A New Model of Chronic Mycobacterium abscessus Lung Infection in Immunocompetent Mice
Abstract
1. Introduction
2. Results
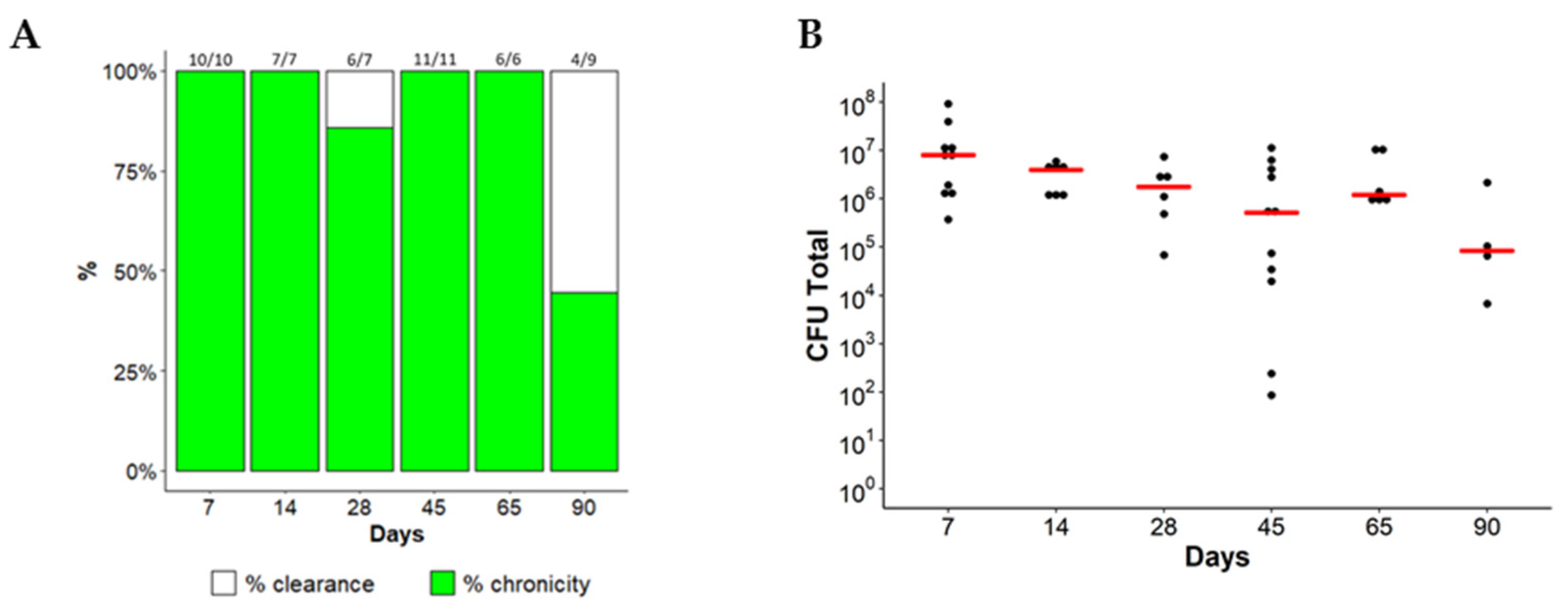
2.1. Chronic Lung Persistence of MA in C57BL/6NCrl Mice
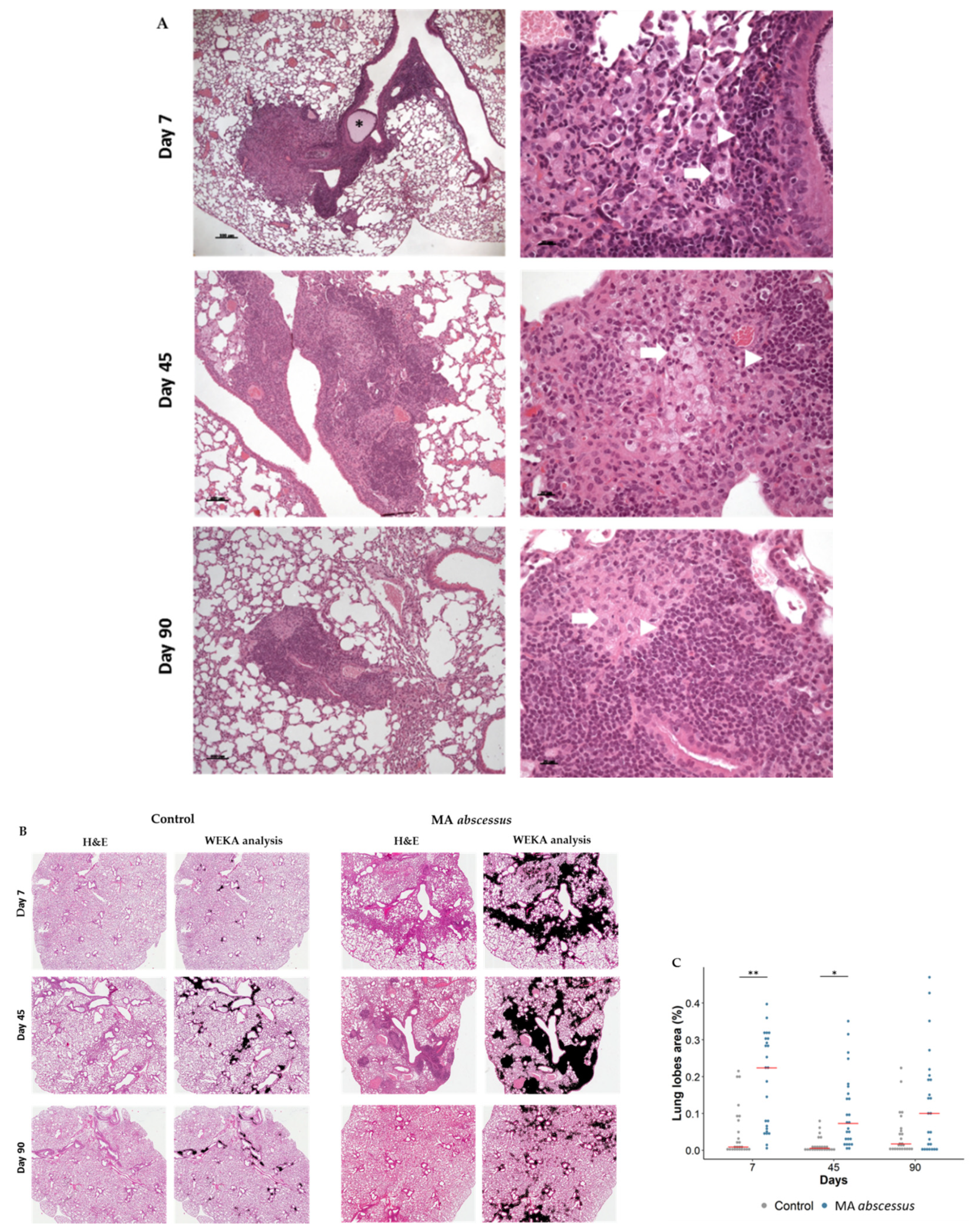
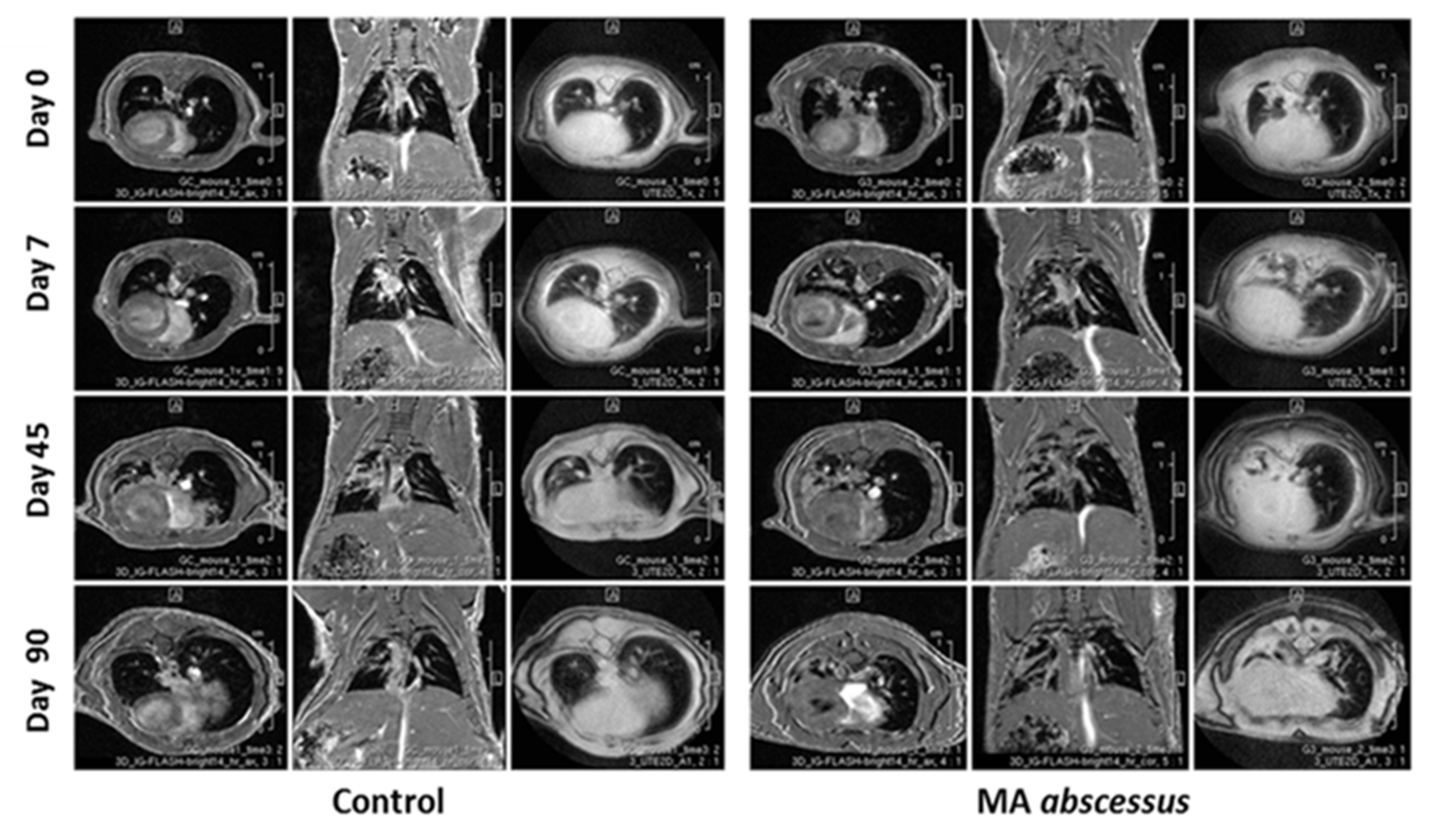
2.2. Lung Lesions and Inflammatory Response during MA Chronic Infection in C57BL/6NCrl Mice
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Bacterial Strains
4.3. Mouse Strains
4.4. Mouse Model of Chronic MA Lung Infection
4.5. Histological Analysis and Lung Damage Quantification
4.6. MRI Longitudinal Monitoring
4.7. Image Analysis
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| NTMs | Nontuberculous mycobacteria |
| COPD | Chronic obstructive pulmonary disease |
| CF | Cystic fibrosis |
| MA | Mycobaterium abscessus |
| GM-CSF mice | Granulocyte-macrophage colony-stimulating factor mice |
| GKO mice | Gamma interferon knockout mice |
| SCID mice | Severe combined immunodeficiency mice |
| CFU | Colony forming units |
| TNF-α | Tumor Necrosis Factor |
| IFN-γ | Interferon-gamma |
References
- Tortoli, E. Clinical manifestations of nontuberculous mycobacteria infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009, 15, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, K.C.; Nichols, E.M.; Caceres, S.M.; Kret, J.E.; Martiniano, S.L.; Sagel, S.D.; Chan, E.D.; Caverly, L.; Solomon, G.M.; Reynolds, P.; et al. Mycobacterium abscessus induces a limited pattern of neutrophil activation that promotes pathogen survival. PLoS ONE 2013, 8, e57402. [Google Scholar] [CrossRef]
