Pathological Responses of Cardiac Mitochondria to Burn Trauma
Abstract
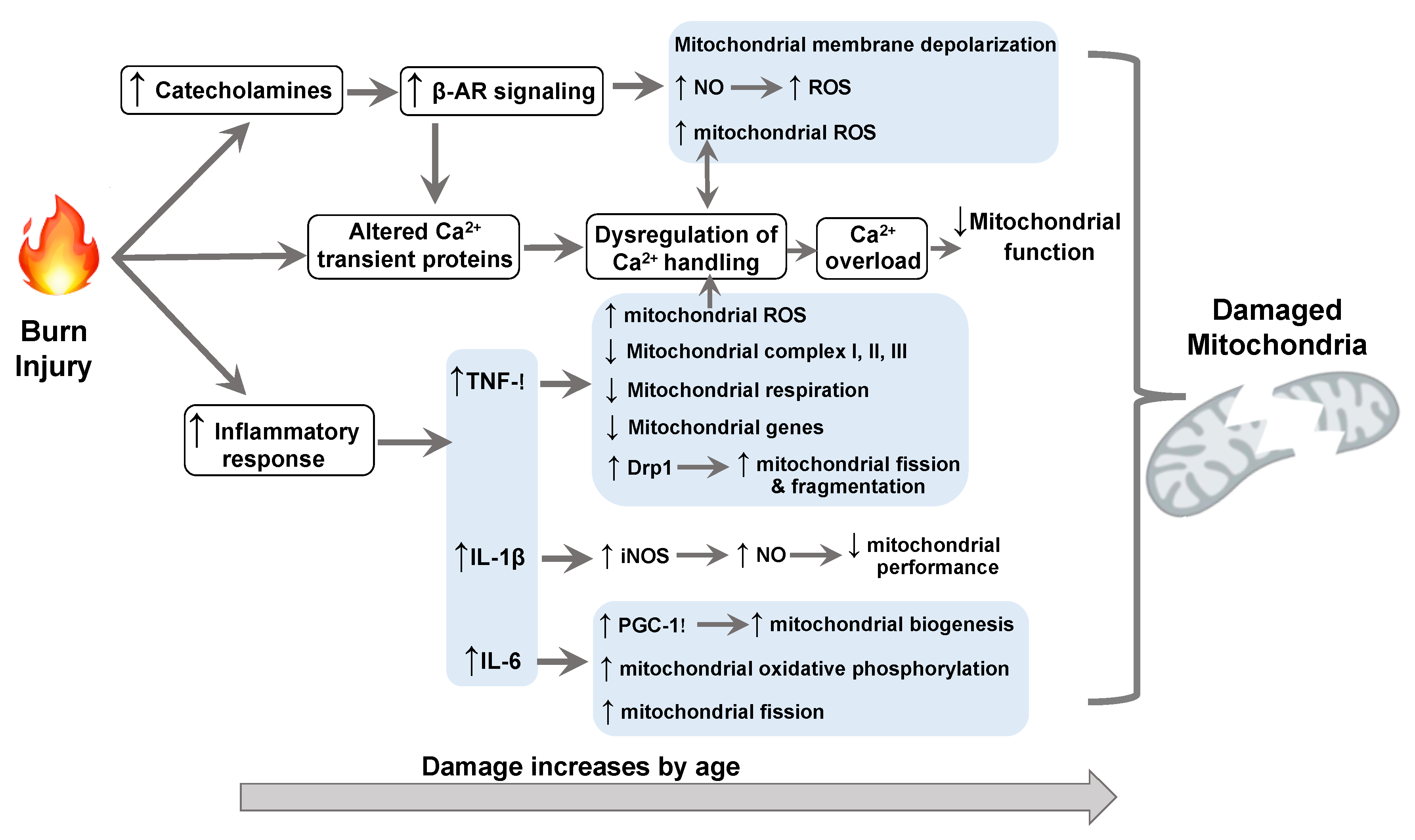
1. Introduction
2. Burn Trauma-Damaged Cardiac Mitochondria
2.1. Burn-Impaired Cardiac Mitochondrial Respiratory Activity and ATP Synthesis
2.2. Burn-Induced Oxidative Stress
2.3. Burn-Increased Cell Death
3. Molecular Mechanisms Underlying Burn-Impaired Cardiac Mitochondrial Performance
3.1. β-Adrenergic Signaling
3.2. TNF-α
3.3. IL-1β
3.4. IL-6
3.5. Cardiac Alterations in Calcium Signaling
4. Other Biological Factors Related to Cardiac Dysfunction and Mitochondrial Dysregulation after Burn
4.1. Aging
4.2. Sex
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AIF | Apoptosis-induced factor |
| AMPK | adenosine monophosphate-activated protein kinase |
| AR | β-adrenergic receptor |
| ATP | adenosine triphosphate |
| BNIP3 | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 |
| cGMP-PKG | cyclic GMP protein kinase G |
| Drp1 | dynamin-related peptide 1 |
| EF-Tumt | mitochondrial translation elongation factor Tu |
| FUNDC1 | FUN14 domain containing 1 |
| GPx | glutathione peroxide |
| H2O2 | hydrogen peroxide |
| iNOS | inducible NO synthase |
| I/R | ischemia/reperfusion |
| LC3 | microtubule associated protein 1 light chain 3 |
| LV | left ventricle |
| MCP-1 | monocyte chemoattractant protein-1 |
| MnSOD | superoxide dismutase |
| mtDNA | mitochondrial DNA |
| NO | nitric oxide |
| PDE5 | phosphodiesterase 5 |
| PGC | peroxisome proliferator-activated receptor-gamma coactivator |
| PKA | protein kinase A |
| PKG | protein kinase G |
| ROS | reactive oxygen species |
| RV | right ventricle |
| RyR | ryanodine receptor |
References
- Toussaint, J.; Singer, A.J. The evaluation and management of thermal injuries: 2014 update. Clin. Exp. Emerg Med. 2014, 1, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Guillory, A.N.; Clayton, R.P.; Herndon, D.N.; Finnerty, C.C. Cardiovascular Dysfunction Following Burn Injury: What We Have Learned from Rat and Mouse Models. Int. J. Mol. Sci. 2016, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sittah, G.S.; Sarhane, K.A.; Dibo, S.A.; Ibrahim, A. Cardiovascular dysfunction in burns: Review of the literature. Ann. Burns Fire Disasters 2012, 25, 26–37. [Google Scholar]
- Carlson, D.L.; Horton, J.W. Cardiac molecular signaling after burn trauma. J. Burn Care Res. 2006, 27, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Fozzard, H.A. Myocardial injury in burn shock. Ann. Surg. 1961, 154, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.T.; Barrow, R.E.; Sterns, A.M.; Hawkins, H.K.; Kimbrough, C.W.; Jeschke, M.G.; Lee, J.O.; Sanford, A.P.; Herndon, D.N. Age-dependent differences in survival after severe burns: A unicentric review of 1,674 patients and 179 autopsies over 15 years. J. Am. Coll. Surg. 2006, 202, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.P.; Angeles Valero Zanuy, M.A.; Leon Sanz, M.L. Post-shock metabolic response. 1942. Nutr. Hosp. 2001, 16, 176–182. [Google Scholar]
- Williams, F.N.; Herndon, D.N.; Suman, O.E.; Lee, J.O.; Norbury, W.B.; Branski, L.K.; Mlcak, R.P.; Jeschke, M.G. Changes in cardiac physiology after severe burn injury. J. Burn Care Res. 2011, 32, 269–274. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic response to severe burn injury. Ann. Surg. 2008, 248, 387–401. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Mlcak, R.P.; Finnerty, C.C.; Norbury, W.B.; Gauglitz, G.G.; Kulp, G.A.; Herndon, D.N. Burn size determines the inflammatory and hypermetabolic response. Crit. Care 2007, 11, R90. [Google Scholar] [CrossRef]
- Howard, T.S.; Hermann, D.G.; McQuitty, A.L.; Woodson, L.C.; Kramer, G.C.; Herndon, D.N.; Ford, P.M.; Kinsky, M.P. Burn-induced cardiac dysfunction increases length of stay in pediatric burn patients. J. Burn Care Res. 2013, 34, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.R.; Baxter, C.R.; Parker, J.L. Contractile function of heart muscle from burned guinea pigs. Circ. Shock 1982, 9, 63–73. [Google Scholar] [PubMed]
- Adams, H.R.; Baxter, C.R.; Izenberg, S.D. Decreased contractility and compliance of the left ventricle as complications of thermal trauma. Am. Heart J. 1984, 108, 1477–1487. [Google Scholar] [CrossRef]
- Brooks, F.; Dragstedt, L.R.; Warner, L.; Knisely, M.H. Sludged blood following severe thermal burns. Arch. Surg. 1950, 61, 387–418. [Google Scholar] [CrossRef]
- Salzberg, A.M.; Evans, E.I. Blood volumes in normal and burned dogs; a comparative study with radioactive phosphorus tagged red cells and T-1824 dye. Ann. Surg. 1950, 132, 746–759. [Google Scholar] [CrossRef]
- Horton, J.W.; Maass, D.L.; White, D.J.; Sanders, B.; Murphy, J. Effects of burn serum on myocardial inflammation and function. Shock 2004, 22, 438–445. [Google Scholar] [CrossRef]
- Horton, J.W.; White, D.J. Diminished cardiac contractile response to burn injury in aged guinea pigs. J. Trauma. 1993, 34, 429–436. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Miller, H.I. Burn shock in untreated and saline-resuscitated guinea pigs. Development of a model. J. Surg. Res. 1976, 21, 269–276. [Google Scholar] [CrossRef]
- Marin-Garcia, J.; Goldenthal, M.J. Understanding the impact of mitochondrial defects in cardiovascular disease: A review. J. Card. Fail. 2002, 8, 347–361. [Google Scholar] [CrossRef]
