Abstract
Environmental factors contribute to autoimmune disease manifestation, and as regarded today, AhR has become an important factor in studies of immunomodulation. Besides immunological aspects, AhR also plays a role in pharmacological, toxicological and many other physiological processes such as adaptive metabolism. In recent years, epigenetic mechanisms have provided new insight into gene regulation and reveal a new contribution to autoimmune disease pathogenesis. DNA methylation, histone modifications, chromatin alterations, microRNA and consequently non-genetic changes in phenotypes connect with environmental factors. Increasing data reveals AhR cross-roads with the most significant in immunology pathways. Although study on epigenetic modulations in autoimmune diseases is still not well understood, therefore future research will help us understand their pathophysiology and help to find new therapeutic strategies. Present literature review sheds the light on the common ground between remodeling chromatin compounds and autoimmune antibodies used in diagnostics. In the proposed review we summarize recent findings that describe epigenetic factors which regulate AhR activity and impact diverse immunological responses and pathological changes.
1. Introduction
Heredity is only the sum of all past environment—this famous statement published in 1906 in the article “The Training of the Human Plant” in Century magazine by Luther Burbank [1] has never been so much reliable and evident as in recent years when epigenetics and its underlying mechanisms have changed classical approach to many topics requiring a thorough understanding of all aspects of genetics, such as stem cells, cloning, aging or synthetic biology [2]. Epigenetic mechanisms, defined as the mitotically heritable changes, regulate or affect gene expression without affecting genome sequence [3] by methylation of DNA, modification by non-coding RNA [4], histone modification, and chromatin remodeling [4,5]. More and more studies reveal that in the case of complex autoinflammatory disorders, non-genetic mechanisms lead to pathophysiological responses. In accordance with the recent literature, there is a clear correlation between environmental factors and diseases [6]. In the case of those kinds of pathological conditions also well-established heritability model is problematic and very often it is a subject of controversy [5]. In this respect, more in-depth research providing epigenetic studies could be a great value in searching for potential biomarkers, molecular mechanisms responsible for maintaining balance in autoimmunity, and crucial in clinical aspects such as efficient drug therapy. Therefore, in recent years, there has been an increasing focus on epigenetic changes in autoimmune diseases [7]. Additionally, it becomes clear that environmental pollutants, diet and lifestyle are linked with epigenetic variations, including changes in DNA methylation, histone modifications and microRNA [5,8].
It has been estimated that autoimmune diseases (ADs) represent a family of at least 80 illnesses that share common pathogenesis [9]. It is not possible within the scope of this article to provide a comprehensive overview of all ADs, therefore we referred to the selected examples which are well described in the aspect of aryl hydrocarbon receptor (AhR) and epigenetic regulation (Table 1).

Table 1.
Characteristic of selected autoimmune diseases and their association with epigenetics mechanisms and aryl hydrocarbon receptor (AhR) presence.
Considering the particular role of AhR in immune responses, increasing data reveals its cross-roads on the other significant in immunology pathways such as NF-κB [10], TGFβ/SMAD3 [11,12,13] and TLRs [14]. For example, the AHR seems to regulate innate inflammatory signaling not only through binding to its cognate response element in association with ARNT but also through direct binding to RELA (also known as p65) and RELB, which are members of the NF-κB family of transcription factors [15,16]. AhR interacts with ERα (estrogen receptor α, present in immune cells) but also directly binds estradiol [17,18,19]. Additionally, cross-talk between ER and NF-κB has been demonstrated in many studies [20]. Recently, Shinde et al. [21] revealed that AhR is activated by TLR9 and DNA from apoptotic cells in systemic lupus erythematosus patients (SLE) [21]. This assumption that TLR ligands and cytokines are potent agonists in various cell types has been pointed even a few years back by other authors [22,23].
1.1. AhR Regulation in Immune System
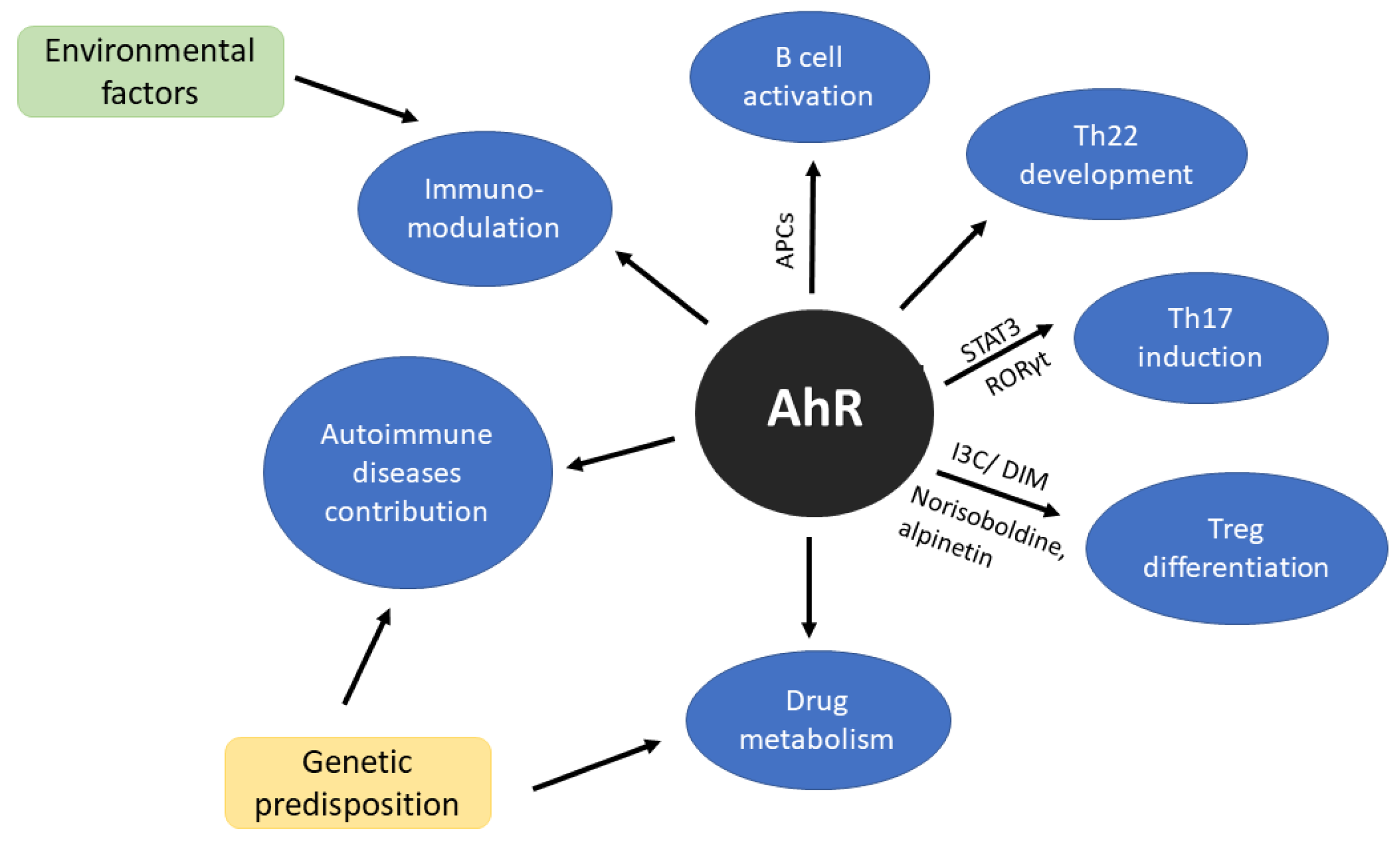
Aryl Hydrocarbon Receptor (AhR) is one of the transcription factors that has been extensively studied in the context of cellular responses to environmental toxins and also plays an important physiological role in T cell immune responses and T cell differentiation (Figure 1) [68]. Together with signal transducer and activator of transcription factor 3 (STAT3) and retinoid-related orphan receptor gamma (RORγt) is crucial in Th22 induction [69]. AhR is a modulator of B cell activity [70,71], furthermore, cells presenting antigen (CPAs) also express elevated level of AhR after stimulation [30,72]. For many years Th22 was considered as a subtype of Th17 but Miyazaki et al. [73] study do not support this hypothesis. Moreover, their research suggests that Th22 may be directly involved in joint destruction [73]. For more detailed information on AhR regulation in immune response, we would recommend recent reviews written by Gutiereez-Vazquez and Qiotana [74] or Trikha and Lee [75].
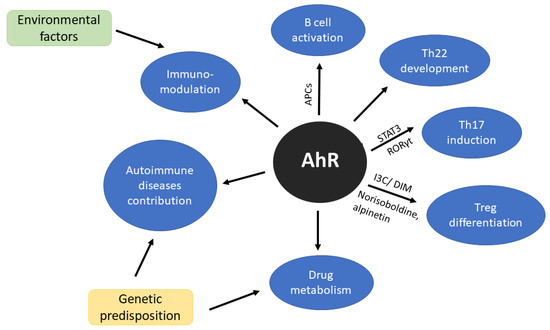
Figure 1.
Scheme of aryl hydrocarbon receptor (AhR) significance in immune system. AhR as a sensor of environmental factors affects immunomodulation. Additionally, genetic compound may trigger the mechanisms of autoimmune diseases development and treatment response.
1.2. AhR Ligands
AhR, a basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) transcription factor [76,77], is best known for mediating of the biotransformation and cancerogenic/teratogenic effects of environmental toxins such as 2,3,7,8-tetrachloro- dibenzo-p-dioxin (TCDD) [77,78] or many polycyclic aromatic hydrocarbons (PAHs) such as benzo-A-pyrene, dimethylbenzanthracene [78]. AhR regulates phase I and II drug-metabolizing enzymes [79] and drug transporters [80]. In accordance with that environmental factors undoubtedly contribute to autoimmune disease manifestation [6], and as regarded today, AhR has become an essential factor in studies of immunomodulation [81]. Moreover, epidemiologic studies suggested that rates of autoimmune diseases are increasing, particularly in industrialized countries [9] and most prevalent in women [82]. Interestingly, xenoestrogens are endocrine disruptors and many of them have an impact on AhR activation [83].