- Tortoli, E.; Kohl, T.A.; Trovato, A.; Baldan, R.; Campana, S.; Cariani, L.; Colombo, C.; Costa, D.; Cristadoro, S.; Di Serio, M.C.; et al. Mycobacterium abscessus in patients with cystic fibrosis: Low impact of inter-human transmission in Italy. Eur. Respir. J. 2017, 50, 1602525. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Sheng, W.H.; Hung, C.C.; Yu, C.J.; Lee, L.N.; Hsueh, P.R. Mycobacterium abscessus Complex Infections in Humans. Emerg. Infect. Dis. 2015, 21, 1638–1646. [Google Scholar] [CrossRef]
- Tissot, A.; Thomas, M.F.; Corris, P.A.; Brodlie, M. NonTuberculous Mycobacteria infection and lung transplantation in cystic fibrosis: A worldwide survey of clinical practice. BMC Pulm. Med. 2018, 18, 86. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.L.; Ordway, D.; Kremer, L. The Diverse Cellular and Animal Models to Decipher the Physiopathological Traits of Mycobacterium abscessus Infection. Front. Cell. Infect. Microbiol. 2017, 7, 100. [Google Scholar] [CrossRef]
- Ordway, D.; Henao-Tamayo, M.; Smith, E.; Shanley, C.; Harton, M.; Troudt, J.; Bai, X.; Basaraba, R.J.; Orme, I.M.; Chan, E.D. Animal model of Mycobacterium abscessus lung infection. J. Leukoc. Biol. 2008, 83, 1502–1511. [Google Scholar] [CrossRef]
- Caverly, L.J.; Caceres, S.M.; Fratelli, C.; Happoldt, C.; Kidwell, K.M.; Malcolm, K.C.; Nick, J.A.; Nichols, D.P. Mycobacterium abscessus morphotype comparison in a murine model. PLoS ONE 2015, 10, e0117657. [Google Scholar] [CrossRef]
- Jeon, B.Y.; Kwak, J.; Lee, S.S.; Cho, S.; Won, C.J.; Kim, J.M.; Shin, S.J. Comparative analysis of immune responses to Mycobacterium abscessus infection and its antigens in two murine models. J. Microbiol. 2009, 47, 633–640. [Google Scholar] [CrossRef]
- De Groote, M.A.; Johnson, L.; Podell, B.; Brooks, E.; Basaraba, R.; Gonzalez-Juarrero, M. GM-CSF knockout mice for preclinical testing of agents with antimicrobial activity against Mycobacterium abscessus. J. Antimicrob. Chemother. 2014, 69, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Obregón-Henao, A.; Arnett, K.A.; Henao-Tamayo, M.; Massoudi, L.; Creissen, E.; Andries, K.; Lenaerts, A.J.; Ordway, D.J. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob. Agents Chemother. 2015, 59, 6904–6912. [Google Scholar] [CrossRef] [PubMed]