- Porter, C.; Herndon, D.N.; Borsheim, E.; Chao, T.; Reidy, P.T.; Borack, M.S.; Rasmussen, B.B.; Chondronikola, M.; Saraf, M.K.; Sidossis, L.S. Uncoupled skeletal muscle mitochondria contribute to hypermetabolism in severely burned adults. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E462–E467. [Google Scholar] [CrossRef]
- Wen, J.J.; Cummins, C.B.; Radhakrishnan, R.S. Burn-Induced Cardiac Mitochondrial Dysfunction via Interruption of the PDE5A-cGMP-PKG Pathway. Int. J. Mol. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.; Gomez, B.I.; Heard, T.C.; Smith, B.W.; Dubick, M.A.; Burmeister, D.M. Burn-induced reductions in mitochondrial abundance and efficiency are more pronounced with small volumes of colloids in swine. Am. J. Physiol. Cell Physiol. 2019, 317, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Chen, K.M.; Shi, Y.; Shi, H.P. Functional changes of the NADH respiratory chain in rat-liver mitochondria and the content changes of high-energy phosphate groups in rat liver and heart during the early phase of burn injury. Burns 1990, 16, 377–380. [Google Scholar] [PubMed]
- Wang, Z.; Cai, B.; Zhou, W. Effects of thermal injuries on electron transport chains of rat myocardial mitochondria. Zhonghua Zheng Xing Shao Shang Wai Ke Za 1999, 15, 56–58. [Google Scholar]
- Ogawa, Y. Changes in adenine nucleotide and mitochondrial metabolism of the kidney of burned rats and their relation to insulin. J. Lab. Clin. Med. 1977, 90, 457–465. [Google Scholar]
- Wen, J.J.; Cummins, C.; Radhakrishnan, R.S. Sildenafil Recovers Burn-Induced Cardiomyopathy. Cells 2020. [Google Scholar] [CrossRef]
- Wen, J.J.; Cummins, C.B.; Szczesny, B.; Radhakrishnan, R.S. Cardiac Dysfunction after Burn Injury: Role of the AMPK-SIRT1-PGC1alpha-NFE2L2-ARE Pathway. J. Am. Coll. Surg. 2020, 230, 562–571. [Google Scholar] [CrossRef]
- Zang, Q.; Maass, D.L.; White, J.; Horton, J.W. Cardiac mitochondrial damage and loss of ROS defense after burn injury: The beneficial effects of antioxidant therapy. J. Appl. Physiol. 2007, 102, 103–112. [Google Scholar] [CrossRef]
- Szczesny, B.; Brunyanszki, A.; Ahmad, A.; Olah, G.; Porter, C.; Toliver-Kinsky, T.; Sidossis, L.; Herndon, D.N.; Szabo, C. Time-Dependent and Organ-Specific Changes in Mitochondrial Function, Mitochondrial DNA Integrity, Oxidative Stress and Mononuclear Cell Infiltration in a Mouse Model of Burn Injury. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Martensson, J.; Goodwin, C.W.; Blake, R. Mitochondrial glutathione in hypermetabolic rats following burn injury and thyroid hormone administration: Evidence of a selective effect on brain glutathione by burn injury. Metabolism 1992, 41, 273–277. [Google Scholar] [CrossRef]
- Zang, Q.S.; Maass, D.L.; Wigginton, J.G.; Barber, R.C.; Martinez, B.; Idris, A.H.; Horton, J.W.; Nwariaku, F.E. Burn serum causes a CD14-dependent mitochondrial damage in primary cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Yan, H.; Hu, J.Y.; Zhang, J.P.; Teng, M.; Tong, D.L.; Xiang, F.; Zhang, Q.; Fang, Y.D.; Liang, G.P.; et al. Identification of mitochondria translation elongation factor Tu as a contributor to oxidative damage of postburn myocardium. J. Proteomics. 2012, 77, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Costantini, T.; Lopez, N.E.; Wolf, P.L.; Hageny, A.M.; Putnam, J.; Eliceiri, B.; Coimbra, R. Vagal nerve stimulation protects cardiac injury by attenuating mitochondrial dysfunction in a murine burn injury model. J. Cell. Mol. Med. 2013, 17, 664–671. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Koenitzer, J.R.; Freeman, B.A. Redox signaling in inflammation: Interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann. N. Y. Acad. Sci. 2010, 1203, 45–52. [Google Scholar] [CrossRef]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Yao, X.; Wigginton, J.G.; Maass, D.L.; Ma, L.; Carlson, D.; Wolf, S.E.; Minei, J.P.; Zang, Q.S. Estrogen-provided cardiac protection following burn trauma is mediated through a reduction in mitochondria-derived DAMPs. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, 882–894. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Chai, J.K.; Chu, W.L.; Duan, H.J.; Ma, L.; Zhang, H.J. Voltage dependent anion channel 2 involved mitochondrial apoptosis and its possible regulatory signal pathway in hearts of rats with severe scalds. Zhonghua Yi Xue Za Zhi 2013, 93, 939–943. [Google Scholar]
- Zhang, D.X.; Huang, Y.S.; Zhao, S.T.; Li, X.D. An experimental study on large fragment deletion of rat myocardial mitochondrial DNA during early postburn stage. Zhonghua Shao Shang Za Zhi 2004, 20, 271–274. [Google Scholar]
- Carlson, D.L.; Lightfoot, E., Jr.; Bryant, D.D.; Haudek, S.B.; Maass, D.; Horton, J.; Giroir, B.P. Burn plasma mediates cardiac myocyte apoptosis via endotoxin. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Hui-qing, X.; Jian-da, Z.; Xin-min, N.; Yan-zhong, Z.; Cheng-qun, L.; Quan-yong, H.; Yi, X.; Pokharel, P.B.; Shao-hua, W.; Dan, X. HSP70 inhibits burn serum-induced apoptosis of cardiomyocytes via mitochondrial and membrane death receptor pathways. J. Burn Care Res. 2008, 29, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Teng, M.; Zhang, Q.; Shi, X.H.; Huang, Y.S. Myocardial autophagy after severe burn in rats. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Kostin, S.; Pool, L.; Elsasser, A.; Hein, S.; Drexler, H.C.; Arnon, E.; Hayakawa, Y.; Zimmermann, R.; Bauer, E.; Klovekorn, W.P.; et al. Myocytes die by multiple mechanisms in failing human hearts. Circ. Res. 2003, 92, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.E.; Arias-Duran, C.; Avalos-Guajardo, Y.; Aedo, G.; Verdejo, H.E.; Parra, V.; Lavandero, S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol. Aspects Med. 2020, 71, 100822. [Google Scholar] [CrossRef]