In the absence of a ligand, AhR is localized in the cytoplasm of the cell and associated with a dimer of Hsp90 and other proteins such as p23, immunophilin-like AhR interacting protein AIP (also known as XAP2 or Ara9) or cytosolic proteins such as kinome chaperone Cdc37 and the non-receptor tyrosine kinase Src [76,77,84]. Ligand binding leads to the conformational changes and as a consequence AhR dissociates from Hsp90, translocates to the nucleus and heterodimerizes with ARNT (another bHLH-PAS protein which constitutively occurs in the nucleus). The AhR-ARNT complex binds to promoters AhR gene responsive elements referred (AhRE), dioxin response elements (DRE), or xenobiotic response elements (XRE) and initiates or regulates target gene transcription [6,77]. Moreover, many studies revealed an alternative pathway of activated AhR signaling and cross-talk or competition for transcription cofactors such as NF- NF-kB, ER or NRF2 [85,86].
AhR activity may be regulated via a few different mechanisms. One of them is degradation by the proteasome. Binding Ahr with ligand induces ubiquitination and subsequent degradation. Another is the auto-regulatory feedback loop. AhR activation induces the expression of negative regulators, which prevent excessive AhR activation. For example, a repressor of AhR–AhRR through competition with AhR-ARNT complex downregulates the expression of AhR dependent genes [77]; or few years ago discovered, more general regulator TiPARP (TCDD-induced poly-ADP-ribose polymerase, ARTD) [87]. Moreover, indirect regulation by the availability of AhR ligands may also suppress activity, for example, by the induction of ligand metabolizing enzymes such as CYP1 and consequently by the prevention of prolonged AhR activation [77]. In recent years, epigenetic mechanisms, which are presented in this paper, have provided new insight into AhR regulation.
It should be mentioned that AhR activation and its target gene networks may be caused not only by agonist or antagonist but also by factors that do not fit well into the AhR ligand-binding domain (LBD) and by numerous stress factors like oxidative stress, shear stress that do not bind at all with the LBD [88]. Nevertheless, as many studies showed, besides different ligand-dependent AhR responses, also the tissue or even cell type determines the level of expression and signaling pathway [6]. However, up to date, a list of diverse AhR ligands is impressive and still growing. Additionally, advances in the microbiota research suggest that microflora and their metabolic products can activate AhR [77] and shape immunity and tolerance [88]. Recent discoveries also complement epigenetic regulation, for example, butyrate (short-chain fatty acid derived from bacterial fermentation of dietary fibers) stimulates AhR-dependent genes probably by decreasing expression of histone deacetylase (HDAC) in the human intestinal cell [89].
In general, according to chemical structure, AhR ligands may be categorized into two major classes. The first one, which was mentioned above, contains aromatic hydrocarbons, which are derived mainly from environmental toxins [90] or also through the medicine or diet [79].
Another class of AhR ligands is composed of tryptophan-based molecules that can be derived from the diet or endogenously produced by the host organism [90] such as 6-formylindolo[3,2-b]carbazole (FICZ), indolo-[3,2-b]-carbazole (ICZ, converted from indole-3-carbinal which is found at especially high levels in cruciferous vegetables [91]) or kynurenine [77], cinnabarinic acid [92], galic acid (3,4,5-trihydroxybenzoic acid–metabolite of shikimate pathway) [93]. Generally, FICZ is an ultraviolet photoproduct of L-tryptophan, but it can also be generated during other metabolic pathways such as tryptamine deamination or tryptophan oxidation by intracellular oxidants [78,91]. As compared to dioxins, FICZ reveals different, rapid kinetic patterns of AhR responses, which lead to low intracellular steady-state levels of FICZ. Its high specificity and observed catalytic efficiencies support the argument that this molecule is an endogenous AhR ligand of high importance [6]. Well-established immunoregulatory IDO activity and tryptophan metabolites participation in the regulation of many cell types, including T cells, DC, monocytes, macrophages, and so forth, has been reviewed by Ngyuenet et al. [22]. In large scale pharmaceuticals screening published by Hu et al. [94] leflunomide appeared to be the most potent AhR agonist. Additionally, nimodipine (a calcium-channel blocker indicated for subarachnoid hemorrhage) and flutamide (an androgen receptor antagonist indicated for prostate cancer) for the first time were reported as compounds which competitively bind to the AhR in vitro [94]. Other studies also showed that some benzimidazole derivatives, antiallergenic drugs, are AhR receptor agonists in vivo and in vitro [95,96]. Recently, more and more evidence shows that norisoboldine (an alkaloid from Radix linderae) is AhR ligand and have an impact on Treg differentiation. As a herb-drug and AhR ligand, norisoboldine attenuates rheumatoid arthritis (RA) and colitis [97,98].
Among endogenous AhR ligands such as bilirubin and biliverdin, prostaglandin G2, indirubin and lipoxin A4 have been identified. In the case of endogenous ligands, rapid metabolism is very often observed, also as a negative feedback loop mechanism. For example, anti-inflammatory lipoxin A4 is degraded by Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) and bilirubin via UDP glucuronosyltransferase family 1 member A1 (UGT1A1), whose expression is induced by AhR [99]. As it was mentioned before, the physiological effect may be produced even by ligands with relatively weak affinity for binding the AhR, such as genistein (flavonoid present in soy), and quercetin (flavonol in apples, tea, onions) because of their wide occurrence in the diet [78].
2. Crosstalk between AhR and DNA Methylation
DNA methylation was the first described covalent DNA modification. In this process methyl group onto the 5’ carbon position of cytosine in CpG islands is added and in consequence access to DNA for transcription factors and RNA polymerases is reduced and transcription is repressed [100]. DNA methylation plays a role in promoting the proinflammatory immune cells, which might depend on a large extent on environmental factors (UV exposure, viral infection) but also individual factors (genetic predisposition, age, diet, etc.) and medications (DMARDs-disease-modifying anti-rheumatic drugs) [101]. Decreased global DNA methylation has been observed in immune cells from SLE patients [102], in PBMC and CD4+ T cells of psoriasis patients [42,103] whereas in Sjögren Syndrome, in epithelial cell of the salivary gland and minor salivary glands hypomethylation and reduced DNA methyltransferase (DNMT) level was observed [47,104].
Research conducted on Atlantic Killifish (Fundulus heteroclitus) that adapted to survive in PCB-contaminated water, revealed an evolved genetic mechanism where methylation status of promoter regions may control the tissue-specific expression of AhR genes. PCB-resistant and PCB-sensitive populations of killifish respectively, exhibit different patterns of DNA methylation in the promoters of AhR genes [105]. It also appears that early life activation of AhR alters the responsive capacity of CD8+ T cells and consequently influences the interaction between DNA methylation and gene expression during infection [106].
In 2006, Mulero-Navarro et al. described for the first time a case of methylated AhR promoter in human acute lymphoblastic leukemia [107]. Methylation of AhR CpG sequences is responsible for AhR activation, however, this regulation seems to be cell-type specific.
Andrade et al. [108] conducted a study showing the connection between methylation of AhR and its clinical implementation. It has been revealed that contrary to normal cells, in acute lymphoblastic leukemia (ALL) AhR promoter gene is methylated and consequently deactivated. Although the majority of patients with ALL respond well to current therapies about 20% of affected children relapse or have a poor prognosis because of the development of drug resistance. The authors showed that DNA demethylation by zebularine (1-[b-D-ribofuranosyl]-1,2-dihydropyrimidin- 2-one; ZB) in primarily resistant cells can sensitize cells to methotrexate (MTX) and re-express the AhR gene. ZB also caused apoptosis and re-expressed the silenced methylated AhR. Additionally, ZB combined with MTX synergistically affected leukemia cell lines by decreasing their growth and clonogenic capacity. Demethylation and activation of AhR may contribute to restoring normal cell phenotype and prevent ALL development [108]. In contrast to studies on leukemia cells, mechanically different AhR silencing has been noted in MCF-7 or -10A breast cancer cells. In this case, AhR silencing probably is not connected with DNA methylation but histone modification, which is another element of epigenetic modulation [109]. Depending on AhR ligands, process of DNA methylation might be modulated as it has been observed for Treg/Th17 differentiation process. In CD4+ T cells from lupus patients, low activity of DNMT1 has been observed after activation of AhR by UVB. AhR suppresses SIRT1 which has a negative effect on DNTM1 activity [110]. Liu et at. [111] revealed that activation of AhR by phenanthrene leads to conversion of primary human Treg cells to Th2 cells, whereas TCDD and FICZ to Th17 cells. Moreover, inhibition of AhR results with significant FOXP3 expression, decrease of CpG sites methylation in its locus and reduce DNA (cytosine-5)-methyltransferase (DNMT)1 and DNMT3b transcripts. Interestingly, additional treatment of Treg cells with transforming growth factor β (TGF-β), partially reverses methylation of FOXP3 and maintains Treg phenotype [111]. TCDD-activated AhR displays to partially demethylate CpG islands of FOXP3 and to hypermethylate IL-17 promoters in colitis [51]. Liu et al. [111] study is significant in understanding the pathophysiology of atopic diseases such as atopic dermatitis, asthma, and hay fever and their association with Th2 and environmental pollution. Although the literature is not consistent on AhR ligand specificity and T cell differentiation [51], the relationship between AhR activity and methylation seems to be in line. Activation of AhR decreases the level of DNMT expression, which has been proved in many studies [112,113]. This hypothesis correlates with demethylation mechanism. Research on hepatocytes indicated that AhR is important in the bare excision repair (BER) pathway where methylated cytosine is replaced by non-methylated in CYP1a1 promoter and increase mRNA level of CYP1a1 [114].
AhR also plays a role in B cell differentiation. Recently, it has been proved that in induce arthritis in mice model, AhR promoter is methylated in B cell and its demethylation by 5’-azacytidine decrease IgG antibody production. Another consequence of reactivation of AhR is the suppression of Aicda (single-stranded DNA-specific cytidine deaminase) expression, crucial for efficient antibody response [115]. Overproduced IgG1 antibodies form immune complexes on the articular cartilage surface and contribute to joint destruction in RA pathogenesis [116]. It has been shown that AhR expression is also downregulated in PBMC and B cells in treatment naïve RA patients. However, hypermethylation was not observed like in mice model of RA in promoter AhR but in the distant intergenic region, precisely 155 kb upstream of AhR transcription start site. Nevertheless, in both experiments, AhR was silenced. Therefore, the authors postulated that treatment with DNA methyltransferase inhibitor may have a therapeutic potential in autoimmune arthritis [115]. However, on the other hand, 5-azacytidine may induce lupus-like disease in rodents [117,118].
