Superparamagnetic Iron Oxide Nanoparticles and Essential Oils: A New Tool for Biological Applications
Abstract
1. Introduction
2. Synthesis of Magnetite Nanoparticles
2.1. Coprecipitation Methods
2.2. Water–Oil Emulsion Methods
2.3. High-Temperature Methods
2.4. Sol–Gel Methods
2.5. Other Methods
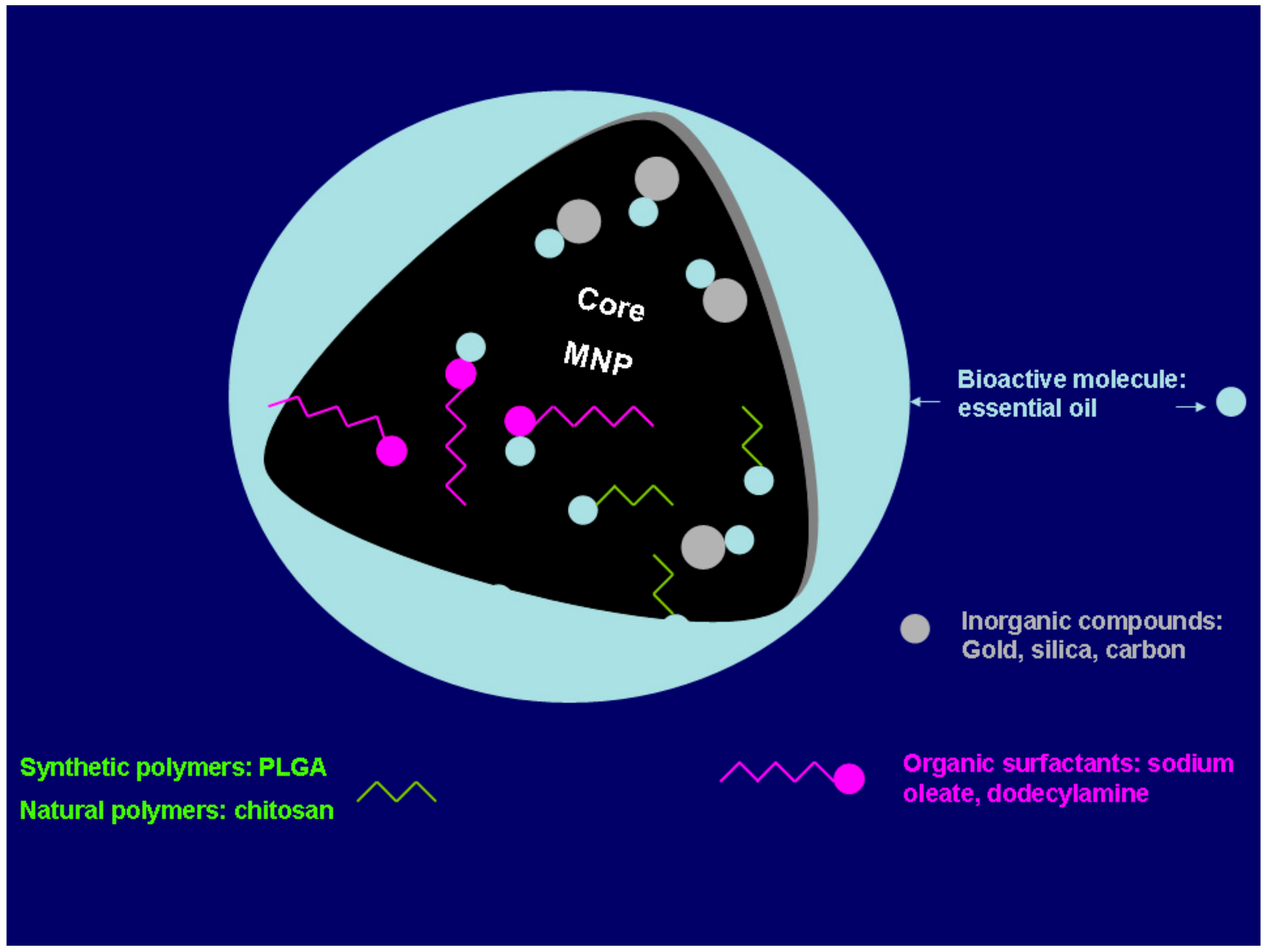
3. Stabilization and Surface Modification
4. Superparamagnetic Iron Oxide Nanoparticles for Extracting Essential Oils versus Essential Oils for Improving or Producing Superparamagnetic Iron Oxide Nanoparticles
5. Superparamagnetic Iron Oxide Nanoparticles Applications
6. Superparamagnetic Iron Oxide Nanoparticles Supplemented with Essential Oils for Combating Microbial Biofilms
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gan, R.Y.; Zhang, J.R.; Farha, A.K.; Li, H. Bin; Zhu, F.; Wang, X.H.; Corke, H. Antivirulence properties and related mechanisms of spice essential oils: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1018–1055. [Google Scholar] [CrossRef]
- Association Francaise de Normalisation AFNOR. NF EN ISO 9235 January 2014 Aromatic Natural Raw Materials-Vocabulary-Matières Premières Aromatiques D’origine Naturelle. Available online: https://www.boutique.afnor.org/norme/nf-en-iso-9235/matieres-premieres-aromatiques-naturelles-vocabulaire/article/802028/fa158620 (accessed on 10 September 2020).
