Ivabradine-Stimulated Microvesicle Release Induces Cardiac Protection against Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
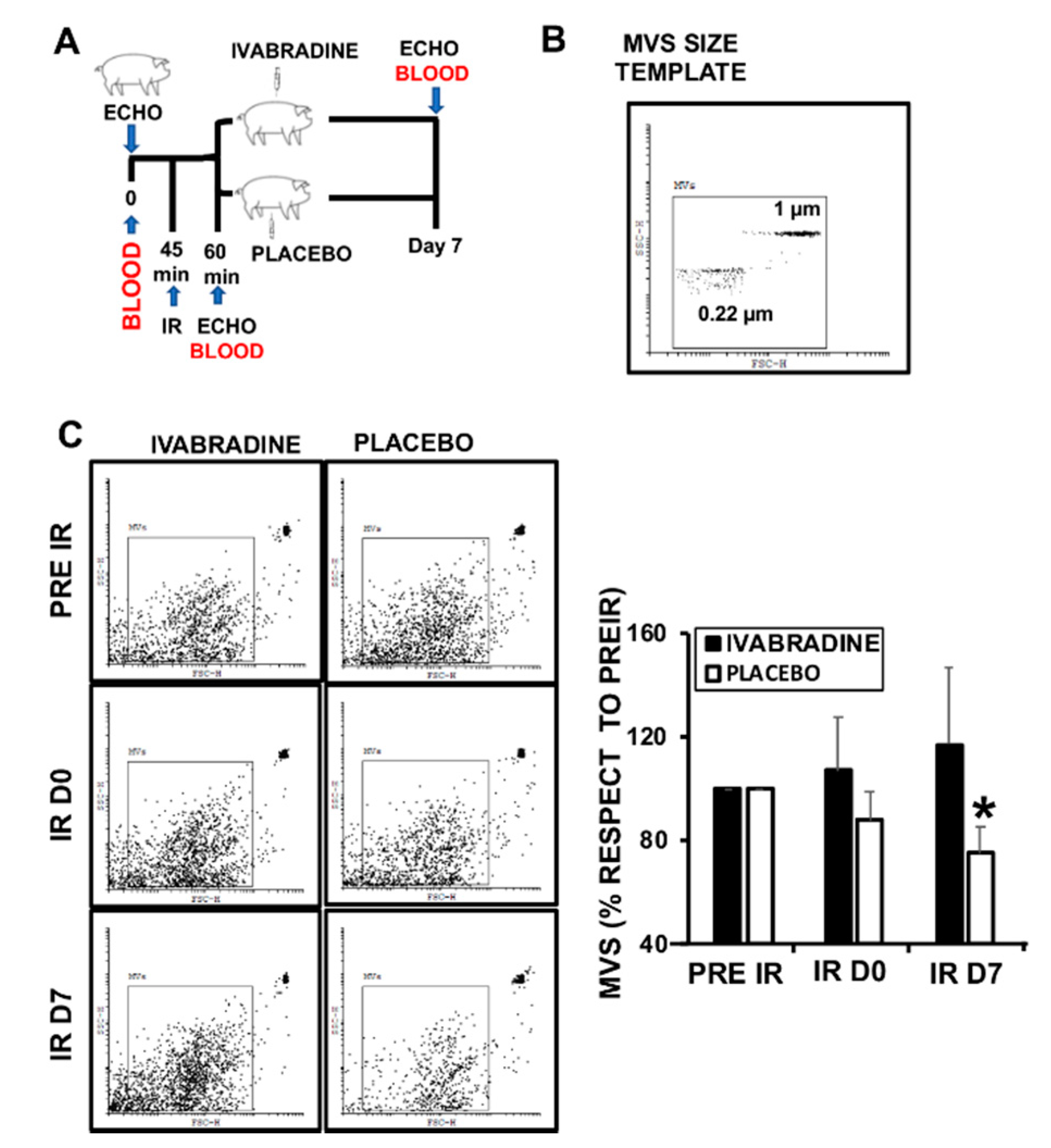
2.1. Ivabradine Increases Microvesicle Release after Ischemia Reperfusion (IR)
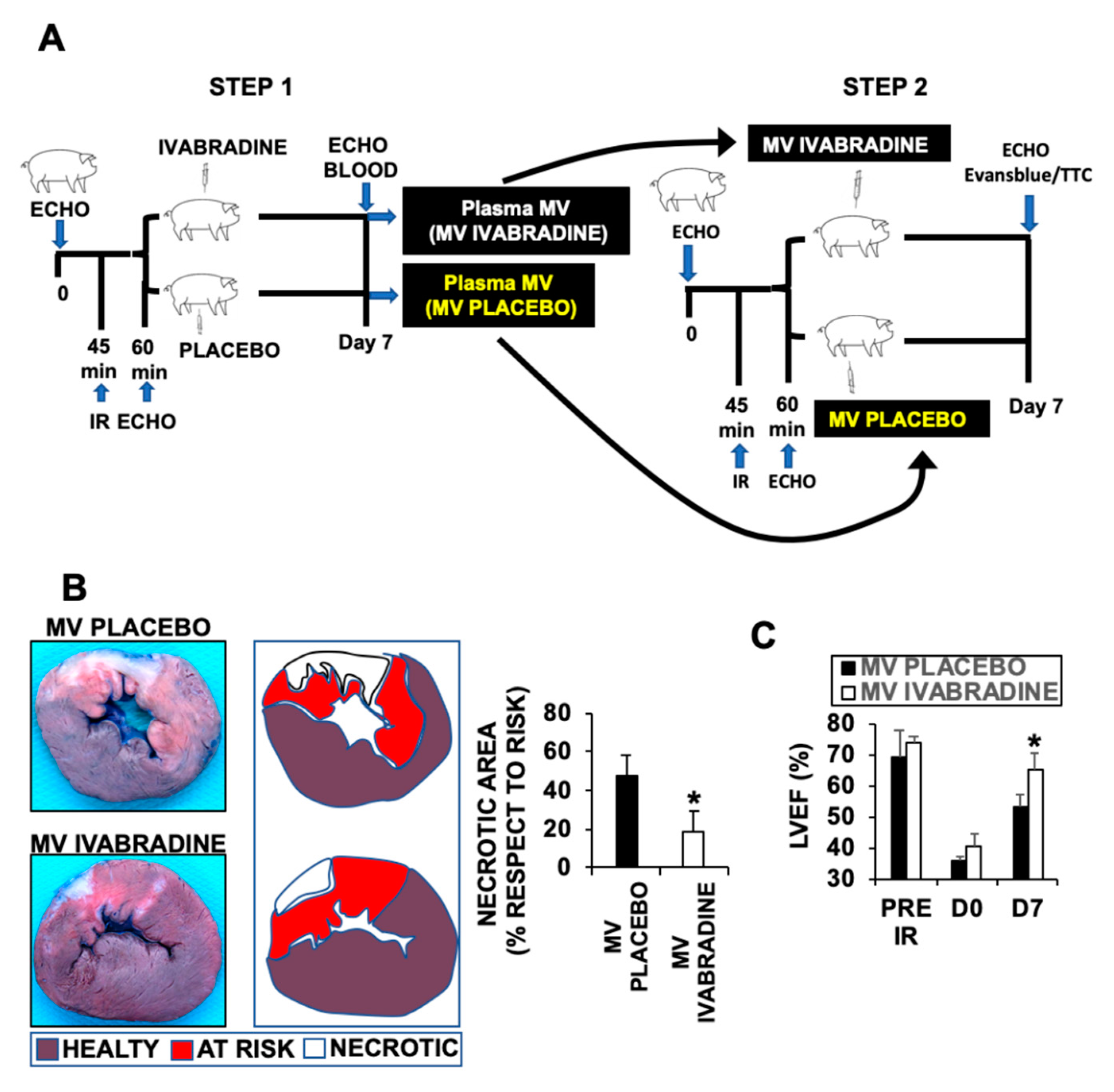
2.2. Ivabradine-Induced Microvesicles Improve Cardiac Function in Pigs Subjected to IR
2.3. Ivabradine Induces the Release of Cardiac Circulating Microvesicles in Pigs Subjected to IR
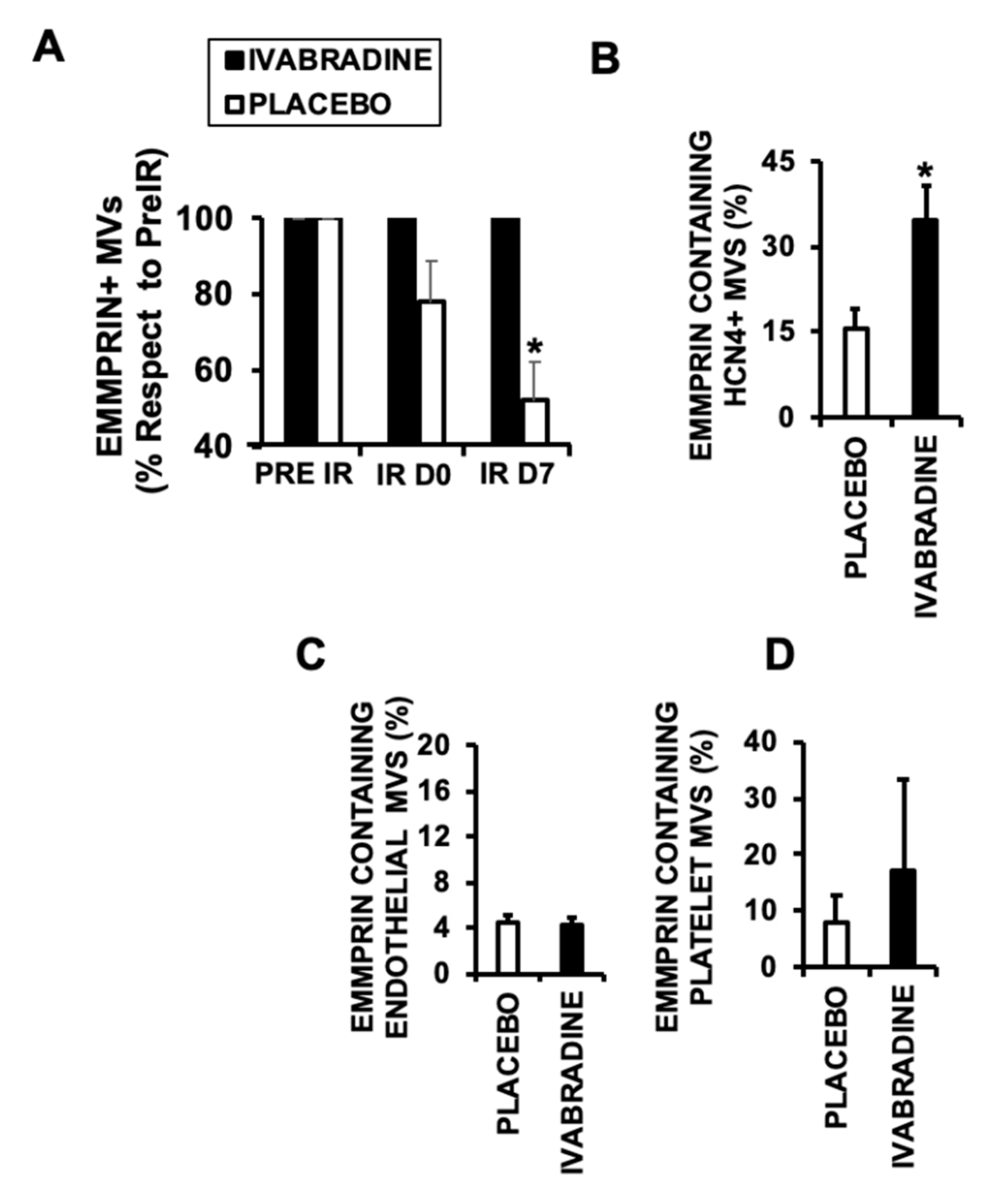
2.4. Ivabradine Increases EMMPRIN-Containing Microvesicles after IR
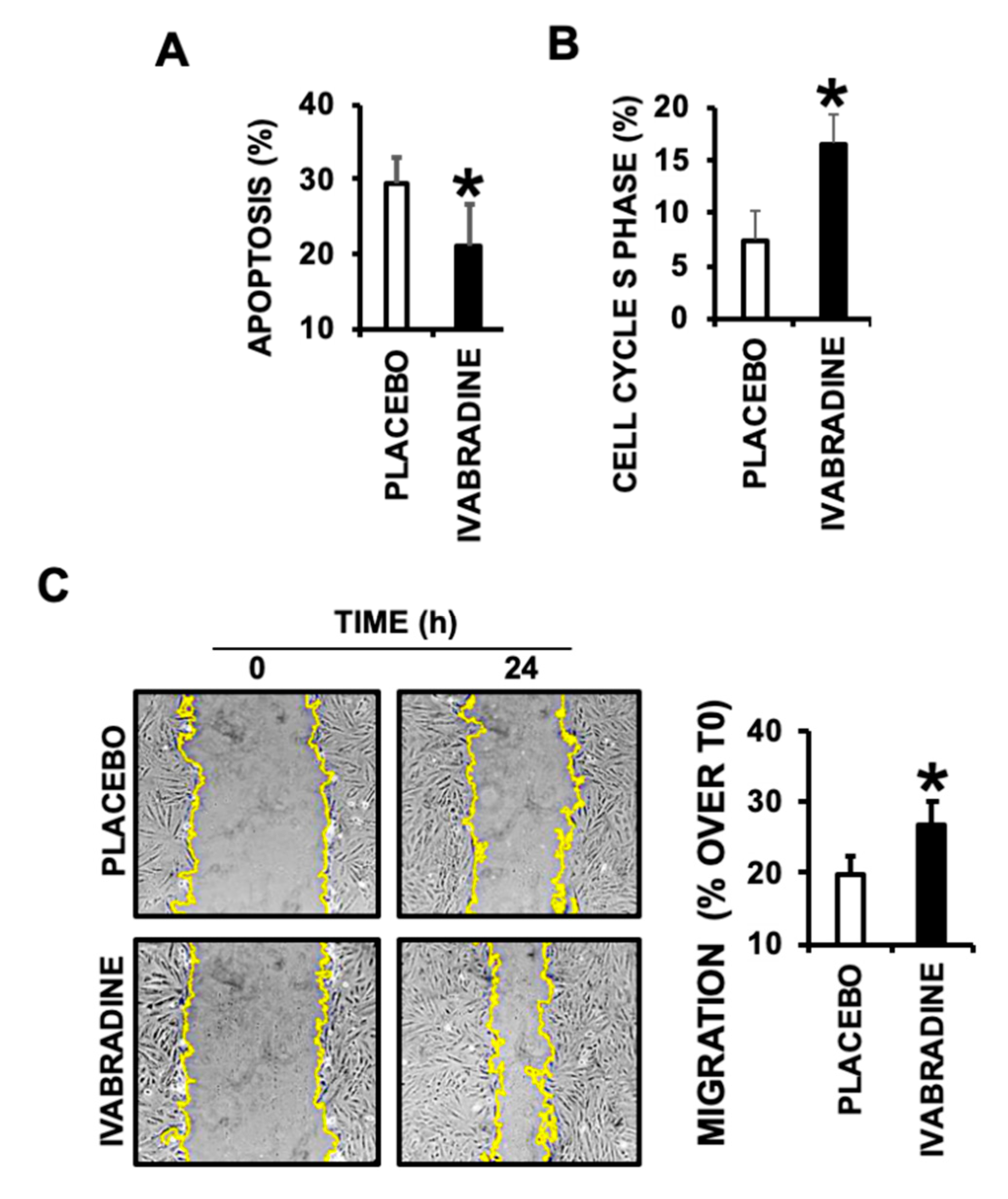
2.5. Ivabradine-Induced Cardiac MVs Promote Cardiac Cell Migration, Proliferation, and Prevent Apoptotic Cell Death
3. Discussion
Limitations
4. Materials and Methods
4.1. Cardiac Ischemia/Reperfusion
4.2. H9c2 Cell Culture
4.3. Extracellular Vesicles Isolation
4.4. Microvesicles Characterization by Flow Cytometry
4.5. Cell Cycle Phase Assay in H9c2
4.6. Wound Healing Assay
4.7. Protein Expression Determination by Immunoblot
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IR | Ischemia/reperfusion |
| AMI | Acute Myocardial Infarction |
| MV | Microvesicle |
| EMMPRIN | Extracellular Matrix Metalloproteinase Inducer |
References
- Fox, K.; Borer, J.S.; Camm, A.J.; Danchin, N.; Ferrari, R.; Lopez Sendon, J.L.; Steg, P.G.; Tardif, J.C.; Tavazzi, L.; Tendera, M. Heart Rate Working Group. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. 