3. Post-Translation Histone Modification (Methylation, Acetylation of Residues in the N-Terminal Tails of Histones)
Chromatin remodeling and histone modification are interrelated processes. Regulation of gene expression is strongly associated with the state of chromatin—1) euchromatin-relaxed with active genes and 2) packed, transcriptionally inactive heterochromatin [119]. The basic structural unit of the chromatin is nucleosome consisted of DNA and histones. Histone modification constitutes of many diverse classes and complex regulations and the histone tails are the targets of changes [100]. Several modifications of histone have been identified as regulators of gene expression—acetylation, methylation, phosphorylation, ubiquitination, ADP-ribosylation, and sumoylation. An additional aspect is that histones variants may contribute to the chromatin changes [120] and typically, each variant has dedicated chaperones [121]. Antibodies against the centromere-specific histone H3-variant, so-called anti-CENP-A, are generated in patients suffering from for example, systemic sclerosis (SSc) but not in healthy people and cancer patients [122].
In the present review, we focused particularly on methylation and acetylation as they have been mostly studied in the aspect of AhR and immune regulations. Histone methylation affects lysine (K) and arginine (R) residues. Lysine can be methylated with one to three methyl groups, while arginine can be methylated with only one methyl group. Histone methylation correlates with gene activation and repression. An active form of chromatin consists with for example, H3K4me; H3K36me, H3K79me while H3K9me2 and H3K27me3 occur in condensed chromatin and silence the activity of genes. Histone methylation is conducted by the histone methyltransferases HKMT and PRMT whereas demethylation by histone demethylases (HDM) [123,124,125]. During histone acetylation acetyl group is attached to the lysine and the reaction is catalyzed by the histone acetyltransferases HATs. Some of the proteins such as p55 or p300/CBP, which have been known of their other biological function before, appeared to have HATs activities. HATs active in the nucleus belongs to HAT A type, whereas those localize in the cytoplasm (e.g., HAT1) belongs to HAT B type [126]. This process leads to chromatin decondensation and consequently activates transcription. The reverse process associated with chromatin condensation and transcription inhibition is deacetylation of histones with histone deacetyltransferases HDACs. Noteworthy is that the modification of histones is dynamic, therefore gene transcription might be epigenetically regulated [127].
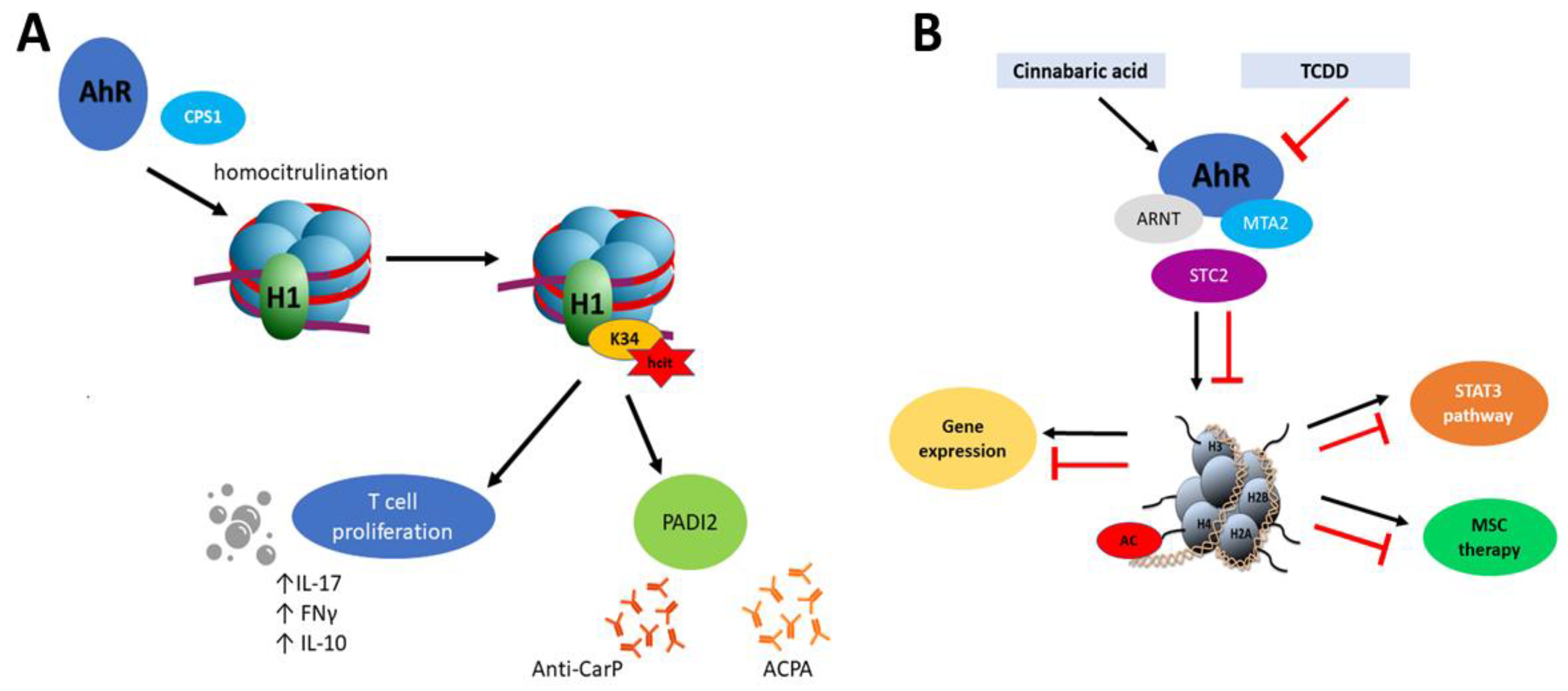
However, epigenetic mechanisms of AhR and the role of this transcription factor in epigenetic modifications are not fully explored. For example, Joshi et al. [127] showed that AhR recruits carbamoyl phosphate synthase 1 (CPS1) which is responsible for homocitrullination (carbamylation) of histone H1 on lysine 34 (H1K34). Moreover, H1K34hcit is implicated in the enhanced expression of the peptidyl arginine deiminase 2 gene (PADI2) [127]. Carbamylation is a post-translational modification of proteins which similarly to citrullination seems to be implicated in the pathogenesis of rheumatoid arthritis [128] but also with Sjögren Syndrome [129]. In the inflammation process, carbamylated lysine affects proliferation of CD4+ T cells, production of IL-10 and the pro-inflammatory cytokines IFN-γ and IL-17 in combination with a response to antibodies (anti-CarP) [130]. The process described by Joshi et al. [127] combines PADI 2- expression in an AhR-dependent manner. It is also noteworthy that PADs proteins, including PADI2, facilitate chromatin decondensation during NET formation (neutrophil cellular trap) which along with DNA and citrullinated histones are one of the significant factors during the developing autoimmunity. Similarly to carbamylation, histone citrullination results in inflammation process by activation of immune T cells response and induction of specific antibodies (ACPA) [131]. This cascade has been particularly described in RA pathogenesis, where NET activates synovial fibroblast with proinflammatory response and production of citrullinated autoantigens [132] (Figure 2A).
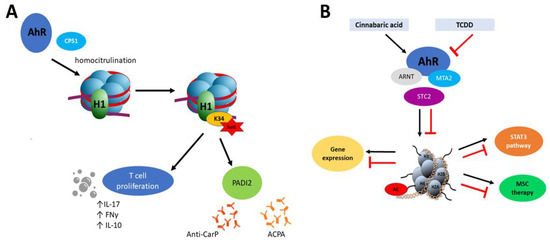
Figure 2.
(A) Mechanism of homocitrulination and anti-CarP and anti-citrullinated protein (ACPA) antibody production. AhR recruits CPS1, which is responsible for homocitrulination of H1K34. H1K34hct enhances PADI2 expression, affects proliferation of T cells and production of IL-10 and the pro-inflammatory cytokines IFN-γ and IL-17. PADI2 plays a role in anti-CarP and ACPA antibody production. (B) Histone acetylation mediated by AhR. Cinnabaric acid (but not 2,3,7,8-tetrachloro- dibenzo-p-dioxin (TCDD)) activates AhR which recruits MTA2. Complex AhR/ARNT -MTA2 leads to transcriptional activation of STC2. MTA2 takes part in acetylation of histone 4. STC2 reveals anti-inflammatory properties which have been implicated into MSC therapy. STC2 also activates STAT3 signaling.
Global hypermethylation (except H3K4 methylation) and also novel methylation marks have been observed in a lupus-prone mouse study [133]. Hypermethylation H3K4me3 along with methylation CpG sites in CD4+ and CD8+ was marked and their association with several T cell signaling genes also in Graves’ disease [67]. Many autoimmune diseases are triggered with IFNα [134,135], according to this, Stefan et al. [136] checked the IFNα effect on epigenetic changes in thyroid cells. It has appeared that IFNα can lead to raised methylation level of H3K4me what is associated with the lower TSHR expression. As a consequence, autoantigen expression in the thymus decreased, which lead to the escape of self-reactive T cells [136]. Epigenetic analysis in case of type 1 diabetes revealed that high level of methylation H3K9me in CTLA4 (T1D susceptibility gene) correlates with T cell activation [63]. Study on SSc revealed a lower level of methylation in H3K27me3 in CD4+ cells [137].
P300, one of the major HAT proteins, has an impact on STAT3 expression and consequently IL10 production and B cell activity, and production of specific autoantibodies in SLE [28]. In T cell of SLE patients, increased level of transcription factor CREMα (cAMP response element modulator) was observed. According to the study by Tenbrock et al. [138], CREMα interacts with HDAC1 and terminates IL-2 production. A similar mechanism has been observed also in monocytes, macrophages and B cells [138]. A recent clinical trial with low-dose IL-2 treatment resulted in a higher rate of complete remission in patients with lupus nephritis. Additionally, low-dose IL-2 therapy promotes regulatory T cells and may also sustain cellular immunity with enhanced natural killer cells [139].
In the histone acetylation process, the electrostatic interaction between histones and DNA is diminished. Consequently, the chromatin structure is decondensed which provides access to the transcription initiation [140]. It appears that histone acetylation is crucial for the activation of AhR promoter. Inhibitors of histone deacetylase 1 (HDAC1), such as n-butyrate and trichostatin A (TSA), may suppress AhR expression [35]. On the other hand, AhR may have an impact on local histone hyperacetylation and methylation by interaction with coactivators or by displacing histone deacetylase (HDAC) complexes [141].
It has been established that in human T cells, HAT and HADC enzymes co-occupy active and aligned genomic loci to maintain proper transcription regulation and uncontrolled binding of RNA polymerase II [142].
