A Multi-Endpoint Approach to Base Excision Repair Incision Activity Augmented by PARylation and DNA Damage Levels in Mice: Impact of Sex and Age
Abstract
1. Introduction
2. Results
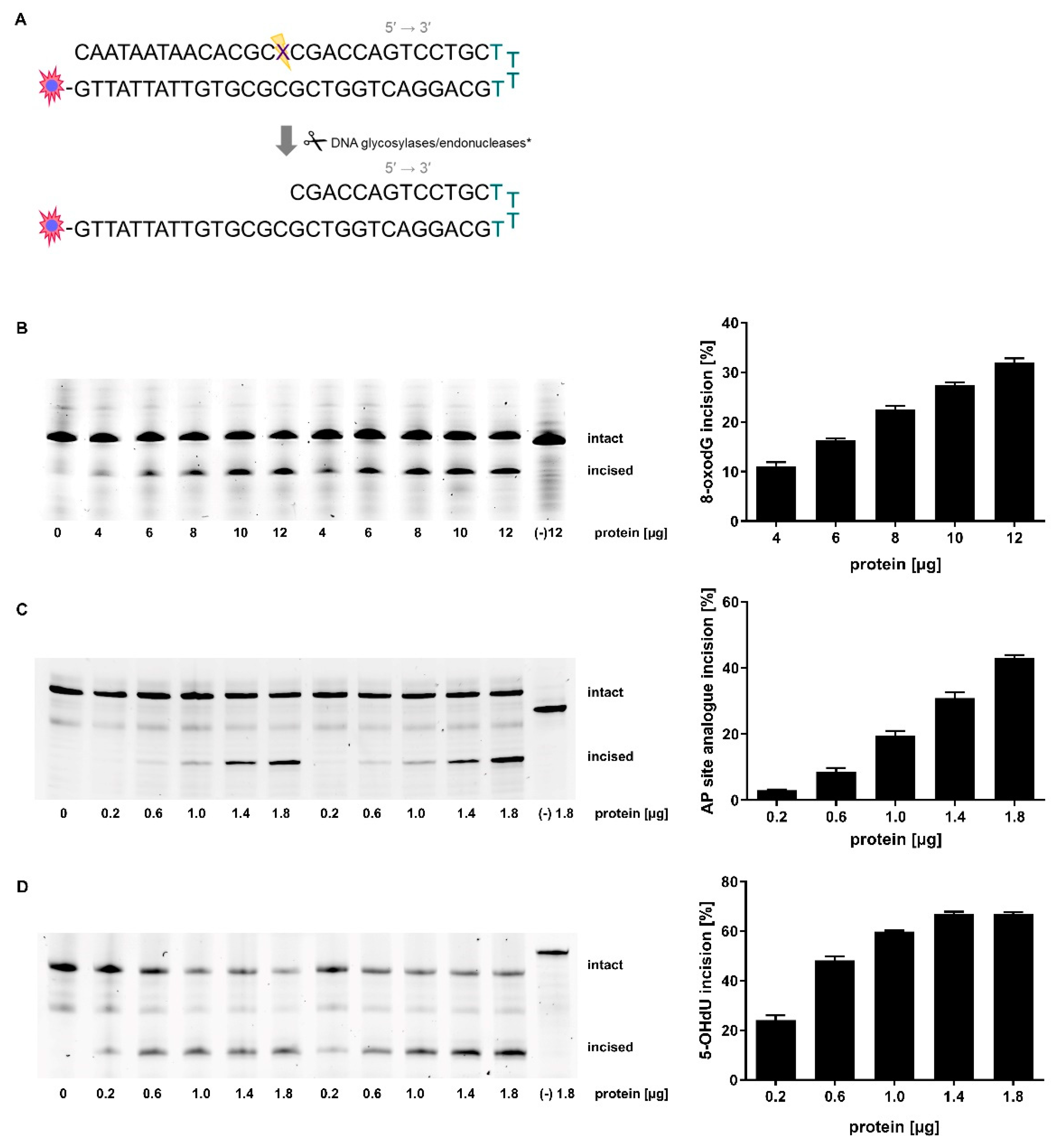
2.1. Adaption of Assay Parameters
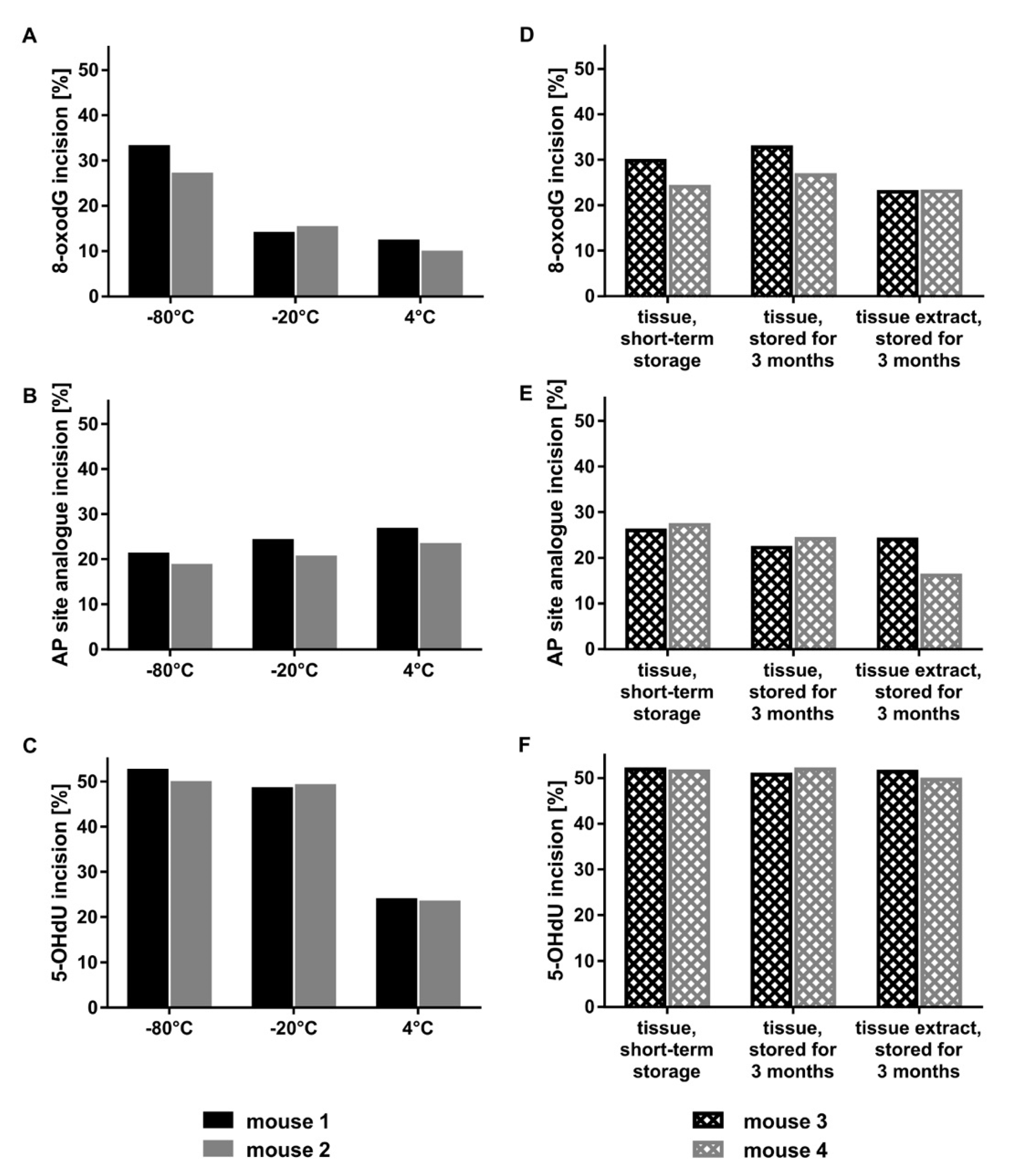
2.2. Optimal Protein Amounts, Storage Conditions, and Tissue Processing
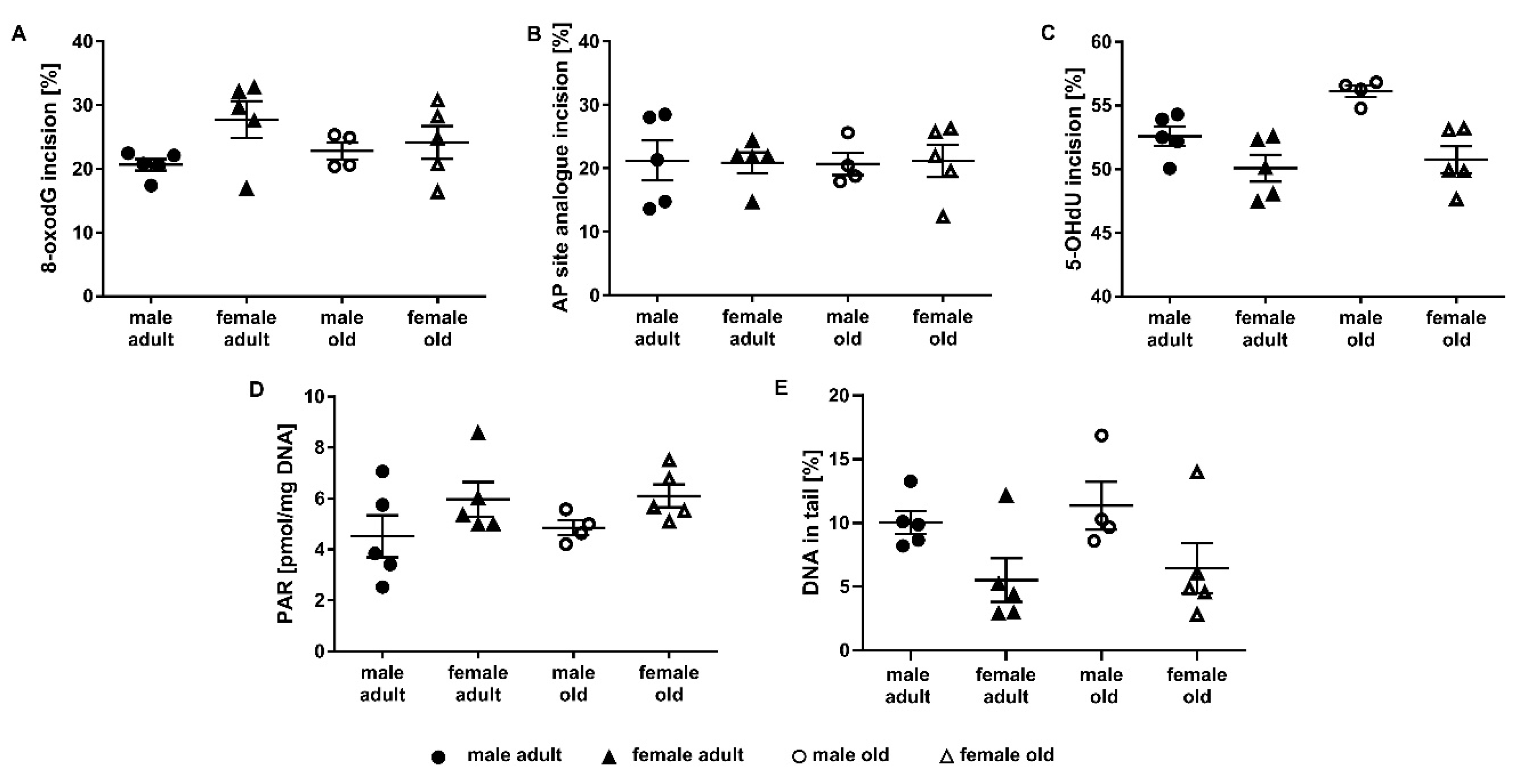
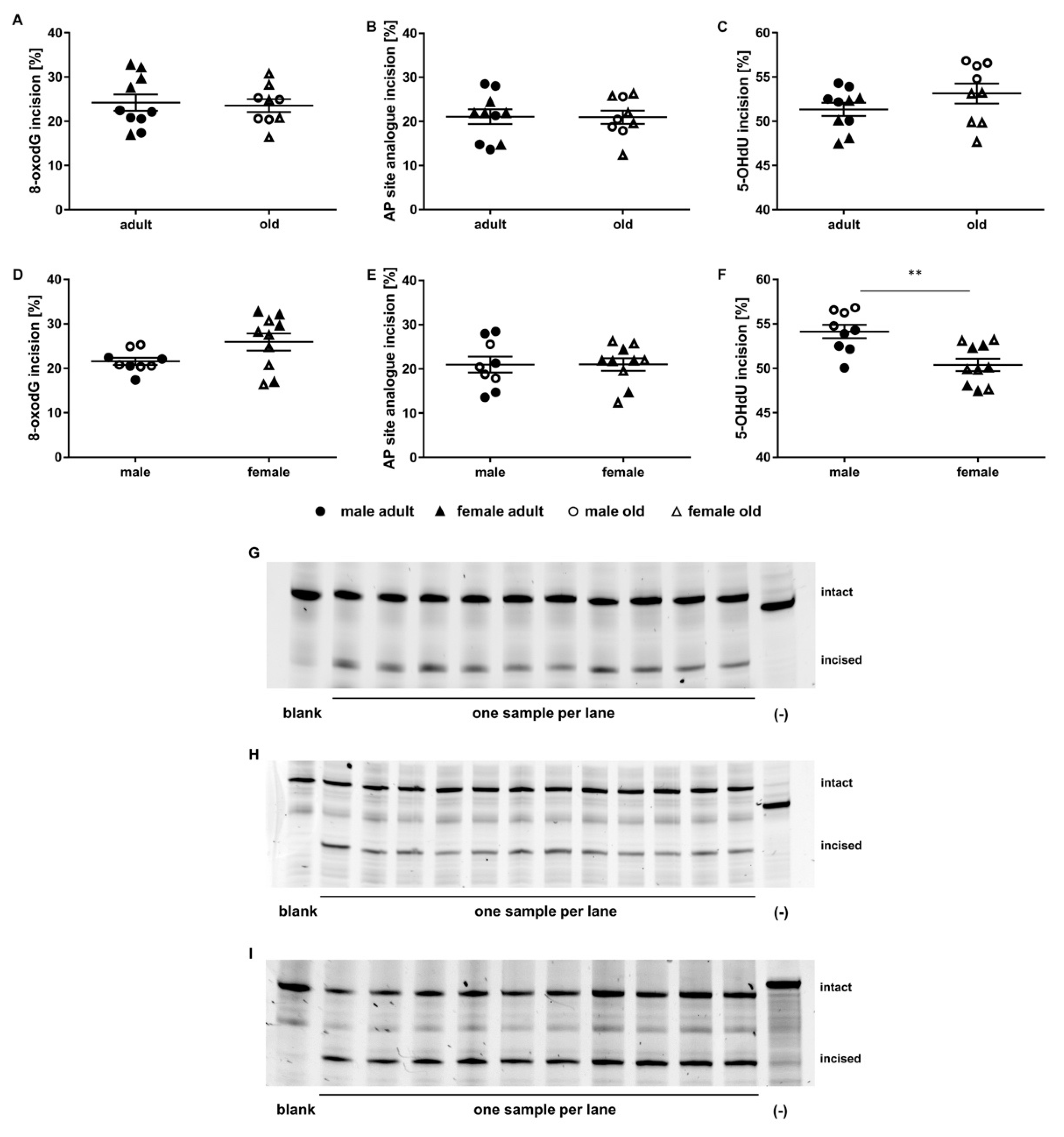
2.3. Application of the Incision Activity Assay to a Cohort of Adult and Old Mice
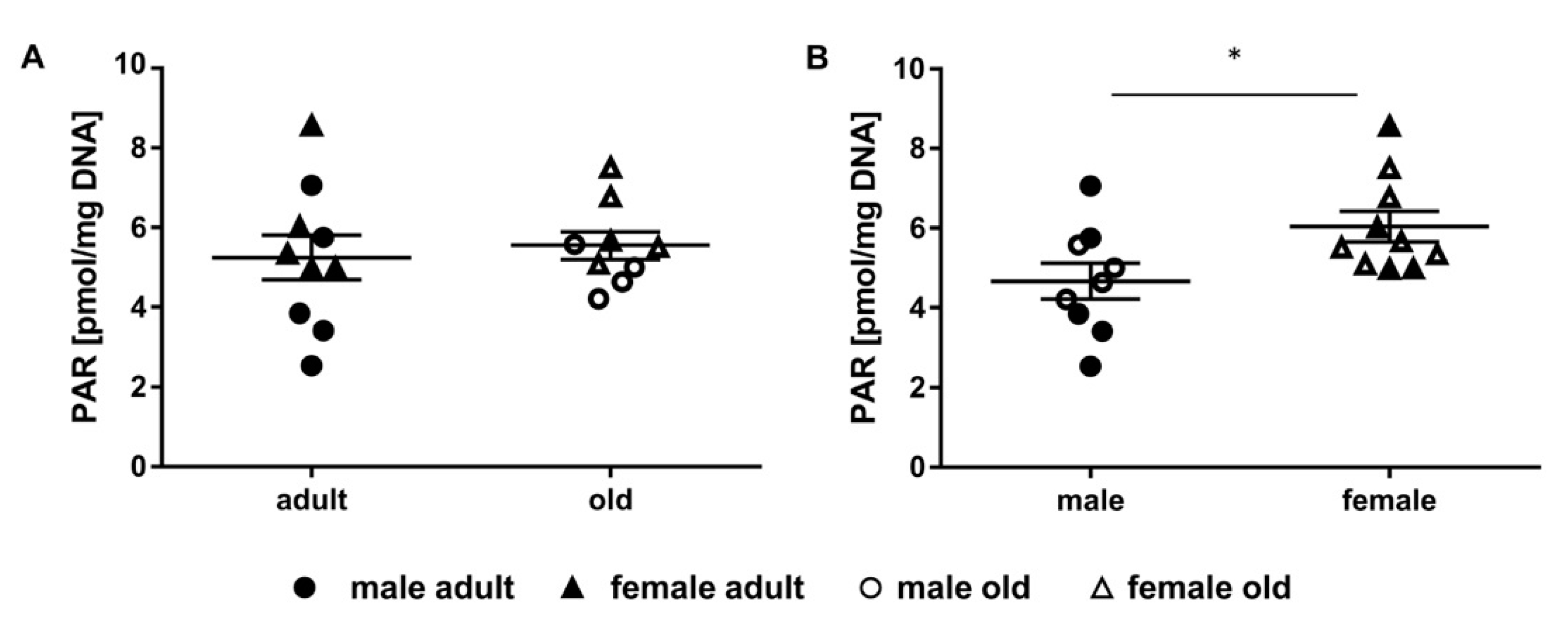
2.4. Determination of Parylation Levels in Murine Liver
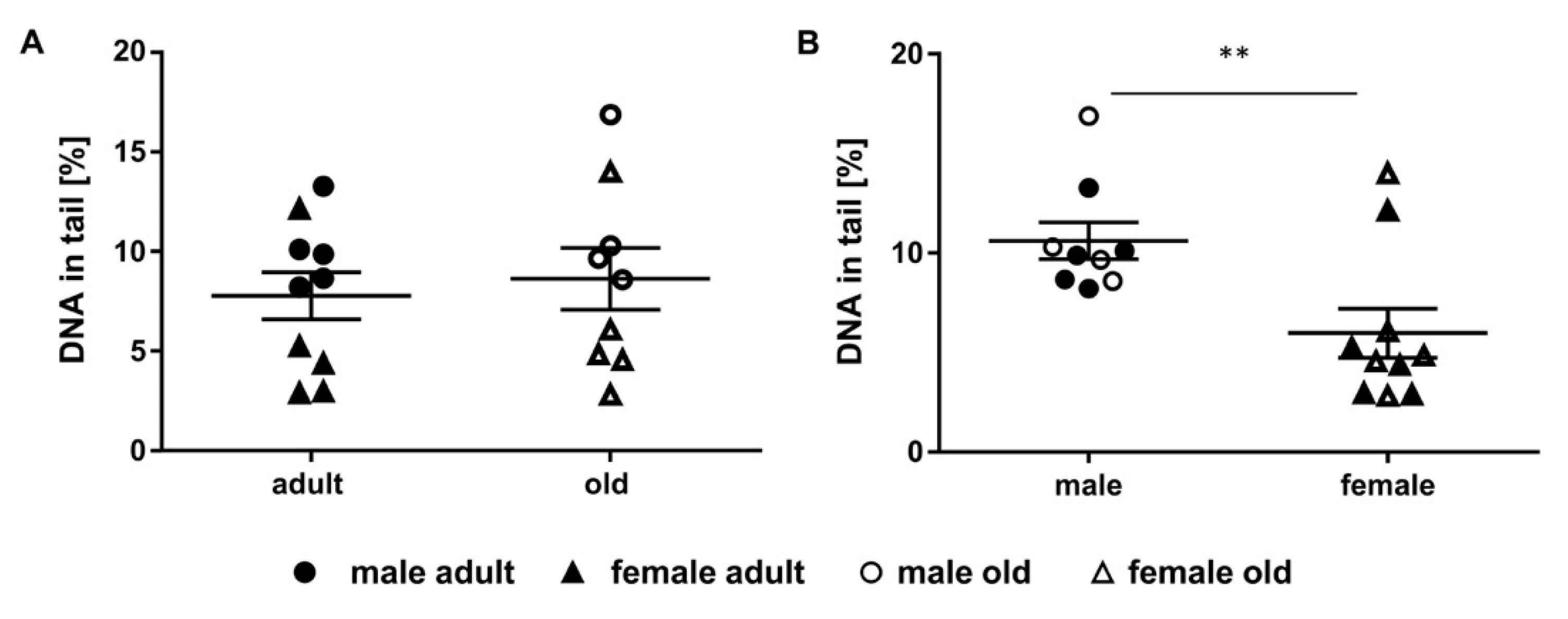
2.5. Assessment of DNA Damage in Murine Liver Samples
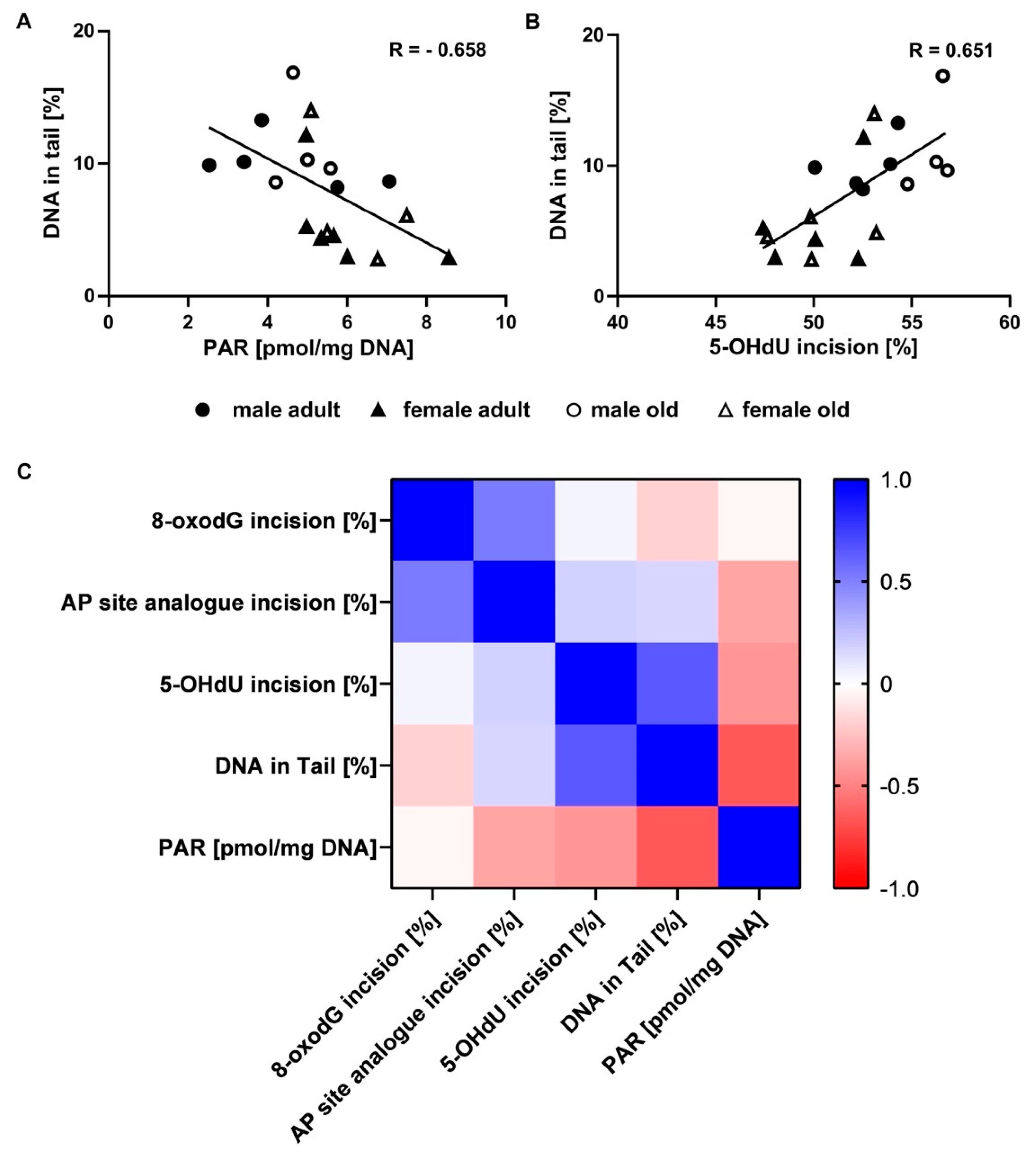
2.6. Correlation of Genomic Stability Endpoints
3. Discussion
4. Materials and Methods
4.1. Animal Husbandry
4.2. Materials
4.3. Incision Activity Assay Principles
4.4. Oligonucleotide Design
4.5. Preparation of Tissue Extracts for Incision Activity
4.6. Incision Activity
4.7. Analysis of PAR Levels
4.8. Quantification of DNA Damage Levels
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-OHdU | 5-hydroxy-2’deoxyuracil |
| 8-oxodG | 8-oxo-2’-deoxyguanosine |
| AP | apurinic/apyrimidinic |
| APC | aphidicolin |
| APE | AP endonuclease |
| APS | ammonium persulfate |
| BCA | bicinchoninic acid |
| BER | base excision repair |
| BSA | bovine serum albumin |
| CCD | charge-coupled device |
| DDR | DNA damage response |
| DMSO | dimethyl sulfoxide |
| DTT | dithiothreitol |
| EtOH | ethanol |
| HCl | hydrochloric acid |
| HEPES | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| KCl | potassium chloride |
| KH2PO4 | potassium dihydrogen phosphate |
| KOH | potassium hydroxide |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| MeOH | methanol |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| NaCl | sodium chloride |
| Na2EDTA | disodium ethylenediaminetetraacetate |
| NaF | sodium fluoride |
| NaOH | sodium hydroxide |
| Na3VO4 | sodium orthovanadate |
| NEIL | Nei-like DNA glycosylase |
| NTH | Nth-like DNA glycosylase |
| OGG1 | 8-oxoguanine DNA glycosylase 1 |