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jurado, F.; Navarro-Cruz, A.R.; Ochoa-Velasco, C.E.; Palou, E.; López-Malo, A.; Ávila-Sosa, R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Gertz, F.; Khitun, A. Biological cell manipulation by magnetic nanoparticles. AIP Adv. 2016, 6, 025308. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Zayed, M.A.; Ahmed, M.A.; Imam, N.G.; El Sherbiny, D.H. Analytical Characterization of Hematite/Magnetite Ferrofluid Nanocomposites for Hyperthermia Purposes. J. Supercond. Nov. Magn. 2016, 29, 2899–2916. [Google Scholar] [CrossRef]
- Bruce, I.J.; Sen, T. Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations. Langmuir 2005, 21, 7029–7035. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Kohler, N.; Sun, C.; Fichtenholtz, A.; Gunn, J.; Fang, C.; Zhang, M. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small 2006, 2, 785–792. [Google Scholar] [CrossRef]
- Sangeetha, J.; Thomas, S.; Arutchelvi, J.; Doble, M.; Philip, J. Functionalization of iron oxide nanoparticles with biosurfactants and biocompatibility studies. J. Biomed. Nanotechnol. 2013, 9, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Seabra, A.B.; Pelegrino, M.T.; Haddad, P.S. Synthesis, characterization and cytotoxicity of glutathione- and PEG-glutathione-superparamagnetic iron oxide nanoparticles for nitric oxide delivery. Appl. Surf. Sci. 2016, 367, 26–35. [Google Scholar] [CrossRef]
- Shabani, F.; Khodayari, A. Structural, compositional, and biological characterization of Fe3O4 nanoparticles synthesized by hydrothermal method. Synth. React. Inorg. Met.-Org. Nano-Metal Chem 2015, 45, 356–362. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, M.; Zhao, T.; Xu, Y.; Lin, J.; Duan, Y.; Gu, H. Low toxicity and long circulation time of Polyampholyte-coated magnetic nanoparticles for blood pool contrast agents. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Tan, X.; Zhang, H.; Sun, G. Construction of doxorubicin-loading magnetic nanocarriers for assaying apoptosis of glioblastoma cells. J. Colloid Interface Sci. 2014, 436, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Gazeau, F. Universal cell labelling with anionic magnetic nanoparticles. Biomaterials 2008, 29, 3161–3174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, F.; Cole, A.J.; Chertok, B.; David, A.E.; Wang, J.; Yang, V.C. Gum arabic-coated magnetic nanoparticles for potential application in simultaneous magnetic targeting and tumor imaging. AAPS J. 2009, 11, 693–699. [Google Scholar] [CrossRef]
- Phong, P.T.; Phuc, N.X.; Nguyen, L.H. Study of specific loss power of magnetic fluids with various viscosities. J. Magn. Magn. Mater. 2017, 428, 36–42. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Moghanaki, A.A. Folate-Conjugated pH-Responsive Nanocarrier Designed for Active Tumor Targeting and Controlled Release of Gemcitabine. Pharm. Res. 2016, 33, 417–432. [Google Scholar] [CrossRef]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in MRI. Appl. Nanosci. 2015, 5, 435–441. [Google Scholar] [CrossRef]
- Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Gurau, D.; Ficai, A.; Ficai, D.; Andronescu, E. Synthesis and Applications of Fe3O4/SiO2 core-shell materials. Curr. Pharm. Des. 2015, 21, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbabi Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef]
- Tartalja, D.M.; Kuzmanović, B.; Bojanić, S.; Radisavljević, I.; Ivanović, N. Calculations of optical properties of some molecules suitable for coating of nanoparticles for biological applications. Opt. Quant. Electron. 2016, 48, 241. [Google Scholar] [CrossRef]
- Couto, D.; Freitas, M.; Carvalho, F.; Fernandes, E. Iron Oxide Nanoparticles: An Insight into their Biomedical Applications. Curr. Med. Chem. 2015, 22, 1808–1828. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Schladt, T.D.; Schneider, K.; Schild, H.; Tremel, W. Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalt. Trans. 2011, 40, 6315–6343. [Google Scholar] [CrossRef]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A.; Khan, M.I. A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Gunduz, U.; Oprea, O.; Ficai, D.; Sonmez, M.; Radulescu, M.; Alexie, M.; Ficai, A. Magnetite: From Synthesis to Applications. Curr. Top. Med. Chem. 2015, 15, 1622–1640. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Risbud, S.; Rabolt, J.F.; Stroeve, P. Synthesis and characterization of nanometer-size Fe3O4 and γ-Fe2O3 particles. Chem. Mater. 1996, 8, 2209–2211. [Google Scholar] [CrossRef]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Qu, S.; Yang, H.; Ren, D.; Kan, S.; Zou, G.; Li, D.; Li, M. Magnetite nanoparticles prepared by precipitation from partially reduced ferric chloride aqueous solutions. J. Colloid Interface Sci. 1999, 215, 190–192. [Google Scholar] [CrossRef]
- Baruwati, B.; Nadagouda, M.N.; Varma, R.S. Bulk synthesis of monodisperse ferrite nanoparticles at water-organic interfaces under conventional and microwave hydrothermal treatment and their surface functionalization. J. Phys. Chem. C 2008, 112, 18399–18404. [Google Scholar] [CrossRef]