Hyperacetylation has been reported in RA patients, which is related to HATs and HDACs disbalance [33]. High acetylation H3ac results with high expression of IL-6 in synovial fibroblast in RA [32]. Similarly, in SSc fibroblast hypoacetylation of H3 and H4 was reported [40]. In the case of Graves’ disease high acetylation of H3K27ac in CD4+ and CD8+ in PBMC [66,67] was observed but low H4ac in PBMC [66]. A concept of a ‘‘transcription cycle’’ model in the epigenetic control, observed in case of CYP1A1 transcriptional activity through histone modifications, has been proposed by Tian [10]. The author focused on AhR–NF-kB interaction and described to the positive and negative regulation of transcription by these two factors. Briefly, PRMT1 is a co-activator of AhR and PRMT1 takes part in arginine methylation of H4R3. Methylation is crucial for transcription but also for acetylation of H4 which blocked methylation of H43R. To activate methylation, deacetylation process of H4 is necessary with the HDAC activity. Interestingly, this mechanism occurs after TCCD treatment whereas exposition to TNFalpha (NF-kB regulator) reveals the opposite cascade of reactions [10]. Likewise, other histone acetylase coactivators such as p300, SRC1 and -2, and p300/CBP-associated factor are triggered after TCDD induction [143,144]].
Based on the earlier study, it seems that HDAC inhibitors may be used in successful autoimmune diseases therapy. It has been shown that the HDAC inhibitors (e.g., trichostatin A) cause changes in T cells and reduces the activity of SLE [145]. Also studies in an animal model of RA have demonstrated anti-inflammatory properties of HDACi in synovial fibroblasts. HDAC2/3-selective inhibitor reduces IL-6 production in RA patients. Interestingly, conventional treatment with TNFalpha inhibitor did not affect HDAC activity in PBMC from RA patients [146]. Other examples of efficient treatment with HDAC inhibitor are largazole in RA [147] or givinostat in juvenile idiopathic arthritis [148]. In the case of SSc this kind of therapy revealed decrease of collagen production [149,150].
Depending on the AhR ligand, a different response might be observed. Joshi et al. [92] revealed that Stc2 (stanniocalcin 2), a target AhR gene, is expressed after activation of AhR by cinnabarinic acid (endogenous ligand, a metabolite of tryptophan in kynurenine pathway) but not after TCDD interaction (Figure 2B). Additionally, in this mechanism, the new complex between AhR-ARNT with MTA2 (metastasis tumor-associated protein 2) has been described. MTA2 recruitment is necessary for histone H4 acetylation at positions K5, -8, -12, and -16. In the aspect of epigenetic regulation, this is interesting because MTA2 is a chromatin-modifying protein and component of the nucleosome remodeling and deacetylation (NuRD) complex. MTA2 may repress and as well activate gene expression [92]. Stanniocalcins (STC) belong to anti-inflammatory proteins and it has been proved that STC2 is significant in MSC (mesenchymal stromal cell) therapy in allergic contact dermatitis [151]. Knockdown of STC2 in MSC was associated with reducing TNF-α- and IFN-γ-producing CD8+ T cells [151], lower production of anti-inflammatory IL10 by MSC, and impaired macrophage polarization from monocytes THP1 cells [152]. mRNA level of STC2 was significantly higher in the intestinal mucosa of CD patients compared to controls [153]. Additionally, STC2 activates also the STAT3 signaling pathway [154].
4. Chromatin Remodeling
Chromatin remodeling is usually initiated by posttranslational modification of the amino acids that make up the histone proteins, but also through the methylation of neighboring DNA. Additionally, proper heterochromatin formation in certain regions of the mammalian genome requires Dicer- an enzyme involved in microRNA maturation [155]. One of the most widely used methods is chromatin immunoprecipitation (ChIP) assay. Chromatin is sheared and immunoprecipitated with a specific antibody directed against a component of the chromatin complex. Other methods that enable analysis of various histones modification within one nucleosome is mass spectrometry, however, due to methodology, it seems that proteomic analysis will be more functional [140].
Chromatin remodeling is a process in which a cascade of protein complexes, transcription factors, coactivators participate and together with RNA polymerase II (Pol II) enables transcription initiation. So-called “bridge model” connects Mediator, large multisubunit polypeptides with the transcription factors and Pol II. Mediator complex might be a master in epigenetic regulation, but also a “coordinator” in non-coding RNA activation or super-enhancer formation [156]. It has been shown that one of the Mediator subunits, Med220 enhances AhR/ARNT-dependent transcription of CYP1A1 in different mechanistically way. Contrary to the CYP1A1 expression with the interaction of AhR and p300, Med220 associate with the enhancer, not with the promoter of CYP1A1. Therefore, these two different regulations of CYP1A1 expression are characterized by different kinetics. Variety of mediator complex in tissues helps to explain the alteration in response to for example, chemical toxicity and different susceptibility of cells/tissues [157]. Recently, a more complex association involving the Mediator subunit Med1 (MED1) and AhR has been proven by Chowdhary et al. [158] who described a mechanism of chromatin remodeling with an additional microRNA involvement. Although the study is specific for hepatocytes and is associated with acetaminophen toxicity, it sheds the light on very complex machinery of epigenetic regulations and strict dividing them into the individual process is sometimes impossible. Chowdhary et al. [158] showed that miR-122 protects hepatocytes from toxicity caused by acetaminophen. Additionally, it appears that miR-122 regulates CYP1A2 expression through AhR, MED1 and another transcription factor CTFC. Both CTFC and MED1 are target for miR-122 and low levels of this microRNA and high activity of AhR-mediated CYP1A2 are observed in the liver failure. AhR or Med1 depletion is associated with downregulation at mRNA and protein levels of Cyp1a2 in mouse liver. However, the regulation of AhR and MED1 expression by mir-122 occurs by distinct mechanisms. Contrary to Med1, mouse AhR does not have a binding site for miR-122 and is not directly regulated by this microRNA [158].
Another complex of proteins SWI/SNF (also known as BRG1/BRM-associated factor (BAF) complexes) with other histone-modifying enzymes may independently or cooperatively mediate remodeling of targeted chromatin structures [159]. Nowadays, we know that chromatin remodeling complexes such as SWI/SNF and NuRD (nucleosome remodeling and deacetylase) are associated with Ikaros. First reports on Ikaros–a zinc-finger DNA binding protein as an enhancer and promoter elements critical for the expression of lymphoid-specific genes has been written in 90’ [160,161]. Ikaros plays a critical role in hematopoiesis and B-cell differentiation, its deficiency caused immunodeficiency type 13 [162]. Moreover, Ikaros is required to limit repressive chromatin modifications at Ahr, Runx1, Rorc, Il17a, and Il22 loci, thus maintaining the potential for expression of the Th17 gene program. It binds directly to the AhR and Runx1 promoter which consequently control expression of Rorc and Il17a, therefore it can be concluded that Th17 development is controlled by Ikaros in direct chromatin modulation in AhR and Runx1 genes [163].
AhR activity is tissue-specific, which might depend on chromatin-accessible regions. Moreover, AhR may regulate its own locus chromatin accessibility [164]. On the other hand, AhR may have an indirect impact by other chromatin regulators. Interaction of AhR with the Brahma/SWI2- related gene 1 (BRG1), a subunit of the SWI/SNF chromatin-remodeling complex, affects local chromatin architecture. Depletion of BRG1 reduces of chromatin accessibility. This result has been shown in the regulation of CYP1A1 expression in mouse hepatoma cells, where BRG1 directly targeted to enhancer region [159]. Another study on human retinal pigment epithelial cells revealed similar observation. Moreover, AhR directly interacts with BRG1 [165] and cannot associate with the enhancer in ARNT-deficient cells [159]. DiNatale et al. [166] showed that the expression of IL6 depends on AhR and Brg1 activity. The authors suggest using of an AhR antagonist to inhibit pro-growth and antiapoptotic signaling and sensitize cells to more aggressive treatment heterogeneous mixture of tumors like in the case of carcinoma of the head and neck [166]. Although the abovementioned experiment was conducted on cancer cell lines, in respect of autoimmunity, these outcomes might be interesting because of the treatment with IL-6 inhibitors of various rheumatic diseases, such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA) [167], lupus, Crohn’s disease [168] and necessity of alternative strategies when resistance to biologics drug develop. Interestingly, study conducted on a mouse model of induced-colitis revealed that FICZ prevents intestinal barrier function via AhR activation by suppressing IL-6 and claudin-2 expression [142].
Another transcriptional factor that has an impact on chromatin architecture is growth factor independence (GFI1). It has been shown that GFI1 mediates transcriptional repression mainly by recruiting histone-modifying enzymes such as histone deacetylases (HDAC-1–3), histone methyltransferases (EHMT2) or histone demethylases (KDM1A/LSD1/) [169]. GFI1 promoted the development of gut innates lymphocyte (ILC2). Study on ILC2 revealed that AhR suppresses GFI1 recruitment. As a consequence, expression of the interleukin-33 (IL-33) receptor ST2 in ILC2s and expression of ILC2 effector molecules IL-5, IL-13 is downregulated. The authors suggest that AhR is not a general chromatin remodeler but seems to be crucial in the regulation of some event in the genome. Although high expression of AhR in Treg cells in gut (higher than Treg cells in the lymphoid organs and other tissue-resident Treg cells in the lung, fat, skin, and liver [170]) remodeling peak dependent on Ahr was absent in gut Treg cells but present in ILC2s. In other words, gut-ILC2-specific open-chromatin events at the Ahr locus were mostly absent in gut Treg cells. These data support a cell-type-specific positive-feedback role of Ahr in its own transcription regulation of expression distinct cytokines [164]. GFI1 is transiently induced during T cell activation. Furthermore, Zhu et al. [171] showed that GFI-1 is important for the epigenetic regulation of Th17 and in the iTreg-related genes in Th2 cells and activation of GFI-1 inhibits both Th17 and iTreg cell differentiation. Moreover, TGF-β stimulation downregulates GFI-1 [171]. Moreover, it has been proved that Gfi1-deficient mice develop autoimmunity and particularly B cell-dependent autoimmunity like lupus [172]. Gfi1 may also inhibits development of Th17 and Treg. Whereas absence of Gfi-1 is associated with significantly higher percentage of Foxp3+ cells [171]. In the case of lupus, in mice model knockdown of Gfi1 developed this entity which might be also associated with the negative regulation of TLR7. Lack of activation of TLR7 or TLR4 results in decrease of IL-6, IFNβ, TNF production and less productive NF-κB phosphorylation [173].