2007, 50, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Lim, P.; Roubille, F. Does ivabradine balance dobutamine effects in cardiogenic shock? A promising new strategy. Acta Physiol. 2016, 218, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- Pascual Izco, M.; Ramírez-Carracedo, R.; Hernández, I.; Osorio, Á.; Castejon, B.; Cuadrado, I.; Largo, C.; Alonso, G.L.; Díez, J.; Saura, M.; et al. Ivabradine in acute heart failure: Effects on heart rate and hemodynamic parameters in a randomized and controlled swine trial. Cardiol. J. 2020, 27, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Pascual Izco, M.; Castejón, B.; Piedras, M.J.; Zamorano, J.L.; Sanmartín, M.; Zaragoza, C. Effects of Ivabradine on Heart Rate and Hemodynamic Parameters in a Swine Model of Cardiogenic Shock. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 1139–1141. [Google Scholar] [CrossRef]
- Yue-Chun, L.; Guang-Yi, C.; Li-Sha, G.; Chao, X.; Xinqiao, T.; Cong, L.; Xiao-Ya, D.; Xiangjun, Y. The Protective Effects of Ivabradine in Preventing Progression from Viral Myocarditis to Dilated Cardiomyopathy. Front. Pharmacol. 2016, 7, 408. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Gao, L.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Kalinec, F. Extracellular Vesicles From Auditory Cells as Nanocarriers for Anti-inflammatory Drugs and Pro-resolving Mediators. Front. Cell. Neurosci. 2019, 13, 530. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef]