- Lerat, I.; Cambau, E.; Roth dit Bettoni, R.; Gaillard, J.L.; Jarlier, V.; Truffot, C.; Veziris, N. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J. Infect. Dis. 2014, 209, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Rottman, M.; Catherinot, E.; Hochedez, P.; Emile, J.F.; Casanova, J.L.; Gaillard, J.L.; Soudais, C. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect. Immun. 2007, 75, 5898–5907. [Google Scholar] [CrossRef] [PubMed]
- Bragonzi, A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. Int. J. Med. Microbiol. 2010, 300, 584–593. [Google Scholar] [CrossRef]
- Cigana, C.; Lorè, N.I.; Riva, C.; De Fino, I.; Spagnuolo, L.; Sipione, B.; Rossi, G.; Nonis, A.; Cabrini, G.; Bragonzi, A. Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci. Rep. 2016, 6, 21465. [Google Scholar] [CrossRef]
- Cigana, C.; Bianconi, I.; Baldan, R.; De Simone, M.; Riva, C.; Sipione, B.; Rossi, G.; Cirillo, D.M.; Bragonzi, A. Staphylococcus aureus impacts Pseudomonas aeruginosa chronic respiratory disease in murine models. J. Infect. Dis. 2018, 217, 933–942. [Google Scholar] [CrossRef]
- Torraca, V.; Masud, S.; Spaink, H.P.; Meijer, A.H. Macrophage-pathogen interactions in infectious diseases: New therapeutic insights from the zebrafish host model. Dis. Models Mech. 2014, 7, 785–797. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 2000, 68, 6511–6518. [Google Scholar] [CrossRef]
- Silva-Gomes, R.; Marcq, E.; Trigo, G.; Goncalves, C.M.; Longatto-Filho, A.; Castro, A.G.; Pedrosa, J.; Fraga, A.G. Spontaneous Healing of Mycobacterium ulcerans Lesions in the Guinea Pig Model. PLoS Negl. Trop. Dis. 2015, 9, e0004265. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saito, H.; Setogawa, T.; Tomioka, H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect. Immun. 1991, 59, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Bragonzi, A.; Worlitzsch, D.; Pier, G.B.; Timpert, P.; Ulrich, M.; Hentzer, M.; Andersen, J.B.; Givskov, M.; Conese, M.; Döring, G. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J. Infect. Dis. 2005, 192, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Le Moigne, V.; Raynaud, C.; Moreau, F.; Dupont, C.; Nigou, J.; Neyrolles, O.; Kremer, L.; Herrmann, J.L. Efficacy of Bedaquiline, Alone or in Combination with Imipenem, against Mycobacterium abscessus in C3HeB/FeJ Mice. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Medjahed, H.; Gaillard, J.L.; Reyrat, J.M. Mycobacterium abscessus: A new player in the mycobacterial field. Trends Microbiol. 2010, 18, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Koh, W.J.; Kim, Y.H.; Jeong, B.H.; Park, H.Y.; Jeon, K.; Kim, J.S.; Cho, S.N.; Shin, S.J. Importance of reciprocal balance of T cell immunity in Mycobacterium abscessus complex lung disease. PLoS ONE 2014, 9, e109941. [Google Scholar] [CrossRef]
- Maggioncalda, E.C.; Story-Roller, E.; Mylius, J.; Illei, P.; Basaraba, R.J.; Lamichhane, G. A mouse model of pulmonary Mycobacteroides abscessus infection. Sci. Rep. 2020, 10, 3690. [Google Scholar] [CrossRef]
- Zaccagnini, G.; Palmisano, A.; Canu, T.; Maimone, B.; Russo, F.M.; Ambrogi, F.; Gaetano, C.; De Cobelli, F.; Del Maschio, A.; Esposito, A.; et al. Magnetic Resonance Imaging Allows the Evaluation of Tissue Damage and Regeneration in a Mouse Model of Critical Limb Ischemia. PLoS ONE 2015, 10, e0142111. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Esserman, D.A.; Gilligan, P.; Kerr, A.; Noone, P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2010, 9, 117–123. [Google Scholar] [CrossRef]