- Chen, D.H.; Wu, S.H. Synthesis of nickel nanoparticles in water-in-oil microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar] [CrossRef]
- Gobe, M.; Kon-No, K.; Kandori, K.; Kitahara, A. Preparation and characterization of monodisperse magnetite sols in W O microemulsion. J. Colloid Interface Sci. 1983, 93, 293–295. [Google Scholar] [CrossRef]
- Lu, T.; Wang, J.; Yin, J.; Wang, A.; Wang, X.; Zhang, T. Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surf. A 2013, 436, 675–683. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.G.; Noh, H.J.; Park, J.H.; Hyeon, T. Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Vidal-Vidal, J.; Rivas, J.; López-Quintela, M.A. Synthesis of monodisperse maghemite nanoparticles by the microemulsion method. Colloids Surf. A 2006, 288, 44–51. [Google Scholar] [CrossRef]
- Shen, K.; Wang, J.; Li, Y.; Wang, Y.; Li, Y. Preparation of magnetite core-shell nanoparticles of Fe3O4 and carbon with aryl sulfonyl acetic acid. Mater. Res. Bull. 2013, 48, 4655–4660. [Google Scholar] [CrossRef]
- Thomas, G.; Demoisson, F.; Chassagnon, R.; Popova, E.; Millot, N. One-step continuous synthesis of functionalized magnetite nanoflowers. Nanotechnology 2016, 27, 135604. [Google Scholar] [CrossRef]
- Dubois, T.; Demazeau, G. Preparation of Fe3O4 fine particles through a solvothermal process. Mater. Lett. 1994, 19, 38–47. [Google Scholar] [CrossRef]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef]
- Liu, X. Di; Chen, H.; Liu, S.S.; Ye, L.Q.; Li, Y.P. Hydrothermal synthesis of superparamagnetic Fe3O4 nanoparticles with ionic liquids as stabilizer. Mater. Res. Bull. 2015, 62, 217–221. [Google Scholar] [CrossRef]
- Hu, A.; Yee, G.T.; Lin, W. Magnetically recoverable chiral catalysts immobilized on magnetite nanoparticles for asymmetric hydrogenation of aromatic ketones. J. Am. Chem. Soc. 2005, 127, 12486–12487. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe(III) precursor by a sucrose bifunctional hydrothermal method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Si, S.; Kotal, A.; Mandal, T.K.; Giri, S.; Nakamura, H.; Kohara, T. Size-controlled synthesis of magnetite nanoparticles in the presence of polyelectrolytes. Chem. Mater. 2004, 16, 3489–3496. [Google Scholar] [CrossRef]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker, J.R.; Banaszak Holl, M.M.; Orr, B.G. Facile hydrothermal synthesis of iron oxide nanoparticles with tunable magnetic properties. J. Phys. Chem. C 2009, 113, 13593–13599. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, B.; Li, X.; Li, K. Facile solvothermal synthesis of monodisperse Fe3O4 nanocrystals with precise size control of one nanometre as potential MRI contrast agents. J. Mater. Chem. 2011, 21, 2476–2481. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Wan, J.; Tang, J.; Zhang, C.; Yuan, R.; Chen, K. Insight into the formation of magnetite mesocrystals from ferrous precursors in ethylene glycol. Chem. Commun. 2015, 51, 15910–15913. [Google Scholar] [CrossRef]
- Liang, J.; Yue, A.; Wang, Q.; Song, S.; Li, L. Tailored synthesis of well-faceted single crystals of Fe3O4 and their application in p-nitrophenol reduction. RSC Adv. 2016, 6, 29497–29503. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.E.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Li, Y.; Ma, F.; Su, X.; Sun, C.; Liu, J.; Sun, Z.; Hou, Y. Synthesis and catalysis of oleic acid-coated Fe3O4 nanocrystals for direct coal liquefaction. Catal. Commun. 2012, 26, 231–234. [Google Scholar] [CrossRef]
- Patsula, V.; Kosinová, L.; Lovrić, M.; Ferhatovic Hamzić, L.; Rabyk, M.; Konefal, R.; Paruzel, A.; Šlouf, M.; Herynek, V.; Gajović, S.; et al. Superparamagnetic Fe3O4 Nanoparticles: Synthesis by Thermal Decomposition of Iron(III) Glucuronate and Application in Magnetic Resonance Imaging. ACS Appl. Mater. Interface 2016, 8, 7238–7247. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Shan, X.; Wang, A.; Li, G. Controlled Synthesis of MFe2O4 (M = Mn, Fe, Co, Ni and Zn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, C.; Bandyopadhyaya, R. Mechanistic study on magnetite nanoparticle formation by thermal decomposition and coprecipitation routes. J. Phys. Chem. C 2011, 115, 1380–1387. [Google Scholar] [CrossRef]
- Belaïd, S.; Laurent, S.; Vermeech, M.; Elst, L. Vander; Perez-Morga, D.; Muller, R.N. A new approach to follow the formation of iron oxide nanoparticles synthesized by thermal decomposition. Nanotechnology 2013, 24, 055705. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.K.O.; Tran, D.L.; Le, T.L.; Pham, D.V.; Pham, H.N.; Ngo, T.H. Le; Do, H.M.; Nguyen, X.P. Synthesis of high-magnetization and monodisperse Fe3O4 nanoparticles via thermal decomposition. Mater. Chem. Phys. 2015, 163, 537–544. [Google Scholar] [CrossRef]