| PAGE | polyacrylamide gel electrophoresis |
| PAR | poly(ADP-ribose) |
| PARP(s) | poly(ADP-ribose) polymerase(s) |
| PARylation | poly(ADP-ribosyl)ation |
| PMSF | phenylmethanesulfonyl fluoride |
| ROS | reactive oxygen species |
| TCA | trichloroacetic acid |
| TEMED | N,N,N′,N′-Tetramethylethylenediamine |
| THF | tetrahydrofuran |
| Tris | 2-amino-2-(hydroxymethyl)-1,3-propanediol |
Appendix A
References
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709. [Google Scholar] [CrossRef]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Vermeij, W.P.; Hoeijmakers, J.H.J.; Pothof, J. Genome integrity in aging: Human syndromes, mouse models, and therapeutic options. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 427–445. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Gurkar, A.U.; Wang, Y.; Vijg, J.; Hoeijmakers, J.H.J.; Robbins, P.D. Nuclear genomic instability and aging. Annu. Rev. Biochem. 2018, 87, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.C.; Corbett, A.H.; Doetsch, P.W. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 2015, 43, 10083–10101. [Google Scholar] [CrossRef]
- Krokan, H.E.; Bjørås, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S. Base excision repair: A critical player in many games. DNA Repair 2014, 19, 14–26. [Google Scholar] [CrossRef]
- Whitaker, A.M.; Schaich, M.A.; Smith, M.R.; Flynn, T.S.; Freudenthal, B.D. Base excision repair of oxidative DNA damage: From mechanism to disease. Front. Biosci. 2017, 22, 1493–1522. [Google Scholar] [CrossRef]
- Seeberg, E.; Eide, L.; Bjørås, M. The base excision repair pathway. Trends Biochem. Sci. 1995, 20, 391–397. [Google Scholar] [CrossRef]
- Paz-Elizur, T.; Elinger, D.; Leitner-Dagan, Y.; Blumenstein, S.; Krupsky, M.; Berrebi, A.; Schechtman, E.; Livneh, Z. Development of an enzymatic DNA repair assay for molecular epidemiology studies: Distribution of OGG activity in healty individuals. DNA Repair 2007, 6, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Hamann, I.; Schwerdtle, T.; Hartwig, A. Establishment of a non-radioactive cleavage assay to assess the DNA repair capacity towards oxidatively damaged DNA in subcellular and cellular systems and the impact of copper. Mutat. Res. 2009, 669, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Astley, S.B.; Southon, S.; Archer, D.B. Measurement of cellular repair activities for oxidative DNA damage. Free Radic. Biol. Med. 2000, 28, 1438–1446. [Google Scholar] [CrossRef]