- Jarand, J.; Levin, A.; Zhang, L.; Huitt, G.; Mitchell, J.D.; Daley, C.L. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, 565–571. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Davidson, R.M.; Nick, J.A. Nontuberculous mycobacteria in cystic fibrosis: Updates and the path forward. Pediatric Pulmonol. 2017, 52, S29–S36. [Google Scholar] [CrossRef]
- Oliver, A.; Maiz, L.; Cantón, R.; Escobar, H.; Baquero, F.; Gómez-Mampaso, E. Nontuberculous mycobacteria in patients with cystic fibrosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 32, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Ballarino, G.J.; Olivier, K.N.; Claypool, R.J.; Holland, S.M.; Prevots, D.R. Pulmonary nontuberculous mycobacterial infections: Antibiotic treatment and associated costs. Respir. Med. 2009, 103, 1448–1455. [Google Scholar] [CrossRef]
- Snouwaert, J.N.; Brigman, K.K.; Latour, A.M.; Iraj, E.; Schwab, U.; Gilmour, M.I.; Koller, B.H. A murine model of cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, S59–S64. [Google Scholar] [CrossRef]
- Zelazny, A.M.; Root, J.M.; Shea, Y.R.; Colombo, R.E.; Shamputa, I.C.; Stock, F.; Conlan, S.; McNulty, S.; Brown-Elliott, B.A.; Wallace, R.J.; et al. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J. Clin. Microbiol. 2009, 47, 1985–1995. [Google Scholar] [CrossRef]
- Catherinot, E.; Clarissou, J.; Etienne, G.; Ripoll, F.; Emile, J.F.; Daffe, M.; Perronne, C.; Soudais, C.; Gaillard, J.L.; Rottman, M. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect. Immun. 2007, 75, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, F.; Pasek, S.; Schenowitz, C.; Dossat, C.; Barbe, V.; Rottman, M.; Macheras, E.; Heym, B.; Herrmann, J.L.; Daffé, M.; et al. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS ONE 2009, 4, e5660. [Google Scholar] [CrossRef]