- Jin, Q.; Li, R.; Hu, N.; Xin, T.; Zhu, P.; Hu, S.; Ma, S.; Zhu, H.; Ren, J.; Zhou, H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018, 14, 576–587. [Google Scholar] [CrossRef]
- Dhingra, A.; Jayas, R.; Afshar, P.; Guberman, M.; Maddaford, G.; Gerstein, J.; Lieberman, B.; Nepon, H.; Margulets, V.; Dhingra, R.; et al. Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes. Free Radic. Biol. Med. 2017, 112, 411–422. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, P.; Guo, J.; Hu, N.; Wang, S.; Li, D.; Hu, S.; Ren, J.; Cao, F.; Chen, Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017, 13, 498–507. [Google Scholar] [CrossRef]
- Sun, T.; Ding, W.; Xu, T.; Ao, X.; Yu, T.; Li, M.; Liu, Y.; Zhang, X.; Hou, L.; Wang, J. Parkin Regulates Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury by Targeting Cyclophilin-D. Antioxid. Redox Signal. 2019, 31, 1177–1193. [Google Scholar] [CrossRef]
- Auger, C.; Samadi, O.; Jeschke, M.G. The biochemical alterations underlying post-burn hypermetabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2633–2644. [Google Scholar] [CrossRef]
- Kulp, G.A.; Herndon, D.N.; Lee, J.O.; Suman, O.E.; Jeschke, M.G. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock 2010, 33, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Sidossis, L.S.; Porter, C.; Saraf, M.K.; Borsheim, E.; Radhakrishnan, R.S.; Chao, T.; Ali, A.; Chondronikola, M.; Mlcak, R.; Finnerty, C.C.; et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015, 22, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.; Herndon, D.H.; Kamolz, L.P.; Frey, M.; Jeschke, M.G. Pathophysiology of burns. Wien. Med. Wochenschr. 2009, 159, 327–336. [Google Scholar] [CrossRef]
- Tapking, C.; Popp, D.; Herndon, D.N.; Branski, L.K.; Hundeshagen, G.; Armenta, A.M.; Busch, M.; Most, P.; Kinsky, M.P. Cardiac Dysfunction in Severely Burned Patients: Current Understanding of Etiology, Pathophysiology and Treatment. Shock 2019. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; He, J.; Sakamoto, T.; Okada, Y. Catecholamines play a role in the production of interleukin-6 and interleukin-1alpha in unburned skin after burn injury in mice. Crit. Care Med. 2001, 29, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Hart, D.W.; Wolf, S.E.; Chinkes, D.L.; Wolfe, R.R. Reversal of catabolism by beta-blockade after severe burns. N. Engl. J. Med. 2001, 345, 1223–1229. [Google Scholar] [CrossRef]
- Liaudet, L.; Calderari, B.; Pacher, P. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail. Rev. 2014, 19, 815–824. [Google Scholar] [CrossRef]
- Bovo, E.; Lipsius, S.L.; Zima, A.V. Reactive oxygen species contribute to the development of arrhythmogenic Ca(2)(+) waves during beta-adrenergic receptor stimulation in rabbit cardiomyocytes. J. Physiol. 2012, 590, 3291–3304. [Google Scholar] [CrossRef]
- Remondino, A.; Kwon, S.H.; Communal, C.; Pimentel, D.R.; Sawyer, D.B.; Singh, K.; Colucci, W.S. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ. Res. 2003, 92, 136–138. [Google Scholar] [CrossRef]
- Menon, B.; Singh, M.; Ross, R.S.; Johnson, J.N.; Singh, K. Beta-Adrenergic receptor-stimulated apoptosis in adult cardiac myocytes involves MMP-2-mediated disruption of beta1 integrin signaling and mitochondrial pathway. Am. J. Physiol. Cell Physiol. 2006, 290, 254–261. [Google Scholar] [CrossRef]
- Andersson, D.C.; Fauconnier, J.; Yamada, T.; Lacampagne, A.; Zhang, S.J.; Katz, A.; Westerblad, H. Mitochondrial production of reactive oxygen species contributes to the beta-adrenergic stimulation of mouse cardiomycytes. J. Physiol. 2011, 589, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Chen, Z.R.; Li, R.; Lou, S.F. Nitric oxide synthesis in myocardium following burn injury in rats. Burns 1998, 24, 455–459. [Google Scholar] [CrossRef]
- White, J.; Carlson, D.L.; Thompson, M.; Maass, D.L.; Sanders, B.; Giroir, B.; Horton, J.W. Molecular and pharmacological approaches to inhibiting nitric oxide after burn trauma. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 1616–1625. [Google Scholar] [CrossRef]
- Seya, K.; Ono, K.; Fujisawa, S.; Okumura, K.; Motomura, S.; Furukawa, K. Cytosolic Ca2+-induced apoptosis in rat cardiomyocytes via mitochondrial NO-cGMP-protein kinase G pathway. J. Pharmacol. Exp. Ther. 2013, 344, 77–84. [Google Scholar] [CrossRef]
- Koupparis, A.J.; Jeremy, J.Y.; Muzaffar, S.; Persad, R.; Shukla, N. Sildenafil inhibits the formation of superoxide and the expression of gp47 NAD[P]H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int. 2005, 96, 423–427. [Google Scholar] [CrossRef]
- Costa, A.D.; Garlid, K.D.; West, I.C.; Lincoln, T.M.; Downey, J.M.; Cohen, M.V.; Critz, S.D. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ. Res. 2005, 97, 329–336. [Google Scholar] [CrossRef]