- Collins, A.R.; Fleming, I.M.; Gedik, C.M. In vitro repair of oxidative and ultraviolet-induced DNA damage in supercoiled nucleoid DNA by human cell extract. Biochim. Biophys. Acta 1994, 1219, 724–727. [Google Scholar] [CrossRef]
- Parsons, J.L.; Dianov, G.L. In vitro base excision repair using mammalian cell extracts. In DNA Repair Protocols; Bjergbæk, L., Ed.; Humana Springer: Totowa, NJ, USA, 2012; pp. 245–262. ISBN 161779998X. [Google Scholar]
- Golato, T.; Brenerman, B.; McNeill, D.R.; Li, J.; Sobol, R.W.; Wilson, D.M. Development of a cell-based assay for measuring base excision repair responses. Sci. Rep. 2017, 7, 13007. [Google Scholar] [CrossRef]
- Chaim, I.A.; Nagel, Z.D.; Jordan, J.J.; Mazzucato, P.; Le Ngo, P.; Samson, L.D. In vivo measurements of interindividual differences in DNA glycosylases and APE1 activities. Proc. Natl. Acad. Sci. USA 2017, 114, E10379–E10388. [Google Scholar] [CrossRef]
- Steenken, S.; Jovanovic, S.V. How Easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Klungland, A.; Rosewell, I.; Hollenbach, S.; Larsen, E.; Daly, G.; Epe, B.; Seeberg, E.; Lindahl, T.; Barnes, D.E. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl. Acad. Sci. USA 1999, 96, 13300–13305. [Google Scholar] [CrossRef]
- Bjorâs, M.; Luna, L.; Johnsen, B.; Hoff, E.; Haug, T.; Rognes, T.; Seeberg, E. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. EMBO J. 1997, 16, 6314–6322. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Holwitt, E.; Hagan, M.P.; Blakely, W.F. Formation of cytosine glycol and 5,6-dihydroxycytosine in deoxyribonucleic acid on treatment with osmium tetroxide. Biochem. J. 1986, 235, 531–536. [Google Scholar] [CrossRef]
- Katafuchi, A.; Nakano, T.; Masaoka, A.; Terato, H.; Iwai, S.; Hanaoka, F.; Ide, H. Differential specificity of human and Escherichia coli endonuclease III and VIII homologues for oxidative base lesions. J. Biol. Chem. 2004, 279, 14464–14471. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Bandaru, V.; Kathe, S.D.; Bond, J.P. The enigma of endonuclease VIII. DNA Repair 2003, 2, 441–453. [Google Scholar] [CrossRef]