5. microRNAs
MicroRNAa (miRNA) are short/small, non-coding RNAs of 19–25 nucleotides long that regulate expression of many genes by the inhibition of translation after complementary base pairing or mRNA degradation after imperfect complementary base pairing with 3’ untranslated regions (3’UTR) of target mRNA [174].
According to the primary public repository and online source for microRNA (http://mirbase.org/), human genome encodes over 2600 miRNAs (miRbase v.22) that are involved in post-transcriptional regulation of thousands of protein-coding genes. One miRNA may regulate the expression of several genes and a group of miRNAs may interact with the expression of one gene. Since miRNA controls the expression of variate of genes, they are involved in important life processes including immune cell differentiation, maturation and function [175]. Dysregulations of miRNA expression could lead to autoimmune disease including rheumatoid arthritis, systemic lupus erythematosus, intestinal bowel disease, Sjögren’s syndrome [176,177,178]. Moreover, Hou et al. [179,180] indicated that exposure to environmental chemicals, including AhR agonist TCDD, may alter miRNAs and gene expression that results in diseases and health problems [179,180]. Al-Ghezi et al. [181] demonstrated that mice treated with PTX (pertussis toxin, a microbial metabolite) were characterized by raised serum level of pro-inflammatory cytokines—IL-17A, IL-6 and TNFγ. Moreover, an increased proportion of CD4+ Th1 and Th17 cells in spleen was noted. Contrary to this, mice treated with PTX and additionally with TCDD revealed decreased serum level of above mentioned pro-inflammatory cytokines and increased level of anti-inflammatory cytokines IL-10 and TGF-β. Furthermore, the ratio of Th1 and Th17 cell decreased while Th2 and Tregs increased. Moreover, in CD4+ T cells from mouse spleens treated with PTX and TCDD high expression of miR-3082-5p that targeted IL-17 was observed and low expression of miR-1224-5p that regulate FoxP3. These results suggested that TCDD through AhR activation and miRNA regulation repress inflammation mediated by PTX [181].
According to the available miRNA databases (MIRTarBASE, miRDB- target score >80, Targetscan- miRNA described as “conserved”), almost a hundred miRNAs are potentially involved in post-transcriptional regulation of AhR. However to date, in the aspect of autoimmune diseases, there is still a paucity data regarding the regulation of AhR by miRNA.
AhR regulates the differentiation of Th17 and Treg cells; therefore, the difference in its expression shifts the balance between these two T cell subtypes and may contribute to the pathogenesis, among others, of Crohn’s diseases (CD). Zhao et al. [182] showed a negative correlation between miRNA and AhR protein level in patients with Crohn’s diseases and that miR-124 may interact with AhR. The expression of miR-124 was high in inflamed colonic and ileac epithelial cells whereas AhR protein level was very low. It was also found that transfection of Caco-2 cells with pre-miR-124 resulted in overexpression of miR-124 and downregulation of AhR. Further analysis confirmed post-transcriptional regulation of AhR by miR-124. Moreover, Caco-2 cells pre-treatment with siRNA for AhR/siAhR and pre-miR-124 stimulated with lipopolysaccharide showed a significantly higher level of few pro-inflammatory cytokines—TNF-α, IL-1β and IL-6. These results suggest that miR-124 promotes the pathogenesis of CD via decreasing AhR [182] and its anti-inflammatory properties have been proved in a clinical trial (ABX464) [183]. Th17 and Treg differentiation may be dependent on AhR ligand and micro-RNA profile. In material isolated from inguinal lymph nodes, Singh et al. [184] observed that dietary AhR ligands such as I3C and DIM promote Treg differentiation and high IL-10 production whereas Th17 promotion was observed after FICZ (endogenous AhR ligand). The observation was interesting because particular signature of microRNA was correlated with the type of T cell. Downregulation of target FOXP3 microRNAs (miR-31, miR-219 and miR-490) and upregulation of miR-495 and miR-1192 (targets of IL-17) was observed in mouse treated with dietary ligands whereas in animals treated with FICZ the opposite expression profile was observed [184].
Recent study conducted by Lu et al. [185] showed that secretion of IL-22, a tissue injured repair cytokine, depends on miR15a/16-1 cluster. It was revealed in a mouse model with immune-mediated liver injury by concanavalin A (ConA) that miR15a/16-1 decreased IL-22 production. In vitro and in vivo study demonstrated that knockout of miR15a/16-1 caused up-regulation of IL-22 in mice CD4+ T cells and ameliorate liver injury via stimulation of cell proliferation and reduced cell apoptosis. It was also noted that AhR, that is essential for IL-22 secretion, is a direct target of miR15a/16-1 in CD4+ T cells. Overexpression of miR15a/16-1 resulted in depletion of IL-22 in CD4+ T cells by inhibiting AhR [185].
There is a lot of evidence that AhR plays an important role in rheumatoid arthritis (RA) pathogenesis. In human synoviocytes, AhR ligands upregulate IL-1β whereas in macrophages upregulation of AhR inhibits expression of cytokines TNF-α, IL-1β and IL-6 [186]. Recently in silico analysis of the interaction between miRNAs and their potential targets become a popular tool in molecular biology studies. Based on this approach Ogando et al. [187] indicated that translocator of AhR - ARNT is a target of miR-233. Further analysis revealed that in RA derived cells (CD14+) miR-223 is up-regulated. However, pre-treatment THP-1 and HEK-293T cells with pre-miR-223 did not alter AhR protein level but ARNT was reduced. Furthermore, synovial tissue from RA patients was characterized by downregulated level of ARNT and AhR-mediated genes which was the result of miR-223 expression. These results imply complex miR-223-ARNT in human diseases and suggest that proinflammatory cytokines mediated by AhR/ARNT are inhibited by miR-223 [187]. On the other hand, another study on miR-233 showed that its low expression ameliorated the disease severity in the mouse model of collagen-induced arthritis [188]. Therefore, it should be noted that the role of miR-233 in RA is not unambiguous.
Another example of possible therapy with miRNA activity was miR-155 in systemic sclerosis treatment. It appeared that the expression of miR-155 in skin of SSc patients is upregulated, therefore, Yan et al. [189] suggested its silencing as a promising therapy [187]. Lupus-like phenotypes mice with silenced miR155 were characterized by lower serum levels of IL-4 and IL-17A and authors of this study concluded that miR-155 deficiency reduces the severity of disease course [190]. A few months ago, clinical trials of miR-155 as a potential MS biomarker were registered. miR-155 appears to regulate AhR in signaling pathway linked to B-cell differentiation [191] and also is required for optimum cytokine production by DC to promote Th17 cells, again this ties into a possible AhR involvement. Moreover, miR-155 might be upregulated by multiple proinflammatory cytokines (IL1β, TGFβ, TNFα, IFNγ), which are all targets of AhR-signaling [192]. Comparative study on the microRNA expression profile in serum of patients with different autoimmune connective tissue diseases showed upregulated levels miR-155 and miR-132 in sera of SSc, RA and SLE but not MCTD (mixed connective tissue disease) patients [50]. Deficiency of the miR-132/212 cluster prevented the enhancement of Th17 differentiation by AhR activation [193]. Moreover, in response to the specificity of the gut’s immune system, AhR and miR-212/132 maintain intestinal balance by negative feedback in differentiation of Tr1 cells (type 1 regulatory T cells, which produce IL-10 in a c-Maf-dependent manner) and regulation of IL-10 production [194]. Whereas studies on cholinergic anti-inflammation, conducted by Alzahrani et al. [195] and Hanieh and Alzahrani [196], revealed that activation of AhR by TCDD or GA (novel AhR ligand) upregulate miR-132 or miR212/132 in colitis-associated colon cancer and autoimmune encephalomyelitis. In both entities, authors indicated that silencing of miR-132 exacerbates inflammation [195,196]. A study on colitis and anti-inflammatory response with AhR has been recently conducted by Lv et al. [112]. The authors revealed that miR-302 is dependent on AhR and activation of AhR by alpinetin influences the differentiation of Treg cells but not Th17 [112]. Regulation of miR-302 by AhR has been earlier confirmed by Hu et al. [197]. miR-21 and miR-148 have also been shown to be regulated by AhR activation [198].
Based on in silico analyzes, Ushakov et al. [199], selected miRNAs which may target AhR [197]. Further analysis of the literature shows an association of selected microRNAs with autoimmune diseases, however, they have not been studied in the context of interactions with AhR in these particular entities (Table 2). Nevertheless, in our opinion, there is a high probability that they may have an impact on AhR in the aspect of the immune response. For example, there is a strong possibility that miR-30c may have an inducing role in Th17 differentiation by targeting negative or positive regulators of Th17 differentiation [200].

Table 2.
microRNA associated with AhR and their impact on selected autoimmune diseases.
6. Conclusions
Epigenetic mechanisms of AhR and the role of this transcription factor in epigenetic modifications have not been fully explored. Based on the above literature, we can see that it may be implicated into epigenetic processes which are significant in immunomodulation. Although it seems that inhibitors of DNA methylation have a therapeutic significance, in the case of variable autoimmune entities like in lupus treatment, this strategy might be more complicated and needs further research.
AhR regulates the differentiation of Th17 and Treg cells, therefore, the difference in its expression shifts the balance between these two T cell subtypes and may contribute to their pathogenesis of autoimmune diseases. Increasing data reveals AhR cross-roads with the most significant in immunology pathways and probably many of other inter-reaction is still unknown. Therefore, AhR is considered a potential target for new therapeutic strategies in autoimmune diseases.
Nevertheless, it has to be considered that the implementation of AhR as a therapeutic target might be very complex and many factors, such as type of ligand and its pharmacokinetics, cell type and cell environment (inflammation, tumor) should be taken into account. Another important aspect of effective treatment using AhR as a therapeutic target is drug interactions through activation of CYP1 or phase II enzymes (UGTs, GSTs), as well as different genetic variants of this transcription factor which can affect drug metabolism by enzymes that are regulated by AhR [79].
In general, implications of AhR in therapy may be directed into its inhibition as well as activation.