- Wetterskog, E.; Agthe, M.; Mayence, A.; Grins, J.; Wang, D.; Rana, S.; Ahniyaz, A.; Salazar-Alvarez, G.; Bergström, L. Precise control over shape and size of iron oxide nanocrystals suitable for assembly into ordered particle arrays. Sci. Technol. Adv. Mater. 2014, 15. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Costo, R.; Grüttner, C.; Westphal, F.; Gehrke, N.; Heinke, D.; Fornara, A.; Pankhurst, Q.A.; Johansson, C.; Veintemillas-Verdaguer, S.; et al. Synthesis methods to prepare single- and multi-core iron oxide nanoparticles for biomedical applications. Dalt. Trans. 2015, 44, 2943–2952. [Google Scholar] [CrossRef]
- Asuha, S.; Suyala, B.; Siqintana, X.; Zhao, S. Direct synthesis of Fe3O4 nanopowder by thermal decomposition of Fe-urea complex and its properties. J. Alloys Compd. 2011, 509, 2870–2873. [Google Scholar] [CrossRef]
- Raileanu, M.; Crisan, M.; Petrache, C.; Crisan, D.; Jitianu, A.; Zaharescu, M.; Predoi, D.; Kuncser, V.; Filoti, G. Sol-gel FexOy-SiO2 nanocomposites. Rom. J. Phys. 2005, 50, 595–606. [Google Scholar]
- Hasanpour, A.; Niyaifar, M.; Asan, M.; Amighian, J. Synthesis and characterization of Fe3O4 and ZnO nanocomposites by the sol-gelmethod. J. Magn. Magn. Mater. 2013, 334, 41–44. [Google Scholar] [CrossRef]
- Raileanu, M.; Crisan, M.; Petrache, C.; Crisan, D.; Zaharescu, M. Fe2O3-SiO2nanocomposites obtained by different sol-gel routes. J. Optoelectron. Adv. Mater. 2003, 5, 693–698. [Google Scholar]
- Chae, H.S.; Kim, S.D.; Piao, S.H.; Choi, H.J. Core-shell structured Fe3O4@SiO2 nanoparticles fabricated by sol–gel method and their magnetorheology. Colloid Polym. Sci. 2016, 294, 647–655. [Google Scholar] [CrossRef]
- Lemine, O.M.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol-gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattice. Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Rosa, R.; Barrera, G.; Tiberto, P.; Allia, P.; Bondioli, F. Microwave-assisted nonaqueous sol-gel synthesis of highly crystalline magnetite nanocrystals. Mater. Chem. Phys. 2014, 148, 117–124. [Google Scholar] [CrossRef]
- Kubrakova, I.V.; Koshcheeva, I.Y.; Pryazhnikov, D.V.; Martynov, L.Y.; Kiseleva, M.S.; Tyutyunnik, O.A. Microwave synthesis, properties and analytical possibilities of magnetite-based nanoscale sorption materials. J. Anal. Chem. 2014, 69, 336–346. [Google Scholar] [CrossRef]
- Abu Mukh-Qasem, R.; Gedanken, A. Sonochemical synthesis of stable hydrosol of Fe3O4 nanoparticles. J. Colloid Interface Sci. 2005, 284, 489–494. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Wang, D.; Wang, M.; Lin, Z.; Tang, H. Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrason. Sonochem. 2010, 17, 526–533. [Google Scholar] [CrossRef]
- Rodríguez-López, A.; Paredes-Arroyo, A.; Mojica-Gomez, J.; Estrada-Arteaga, C.; Cruz-Rivera, J.J.; Elías Alfaro, C.G.; Antaño-López, R. Electrochemical synthesis of magnetite and maghemite nanoparticles using dissymmetric potential pulses. J. Nanoparticle Res. 2012, 14, 993. [Google Scholar] [CrossRef]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.P.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta 2008, 53, 3436–3441. [Google Scholar] [CrossRef]
- Ozcelik, B.K.; Ergun, C. Synthesis and characterization of iron oxide particles using spray pyrolysis technique. Ceram. Int. 2015, 41, 1994–2005. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, S.H.; Kang, Y.C.; Lee, J.K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: A promising support for enzyme immobilization. Nanoscale 2016, 8, 6728–6738. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, S.H.; Kang, Y.C.; Lee, J.K. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl. Mater. Interfaces 2017, 9, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Majerič, P.; Feizpour, D.; Friedrich, B.; Jelen, Ž.; Anžel, I.; Rudolf, R. Morphology of composite Fe@Au submicron particles, produced with ultrasonic spray pyrolysis and potential for synthesis of Fe@Au core-shell particles. Materials 2019, 12, 3326. [Google Scholar] [CrossRef] [PubMed]
- Strobel, R.; Pratsinis, S.E. Direct synthesis of maghemite, magnetite and wustite nanoparticles by flame spray pyrolysis. Adv. Powder Technol. 2009, 20, 190–194. [Google Scholar] [CrossRef]
- Veintemillas-Verdaguer, S.; Del Puerto Morales, M.; Bomati-Miguel, O.; Bautista, C.; Zhao, X.; Bonville, P.; De Alejo, R.P.; Ruiz-Cabello, J.; Santos, M.; Tendillo-Cortijo, F.J.; et al. Colloidal dispersions of maghemite nanoparticles produced by laser pyrolysis with application as NMR contrast agents. J. Phys. D Appl. Phys. 2004, 37, 2054–2059. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Long, N.J.; Aboagye, E.O. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 2013, 42, 7816–7833. [Google Scholar] [CrossRef]
- Tombácz, E.; Turcu, R.; Socoliuc, V.; Vékás, L. Magnetic iron oxide nanoparticles: Recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem. Biophys. Res. Commun. 2015, 468, 442–453. [Google Scholar] [CrossRef]
- Li, H.; Lu, Z.; Cheng, G.; Rong, K.; Chen, F.; Chen, R. HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological application. RSC Adv. 2015, 5, 5059–5067. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Bankova, V.; Lourenço, J.P.; Rosa Costa, A.M.; Mariano, J.F.; Miguel, M.G.; Faleiro, M.L. Impact of biohybrid magnetite nanoparticles and moroccan propolis on adherence of methicillin resistant strains of staphylococcus aureus. Molecules 2016, 21, 1208. [Google Scholar] [CrossRef]