- Hazra, T.K.; Izumi, T.; Boldogh, I.; Imhoff, B.; Kow, Y.W.; Jaruga, P.; Dizdaroglu, M.; Mitra, S. Identification and characterization of a human DNA glycosylase for repair of modified bases in oxidatively damaged DNA. Proc. Natl. Acad. Sci. USA 2002, 99, 3523–3528. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Mitra, S.; Hazra, T.K. Repair of Oxidized Bases in DNA Bubble Structures by Human DNA Glycosylases NEIL1 and NEIL2. J. Biol. Chem. 2003, 278, 49679–49684. [Google Scholar] [CrossRef]
- Bohr, V.A. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic. Biol. Med. 2002, 32, 804–812. [Google Scholar] [CrossRef]
- Robson, C.N.; Hickson, I.D. Isolation of cDNA clones encoding a human apurini/apyrimidinic endonuclease that corects DNA repair and mutagenisis defects in E.coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991, 19, 5519–5523. [Google Scholar] [CrossRef]
- Dizdaroglu, M. Oxidatively induced DNA damage and its repair in cancer. Mutat. Res. Rev. Mutat. Res. 2015, 763, 212–245. [Google Scholar] [CrossRef]
- Brenerman, B.M.; Illuzzi, J.L.; Wilson, D.M. Base excision repair capacity in informing healthspan. Carcinogenesis 2014, 35, 2643–2652. [Google Scholar] [CrossRef]
- Chaudhuri, A.R.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Beneke, S.; Bürkle, A. Poly(ADP-ribosyl)ation, PARP, and aging. SAGE KE 2004, 2004, re9. [Google Scholar] [CrossRef][Green Version]
- Brochu, G.; Duchaine, C.; Thibeault, L.; Lagueux, J.; Shah, G.M.; Poirier, G.G. Mode of action of poly(ADP-ribose) glycohydrolase. Biochim. Biophys. Acta 1994, 1219, 342–350. [Google Scholar] [CrossRef]
- Luo, X.; Kraus, W.L. On PAR with PARP: Cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012, 26, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Muller, M.; Beneke, S.; Kupper, J.H.; Burkle, A. Negative regulation of alkylation-induced sister-chromatid exchange by poly(ADP-ribose) polymerase-1 activity. Int. J. Cancer 2000, 88, 351–355. [Google Scholar] [CrossRef]