Studies on cancer cell lines revealed upregulated AhR levels [15]. Higher AhR expression has been observed in stomach, thyroid, colon and pancreatic tumors whereas in breast, lung, prostate and cervical cancer AhR mRNA level was similar to non-tumor tissue. In patients with pancreatic tumor increased expression was correlated with patients survival [237]. The intracellular location of AhR activity (nuclear vs. cytosolic) is also important. It seems that nuclear location is associated with disease development and its negative prognosis (kidney tumor [238], urothelial cancer [239]). AhR contributes to carcinogenesis due to CYP1 expression [109]. Moreover, the induction of Treg cells development and differentiation may play a role in immune tolerance in cancer [15]. However, it has been revealed that AhR has both pro-oncogenic and suppressor-like functions depending on the type of tumor [240]. Therefore in the aspect of cancer therapy, different strategies are tried, nevertheless, selective AhR modulators would be preferable in the individualized therapy. Currently conducted clinical trials concern AhR inhibition in tumors, for example, BAY2416964 [241], IK-175 (formerly KYN-175) [242]. Blockage of AhR prevents the activation of immune-tolerant dendritic cells and Treg in tumor microenvironment. On the other hand upregulation of AhR, for example, in the esophageal squamous cell carcinoma cell lines [243], prostate cancer [244] or pancreatic cancer [245] reveals suppressive effect toward cancer. Activation of AhR may be also associated with overcoming of the cell resistance such as methotrexate resistance in acute lymphoblastic leukemia cell [108].
Recent identification of structurally-diverse AhR ligands that include salutary phytochemicals and microbial metabolites has changed the course of underlying clinical trials. Nowadays, there is a trend in research on the importance of gut microbiota, their metabolites (including AhR ligands) and various diseases such as MS, nonalcoholic fatty liver disease. The role of diet particularly dietary tryptophan on AhR activation is also comprehensively studied. In the aspect of autoimmune diseases, AhR upregulation and modulation toward increased Treg cell ratio seems to ameliorate of inflammation and inhibit autoimmune response. Particularly in barrier organs regulation of AhR activity reveals high potential of effective therapy.
AhR ligands, such as coal tar, FICZ and tapinarof via the AhR pathway, restore barrier dysfunction and have been implicated into atopic dermatitis or psoriasis therapy [246,247,248,249,250]. FICZ and UV therapy are recommended as a potential therapeutic agent for scleroderma [251]. Therefore, one of the concepts assumes that the activation of AhR by endogenous ligands may support skin barrier formations, but constitutive, abnormal expression of AhR causes inflammatory skin lesions [252]. Recently, it has been proved that unimpaired AhR signaling is crucial for establishment and maintenance in the neonatal epidermis but also the inflammatory profile of dendritic epithelial T cell in healthy skin [253]. Another well-known example of AhR agonist with a clinical implication is a disease modifying anti-rheumatic drug–leflunomide [254]. Treatment of inflammatory bowel diseases with the AhR agonist, diet and balanced gut microbiota promotes Treg differentiation and attenuates inflammation [91,112,255].
Moreover, in the present article, the association between AhR pathway and autoantibodies against complexes such as ACPA or anti-CarP has been described. These autoantibodies are involved in epigenetic modulation and are used in contemporary diagnostic for autoimmune diseases. Noteworthy is that today, in the diagnostics of autoimmune diseases there is a need for more specific and sensitive biomarkers.
Author Contributions
Conceptualization, A.W., J.Ł.-R. and A.P.-G.; writing—original draft preparation, A.W. and J.Ł.-R.; writing—review and editing, A.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
Grant S/1: National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Weinhold, B. Epigenetics: The science of change. Environ. Health Perspect. 2006, 114, A160-7. [Google Scholar] [CrossRef]
- Wu, D.; Potluri, N.; Kim, Y.; Rastinejad, F. Structure and dimerization properties of the aryl hydrocarbon receptor PAS-A domain. Mol. Cell. Biol. 2013, 33, 4346–4356. [Google Scholar] [CrossRef] [PubMed]
- Niederhuth, C.E.; Schmitz, R.J. Putting DNA methylation in context: From genomes to gene expression in plants. Biochim. Biophys. Acta - Gene Regul. Mech. 2017, 1860, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Errico, D.; Vento-Tormo, R.; Ballestar, E. Genetic and epigenetic determinants in autoinflammatory diseases. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A.; Stockinger, B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009, 30, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, Z.; Ma, J.; Ma, J.; Liu, F.; Wu, R. Foxp3 methylation status in children with primary immune thrombocytopenia. Hum. Immunol. 2014, 75, 1115–1119. [Google Scholar] [CrossRef]
- Breton CV, M.A. Air Pollution and Epigenetics: Recent Findings. Curr. Environ. Heal. Rep. 2014, 1, 35–45. [Google Scholar] [CrossRef]
- Rose, N.R. Prediction and prevention of autoimmune disease in the 21st Century: A review and preview. Am. J. Epidemiol. 2016, 183, 403–406. [Google Scholar] [CrossRef]
- Tian, Y. Ah receptor and NF-κB interplay on the stage of epigenome. Biochem. Pharmacol. 2009, 77, 670–680. [Google Scholar] [CrossRef]
- Sarić, N.; Selby, M.; Ramaswamy, V.; Kool, M.; Stockinger, B.; Hogstrand, C.; Williamson, D.; Marino, S.; Taylor, M.D.; Clifford, S.C.; et al. The AHR pathway represses TGFβ-SMAD3 signalling and has a potent tumour suppressive role in SHH medulloblastoma. Sci. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gramatzki, D.; Pantazis, G.; Schittenhelm, J.; Tabatabai, G.; Köhle, C.; Wick, W.; Schwarz, M.; Weller, M.; Tritschler, I. Aryl hydrocarbon receptor inhibition downregulates the TGF-Β/Smad pathway in human glioblastoma cells. Oncogene 2009, 28, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Sakata, N.; Katsu, Y.; Nochise, D.; Sato, E.; Takahashi, Y.; Yamaguchi, S.; Haga, Y.; Ikeno, S.; Motizuki, M.; et al. Dissociation of the AhR-ARNT complex by TGF-β-Smad signaling represses CYP1A1 gene expression and inhibits benze[a]pyrene-mediated cytotoxicity. J. Biol. Chem. 2020, 295, 9033–9051. [Google Scholar] [CrossRef] [PubMed]
- Kado, S.; Chang, W.L.W.; Chi, A.N.; Wolny, M.; Shepherd, D.M.; Vogel, C.F.A. Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells. Arch. Toxicol. 2017. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Øvrevik, J.; Låg, M.; Lecureur, V.; Gilot, D.; Lagadic-Gossmann, D.; Refsnes, M.; Schwarze, P.E.; Skuland, T.; Becher, R.; Holme, J.A. AhR and Arnt differentially regulate NF-κB signaling and chemokine responses in human bronchial epithelial cells. Cell Commun. Signal. 2014, 12, 48. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Ariazi, E.; Kim, K.; Khan, S.; Barhoumi, R.; Burghardt, R.; Liu, S.; Hill, D.; Finnell, R.; Wlodarczyk, B.; et al. 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor α. Cancer Res. 2006. [Google Scholar] [CrossRef]
- Liu, S.; Abdelrahim, M.; Khan, S.; Ariazi, E.; Jordan, V.C.; Safe, S. Aryl hydrocarbon receptor agonists directly activate estrogen receptor α in MCF-7 breast cancer cells. Biol. Chem. 2006. [Google Scholar] [CrossRef]
- Mortensen, A.S.; Arukwe, A. Interactions between estrogen- and ah-receptor signalling pathways in primary culture of salmon hepatocytes exposed to nonylphenol and 3,3′,4,4′-tetrachlorobiphenyl (Congener 77). Comp. Hepatol. 2007. [Google Scholar] [CrossRef]
- Sas, L.; Lardon, F.; Vermeulen, P.B.; Hauspy, J.; Van Dam, P.; Pauwels, P.; Dirix, L.Y.; Van Laere, S.J. The interaction between ER and NFκB in resistance to endocrine therapy. Breast Cancer Res. 2012, 14, 212. [Google Scholar] [CrossRef]
- Shinde, R.; Hezaveh, K.; Halaby, M.J.; Kloetgen, A.; Chakravarthy, A.; Da Silva Medina, T.; Deol, R.; Manion, K.P.; Baglaenko, Y.; Eldh, M.; et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans article. Nat. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nakahama, T.; Le, D.H.; Van Son, L.; Chu, H.H.; Kishimoto, T. Aryl hydrocarbon receptor and kynurenine: Recent advances in autoimmune disease research. Front. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Sherr, D.H. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 2013, 65, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R. Autoimmune Diseases. In International Encyclopedia of Public Health; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128037089. [Google Scholar]
- Yu, H.; Jiang, L.; Liu, R.; Yang, A.; Yang, X.; Wang, L.; Zhang, W.; Che, T. Association between the ratio of aryl hydrocarbon receptor (AhR) in Th17 cells to AhR in Treg cells and SLE skin lesions. Int. Immunopharmacol. 2019, 69, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Luo, Y.; Yan, K.; Zhao, S.; Li, Y.; Qiu, X.; Zhou, Y.; Long, H.; Zhao, M.; Liang, Y.; et al. Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand. J. Rheumatol. 2009. [Google Scholar] [CrossRef]
- Coit, P.; Yalavarthi, S.; Ognenovski, M.; Zhao, W.; Hasni, S.; Wren, J.D.; Kaplan, M.J.; Sawalha, A.H. Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J. Autoimmun. 2015. [Google Scholar] [CrossRef]