- Maleki, H.; Simchi, A.; Imani, M.; Costa, B.F.O. Size-controlled synthesis of superparamagnetic iron oxide nanoparticles and their surface coating by gold for biomedical applications. J. Magn. Magn. Mater. 2012, 324, 3997–4005. [Google Scholar] [CrossRef]
- Sarkar, T.; Tiwari, S.; Rawat, K.; Solanki, P.R.; Bohidar, H.B. Hydrophilic, fluorescent and superparamagnetic iron oxide-carbon composite nanoparticles. Colloids Surf. A 2017, 514, 218–225. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ramakrishna, S.; Esmaeili, H.; Bahrani, S.; Koosha, M.; Babapoor, A. Green synthesis of supermagnetic Fe3O4–MgO nanoparticles via Nutmeg essential oil toward superior anti-bacterial and anti-fungal performance. J. Drug Deliv. Sci. Technol. 2019, 54, 101352. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Mejri, J.; Aydi, A.; Abderpabba, M.; Mejri, M. Emerging extraction processes of essential oils: A review. Asian J. Green Chem. 2018, 2, 246–267. [Google Scholar] [CrossRef]
- Ye, Q.; Zheng, D. Rapid analysis of the essential oil components of dried Perilla frutescens (L.) by magnetic nanoparticle-assisted microwave distillation and simultaneous headspace solid-phase microextraction followed by gas chromatography-mass spectrometry. Anal. Methods 2009, 1, 39–44. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Mohammdhosseini, M.; Salar, M. Chemical composition of the essential oils from the hulls of Pistacia vera L. by using magnetic nanoparticle-assisted microwave (MW) distillation: Comparison with routine MW and conventional hydrodistillation. Anal. Methods 2014, 6, 2572–2579. [Google Scholar] [CrossRef]
- Oshtrakh, I.; Rodriguez, R.; Semionkin, A.; Santos, G.; Milder, B.; Silveira, B.; Marmolejo, M.; Ushakov, V.; De Souza-Parise, M.; Morais, C. Magnetic fluid: Comparative study of nanosized Fe3O4 and Fe3O4 suspended in Copaiba oil using Mössbauer spectroscopy with a high velocity resolution. J. Phys. Conf. Ser. 2010, 217, 012018. [Google Scholar] [CrossRef]
- Oshtrakh, M.I.; Šepelák, V.; Rodriguez, A.F.R.; Semionkin, V.A.; Ushakov, M.V.; Santos, J.G.; Silveira, L.B.; Marmolejo, E.M.; Parise, M.D.S.; Morais, P.C. Comparative study of iron oxide nanoparticles as-prepared and dispersed in Copaiba oil using Mössbauer spectroscopy with low and high velocity resolution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 100, 94–100. [Google Scholar] [CrossRef]
- Santos, J.G.; Silveira, L.B.; Ferreira, Q.S.; Garg, V.K.; Oliveira, A.C.; Parise, M.S.; Morais, P.C. The stability of magnetic colloid based from copaiba oil. J. Phys. Conf. Ser. 2010, 214, 012133. [Google Scholar] [CrossRef]
- Gaspar, A.S.; Wagner, F.E.; Amaral, V.S.; Costa Lima, S.A.; Khomchenko, V.A.; Santos, J.G.; Costa, B.F.O.; Durães, L. Development of a biocompatible magnetic nanofluid by incorporating SPIONs in Amazonian oils. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 172, 135–146. [Google Scholar] [CrossRef]
- Cabral, E.C.; Da Cruz, G.F.; Simas, R.C.; Sanvido, G.B.; Gonçalves, D.L.V.; Leal, R.V.P.; Da Silva, R.C.F.; Da Silva, J.C.T.; Barata, L.E.S.; Da Cunha, V.S.; et al. Typification and quality control of the Andiroba (Carapa guianensis) oil via mass spectrometry fingerprinting. Anal. Methods 2013, 5, 1385–1391. [Google Scholar] [CrossRef]
- Atoche-Medrano, J.J.; León-Felix, L.; Faria, F.S.E.D.V.; Rodríguez, A.F.R.; Cunha, R.M.; Aragón, F.H.; Sousa, M.H.; Coaquira, J.A.H.; Azevedo, R.B.; Morais, P.C. Magnetite-based nanobioplatform for site delivering Croton cajucara Benth essential oil. Mater. Chem. Phys. 2018, 207, 243–252. [Google Scholar] [CrossRef]
- Medrano, J.J.A.; Aragón, F.F.H.; Leon-Felix, L.; Coaquira, J.A.H.; Rodríguez, A.F.R.; Faria, F.S.E.D.V.; Sousa, M.H.; Ochoa, J.C.M.; Morais, P.C. Evidence of particle-particle interaction quenching in nanocomposite based on oleic acid-coated Fe3O4 nanoparticles after over-coating with essential oil extracted from Croton cajucara Benth. J. Magn. Magn. Mater. 2018, 466, 359–367. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Miranda Salvado, I.M.; Ferreira, J.M.F.; Pullar, R.C. Clove and cinnamon: Novel anti–oxidant fuels for preparing magnetic iron oxide particles by the sol–gel auto–ignition method. J. Alloys Compd. 2019, 786, 71–76. [Google Scholar] [CrossRef]
- Bao, Y.; Wen, T.; Samia, A.C.S.; Khandhar, A.; Krishnan, K.M. Magnetic Nanoparticles: Material Engineering and Emerging Applications in Lithography and Biomedicine. J Mater. Sci. 2016, 51, 513–553. [Google Scholar] [CrossRef]
- Sadhasivam, J.; Sugumaran, A. Magnetic nanocarriers: Emerging tool for the effective targeted treatment of lung cancer. J. Drug Deliv. Sci. Technol. 2020, 55, 101493. [Google Scholar] [CrossRef]
- Mukherjee, S.; Liang, L.; Veiseh, O. Recent advancements of magnetic nanomaterials in cancer therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.; Cao, H.; Wu, Y. Design of Functional Magnetic Nanocomposites for Bioseparation. Colloid Surface. B 2020, 191, 111014. [Google Scholar] [CrossRef]