- Beneke, S.; Bürkle, A. Poly(ADP-ribosyl)ation in mammalian ageing. Nucleic Acids Res. 2007, 35, 7456–7465. [Google Scholar] [CrossRef]
- Piskunova, T.S.; Yurova, M.N.; Ovsyannikov, A.I.; Semenchenko, A.V.; Zabezhinski, M.A.; Popovich, I.G.; Wang, Z.-Q.; Anisimov, V.N. Deficiency in poly(ADP-ribose) polymerase-1 (PARP-1) accelerates aging and spontaneous carcinogenesis in mice. Curr. Gerontol. Geriatr. Res. 2008, 2008. [Google Scholar] [CrossRef]
- Martello, R.; Mangerich, A.; Sass, S.; Dedon, P.C.; Bürkle, A. Quantification of cellular poly(ADP-ribosyl)ation by stable isotope dilution mass spectrometry reveals tissue- and drug-dependent stress response dynamics. ACS Chem. Biol. 2013, 8, 1567–1575. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A.R. The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef]
- OECD. Test No. 489: In Vivo Mammalian Alkaline Comet Assay, OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Yin, J.; Zhang, N.; Wang, H. Liquid chromatography- mass spectrometry for analysis of DNA damages induced by environmental exposure. TrAC Trends Anal. Chem. 2019, 120, 115645. [Google Scholar] [CrossRef]
- Koley, D.; Bard, A.J. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783–16787. [Google Scholar] [CrossRef]
- Langie, S.A.S.; Cameron, K.M.; Waldron, K.J.; Fletcher, K.P.R.; von Zglinicki, T.; Mathers, J.C. Measuring DNA repair incision activity of mouse tissue extracts towards singlet oxygen-induced DNA damage: A comet-based in vitro repair assay. Mutagenesis 2011, 26, 461–471. [Google Scholar] [CrossRef]
- Coudore, F.; Calsou, P.; Salles, B. DNA repair activity in protein extracts from rat tissues. FEBS Lett. 1997, 414, 581–584. [Google Scholar] [CrossRef]
- Salles, B.; Frit, P.; Provot, C.; Jaeg, J.-P.; Calsou, P. In vitro eukaryotic DNA excision repair assays: An overview. Biochimie 1995, 77, 796–802. [Google Scholar] [CrossRef]
- Slyskova, J.; Langie, S.A.S.; Collins, A.R.; Vodicka, P. Functional evaluation of DNA repair in human biopsies and their relation to other cellular biomarkers. Front. Genet. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Cabelof, D.C.; Raffoul, J.J.; Yanamadala, S.; Ganir, C.; Guo, Z.; Heydari, A.R. Attenuation of DNA polymerase β-dependent base excision repair and increased DMS-induced mutagenicity in aged mice. Mutat. Res. 2002, 500, 135–145. [Google Scholar] [CrossRef]
