSCs. Human AECs superior epithelial cells phenotype, WJMSCs superior angiogenic potential and fibroblastic phenotype. Uniform cell distribution. Cell viability > 95% |
| Giuseppe M.D. 2018 [47] | Performance of different alginate/gelatin blends, i.e., 9% Alg/6% Gel; 5% Alg/10% Gel; 7% Alg/8% Gel | sMSCs | YES | Nozzle Ø: 27 G, Speed: 5 mm/s, Temp: 25 °C | CaCl2 (300 mM, 15 min) | Optimized printability with alginate (7%)/gelatin (8%) (POI determination). Compressive modulus. Cell survival 92% |
| Li Z. 2018 [48] | Alginate (2.4%) Gelatin (12%) with varying solvent strengths | mESCs (1 × 107 cells) | YES | Pre-cooling of the bioink at 0 °C for 30 min, Printing temp: 10 °C | CaCl2 (10%, 0 °C, 10 min) | Description of the effect of solvents on printability, mechanical properties, and cell behavior (viability, proliferation, aggregation, differentiation). Bioink designed for regenerating sweat glands |
| Liu W. 2018 [49] | Sheath: Alginate (1%), Core: GelMA, photoinitiator (0.2%) and CaCl2 (1%) | HUVECs, MCF-7, mFBs | YES | Coaxial system. 23 G core Ø: 23 G, Ø sheath: 28 G. Speed: 500 mm/min | UV (3.95 W/cm2) | Development of cell-laden constructs at low concentrations of GelMa (< 2%) Mechanical properties. Cell survival and proliferation |
| He Y. 2016 [50] | Alginate (2.5%) and Gelatin (8%) | L929 mFBs (1 × 106 cells/mL) | YES | Temp: 37 °C nozzle and 5 °C substrate. Pressure: 20 KPa, nozzle Ø: 0.3 mm. Speed: 4.45 mm/s | CaCl2 (2% w/v, 5 min) | Identification of the most important parameters for good printability: viscosity range, air pressure, nozzle Ø, distance between nozzle and substrate. Control of printing quality. Diffusion within and between layers. Cell viability |
| Ouyang L. 2016 [51] | Gelatin (7.5% w/v) and Alginate (1% w/v) | mESCs | YES | Stainless steel needle Ø: 25 G, Extrusion flux: 0.68 uL/s. Temp: nozzle at 30 °C, chamber at 22.5 °C | CaCl2 (100 mM, 3 min) | Assessment of printability of gelatin/alginate bioinks. Shear stress determination. ESC viability: 95%, cell spreading |
| Wu Z. 2016 [52] | Alginate (1%), Gelatin (10%) and Collagen (from bovine Achilles tendon, 0.82 mg/mL) | hCECs (1 × 106 cells/mL) | NO | Not reported | CaCl2 (3%, 37 °C, 3 min) | Incorporation of collagen to the bioink to precisely mimic tissue ECM yielding high cell viability and good printability. Effect of sodium citrate on degradation. Cell viability |
| Others | ||||||
| Reference | Biomaterial | Cell Phenotypes (Source, Density) | Rheology | Bioprinting Conditions | Post-Printing Processing | Bioprinted Construct Application/Evaluation |
| Cellulose | ||||||
| Montheil T. 2020 [53] | HPMC | hMSCs (1 × 106 cells/mL) | YES | Pressure: 45 ± 5 psi; Conical tip Ø: 27 G, Temp: 37 °C, Speed: 10 mm/s | 24 h, 37 °C | Determination of the printing window, Physicochemical analyses |
| Zidaric T. 2020 [54] | Alginate (3 wt %), CMC (3 wt %) and NFC (1.5 wt %) | hDFs (106 cells/mL) | YES | Nozzle Ø: 0.25 mm | CaCl2 (pouring 2 wt % for 1 min) | Wettability, Swelling ratio, In vitro degradation, Cell viability |
| Mendes B.B. 2019 [55] | Aldehyde-CNC (2.88 wt %) and platelet lysate (160 mg/mL of total dry mass) | hASCs(2 × 106/mL PL) | YES | Dual-extrusor with a static mixer, Stainless steel needle Ø: 27 G, Speed: 5 mm/s, Temp: 20 °C | h-thrombin from plasma (5 U/mL) CaCl2 (10 mM, 1 h, 37 °C) | Free-form fabrication, Hierarchical fibrillary architecture, Molecular diffusion, Cell viability > 90%, Metabolic activity, Collagen synthesis after 9 days |