- Oggero, S.; Austin-Williams, S.; Norling, L.V. The Contrasting Role of Extracellular Vesicles in Vascular Inflammation and Tissue Repair. Front. Pharmacol. 2019, 10, 1479. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac Extracellular Vesicles in Normal and Infarcted Heart. Int. J. Mol. Sci. 2016, 17, 63. [Google Scholar] [CrossRef]
- Ramirez-Carracedo, R.; Tesoro, L.; Hernandez, I.; Diez-Mata, J.; Piñeiro, D.; Hernandez-Jimenez, M.; Zamorano, J.L.; Zaragoza, C. Targeting TLR4 with ApTOLL Improves Heart Function in Response to Coronary Ischemia Reperfusion in Pigs Undergoing Acute Myocardial Infarction. Biomolecules 2020, 10, 1167. [Google Scholar] [CrossRef]
- Julich-Haertel, H.; Urban, S.K.; Krawczyk, M.; Willms, A.; Jankowski, K.; Patkowski, W.; Kruk, B.; Krasnodębski, M.; Ligocka, J.; Schwab, R.; et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J. Hepatol. 2017, 67, 282–292. [Google Scholar] [CrossRef]
- Menck, K.; Scharf, C.; Bleckmann, A.; Dyck, L.; Rost, U.; Wenzel, D.; Dhople, V.M.; Siam, L.; Pukrop, T.; Binder, C.; et al. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J. Mol. Cell Biol. 2015, 7, 143–153. [Google Scholar] [CrossRef]
- Aoki, M.; Koga, K.; Hamasaki, M.; Egawa, N.; Nabeshima, K. Emmprin, released as a microvesicle in epithelioid sarcoma, interacts with fibroblasts. Int. J. Oncol. 2017, 50, 2229–2235. [Google Scholar] [CrossRef]
- Bravo-Miana, R.D.C.; Della Vedova, A.B.; De Paul, A.L.; Remedi, M.M.; Guantay, M.L.; Gilardoni, M.B.; Pellizas, C.G.; Donadio, A.C. Thyroid tumor cells-fibroblasts crosstalk: Role of extracellular vesicles. Endocr. Connect. 2020, 9, 506–518. [Google Scholar] [CrossRef]