- Costa, A.D.; Pierre, S.V.; Cohen, M.V.; Downey, J.M.; Garlid, K.D. cGMP signalling in pre- and post-conditioning: The role of mitochondria. Cardiovasc. Res. 2008, 77, 344–352. [Google Scholar] [CrossRef]
- Wang, C.; Martyn, J.A. Burn injury alters beta-adrenergic receptor and second messenger function in rat ventricular muscle. Crit. Care Med. 1996, 24, 118–124. [Google Scholar] [CrossRef]
- Guillory, A.N.; Clayton, R.P.; Prasai, A.; El Ayadi, A.; Herndon, D.N.; Finnerty, C.C. Biventricular differences in beta-adrenergic receptor signaling following burn injury. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Stanojcic, M.; Abdullahi, A.; Rehou, S.; Parousis, A.; Jeschke, M.G. Pathophysiological Response to Burn Injury in Adults. Ann. Surg. 2018, 267, 576–584. [Google Scholar] [CrossRef]
- Niederbichler, A.D.; Papst, S.; Claassen, L.; Jokuszies, A.; Steinstraesser, L.; Hirsch, T.; Altintas, M.A.; Ipaktchi, K.R.; Reimers, K.; Kraft, T.; et al. Burn-induced organ dysfunction: Vagus nerve stimulation attenuates organ and serum cytokine levels. Burns 2009, 35, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Bruns, B.; Maass, D.; Barber, R.; Horton, J.; Carlson, D. Alterations in the cardiac inflammatory response to burn trauma in mice lacking a functional Toll-like receptor 4 gene. Shock 2008, 30, 740–746. [Google Scholar] [CrossRef]
- Ballard-Croft, C.; White, D.J.; Maass, D.L.; Hybki, D.P.; Horton, J.W. Role of p38 mitogen-activated protein kinase in cardiac myocyte secretion of the inflammatory cytokine TNF-alpha. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sankula, R.; Tsai, B.M.; Meldrum, K.K.; Turrentine, M.; March, K.L.; Brown, J.W.; Dinarello, C.A.; Meldrum, D.R. P38 MAPK mediates myocardial proinflammatory cytokine production and endotoxin-induced contractile suppression. Shock 2004, 21, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Markel, T.; Crisostomo, P.; Herring, C.; Meldrum, K.K.; Lillemoe, K.D.; Meldrum, D.R. Deficiency of TNFR1 protects myocardium through SOCS3 and IL-6 but not p38 MAPK or IL-1beta. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 1694–1699. [Google Scholar] [CrossRef]
- Wang, M.; Tsai, B.M.; Crisostomo, P.R.; Meldrum, D.R. Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation 2006, 114, 282–289. [Google Scholar] [CrossRef]
- Sando, I.C.; Wang, Y.; Crisostomo, P.R.; Markel, T.A.; Sharma, R.; Erwin, G.S.; Guzman, M.J.; Meldrum, D.R.; Wang, M. Females exhibit relative resistance to depressive effects of tumor necrosis factor-alpha on the myocardium. J. Surg. Res. 2008, 150, 92–99. [Google Scholar] [CrossRef]
- Drosatos, K.; Lymperopoulos, A.; Kennel, P.J.; Pollak, N.; Schulze, P.C.; Goldberg, I.J. Pathophysiology of sepsis-related cardiac dysfunction: Driven by inflammation, energy mismanagement, or both? Curr. Heart Fail. Rep. 2015, 12, 130–140. [Google Scholar] [CrossRef]
- Cain, B.S.; Meldrum, D.R.; Dinarello, C.A.; Meng, X.; Joo, K.S.; Banerjee, A.; Harken, A.H. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit. Care Med. 1999, 27, 1309–1318. [Google Scholar] [CrossRef]
- Beutler, B.; Milsark, I.W.; Cerami, A.C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 1985, 229, 869–871. [Google Scholar] [CrossRef]
- Peng, T.; Lu, X.; Lei, M.; Feng, Q. Endothelial nitric-oxide synthase enhances lipopolysaccharide-stimulated tumor necrosis factor-alpha expression via cAMP-mediated p38 MAPK pathway in cardiomyocytes. J. Biol. Chem. 2003, 278, 8099–8105. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.; Lee, J.; Torre-Amione, G.; Birdsall, H.H.; Ma, T.S.; Mann, D.L. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration. J. Clin. Investig. 1995, 96, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Tavener, S.A.; Long, E.M.; Robbins, S.M.; McRae, K.M.; Van Remmen, H.; Kubes, P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ. Res. 2004, 95, 700–707. [Google Scholar] [CrossRef]
- Duprez, L.; Takahashi, N.; Van Hauwermeiren, F.; Vandendriessche, B.; Goossens, V.; Vanden Berghe, T.; Declercq, W.; Libert, C.; Cauwels, A.; Vandenabeele, P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 2011, 35, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Ma, X.; Liu, H.; Murphy, J.; Barger, P.M.; Mann, D.L.; Diwan, A. Tumor necrosis factor receptor-associated factor 2 mediates mitochondrial autophagy. Circ. Heart Fail. 2015, 8, 175–187. [Google Scholar] [CrossRef]
- Orvedahl, A.; McAllaster, M.R.; Sansone, A.; Dunlap, B.F.; Desai, C.; Wang, Y.T.; Balce, D.R.; Luke, C.J.; Lee, S.; Orchard, R.C.; et al. Autophagy genes in myeloid cells counteract IFNgamma-induced TNF-mediated cell death and fatal TNF-induced shock. Proc. Natl. Acad. Sci. USA 2019, 116, 16497–16506. [Google Scholar] [CrossRef]
- Suematsu, N.; Tsutsui, H.; Wen, J.; Kang, D.; Ikeuchi, M.; Ide, T.; Hayashidani, S.; Shiomi, T.; Kubota, T.; Hamasaki, N.; et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003, 107, 1418–1423. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.M.; Chaiswing, L.; Yen, H.C.; Oberley, T.D.; Lien, Y.C.; Lin, S.M.; Mattson, M.P.; St Clair, D. Tamoxifen protects against acute tumor necrosis factor alpha-induced cardiac injury via improving mitochondrial functions. Free Radic. Biol. Med. 2006, 40, 1234–1241. [Google Scholar] [CrossRef]