| Law N. 2018 [56] | Hyaluronic acid-7 (0.25–2 wt %) and Methylcellulose (0.5–9 wt %) | sMSCs | YES | Pressure: 160–175 kpa, Ø: 23 G, Speed: 3 mm/s speed, Temp: extruder at 4 °C, plate at 37 °C | 37 °C, 5% CO2, 1 h | Swelling and stability, Compression behavior, Cell viability post-printing, Long-term cell viability (2 weeks) |
| Li H. 2017 [57] | Alginate (3%), methylcellulose (9%) and CaCl2, Trisodium citrate to enhance interfacial adhesion | L929 mFBs (3 × 106 cells/mL) in 15 mg/mL trisodium citrate | YES | Syringe 1: nozzle Ø: 25 G, Pressure: 4 bar; Syringe 2: nozzle Ø: 27 G, Pressure < 0.1 bar; Speed: 7.6–156.7 mm/s; Temp: 20 °C | CaCl2 bath (40 mg/mL, 10 min) | Printability, Mechanical properties, Degradation behavior, Thixotropic properties, Morphology, Cell viability > 95% |
| Chitosan | ||||||
| Pisani S. 2020 [58] | Chitosan (4.5–6% w/v) and Gamma-PGA (2% w/v) | hDFs (2 × 105 cell/mL) | YES | Needle Ø: 22 G and Ø: 25 G. Pressure (chitosan): 25–40 kPa and 5–10 kPa (Gamma-PGA). Speed: 600 mm/min Temp: 37 °C | No | Morphology, Stability (up to 35 d), Physicochemical characterization, Cell viability > 60% |
| Li Y. 2018 [59] | Hydroxypropil chitin (HPCH, 5 wt %, 0.4–0.6 mL) and Matrigel (0–0.3 mL) | hiPSCs (1 × 106 cells/mL) | YES | Nozzle Ø: 260 μm (160–360 μm), Speed: 2–6 mm/s, Temp: 15 °C–37 °C | CaCl2 (1% w/v, 37 °C, 3 min) | Thermal sensitive hydrogel printability, Cell viability (day 0), Proliferation (day 7), Morphology (0–7 d), Aggregation (10 d), Apoptosis (day 1), Pluripotency (qRT-PCR, day 10) |
| Hyaluronic Acid | ||||||
| Wang L.L. 2018 [60] | Nor-HA, HA-HYD, HA-ALD, I2959 photoinitiator (0.05%) and PETMA crosslinker | mFBs (2 × 106 cells/mL) | YES | Nozzle Ø: 25 G, Speed: 40 mm/s | UV irradiation (365 nm, 10 mW/cm2, 2 min). | HA-HYD and HA-ALD characterization, Mechanical properties, Cell viability > 80% |
| Pectin | ||||||
| Pereira R.F. 2018 [61] | PECMA (macromere conc. 1.5 or 2.5 wt %), I2959 (0.05 wt %), CaCl2 (0–5 mM) | hDFs | YES | Metal cylindrical nozzle Ø: 23 G; Temp: 20 °C; Construct, 15 layers | Dual crosslinking: UV photopolimerization (160 s, 7 mW/cm2), Ionic gelation (CaCl2, 5 mM 1 h under agitation) | Biofunctionalization of PECMA, Mechanical properties, Swelling, Cell viability and spreading, Deposition of ECM (fibronectin) |
| Polyethylene Glycol | ||||||
| Rutz A.L. 2019 [62] | PEG-SH/PEG-NH2 inks (base polymer (20%) + PEG crosslinker (10%)) | hDFs (2 × 106 cells/mL) | YES | Stainless steel nozzle Ø: 200 µm, 2 mm length. Pressure: 5 bar | Covalent amine-activated ester crosslinking | Optimization of PEG bioinks, Mechanical properties, Cell viability |
| Xin S. 2019 [63] | PEG microgel produced by electrospraying and thiol-ene click chemistry | hMSCs (5 × 106 cells/mL) | YES | Nozzles Ø: 840 and 600 µm | UV (60 mW/cm2, 365 nm, 3 min) | Gel morphology, Printability of complex structures, Cell viability up to 10 d |
 Indicates how far has evolved skin extrusion bioprinting; ●/○ indicates research category implementation/ non-implementation. The different colors group together, in more general terms, the different TRLs.