- Hedrich, C.M.; Rauen, T.; Apostolidis, S.A.; Grammatikos, A.P.; Rodriguez, N.R.; Ioannidis, C.; Kyttaris, V.C.; Crispin, J.C.; Tsokos, G.C. Stat3 promotes IL-10 expression in lupus T cells through trans-activation and chromatin remodeling. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, A.; Tesmer, L.; Ray, D.; Yousif, N.; Richardson, B. Demethylation of CD40LG on the Inactive X in T Cells from Women with Lupus. J. Immunol. 2007. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009, 206, 2027–2035. [Google Scholar] [CrossRef]
- Araki, Y.; Tsuzuki Wada, T.; Aizaki, Y.; Sato, K.; Yokota, K.; Fujimoto, K.; Kim, Y.T.; Oda, H.; Kurokawa, R.; Mimura, T. Histone Methylation and STAT-3 Differentially Regulate Interleukin-6-Induced Matrix Metalloproteinase Gene Activation in Rheumatoid Arthritis Synovial Fibroblasts. Arthritis Rheumatol. 2016. [Google Scholar] [CrossRef]
- Wada, T.T.; Araki, Y.; Sato, K.; Aizaki, Y.; Yokota, K.; Kim, Y.T.; Oda, H.; Kurokawa, R.; Mimura, T. Aberrant histone acetylation contributes to elevated interleukin-6 production in rheumatoid arthritis synovial fibroblasts. Biochem. Biophys. Res. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.C.; Brock, M.; Hemmatazad, H.; Giger, O.T.; Moritz, F.; Trenkmann, M.; Distler, J.H.W.; Gay, R.E.; Kolling, C.; Moch, H.; et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007, 56, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.; Scheinbart, L.; Strahler, J.; Gross, L.; Hanash, S.; Johnson, M. Evidence for impaired t cell dna methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R. The AH Receptor in Biology and Toxicology. AH Recept. Biol. Toxicol. 2011. [Google Scholar] [CrossRef]
- Takei, H.; Yasuoka, H.; Yoshimoto, K.; Yamaoka, K.; Takeuchi, T. FRI0413 Aryl hydrocarbon receptor expression is associated with lung involvement in systemic sclerosis. In Proceedings of the Annals of the Rheumatic Diseases. BMJ 2018, 77, 738. [Google Scholar]
- Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000, 342, 1350–1358. [Google Scholar] [CrossRef]
- Altorok, N.; Almeshal, N.; Wang, Y.; Kahaleh, B. Epigenetics, the holy grail in the pathogenesis of systemic sclerosis. Rheumatology (UK) 2015, 54, 1759–1770. [Google Scholar] [CrossRef]
- Kuwatsuka, Y.; Ogawa, F.; Iwata, Y.; Komura, K.; Muroi, E.; Hara, T.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. Decreased levels of autoantibody against histone deacetylase 3 in patients with systemic sclerosis. Autoimmunity 2009. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, P.S.; Kahaleh, B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006, 54, 2271–2279. [Google Scholar] [CrossRef]
- DiMeglio, P.; Duarte, J.H.; Ahlfors, H.; Owens, N.D.L.; Li, Y.; Villanova, F.; Tosi, I.; Hirota, K.; Nestle, F.O.; Mrowietz, U.; et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity 2014. [Google Scholar] [CrossRef]
- Zhang, P.; Su, Y.; Chen, H.; Zhao, M.; Lu, Q. Abnormal DNA methylation in skin lesions and PBMCs of patients with psoriasis vulgaris. J. Dermatol. Sci. 2010, 60, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Castillo, L.E.; Cancino-Díaz, J.C.; García-Vázquez, F.; Cancino-Gómez, F.G.; León-Dorantes, G.; Blancas-González, F.; Jiménez-Zamudio, L.; García-Latorre, E.; Cancino-Díaz, M.E. Under-expression of VHL and over-expression of HDAC-1, HIF-1α, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int. J. Dermatol. 2007. [Google Scholar] [CrossRef]
- Ovejero-Benito, M.C.; Reolid, A.; Sánchez-Jiménez, P.; Saiz-Rodríguez, M.; Muñoz-Aceituno, E.; Llamas-Velasco, M.; Martín-Vilchez, S.; Cabaleiro, T.; Román, M.; Ochoa, D.; et al. Histone modifications associated with biological drug response in moderate-to-severe psoriasis. Exp. Dermatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Mishima, K.; Yamamoto-Yoshida, S.; Ushikoshi-Nakayama, R.; Nakagawa, Y.; Yamamoto, K.; Ryo, K.; Ide, F.; Saito, I. Aryl Hydrocarbon Receptor-Mediated Induction of EBV Reactivation as a Risk Factor for Sjögren’s Syndrome. J. Immunol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Shimizu, T.; Kawakami, A. Role of Viral Infections in the Pathogenesis of Sjögren’s Syndrome: Different Characteristics of Epstein-Barr Virus and HTLV-1. J. Clin. Med. 2020, 9, 1459. [Google Scholar] [CrossRef]
- Thabet, Y.; Le Dantec, C.; Ghedira, I.; Devauchelle, V.; Cornec, D.; Pers, J.O.; Renaudineau, Y. Epigenetic dysregulation in salivary glands from patients with primary Sjögren’s syndrome may be ascribed to infiltrating B cells. J. Autoimmun. 2013. [Google Scholar] [CrossRef]
- Yu, X.; Liang, G.; Yin, H.; Ngalamika, O.; Li, F.; Zhao, M.; Lu, Q. DNA hypermethylation leads to lower FOXP3 expression in CD4+ T cells of patients with primary Sjögren’s syndrome. Clin. Immunol. 2013, 148, 254–257. [Google Scholar] [CrossRef]
- Carnero-Montoro, E.; Barturen, G.; Povedano, E.; Kerick, M.; Martinez-Bueno, M.; Ballestar, E.; Martin, J.; Teruel, M.; Alarcón-Riquelme, M.E. Epigenome-wide comparative study reveals key differences between mixed connective tissue disease and related systemic autoimmune diseases. Front. Immunol. 2019. [Google Scholar] [CrossRef]
- Stypinska, B.; Wajda, A.; Walczuk, E.; Olesinska, M.; Lewandowska, A.; Walczyk, M.; Paradowska-Gorycka, A. The Serum Cell-Free microRNA Expression Profile in MCTD, SLE, SSc, and RA Patients. J. Clin. Med. 2020, 9, 161. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Activation of Aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of Foxp3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Prager, M.; Büttner, J.; Grunert, P.; Ellinghaus, D.; Büning, C. A Promoter Variant Within the Aryl Hydrocarbon Receptor Gene Is Associated with an Epithelial Barrier Defect in Smokers with Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2356–2368. [Google Scholar] [CrossRef]
- Moret-Tatay, I.; Cerrillo, E.; Sáez-González, E.; Hervás, D.; Iborra, M.; Sandoval, J.; Busó, E.; Tortosa, L.; Nos, P.; Beltrán, B. Identification of Epigenetic Methylation Signatures With Clinical Value in Crohn’s Disease. Clin. Transl. Gastroenterol. 2019. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, E.S.; Moon, C.M.; Park, J.J.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Genetic polymorphisms of IL-23R and IL-17A and novel insights into their associations with inflammatory bowel disease. Gut 2011. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, E.S.; Moon, C.M.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Abnormal genetic and epigenetic changes in signal transducer and activator of transcription 4 in the pathogenesis of inflammatory bowel diseases. Dig. Dis. Sci. 2012. [Google Scholar] [CrossRef]
- McDermott, E.; Ryan, E.J.; Tosetto, M.; Gibson, D.; Burrage, J.; Keegan, D.; Byrne, K.; Crowe, E.; Sexton, G.; Malone, K.; et al. DNA methylation profiling in inflammatory bowel disease provides new insights into disease pathogenesis. J. Crohn’s Colitis 2016. [Google Scholar] [CrossRef]
- Gonsky, R.; Deem, R.L.; Landers, C.J.; Derkowski, C.A.; Berel, D.; McGovern, D.P.B.; Targan, S.R. Distinct IFNG methylation in a subset of ulcerative colitis patients based on reactivity to microbial antigens. Inflamm. Bowel Dis. 2011. [Google Scholar] [CrossRef]
- Glória, L.; Cravo, M.; Pinto, A.; de Sousa, L.S.; Chaves, P.; Leitão, C.N.; Quina, M.; Mira, F.C.; Soares, J. DNA hypomethylation and proliferative activity are increased in the rectal mucosa of patients with long-standing ulcerative colitis. Cancer 1996, 78, 2300–2306. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Ehrlich, A.K.; Pennington, J.M.; Wang, X.; Rohlman, D.; Punj, S.; Löhr, C.V.; Newman, M.T.; Kolluri, S.K.; Kerkvliet, N.I. Activation of the Aryl Hydrocarbon Receptor by 10-Cl-BBQ Prevents Insulitis and Effector T Cell Development Independently of Foxp3 + Regulatory T Cells in Nonobese Diabetic Mice. J. Immunol. 2016. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Hou, C.; Liang, G.; Yang, L.; Tan, Y.; Wang, Z.; Yin, H.; Zhou, Z.; Lu, Q. Abnormal DNA methylation in CD4+ T cells from people with latent autoimmune diabetes in adults. Diabetes Res. Clin. Pract. 2011. [Google Scholar] [CrossRef]
- Miao, F.; Smith, D.D.; Zhang, L.; Min, A.; Feng, W.; Natarajan, R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation an epigenetic study in diabetes. Diabetes 2008, 57, 3189–3198. [Google Scholar] [CrossRef]
- Song, R.H.; Yu, Z.Y.; Qin, Q.; Wang, X.; Muhali, F.S.; Shi, L.F.; Jiang, W.J.; Xiao, L.; Li, D.F.; Zhang, J.A. Different levels of circulating Th22 cell and its related molecules in graves’ disease and Hashimoto’s thyroiditis. Int. J. Clin. Exp. Pathol. 2014, 7, 4024–4031. [Google Scholar]
- Arakawa, Y.; Watanabe, M.; Inoue, N.; Sarumaru, M.; Hidaka, Y.; Iwatani, Y. Association of polymorphisms in DNMT1, DNMT3A, DNMT3B, MTHFR and MTRR genes with global DNA methylation levels and prognosis of autoimmune thyroid disease. Clin. Exp. Immunol. 2012. [Google Scholar] [CrossRef]
- Yan, N.; Zhou, J.Z.; Zhang, J.A.; Cai, T.; Zhang, W.; Wang, Y.; Muhali, F.S.; Guan, L.; Song, R.H. Histone hypoacetylation and increased histone deacetylases in peripheral blood mononuclear cells from patients with Graves’ disease. Mol. Cell. Endocrinol. 2015. [Google Scholar] [CrossRef]
- Limbach, M.; Saare, M.; Tserel, L.; Kisand, K.; Eglit, T.; Sauer, S.; Axelsson, T.; Syvänen, A.C.; Metspalu, A.; Milani, L.; et al. Epigenetic profiling in CD4+ and CD8+ T cells from Graves’ disease patients reveals changes in genes associated with T cell receptor signaling. J. Autoimmun. 2016. [Google Scholar] [CrossRef]