- Kaliamurthi, S.; Demir-Korkmaz, A.; Selvaraj, G.; Gokce-Polat, E.; Wei, Y.-K.; Almessiere, M.A.; Baykal, A.; Gu, K.; Wei, D.-Q. Viewing the Emphasis on State-of-the-Art Magnetic Nanoparticles: Synthesis, Physical Properties, and Applications in Cancer Theranostics. Curr. Pharm. Des. 2019, 25, 1505–1523. [Google Scholar] [CrossRef]
- Nahar, K.; Absar, S.; Patel, B.; Ahsan, F. Starch-coated magnetic liposomes as an inhalable carrier for accumulation of fasudil in the pulmonary vasculature. Int. J. Pharm. 2014, 464, 185–195. [Google Scholar] [CrossRef]
- Domracheva, N.E.; Pyataev, A.V.; Manapov, R.A.; Gruzdev, M.S. Magnetic resonance and Mössbauer studies of superparamagnetic γ-Fe2O3 nanoparticles encapsulated into liquid-crystalline poly(propylene imine) dendrimers. ChemPhysChem 2011, 12, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K.; De, M.; Chou, S.S.; Vasavada, S.; Bleher, R.; Prasad, P.V.; Bahadur, D.; Dravid, V.P. Thermoresponsive magnetic hydrogels as theranostic nanoconstructs. ACS Appl. Mater. Interfaces 2014, 6, 6237–6247. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Lee, J.S.; Choi, J.H.; Park, K.M.; Lee, Y.; Park, K.D. Hierarchical self-assembly of magnetic nanoclusters for theranostics: Tunable size, enhanced magnetic resonance imagability, and controlled and targeted drug delivery. Acta Biomater. 2016, 35, 109–117. [Google Scholar] [CrossRef]
- Fakhimikabir, H.; Tavakoli, M.B.; Zarrabi, A.; Amouheidari, A.; Rahgozar, S. Could FA-PG-SPIONs act as a hyperthermia sensitizing agent? An in vitro study. J. Therm. Biol. 2018, 78, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR effect: Combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- Yun, W.S.; Aryal, S.; Ahn, Y.J.; Seo, Y.J.; Key, J. Engineered iron oxide nanoparticles to improve regenerative effects of mesenchymal stem cells. Biomed. Eng. Lett. 2020, 10, 259–273. [Google Scholar] [CrossRef]
- Suciu, M.; Ionescu, C.M.; Ciorita, A.; Tripon, S.C.; Nica, D.; Al-Salami, H.; Barbu-Tudoran, L. Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery, and biotechnological advancements. Beilstein J. Nanotechnol. 2020, 11, 1092–1109. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T 2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef]
- Park, J.H.; von Maltzahn, G.; Zhang, L.; Schwartz, M.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv. Mater. 2008, 20, 1630–1635. [Google Scholar] [CrossRef]
- Uthaman, S.; Lee, S.J.; Cherukula, K.; Cho, C.S.; Park, I.K. Polysaccharide-coated magnetic nanoparticles for imaging and gene therapy. Biomed Res. Int. 2015, 2015, 959175. [Google Scholar] [CrossRef]
- Aliakbari, M.; Mohammadian, E.; Esmaeili, A.; Pahlevanneshan, Z. Differential effect of polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles on BT-474 human breast cancer cell viability. Toxicol. Vitr 2019, 54, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, K.R.; Xu, Y.; Gray, M.; Fair, D.; Hayles, H.; Milad, L.; Montes, A.; Sherwood, J.; Bao, Y.; Rasco, J.F. Short- and long-term effects of prenatal exposure to iron oxide nanoparticles: Influence of surface charge and dose on developmental and reproductive toxicity. Int. J. Mol. Sci. 2015, 16, 30251–30268. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Saha, R.; Liu, J.; Chugh, V.K.; Wang, J.P. Magnetic Particle Spectroscopy: A Short Review of Applications Using Magnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 4972–4989. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Saha, R.; Su, D.; Krishna, V.D.; Cheeran, M.C.J.; Wang, J.P. Magnetic Particle Spectroscopy for Detection of Influenza A Virus Subtype H1N1. ACS Appl. Mater. Interfaces 2020, 12, 13686–13697. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Cristescu, R.; Chifiriuc, M.C.; Dorcioman, G.; Socol, G.; Mihailescu, I.N.; Mihaiescu, D.E.; Ficai, A.; Vasile, O.R.; Enculescu, M.; et al. Fabrication of magnetite-based core-shell coated nanoparticles with antibacterial properties. Biofabrication 2015, 7, 015014. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef]
- El-Zowalaty, M.E.; Al-Ali, S.H.H.; Husseiny, M.I.; Geilich, B.M.; Webster, T.J.; Hussein, M.Z. The ability of streptomycin-loaded chitosan-coated magnetic nanocomposites to possess antimicrobial and antituberculosis activities. Int. J. Nanomed. 2015, 10, 3269–3274. [Google Scholar] [CrossRef]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazăr, V.; Andronescu, E. Hybrid magnetite nanoparticles/rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett. 2012, 7, 1–7. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent advances in magnetite nanoparticle functionalization for nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano. Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Wang, P.; Shi, Q.; Shi, Y.; Clark, K.K.; Stucky, G.D.; Keller, A.A. Magnetic permanently confined micelle arrays for treating hydrophobic organic compound contamination. J. Am. Chem. Soc. 2009, 131, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.; Kim, K.S. Water-Dispersable Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.T.; Yavuz, C.; Yean, S.; Cong, L.; Shipley, H.; Yu, W.; Falkner, J.; Kan, A.; Tomson, M.; Colvin, V.L. The effect of nanocrystalline magnetite size on arsenic removal. Sci. Technol. Adv. Mater. 2007, 8, 71–75. [Google Scholar] [CrossRef]