- Mariappan, N.; Elks, C.M.; Haque, M.; Francis, J. Interaction of TNF with angiotensin II contributes to mitochondrial oxidative stress and cardiac damage in rats. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Lkhagva, B.; Kao, Y.H.; Lee, T.I.; Lee, T.W.; Cheng, W.L.; Chen, Y.J. Activation of Class I histone deacetylases contributes to mitochondrial dysfunction in cardiomyocytes with altered complex activities. Epigenetics 2018, 13, 376–385. [Google Scholar] [CrossRef]
- Shen, Y.L.; Shi, Y.Z.; Chen, G.G.; Wang, L.L.; Zheng, M.Z.; Jin, H.F.; Chen, Y.Y. TNF-alpha induces Drp1-mediated mitochondrial fragmentation during inflammatory cardiomyocyte injury. Int. J. Mol. Med. 2018, 41, 2317–2327. [Google Scholar] [PubMed]
- Zuo, S.; Li, L.L.; Ruan, Y.F.; Jiang, L.; Li, X.; Li, S.N.; Wen, S.N.; Bai, R.; Liu, N.; Du, X.; et al. Acute administration of tumour necrosis factor-alpha induces spontaneous calcium release via the reactive oxygen species pathway in atrial myocytes. Europace 2018, 20, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Last-Barney, K.; Homon, C.A.; Faanes, R.B.; Merluzzi, V.J. Synergistic and overlapping activities of tumor necrosis factor-alpha and IL-1. J. Immunol. 1988, 141, 527–530. [Google Scholar]
- Okusawa, S.; Gelfand, J.A.; Ikejima, T.; Connolly, R.J.; Dinarello, C.A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J. Clin. Investig. 1988, 81, 1162–1172. [Google Scholar] [CrossRef]
- Kumar, A.; Thota, V.; Dee, L.; Olson, J.; Uretz, E.; Parrillo, J.E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 1996, 183, 949–958. [Google Scholar] [CrossRef]
- Muller-Werdan, U.; Buerke, M.; Ebelt, H.; Heinroth, K.M.; Herklotz, A.; Loppnow, H.; Russ, M.; Schlegel, F.; Schlitt, A.; Schmidt, H.B.; et al. Septic cardiomyopathy—A not yet discovered cardiomyopathy? Exp. Clin. Cardiol. 2006, 11, 226–236. [Google Scholar]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef]
- Radin, M.J.; Holycross, B.J.; Dumitrescu, C.; Kelley, R.; Altschuld, R.A. Leptin modulates the negative inotropic effect of interleukin-1beta in cardiac myocytes. Mol. Cell. Biochem. 2008, 315, 179–184. [Google Scholar] [CrossRef]
- Tsujino, M.; Hirata, Y.; Imai, T.; Kanno, K.; Eguchi, S.; Ito, H.; Marumo, F. Induction of nitric oxide synthase gene by interleukin-1 beta in cultured rat cardiocytes. Circulation 1994, 90, 375–383. [Google Scholar] [CrossRef]
- Chung, M.K.; Gulick, T.S.; Rotondo, R.E.; Schreiner, G.F.; Lange, L.G. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic AMP in rat cardiac myocytes. Impairment of signal transduction. Circ. Res. 1990, 67, 753–763. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhou, W.; Kennedy, R.H. Suppression of beta-adrenergic responsiveness of L-type Ca2+ current by IL-1beta in rat ventricular myocytes. Am. J. Physiol. 1999, 276, 141–148. [Google Scholar]
- Schreur, K.D.; Liu, S. Involvement of ceramide in inhibitory effect of IL-1 beta on L-type Ca2+ current in adult rat ventricular myocytes. Am. J. Physiol. 1997, 272, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Schreur, K.D. G protein-mediated suppression of L-type Ca2+ current by interleukin-1 beta in cultured rat ventricular myocytes. Am. J. Physiol. 1995, 268, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Panas, D.L.; Catena, R.; Moncada, S.; Olley, P.M.; Lopaschuk, G.D. The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. Br. J. Pharmacol. 1995, 114, 27–34. [Google Scholar] [CrossRef]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol. Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Oddis, C.V.; Finkel, M.S. Cytokine-stimulated nitric oxide production inhibits mitochondrial activity in cardiac myocytes. Biochem. Biophys. Res. Commun. 1995, 213, 1002–1009. [Google Scholar] [CrossRef]
- Tatsumi, T.; Matoba, S.; Kawahara, A.; Keira, N.; Shiraishi, J.; Akashi, K.; Kobara, M.; Tanaka, T.; Katamura, M.; Nakagawa, C.; et al. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J. Am. Coll. Cardiol. 2000, 35, 1338–1346. [Google Scholar] [CrossRef]
- McTiernan, C.F.; Lemster, B.H.; Frye, C.; Brooks, S.; Combes, A.; Feldman, A.M. Interleukin-1 beta inhibits phospholamban gene expression in cultured cardiomyocytes. Circ. Res. 1997, 81, 493–503. [Google Scholar] [CrossRef]
- Maass, D.L.; White, J.; Horton, J.W. IL-1beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma. Shock 2002, 18, 360–366. [Google Scholar] [CrossRef]
- Lang, C.H.; Frost, R.A.; Vary, T.C. Thermal injury impairs cardiac protein synthesis and is associated with alterations in translation initiation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, 740–750. [Google Scholar] [CrossRef]
- Pileri, D.; Accardo Palombo, A.; D’Amelio, L.; D’Arpa, N.; Amato, G.; Masellis, A.; Cataldo, V.; Mogavero, R.; Napoli, B.; Lombardo, C.; et al. Concentrations of cytokines IL-6 and IL-10 in plasma of burn patients: Their relationship to sepsis and outcome. Ann. Burns Fire Disasters 2008, 21, 182–185. [Google Scholar] [PubMed]
- Matsuura, H.; Matsumoto, H.; Osuka, A.; Ogura, H.; Shimizu, K.; Kang, S.; Tanaka, T.; Ueyama, M.; Shimazu, T. Clinical Importance of a Cytokine Network in Major Burns. Shock 2019, 51, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Yang, H.T.; Chun, W.; Kim, J.H.; Shin, S.H.; Kang, H.J.; Kim, H.S. Inflammatory cytokines and their prognostic ability in cases of major burn injury. Ann. Lab. Med. 2015, 35, 105–110. [Google Scholar] [CrossRef]