- Caverly, L.J.; Spilker, T.; LiPuma, J.J. Complete Genome Sequence of Mycobacterium abscessus subsp. bolletii. Genome Announc. 2016, 4. [Google Scholar] [CrossRef]
- Adékambi, T.; Reynaud-Gaubert, M.; Greub, G.; Gevaudan, M.J.; La Scola, B.; Raoult, D.; Drancourt, M. Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J. Clin. Microbiol. 2004, 42, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Facchini, M.; De Fino, I.; Riva, C.; Bragonzi, A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.M.; Ware, J.H. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, C.; Tortoli, E.; Cugnata, F.; Sanvito, F.; Esposito, A.; Rossi, M.; Colarieti, A.; Canu, T.; Cigana, C.; Bragonzi, A.; et al. A New Model of Chronic Mycobacterium abscessus Lung Infection in Immunocompetent Mice. Int. J. Mol. Sci. 2020, 21, 6590. https://doi.org/10.3390/ijms21186590
Riva C, Tortoli E, Cugnata F, Sanvito F, Esposito A, Rossi M, Colarieti A, Canu T, Cigana C, Bragonzi A, et al. A New Model of Chronic Mycobacterium abscessus Lung Infection in Immunocompetent Mice. International Journal of Molecular Sciences. 2020; 21(18):6590. https://doi.org/10.3390/ijms21186590
Chicago/Turabian StyleRiva, Camilla, Enrico Tortoli, Federica Cugnata, Francesca Sanvito, Antonio Esposito, Marco Rossi, Anna Colarieti, Tamara Canu, Cristina Cigana, Alessandra Bragonzi, and et al. 2020. "A New Model of Chronic Mycobacterium abscessus Lung Infection in Immunocompetent Mice" International Journal of Molecular Sciences 21, no. 18: 6590. https://doi.org/10.3390/ijms21186590
APA StyleRiva, C., Tortoli, E., Cugnata, F., Sanvito, F., Esposito, A., Rossi, M., Colarieti, A., Canu, T., Cigana, C., Bragonzi, A., Loré, N. I., Miotto, P., & Cirillo, D. M. (2020). A New Model of Chronic Mycobacterium abscessus Lung Infection in Immunocompetent Mice. International Journal of Molecular Sciences, 21(18), 6590. https://doi.org/10.3390/ijms21186590