- Tarin, C.; Lavin, B.; Gomez, M.; Saura, M.; Diez-Juan, A.; Zaragoza, C. The extracellular matrix metalloproteinase inducer EMMPRIN is a target of nitric oxide in myocardial ischemia/reperfusion. Free Radic. Biol. Med. 2011, 51, 387–395. [Google Scholar] [CrossRef]
- Ramírez, R.; Díez, J.; Sanmartín, M.; Saura, M.; Zamorano, J.L.; Zaragoza, C. Nanotechnology Applied to Preserve Extracelular Matrix as Teranostic Tool in Acute Myocardial Infarction. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 171–174. [Google Scholar] [CrossRef]
- Ramírez, R.; Tesoro, L.; Hernandez, I.; Diez-Mata, J.; Filice, M.; Toro, R.; Rodriguez-Piñeiro, M.; Zamorano, J.L.; Saura, M.; Zaragoza, C. Non-invasive detection of extracellular matrix metalloproteinase inducer EMMPRIN, a new therapeutic target against atherosclerosis, inhibited by endothelial nitric oxide. Int. J. Mol. Sci. 2018, 19, 3248. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, W.; Hu, S.; Xia, Y.; Wang, Y.; Zhang, Q.; Chen, L. A Meta-Analysis of Circulating Microvesicles in Patients with Myocardial Infarction. Arq. Bras. Cardiol. 2017, 109, 156–164. [Google Scholar] [CrossRef]
- Loyer, X.; Zlatanova, I.; Devue, C.; Yin, M.; Howangyin, K.Y.; Klaihmon, P.; Guerin, C.L.; Kheloufi, M.; Vilar, J.; Zannis, K.; et al. Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction. Circ. Res. 2018, 123, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Ridger, V.C.; Boulanger, C.M.; Angelillo-Scherrer, A.; Badimon, L.; Blanc-Brude, O.; Bochaton-Piallat, M.L.; Boilard, E.; Buzas, E.I.; Caporali, A.; Dignat-George, F.; et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb. Haemost. 2017, 117, 1296–1316. [Google Scholar]
- Chiva-Blanch, G.; Laake, K.; Myhre, P.; Bratseth, V.; Arnesen, H.; Solheim, S.; Badimon, L.; Seljeflot, I. Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity. PLoS ONE 2017, 12, e0172558. [Google Scholar] [CrossRef]
- Abbas, M.; Jesel, L.; Auger, C.; Amoura, L.; Messas, N.; Manin, G.; Rumig, C.; León-González, A.J.; Ribeiro, T.P.; Silva, G.C.; et al. Endothelial Microparticles from Acute Coronary Syndrome Patients Induce Premature Coronary Artery Endothelial Cell Aging and Thrombogenicity: Role of the Ang II/AT1 Receptor/NADPH Oxidase-Mediated Activation of MAPKs and PI3-Kinase Pathways. Circulation 2017, 135, 280–296. [Google Scholar] [CrossRef]
- Femminò, S.; Penna, C.; Margarita, S.; Comità, S.; Brizzi, M.F.; Pagliaro, P. Extracellular vesicles and cardiovascular system: Biomarkers and Cardioprotective effectors. Vascul. Pharmacol. 2020, 27, 106790. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Dayger, C.A.; Mehrotra, P.; Belton, R.J., Jr.; Nowak, R.A. EMMPRIN is secreted by human uterine epithelial cells in microvesicles and stimulates metalloproteinase production by human uterine fibroblast cells. Reprod. Sci. 2012, 19, 1292–1301. [Google Scholar] [CrossRef]