- White, J.; Maass, D.L.; Giroir, B.; Horton, J.W. Development of an acute burn model in adult mice for studies of cardiac function and cardiomyocyte cellular function. Shock 2001, 16, 122–129. [Google Scholar] [CrossRef]
- Carlson, D.L.; Maass, D.L.; White, J.; Sikes, P.; Horton, J.W. Caspase inhibition reduces cardiac myocyte dyshomeostasis and improves cardiac contractile function after major burn injury. J. Appl. Physiol. 2007, 103, 323–330. [Google Scholar] [CrossRef]
- Tan, J.; Maass, D.L.; White, D.J.; Horton, J.W. Effects of burn injury on myocardial signaling and cytokine secretion: Possible role of PKC. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, 887–896. [Google Scholar] [CrossRef]
- Melendez, G.C.; McLarty, J.L.; Levick, S.P.; Du, Y.; Janicki, J.S.; Brower, G.L. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 2010, 56, 225–231. [Google Scholar] [CrossRef]
- Pedroso, F.E.; Spalding, P.B.; Cheung, M.C.; Yang, R.; Gutierrez, J.C.; Bonetto, A.; Zhan, R.; Chan, H.L.; Namias, N.; Koniaris, L.G.; et al. Inflammation, organomegaly, and muscle wasting despite hyperphagia in a mouse model of burn cachexia. J. Cachexia Sarcopenia Muscle. 2012, 3, 199–211. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Ye, J. IL-6: A Potential Role in Cardiac Metabolic Homeostasis. Int. J. Mol. Sci. 2018. [Google Scholar] [CrossRef]
- Bonda, T.A.; Szynaka, B.; Sokolowska, M.; Dziemidowicz, M.; Waszkiewicz, E.; Winnicka, M.M.; Bernaczyk, P.; Wawrusiewicz-Kurylonek, N.; Kaminski, K.A. Interleukin 6 modulates PPARalpha and PGC-1alpha and is involved in high-fat diet induced cardiac lipotoxicity in mouse. Int. J. Cardiol. 2016, 219, 1–8. [Google Scholar] [CrossRef]
- Chen, F.; Chen, D.; Zhao, X.; Yang, S.; Li, Z.; Sanchis, D.; Jin, L.; Qiang, X.; Wang, K.; Xu, Y.; et al. Interleukin-6 deficiency facilitates myocardial dysfunction during high fat diet-induced obesity by promoting lipotoxicity and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3128–3141. [Google Scholar] [CrossRef]
- Su, X.; Han, X.; Mancuso, D.J.; Abendschein, D.R.; Gross, R.W. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: Identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry 2005, 44, 5234–5245. [Google Scholar] [CrossRef] [PubMed]
- Sehat, A.; Huebinger, R.M.; Carlson, D.L.; Zang, Q.S.; Wolf, S.E.; Song, J. Burn Serum Stimulates Myoblast Cell Death Associated with IL-6-Induced Mitochondrial Fragmentation. Shock 2017, 48, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Preacher, K.J.; MacCallum, R.C.; Atkinson, C.; Malarkey, W.B.; Glaser, R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA 2003, 100, 9090–9095. [Google Scholar] [CrossRef]
- Wang, L.; Quan, J.; Johnston, W.E.; Maass, D.L.; Horton, J.W.; Thomas, J.A.; Tao, W. Age-dependent differences of interleukin-6 activity in cardiac function after burn complicated by sepsis. Burns 2010, 36, 232–238. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Blatter, L.A. Calcium signaling in cardiac mitochondria. J. Mol. Cell. Cardiol. 2013, 58, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Bose, S.; French, S.A.; Territo, P.R. Role of calcium in metabolic signaling between cardiac sarcoplasmic reticulum and mitochondria in vitro. Am. J. Physiol. Cell Physiol. 2003, 284, 285–293. [Google Scholar] [CrossRef]
- Territo, P.R.; Mootha, V.K.; French, S.A.; Balaban, R.S. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: Role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000, 278, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Balaban, R.S. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012, 51, 2959–2973. [Google Scholar] [CrossRef]
- Nemani, N.; Shanmughapriya, S.; Madesh, M. Molecular regulation of MCU: Implications in physiology and disease. Cell Calcium. 2018, 74, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L.; Enkhbaatar, P.; Traber, D.L.; Buja, L.M.; Jonkam, C.C.; Poindexter, B.J.; Bick, R.J. Cardiovascular distribution of the calcium sensing receptor before and after burns. Burns 2008, 34, 370–375. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; Maass, D.L.; Sanders, B.; Horton, J.W. Cardiomyocyte intracellular calcium and cardiac dysfunction after burn trauma. Crit. Care Med. 2002, 30, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, W.; Deng, J.; Lan, L.; Xue, X.; Zhang, C.; Cai, G.; Luo, X.; Liu, J. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic. Biol. Med. 2013, 60, 292–299. [Google Scholar] [CrossRef]
- Maass, D.L.; White, J.; Sanders, B.; Horton, J.W. Role of cytosolic vs. mitochondrial Ca2+ accumulation in burn injury-related myocardial inflammation and function. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, 744–751. [Google Scholar] [CrossRef]
- Liang, W.Y.; Tang, L.X.; Yang, Z.C.; Huang, Y.S. Calcium induced the damage of myocardial mitochondrial respiratory function in the early stage after severe burns. Burns 2002, 28, 143–146. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Blatter, L.A. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J. Physiol. 2009, 587, 851–872. [Google Scholar] [CrossRef]