- Swain, U.; Subba Rao, K. Study of DNA damage via the comet assay and base excision repair activities in rat brain neurons and astrocytes during aging. Mech. Ageing Dev. 2011, 132, 374–381. [Google Scholar] [CrossRef]
- Gorniak, J.P.; Cameron, K.M.; Waldron, K.J.; von Zglinicki, T.; Mathers, J.C.; Langie, S.A.S. Tissue differences in BER-related incision activity and non-specific nuclease activity as measured by the comet assay. Mutagenesis 2013, 28, 673–681. [Google Scholar] [CrossRef]
- Hamilton, M.L.; van Remmen, H.; Drake, J.A.; Yang, H.; Guo, Z.M.; Kewitt, K.; Walter, C.A.; Richardson, A. Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. USA 2001, 98, 10469–10474. [Google Scholar] [CrossRef]
- De Souza-Pinto, N.; Hogue, B.A.; Bohr, V.A. DNA repair and aging in mouse liver: 8-oxoG glycosylase activity increase in mitochondrial but not in nuclear extracts. Free Radic. Biol. Med. 2001, 30, 916–923. [Google Scholar] [CrossRef]
- Szczesny, B.; Mitra, S. Effect of aging on intracellular distribution of abasic (AP) endonuclease 1 in the mouse liver. Mech. Ageing Dev. 2005, 126, 1071–1078. [Google Scholar] [CrossRef]
- Noren Hooten, N.; Kompaniez, K.; Barnes, J.; Lohani, A.; Evans, M.K. Poly(ADP-ribose) polymerase 1 (PARP-1) binds to 8-oxoguanine-DNA glycosylase (OGG1). J. Biol. Chem. 2011, 286, 44679–44690. [Google Scholar] [CrossRef]
- Noren Hooten, N.; Fitzpatrick, M.; Kompaniez, K.; Jacob, K.D.; Moore, B.R.; Nagle, J.; Barnes, J.; Lohani, A.; Evans, M.K. Coordination of DNA repair by NEIL1 and PARP-1: A possible link to aging. Aging 2012, 4, 674–685. [Google Scholar] [CrossRef]
- Jacob, K.D.; Noren Hooten, N.; Trzeciak, A.R.; Evans, M.K. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 2013, 134, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Maslov, A.Y.; Ganapathi, S.; Westerhof, M.; Quispe-Tintaya, W.; White, R.R.; van Houten, B.; Reiling, E.; Dollé, M.E.T.; van Steeg, H.; Hasty, P.; et al. DNA damage in normally and prematurely aged mice. Aging Cell 2013, 12, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Cartiglia, C.; Taningher, M.; de Flora, S.; Balansky, R. Age-related increases of 8-hydroxy-2′-deoxyguanosine and DNA–protein crosslinks in mouse organs. Mutat. Res. 1999, 446, 215–223. [Google Scholar] [CrossRef]
- Smith, R.; Lebeaupin, T.; Juhász, S.; Chapuis, C.; D’Augustin, O.; Dutertre, S.; Burkovics, P.; Biertümpfel, C.; Timinszky, G.; Huet, S. Poly(ADP-ribose)-dependent chromatin unfolding facilitates the association of DNA-binding proteins with DNA at sites of damage. Nucleic Acids Res. 2019, 47, 11250–11267. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Wang, L.; Luo, X.; Huang, K.; Wang, C.; Du, M.; Liu, F.; Luo, T.; Huang, D.; et al. Poly(ADP-ribose) polymerase 1 is a key regulator of estrogen receptor α-dependent gene transcription. J. Biol. Chem. 2013, 288, 11348–11357. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.E.; Vandenput, L.; Tivesten, Å.; Norlén, A.-K.; Lagerquist, M.K.; Windahl, S.H.; Börjesson, A.E.; Farman, H.H.; Poutanen, M.; Benrick, A.; et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology 2015, 156, 2492–2502. [Google Scholar] [CrossRef]
- Beard, W.A.; Horton, J.K.; Prasad, R.; Wilson, S.H. Eukaryotic Base Excision Repair: New Approaches Shine Light on Mechanism. Annu. Rev. Biochem. 2019, 88, 137–162. [Google Scholar] [CrossRef]
- Prasad, R.; Dianov, G.L.; Bohr, V.A.; Wilson, S.H. FEN1 stimulation of DNA polymerase beta mediates an excision step in mammalian long patch base excision repair. J. Biol. Chem. 2000, 275, 4460–4466. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Bergtold, D.S. Characterization of free radical-induced base damage in DNA at biologically relevant levels. Anal. Biochem. 1986, 156, 182–188. [Google Scholar] [CrossRef]
- Hamann, I.; König, C.; Richter, C.; Jahnke, G.; Hartwig, A. Impact of cadmium on hOGG1 and APE1 as a function of the cellular p53 status. Mutat. Res. 2011, 736, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Tulin, A.V. Poly(ADP-Ribose) Polymerase; Springer: New York, NY, USA, 2017. [Google Scholar]