- Lian, C.; Guo, Y.; Zhang, J.; Chen, X.; Peng, C. Targeting CD147 is a Novel Strategy for Antitumor Therapy. Curr. Pharm. Des. 2017, 23, 4410–4421. [Google Scholar] [CrossRef]
- Unno, K.; Jain, M.; Liao, R. Cardiac side population cells: Moving toward the center stage in cardiac regeneration. Circ. Res. 2012, 110, 1355–1363. [Google Scholar] [CrossRef]
- Kimes, B.W.; Brandt, B.L. Properties of a clonal muscle cell line from rat heart. Exp. Cell Res. 1976, 98, 367–381. [Google Scholar] [CrossRef]
- Branco, A.F.; Pereira, S.P.; Gonzalez, S.; Gusev, O.; Rizvanov, A.A.; Oliveira, P.J. Gene expression profiling of H9c2 myoblast differentiation towards a cardiac-like phenotype. PLoS ONE 2015, 10, e0129303. [Google Scholar] [CrossRef]
- Robert, S.; Poncelet, P.; Lacroix, R.; Arnaud, L.; Giraudo, L.; Hauchard, A. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: A first step towards multicenter studies? J. Thromb. Haemost. 2009, 7, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Alkhatatbeh, M.J.; Enjeti, A.K.; Baqar, S.; Ekinci, E.I.; Liu, D.; Thorne, R.F. Strategies for enumeration of circulating microvesicles on a conventional flow cytometer: Counting beads and scatter parameters. J. Circ. Biomark. 2018, 7, 1849454418766966. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Bodega, G.; Corchete, E.; García-Menéndez, E.; De Sequera, P.; Luque, R.; Rodríguez-Padrón, D.; Marqués, M.; Portolés, J.; Carracedo, J.; et al. Microvesicles from indoxyl sulfate-treated endothelial cells induce vascular calcification in vitro. Comput. Struct. Biotechnol. J. 2020, 18, 953–966. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Carracedo, R.; Tesoro, L.; Hernandez, I.; Diez-Mata, J.; Botana, L.; Saura, M.; Sanmartin, M.; Zamorano, J.L.; Zaragoza, C. Ivabradine-Stimulated Microvesicle Release Induces Cardiac Protection against Acute Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 6566. https://doi.org/10.3390/ijms21186566
Ramirez-Carracedo R, Tesoro L, Hernandez I, Diez-Mata J, Botana L, Saura M, Sanmartin M, Zamorano JL, Zaragoza C. Ivabradine-Stimulated Microvesicle Release Induces Cardiac Protection against Acute Myocardial Infarction. International Journal of Molecular Sciences. 2020; 21(18):6566. https://doi.org/10.3390/ijms21186566
Chicago/Turabian StyleRamirez-Carracedo, Rafael, Laura Tesoro, Ignacio Hernandez, Javier Diez-Mata, Laura Botana, Marta Saura, Marcelo Sanmartin, Jose Luis Zamorano, and Carlos Zaragoza. 2020. "Ivabradine-Stimulated Microvesicle Release Induces Cardiac Protection against Acute Myocardial Infarction" International Journal of Molecular Sciences 21, no. 18: 6566. https://doi.org/10.3390/ijms21186566
APA StyleRamirez-Carracedo, R., Tesoro, L., Hernandez, I., Diez-Mata, J., Botana, L., Saura, M., Sanmartin, M., Zamorano, J. L., & Zaragoza, C. (2020). Ivabradine-Stimulated Microvesicle Release Induces Cardiac Protection against Acute Myocardial Infarction. International Journal of Molecular Sciences, 21(18), 6566. https://doi.org/10.3390/ijms21186566