- Wan, Y.L.; Hui, T.; Zong-Cheng, Y.; Yue-Sheng, H. Changes of myocardial mitochondrial Ca(2+) transport and mechanism in the early stage after severe burns. Burns 2002, 28, 431–434. [Google Scholar]
- Ballard-Croft, C.; Carlson, D.; Maass, D.L.; Horton, J.W. Burn trauma alters calcium transporter protein expression in the heart. J. Appl. Physiol. 2004, 97, 1470–1476. [Google Scholar] [CrossRef]
- Gambardella, J.; Sorriento, D.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; Napolitano, L.; Trimarco, B.; Iaccarino, G.; Santulli, G. Functional Role of Mitochondria in Arrhythmogenesis. Adv. Exp. Med. Biol. 2017, 982, 191–202. [Google Scholar]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.X.; Marks, A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Ernst, P.; Song, J.; Liu, X.M.; Huke, S.; Wang, S.; Zhang, J.J.; Zhou, L. Mitochondrial-Mediated Oxidative Ca(2+)/Calmodulin-Dependent Kinase II Activation Induces Early Afterdepolarizations in Guinea Pig Cardiomyocytes: An In Silico Study. J. Am. Heart Assoc. 2018. [Google Scholar] [CrossRef]
- Huang, Y.S.; Yang, Z.C.; Yan, B.G.; Yang, J.M.; Chen, F.M.; Crowther, R.S.; Li, A. Pathogenesis of early cardiac myocyte damage after severe burns. J. Trauma 1999, 46, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.S.; Li, G.H.; Zhang, J.; Yu, L.H.; Zhao, X.M. Protective effects of taurine on myocardial mitochondria and their enzyme activities in rate with severe burn. Zhonghua Shao Shang Za Zhi 2008, 24, 171–174. [Google Scholar] [PubMed]
- Chaudhary, K.R.; El-Sikhry, H.; Seubert, J.M. Mitochondria and the aging heart. J. Geriatr. Cardiol. 2011, 8, 159–167. [Google Scholar] [PubMed]
- Lesnefsky, E.J.; Chen, Q.; Hoppel, C.L. Mitochondrial Metabolism in Aging Heart. Circ. Res. 2016, 118, 1593–1611. [Google Scholar] [CrossRef]
- Atlante, A.; Seccia, T.M.; Marra, E.; Passarella, S. The rate of ATP export in the extramitochondrial phase via the adenine nucleotide translocator changes in aging in mitochondria isolated from heart left ventricle of either normotensive or spontaneously hypertensive rats. Mech. Ageing Dev. 2011, 132, 488–495. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Gudz, T.I.; Migita, C.T.; Ikeda-Saito, M.; Hassan, M.O.; Turkaly, P.J.; Hoppel, C.L. Ischemic injury to mitochondrial electron transport in the aging heart: Damage to the iron-sulfur protein subunit of electron transport complex III. Arch. Biochem. Biophys. 2001, 385, 117–128. [Google Scholar] [CrossRef]
- Duicu, O.M.; Mirica, S.N.; Gheorgheosu, D.E.; Privistirescu, A.I.; Fira-Mladinescu, O.; Muntean, D.M. Ageing-induced decrease in cardiac mitochondrial function in healthy rats. Can. J. Physiol. Pharmacol. 2013, 91, 593–600. [Google Scholar] [CrossRef]
- Poulose, N.; Raju, R. Aging and injury: Alterations in cellular energetics and organ function. Aging Dis. 2014, 5, 101–108. [Google Scholar]
- Jian, B.; Yang, S.; Chen, D.; Zou, L.; Chatham, J.C.; Chaudry, I.; Raju, R. Aging influences cardiac mitochondrial gene expression and cardiovascular function following hemorrhage injury. Mol. Med. 2011, 17, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Patsouris, D.; Stanojcic, M.; Abdullahi, A.; Rehou, S.; Pinto, R.; Chen, P.; Burnett, M.; Amini-Nik, S. Pathophysiologic Response to Burns in the Elderly. EBioMedicine 2015, 2, 1536–1548. [Google Scholar] [CrossRef]
- Auger, C.; Sivayoganathan, T.; Abdullahi, A.; Parousis, A.; Jeschke, M.G. Hepatic mitochondrial bioenergetics in aged C57BL/6 mice exhibit delayed recovery from severe burn injury. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Frink, M.; Pape, H.C.; van Griensven, M.; Krettek, C.; Chaudry, I.H.; Hildebrand, F. Influence of sex and age on mods and cytokines after multiple injuries. Shock 2007, 27, 151–156. [Google Scholar] [CrossRef] [PubMed]
- George, R.L.; McGwin, G., Jr.; Windham, S.T.; Melton, S.M.; Metzger, J.; Chaudry, I.H.; Rue, L.W., 3rd. Age-related gender differential in outcome after blunt or penetrating trauma. Shock 2003, 19, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Guidry, C.A.; Swenson, B.R.; Davies, S.W.; Dossett, L.A.; Popovsky, K.A.; Bonatti, H.; Evans, H.L.; Metzger, R.; Hedrick, T.L.; Tache-Leon, C.A.; et al. Sex- and diagnosis-dependent differences in mortality and admission cytokine levels among patients admitted for intensive care. Crit. Care Med. 2014, 42, 1110–1120. [Google Scholar] [CrossRef]
- Haider, A.H.; Crompton, J.G.; Oyetunji, T.; Stevens, K.A.; Efron, D.T.; Kieninger, A.N.; Chang, D.C.; Cornwell, E.E., 3rd; Haut, E.R. Females have fewer complications and lower mortality following trauma than similarly injured males: A risk adjusted analysis of adults in the National Trauma Data Bank. Surgery 2009, 146, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.; Huynh, T.; Sing, R.F.; Miles, W.S.; Norton, H.J.; Thomason, M.H. Gender-related outcomes in trauma. J. Trauma. 2002, 53, 430–434. [Google Scholar] [CrossRef]
- Deitch, E.A.; Livingston, D.H.; Lavery, R.F.; Monaghan, S.F.; Bongu, A.; Machiedo, G.W. Hormonally active women tolerate shock-trauma better than do men: A prospective study of over 4000 trauma patients. Ann. Surg. 2007, 246, 447–453. [Google Scholar] [CrossRef]