- Ó Dálaigh, C.; Corr, S.A.; Gun’ko, Y.; Connon, S.J. A magnetic-nanoparticle-supported 4-N,N-dialkylaminopyridine catalyst: Excellent reactivity combined with facile catalyst recovery and recyclability. Angew. Chem. Int. Ed. 2007, 46, 4329–4332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.S.; Wen, L.; Lu, G.Q.; Cheng, H.M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Xu, H.L.; Shen, Y.; Bi, H. Reduced graphene oxide decorated with Fe3O4 nanoparticles as high performance anode for lithium ion batteries. Key Eng. Mater. 2012, 519, 108–112. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, X.; Liu, J.; Huang, Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 2020, 188, 106943. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Effects of Magnetite and Maghemite Nanoparticles on Bone Cell and Staphylococcus Aureus Functions. Technol. Innov. 2011, 13, 39–50. [Google Scholar] [CrossRef]
- Saviuc, C.; Grumezescu, A.M.; Chifiriuc, M.C.; Bleotu, C.; Stanciu, G.; Hristu, R.; Mihaiescu, D.; Lazǎr, V. In vitro methods for the study of microbial biofilms. Biointerface. Res. Appl. Chem. 2011, 1, 31–40. [Google Scholar]
- Darwish, M.S.A.; Nguyen, N.H.A.; Ševců, A.; Stibor, I.; Smoukov, S.K. Dual-modality self-heating and antibacterial polymer-coated nanoparticles for magnetic hyperthermia. Mater. Sci. Eng. C 2016, 63, 88–95. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Gesta, M.C.; Holban, A.M.; Grumezescu, V.; Vasile, B.S.; Mogoanta, L.; Iordache, F.; Bleotu, C.; Dan Mogosanu, G. Biocompatible Fe3O4 increases the efficacy of amoxicillin delivery against gram-positive and gram-negative bacteria. Molecules 2014, 19, 5013–5027. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vizuete, P.; Orgaz, B.; Aymerich, S.; Le Coq, D.; Briandet, R. Pathogens protection against the action of disinfectants in multispecies biofilms. Front. Microbiol. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Lazǎr, V.; Chifiriuc, M.C. Modified wound dressing with phytonanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Apolónio, J.; Faleiro, M.L.; Miguel, M.G.; Neto, L. No induction of antimicrobial resistance in Staphylococcus aureus and Listeria monocytogenes during continuous exposure to eugenol and citral. FEMS Microbiol. Lett. 2014, 354, 92–101. [Google Scholar] [CrossRef]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef]
- Niu, C.; Gilbert, E.S. Colorimetric Method for Identifying Plant Essential Oil Components That Affect Biofilm Formation and Structure. Appl. Environ. Microbiol. 2004, 70, 6951–6956. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Alibi, S.; Ben Selma, W.; Ramos-Vivas, J.; Smach, M.A.; Touati, R.; Boukadida, J.; Navas, J.; Ben Mansour, H. Anti-oxidant, antibacterial, anti-biofilm, and anti-quorum sensing activities of four essential oils against multidrug-resistant bacterial clinical isolates. Curr. Res. Transl. Med. 2020, 68, 59–66. [Google Scholar] [CrossRef]
- Mizan, M.F.R.; Ashrafudoulla, M.; Hossain, M.I.; Cho, H.R.; Ha, S. Do Effect of essential oils on pathogenic and biofilm-forming Vibrio parahaemolyticus strains. Biofouling 2020, 36, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, X.; Yang, H.; Niu, X.; Li, D.; Yang, C.; Li, L.; Zou, L.; Qiu, Z.; Wu, S.; et al. Antibacterial activity and anti-quorum sensing mediated phenotype in response to essential oil from Melaleuca bracteata leaves. Int. J. Mol. Sci. 2019, 20, 5696. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, J.; Liu, C.; Yang, A.; Qiao, J. Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cell. Mol. Life Sci. 2020, 77, 1319–1343. [Google Scholar] [CrossRef] [PubMed]
- Homer, C.M.; Summers, D.K.; Goranov, A.I.; Clarke, S.C.; Wiesner, D.L.; Diedrich, J.K.; Moresco, J.J.; Toffaletti, D.; Upadhya, R.; Caradonna, I.; et al. Intracellular Action of a Secreted Peptide Required for Fungal Virulence. Cell Host Microbe 2016, 19, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between viruses guides lysis-lysogeny decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef]
- Silpe, J.E.; Bridges, A.A.; Huang, X.; Coronado, D.R.; Duddy, O.P.; Bassler, B.L. Separating Functions of the Phage-Encoded Quorum-Sensing-Activated Antirepressor Qtip. Cell Host Microbe 2020, 27, 629–641. [Google Scholar] [CrossRef]
- Rojas, F.; Matthews, K.R. Quorum sensing in African trypanosomes. Curr. Opin. Microbiol. 2019, 52, 124–129. [Google Scholar] [CrossRef]