Indicates how far has evolved skin extrusion bioprinting; ●/○ indicates research category implementation/ non-implementation. The different colors group together, in more general terms, the different TRLs.
 Indicates how far has evolved skin extrusion bioprinting; ●/○ indicates research category implementation/ non-implementation. The different colors group together, in more general terms, the different TRLs.
Indicates how far has evolved skin extrusion bioprinting; ●/○ indicates research category implementation/ non-implementation. The different colors group together, in more general terms, the different TRLs.| TRL | Technology Readiness Level Scale Proposed by H2020 | Bioprinting TRL Adaptation | Research | |||
|---|---|---|---|---|---|---|
| In Vitro | In Vivo Animal | In Vivo Human | ||||
| Basic research |  | Basic principles observed | Review of the scientific literature to establish the starting point for the characterization of the new technology and procedure. | ○ | ○ | ○ |
| Technology concept formulated | Development of hypothesis and experimental designs for addressing the related scientific issues. | ○ | ○ | ○ | ||
| Applied research | Experimental proof of concept | Beginning of the research. Identification of candidate and/or target. In vitro demonstration of activity of skin constructs. Generation of preliminary in vivo proof-of-concept efficacy data (non-GLP (Good Laboratory Practice)). | ● | ● | ○ | |
| Technology validated in lab | Optimization and Non-GLP in vivo demonstration of activity, toxicity and efficacy of the skin construct. Manufacture of the product at laboratory-scale (i.e., non-GMP (Good Manufacturing Practice)) | ● | ● | ○ | ||
| Product demonstration | Technology validated in relevant environment | Advanced characterization of skin constructs (non-GLP in vivo studies, animal model, and assay development) Establishment of preliminary target product profiles. Initiation of the development of a scalable and reproducible manufacturing process according to GMP standards. | ● | ● | ○ | |
| Technology demonstrated in relevant environment | GMP Pilot Lot Production. Phase 1 clinical trial(s): Determination of an initial safety pharmacokinetics and immunogenicity as well as other properties of the clinical product. | ● | ● | ● | ||
| System prototype demonstration in operational environment | Scale-up and initiation of GMP process validation. Phase 2 clinical trial(s). | ● | ● | ● | ||
| System complete and qualified | Completion of GMP validation and consistency lot manufacturing. Animal efficacy studies, Phase 3 clinical trials, as well as any other extended clinical safety trials that are appropriate for the product Regulatory issues or product licensure. | ○ | ● | ● | ||
| Competitive manufacturing | Actual system proven in operational environment | Phase 4 studies (post-marketing commitments), safety surveillance, studies to support use in special populations, and clinical trials to confirm safety and efficacy as feasible and appropriate. Maintenance of manufacturing capability. | ○ | ○ | ○ | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Valle, A.; Del Amo, C.; Andia, I. Overview of Current Advances in Extrusion Bioprinting for Skin Applications. Int. J. Mol. Sci. 2020, 21, 6679. https://doi.org/10.3390/ijms21186679
Perez-Valle A, Del Amo C, Andia I. Overview of Current Advances in Extrusion Bioprinting for Skin Applications. International Journal of Molecular Sciences. 2020; 21(18):6679. https://doi.org/10.3390/ijms21186679
Chicago/Turabian StylePerez-Valle, Arantza, Cristina Del Amo, and Isabel Andia. 2020. "Overview of Current Advances in Extrusion Bioprinting for Skin Applications" International Journal of Molecular Sciences 21, no. 18: 6679. https://doi.org/10.3390/ijms21186679
APA StylePerez-Valle, A., Del Amo, C., & Andia, I. (2020). Overview of Current Advances in Extrusion Bioprinting for Skin Applications. International Journal of Molecular Sciences, 21(18), 6679. https://doi.org/10.3390/ijms21186679