- McGwin, G., Jr.; George, R.L.; Cross, J.M.; Reiff, D.A.; Chaudry, I.H.; Rue, L.W., 3rd. Gender differences in mortality following burn injury. Shock 2002, 18, 311–315. [Google Scholar] [CrossRef]
- O’Keefe, G.E.; Hunt, J.L.; Purdue, G.F. An evaluation of risk factors for mortality after burn trauma and the identification of gender-dependent differences in outcomes. J. Am. Coll. Surg. 2001, 192, 153–160. [Google Scholar] [CrossRef]
- Fazeli, S.; Karami-Matin, R.; Kakaei, N.; Pourghorban, S.; Safari-Faramani, R.; Safari-Faramani, B. Predictive factors of mortality in burn patients. Trauma Mon. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.I.; Ziembicki, J.A.; Corcos, A.C.; Peitzman, A.B.; Billiar, T.R.; Sperry, J.L. Characterization of sex dimorphism following severe thermal injury. J. Burn Care Res. 2014, 35, 484–490. [Google Scholar] [CrossRef]
- Kerby, J.D.; McGwin, G., Jr.; George, R.L.; Cross, J.A.; Chaudry, I.H.; Rue, L.W., 3rd. Sex differences in mortality after burn injury: Results of analysis of the National Burn Repository of the American Burn Association. J. Burn Care Res. 2006, 27, 452–456. [Google Scholar] [CrossRef]
- Gregory, M.S.; Faunce, D.E.; Duffner, L.A.; Kovacs, E.J. Gender difference in cell-mediated immunity after thermal injury is mediated, in part, by elevated levels of interleukin-6. J. Leukoc. Biol. 2000, 67, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Plackett, T.P.; Gamelli, R.L.; Kovacs, E.J. Gender-based differences in cytokine production after burn injury: A role of interleukin-6. J. Am. Coll. Surg. 2010, 210, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.S.; Duffner, L.A.; Faunce, D.E.; Kovacs, E.J. Estrogen mediates the sex difference in post-burn immunosuppression. J. Endocrinol. 2000, 164, 129–138. [Google Scholar] [CrossRef]
- Deitch, E.A.; Ananthakrishnan, P.; Cohen, D.B.; Xu, D.Z.; Feketeova, E.; Hauser, C.J. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 1456–1465. [Google Scholar] [CrossRef]
- Chang, E.J.; Edelman, L.S.; Morris, S.E.; Saffle, J.R. Gender influences on burn outcomes in the elderly. Burns 2005, 31, 31–35. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Mlcak, R.P.; Finnerty, C.C.; Norbury, W.B.; Przkora, R.; Kulp, G.A.; Gauglitz, G.G.; Zhang, X.J.; Herndon, D.N. Gender differences in pediatric burn patients: Does it make a difference? Ann. Surg. 2008, 248, 126–136. [Google Scholar] [CrossRef]
- Steinvall, I.; Fredrikson, M.; Bak, Z.; Sjoberg, F. Mortality after thermal injury: No sex-related difference. J. Trauma. 2011, 70, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ikeda, H.; Higuchi, R.; Nozaki, M.; Yamamoto, Y.; Urabe, M.; Shimazaki, S.; Sugamata, A.; Aikawa, N.; Ninomiya, N.; et al. Epidemiological and outcome characteristics of major burns in Tokyo. Burns 2005, 31, 3–11. [Google Scholar] [CrossRef]
- Wang, M.; Crisostomo, P.R.; Markel, T.A.; Wang, Y.; Meldrum, D.R. Mechanisms of sex differences in TNFR2-mediated cardioprotection. Circulation 2008, 118, 38–45. [Google Scholar] [CrossRef]
- Wang, M.; Baker, L.; Tsai, B.M.; Meldrum, K.K.; Meldrum, D.R. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Lagranha, C.J.; Deschamps, A.; Aponte, A.; Steenbergen, C.; Murphy, E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 2010, 106, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tsai, B.M.; Reiger, K.M.; Brown, J.W.; Meldrum, D.R. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J. Mol. Cell. Cardiol. 2006, 40, 205–212. [Google Scholar] [CrossRef]
- Shinohara, T.; Takahashi, N.; Ooie, T.; Ichinose, M.; Hara, M.; Yonemochi, H.; Saikawa, T.; Yoshimatsu, H. Estrogen inhibits hyperthermia-induced expression of heat-shock protein 72 and cardioprotection against ischemia/reperfusion injury in female rat heart. J. Mol. Cell. Cardiol. 2004, 37, 1053–1061. [Google Scholar] [CrossRef]
- Wang, M.; Smith, K.; Yu, Q.; Miller, C.; Singh, K.; Sen, C.K. Mitochondrial connexin 43 in sex-dependent myocardial responses and estrogen-mediated cardiac protection following acute ischemia/reperfusion injury. Basic Res. Cardiol. 2019, 115, 1. [Google Scholar] [CrossRef]
- Horton, J.W.; White, D.J.; Maass, D.L. Gender-related differences in myocardial inflammatory and contractile responses to major burn trauma. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, 202–213. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Scott, S.R.; Koniaris, L.G.; Zimmers, T.A. Pathological Responses of Cardiac Mitochondria to Burn Trauma. Int. J. Mol. Sci. 2020, 21, 6655. https://doi.org/10.3390/ijms21186655
Wang M, Scott SR, Koniaris LG, Zimmers TA. Pathological Responses of Cardiac Mitochondria to Burn Trauma. International Journal of Molecular Sciences. 2020; 21(18):6655. https://doi.org/10.3390/ijms21186655
Chicago/Turabian StyleWang, Meijing, Susan R. Scott, Leonidas G. Koniaris, and Teresa A. Zimmers. 2020. "Pathological Responses of Cardiac Mitochondria to Burn Trauma" International Journal of Molecular Sciences 21, no. 18: 6655. https://doi.org/10.3390/ijms21186655
APA StyleWang, M., Scott, S. R., Koniaris, L. G., & Zimmers, T. A. (2020). Pathological Responses of Cardiac Mitochondria to Burn Trauma. International Journal of Molecular Sciences, 21(18), 6655. https://doi.org/10.3390/ijms21186655