- Sampriti Mukherjee and Bonnie, L. Bassler Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Chifiriuc, M.C.; Saviuc, C.; Grumezescu, V.; Hristu, R.; Mihaiescu, D.E.; Stanciu, G.A.; Andronescu, E. Hybrid nanomaterial for stabilizing the antibiofilm activity of eugenia carryophyllata essential oil. IEEE Trans. Nanobioscience 2012, 11, 360–365. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Ficai, A.; Chifiriuc, C.M.; Lăzar, V.; Rădulescu, R. biofilmului de candida tropicalis Fe3O4 @ C 18 -carvone to prevent candida tropicalis biofilm development. Rom. J. Mater. 2013, 43, 300–305. [Google Scholar]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and Satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef] [PubMed]
- Bilcu, M.; Grumezescu, A.M.; Oprea, A.E.; Popescu, R.C.; Mogoanu, G.D.; Hristu, R.; Stanciu, G.A.; Mihailescu, D.F.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of vanilla, patchouli and ylang ylang essential oils stabilized by iron oxide@C14 nanostructures against bacterial adherence and biofilms formed by staphylococcus aureus and klebsiella pneumoniae clinical strains. Molecules 2014, 19, 17943–17956. [Google Scholar] [CrossRef] [PubMed]
- Rădulescu, M.; Andronescu, E.; Holban, A.M.; Vasile, B.S.; Iordache, F.; Mogoantă, L.; Dan Mogoșanu, G.; Grumezescu, A.M.; Georgescu, M.; Chifiriuc, M.C. Antimicrobial nanostructured bioactive coating based on Fe3O4 and patchouli oil for wound dressing. Metals 2016, 6, 103. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Andronescu, E.; Oprea, A.E.; Holban, A.M.; Socol, G.; Grumezescu, V.; Chifiriuc, M.C.; Iordache, F.; Maniu, H. MAPLE fabricated magnetite@Melissa officinalis and poly lactic acid: Chitosan coated surfaces with anti-staphylococcal properties. J. Sol-Gel Sci. Technol. 2015, 73, 612–619. [Google Scholar] [CrossRef]
- Iordache, F.; Oprea, A.E.; Grumezescu, V.; Andronescu, E.; Socol, G.; Grumezescu, A.M.; Popa, M.; Mogoşanu, G.D.; Holban, A.M.; Maniu, H. Poly(lactic-co-glycolic) acid/chitosan microsphere thin films functionalized with Cinnamomi aetheroleum and magnetite nanoparticles for preventing the microbial colonization of medical surfaces. J. Sol-Gel Sci. Technol. 2015, 73, 679–686. [Google Scholar] [CrossRef]

| Method | Advantages | Disadvantages | Size and Size Distribution | Morphology |
|---|---|---|---|---|
| Coprecipitation | Large quantities can be synthesized. Simple experimental procedure. | Limited control over the size distribution. Possible oxidation of magnetite to maghemite. | Typically below 50 nm, with broad size distribution. | Spherical with aggregates. |
| Microemulsion | Good control over the size and shape of the nanoparticles. Low temperature of synthesis. | Limited quantities produced. Use of organic solvents and surfactants that can be difficult to remove. | Usually below 15–20 nm with very narrow size distribution. | Spherical with no aggregates. |
| High temperature | Very good control over the size, shape, and size distribution of the nanoparticles. | Need high-temperature equipment and, depending on the method, metal organic precursors could be used. | Variable with the method and the precursor. Very small particles can be prepared (ca. 2–3 nm). Very narrow size distribution. | Very different shapes can be prepared, including unusual morphologies as nanopolyhedra, core–shell structures, aggregate nanoflowers, hollow nanoparticles, nanocapsules. |
| Sol–gel | Particles of desired shape and length can be synthesized. Adequate for the preparation of core–shell nanoparticles and organic–inorganic composites involving magnetic oxides. | The reactions are performed at low temperature, but further treatments at higher temperature are needed to obtain the final structure. Sol–gel matrix residues may remain in the final products. | Nanoparticles smaller than 20 nm are usually prepared, but larger particles (up to 200 nm) have been reported. Usually narrow size distribution. | Usually spherical. High porosity may be introduced. |
| Spray pyrolysis | High production rate. Cost-effective. High chemical flexibility. Easy control of process parameters. | Large aggregates could be formed. | Particles up to ca. 700 nm depending on the process parameters. | Usually spherical, but aggregates could have different shapes. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, M.G.; Lourenço, J.P.; Faleiro, M.L. Superparamagnetic Iron Oxide Nanoparticles and Essential Oils: A New Tool for Biological Applications. Int. J. Mol. Sci. 2020, 21, 6633. https://doi.org/10.3390/ijms21186633
Miguel MG, Lourenço JP, Faleiro ML. Superparamagnetic Iron Oxide Nanoparticles and Essential Oils: A New Tool for Biological Applications. International Journal of Molecular Sciences. 2020; 21(18):6633. https://doi.org/10.3390/ijms21186633
Chicago/Turabian StyleMiguel, Maria Graça, João Paulo Lourenço, and Maria Leonor Faleiro. 2020. "Superparamagnetic Iron Oxide Nanoparticles and Essential Oils: A New Tool for Biological Applications" International Journal of Molecular Sciences 21, no. 18: 6633. https://doi.org/10.3390/ijms21186633
APA StyleMiguel, M. G., Lourenço, J. P., & Faleiro, M. L. (2020). Superparamagnetic Iron Oxide Nanoparticles and Essential Oils: A New Tool for Biological Applications. International Journal of Molecular Sciences, 21(18), 6633. https://doi.org/10.3390/ijms21186633