- Neumann, C.; Baesler, J.; Steffen, G.; Nicolai, M.M.; Zubel, T.; Aschner, M.; Bürkle, A.; Mangerich, A.; Schwerdtle, T.; Bornhorst, J. The role of poly(ADP-ribose) polymerases in manganese exposed Caenorhabditis elegans. J. Trace Elem. Med. Biol. 2019, 57, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Pedersen, L.M.; Kyjovska, Z.O.; Jacobsen, N.R.; Saber, A.T.; Hougaard, K.S.; Vogel, U.; Wallin, H. Validation of freezing tissues and cells for analysis of DNA strand break levels by comet assay. Mutagenesis 2013, 28, 699–707. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkelbeiner, N.; Wandt, V.K.; Ebert, F.; Lossow, K.; Bankoglu, E.E.; Martin, M.; Mangerich, A.; Stopper, H.; Bornhorst, J.; Kipp, A.P.; et al. A Multi-Endpoint Approach to Base Excision Repair Incision Activity Augmented by PARylation and DNA Damage Levels in Mice: Impact of Sex and Age. Int. J. Mol. Sci. 2020, 21, 6600. https://doi.org/10.3390/ijms21186600
Winkelbeiner N, Wandt VK, Ebert F, Lossow K, Bankoglu EE, Martin M, Mangerich A, Stopper H, Bornhorst J, Kipp AP, et al. A Multi-Endpoint Approach to Base Excision Repair Incision Activity Augmented by PARylation and DNA Damage Levels in Mice: Impact of Sex and Age. International Journal of Molecular Sciences. 2020; 21(18):6600. https://doi.org/10.3390/ijms21186600
Chicago/Turabian StyleWinkelbeiner, Nicola, Viktoria K. Wandt, Franziska Ebert, Kristina Lossow, Ezgi E. Bankoglu, Maximilian Martin, Aswin Mangerich, Helga Stopper, Julia Bornhorst, Anna P. Kipp, and et al. 2020. "A Multi-Endpoint Approach to Base Excision Repair Incision Activity Augmented by PARylation and DNA Damage Levels in Mice: Impact of Sex and Age" International Journal of Molecular Sciences 21, no. 18: 6600. https://doi.org/10.3390/ijms21186600
APA StyleWinkelbeiner, N., Wandt, V. K., Ebert, F., Lossow, K., Bankoglu, E. E., Martin, M., Mangerich, A., Stopper, H., Bornhorst, J., Kipp, A. P., & Schwerdtle, T. (2020). A Multi-Endpoint Approach to Base Excision Repair Incision Activity Augmented by PARylation and DNA Damage Levels in Mice: Impact of Sex and Age. International Journal of Molecular Sciences, 21(18), 6600. https://doi.org/10.3390/ijms21186600