- Ramsay, G.; Cantrell, D. Environmental and metabolic sensors that control T cell biology. Front. Immunol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Yeste, A.; Mascanfroni, I.D.; Nadeau, M.; Burns, E.J.; Tukpah, A.M.; Santiago, A.; Wu, C.; Patel, B.; Kumar, D.; Quintana, F.J. IL-21 induces IL-22 production in CD4+ T cells. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- North, C.M.; Crawford, R.B.; Lu, H.; Kaminski, N.E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated suppression of toll-like receptor stimulated B-lymphocyte activation and initiation of plasmacytic differentiation. Toxicol. Sci. 2010. [Google Scholar] [CrossRef]
- Villa, M.; Gialitakis, M.; Tolaini, M.; Ahlfors, H.; Henderson, C.J.; Wolf, C.R.; Brink, R.; Stockinger, B. Aryl hydrocarbon receptor is required for optimal B-cell proliferation. EMBO J. 2017, 36, 116–128. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 2010. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Nakayamada, S.; Kubo, S.; Nakano, K.; Iwata, S.; Miyagawa, I.; Ma, X.; Trimova, G.; Sakata, K.; Tanaka, Y. Th22 Cells Promote Osteoclast Differentiation via Production of IL-22 in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 2901. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Trikha, P.; Lee, D.A. The role of AhR in transcriptional regulation of immune cell development and function. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188335. [Google Scholar] [CrossRef]
- Hao, N.; Whitelaw, M.L. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 2013, 86, 561–570. [Google Scholar] [CrossRef]
- Zhou, L. AHR Function in Lymphocytes: Emerging Concepts. Trends Immunol. 2016, 37, 17–31. [Google Scholar] [CrossRef]
- Kerkvliet, N.I. AHR-mediated immunomodulation: The role of altered gene transcription. Biochem. Pharmacol. 2009, 77, 746–760. [Google Scholar] [CrossRef]
- Ramadoss, P.; Marcus, C.; Perdew, G.H. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2005, 1, 9–21. [Google Scholar] [CrossRef]
- Vee, M.L.; Jouan, E.; Stieger, B.; Lecureur, V.; Fardel, O. Regulation of human hepatic drug transporter activity and expression by diesel exhaust particle extract. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nakahama, T.; Kishimoto, T. Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin. Immunopathol. 2013, 35, 637–644. [Google Scholar] [CrossRef]
- Merrheim, J.; Villegas, J.; Van Wassenhove, J.; Khansa, R.; Berrih-Aknin, S.; le Panse, R.; Dragin, N. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun. Rev. 2020, 19, 102468. [Google Scholar] [CrossRef] [PubMed]
- Hutz, R.J.; Carvan, M.J.; Larson, J.K.; Liu, Q.; Stelzer, R.V.; King-Heiden, T.C.; Baldridge, M.G.; Shahnoor, N.; Julien, K. Familiar and novel reproductive endocrine disruptors: Xenoestrogens, dioxins and nanoparticles. Curr. trends Endocrinol. 2014, 7, 111–122. [Google Scholar]
- Park, S.; Dong, B.; Matsumura, F. Rapid Activation of c-Src Kinase by Dioxin Is Mediated by the Cdc37−HSP90 Complex as Part of Ah Receptor Signaling in MCF10A Cells†. Biochemistry 2006. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Khan, E.M.; Leung, P.S.C.; Gershwin, M.E.; Chang, W.L.W.; Wu, D.; Haarmann-Stemmann, T.; Hoffmann, A.; Denison, M.S. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: A role for nuclear factor-κB. J. Biol. Chem. 2014, 289, 1866–1875. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; McMahon, M. Cross-talk between Transcription Factors AhR and Nrf2: Lessons for Cancer Chemoprevention from Dioxin. Toxicol. Sci. 2009, 111, 199–201. [Google Scholar] [CrossRef]
- MacPherson, L.; Ahmed, S.; Tamblyn, L.; Krutmann, J.; Förster, I.; Weighardt, H.; Matthews, J. Aryl hydrocarbon receptor repressor and TiPARP (ARTD14) use similar, but also distinct mechanisms to repress aryl hydrocarbon receptor signaling. Int. J. Mol. Sci. 2014, 15, 7939–7957. [Google Scholar] [CrossRef]
- Esser, C.; Rannug, A. The Aryl Hydrocarbon Receptor in Barrier Organ Physiology, Immunology, and Toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Rothhammer, V.; Quintana, F.J. Control of immune-mediated pathology via the aryl hydrocarbon receptor. J. Biol. Chem. 2017, 12383–12389. [Google Scholar] [CrossRef]
- Hooper, L.V.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.-C.; Stockinger, B.; Oukka, M.; Weiner, H.L.; Yarovinsky, F.; et al. You AhR what you eat: Linking diet and immunity. Cell 2011, 147, 489–491. [Google Scholar] [CrossRef]
- Joshi, A.D.; Hossain, E.; Elferink, C.J. Epigenetic Regulation by Agonist-Specific Aryl Hydrocarbon Receptor Recruitment of Metastatic Associated Protein 2 Selectively Induces Stanniocalcin 2 Expression. Mol. Pharmacol. 2017. [Google Scholar] [CrossRef]
- Abdullah, A.; Maged, M.; Ibrahim Hairul-Islam, M.; Alwassil Osama, I.; Maha, H.; Manal, A.; Hamza, H. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS ONE 2019. [Google Scholar] [CrossRef]
- Hu, W.; Sorrentino, C.; Denison, M.S.; Kolaja, K.; Fielden, M.R. Induction of Cyp1a1 Is a Nonspecific Biomarker of Aryl Hydrocarbon Receptor Activation: Results of Large Scale Screening of Pharmaceuticals and Toxicants in Vivo and in Vitro. Mol. Pharmacol. 2007, 71, 1475–1486. [Google Scholar] [CrossRef]
- Negishi, T.; Kato, Y.; Ooneda, O.; Mimura, J.; Takada, T.; Mochizuki, H.; Yamamoto, M.; Fujii-Kuriyama, Y.; Furusako, S. Effects of Aryl Hydrocarbon Receptor Signaling on the Modulation of Th1/Th2 Balance. J. Immunol. 2005, 175, 7348–7356. [Google Scholar] [CrossRef]
- Morales, J.L.; Krzeminski, J.; Amin, S.; Perdew, G.H. Characterization of the antiallergic drugs 3-[2-(2-phenylethyl) benzoimidazole-4-yl]-3-hydroxypropanoic acid and ethyl 3-hydroxy-3-[2-(2-phenylethyl)benzoimidazol-4-yl]propanoate as full aryl hydrocarbon receptor agonists. Chem. Res. Toxicol. 2008, 21, 472–482. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, K.; Qiao, S.; Yang, L.; Xin, Y.; Dai, Y.; Wei, Z. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD+/SIRT1/SUV39H1/H3K9me3 signaling pathway article. Cell Death Dis. 2018. [Google Scholar] [CrossRef]
- Duan, C.; Guo, J.M.; Dai, Y.; Xia, Y.F. The absorption enhancement of norisoboldine in the duodenum of adjuvant-induced arthritis rats involves the impairment of P-glycoprotein. Biopharm. Drug Dispos. 2017. [Google Scholar] [CrossRef]
- Bock, K.W.; Köhle, C. The mammalian aryl hydrocarbon (Ah) receptor: From mediator of dioxin toxicity toward physiological functions in skin and liver. Biol. Chem. 2009, 390, 1225–1235. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Surace, A.E.A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Javierre, B.M.; Fernandez, A.F.; Richter, J.; Al-Shahrour, F.; Ignacio Martin-Subero, J.; Rodriguez-Ubreva, J.; Berdasco, M.; Fraga, M.F.; O’Hanlon, T.P.; Rider, L.G.; et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010, 20, 170–179. [Google Scholar] [CrossRef]
- Park, G.T.; Han, J.; Park, S.G.; Kim, S.; Kim, T.Y. DNA methylation analysis of CD4+ T cells in patients with psoriasis. Arch. Dermatol. Res. 2014, 306, 259–268. [Google Scholar] [CrossRef]
- Konsta, O.D.; Le Dantec, C.; Charras, A.; Cornec, D.; Kapsogeorgou, E.K.; Tzioufas, A.G.; Pers, J.O.; Renaudineau, Y. Defective DNA methylation in salivary gland epithelial acini from patients with Sjögren’s syndrome is associated with SSB gene expression, anti-SSB/LA detection, and lymphocyte infiltration. J. Autoimmun. 2016, 68, 30–38. [Google Scholar] [CrossRef]
- Aluru, N.; Karchner, S.I.; Hahn, M.E. Role of DNA methylation of AHR1 and AHR2 promoters in differential sensitivity to PCBs in Atlantic Killifish, Fundulus heteroclitus. Aquat. Toxicol. 2011, 101, 288–294. [Google Scholar] [CrossRef]
- Winans, B.; Nagari, A.; Chae, M.; Post, C.M.; Ko, C.-I.; Puga, A.; Kraus, W.L.; Lawrence, B.P. Linking the Aryl Hydrocarbon Receptor with Altered DNA Methylation Patterns and Developmentally Induced Aberrant Antiviral CD8 + T Cell Responses. J. Immunol. 2015, 194, 4446–4457. [Google Scholar] [CrossRef]
- Mulero-Navarro, S.; Carvajal-Gonzalez, J.M.; Herranz, M.; Ballestar, E.; Fraga, M.F.; Ropero, S.; Esteller, M.; Fernandez-Salguero, P.M. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis 2006. [Google Scholar] [CrossRef]
- Andrade, A.F.; Borges, K.S.; Castro-Gamero, A.M.; Silveira, V.S.; Suazo, V.K.; Oliveira, J.C.; Moreno, D.A.; De Paula Queiroz, R.G.; Scrideli, C.A.; Tone, L.G. Zebularine induces chemosensitization to methotrexate and efficiently decreases AhR gene methylation in childhood acute lymphoblastic leukemia cells. Anticancer. Drugs 2013. [Google Scholar] [CrossRef]
- Englert, N.A.; Turesky, R.J.; Han, W.; Bessette, E.E.; Spivack, S.D.; Caggana, M.; Spink, D.C.; Spink, B.C. Genetic and epigenetic regulation of AHR gene expression in MCF-7 breast cancer cells: Role of the proximal promoter GC-rich region. Biochem. Pharmacol. 2012. [Google Scholar] [CrossRef]
- Wu, Z.; Mei, X.; Ying, Z.; Sun, Y.; Song, J.; Shi, W. Ultraviolet B inhibition of DNMT1 activity via AhR activation dependent SIRT1 suppression in CD4+ T cells from systemic lupus erythematosus patients. J. Dermatol. Sci. 2017, 86, 230–237. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Winterroth, L.C.; Garcia, M.; Weiman, S.; Wong, J.W.; Sunwoo, J.B.; Nadeau, K.C. Epigenetically mediated pathogenic effects of phenanthrene on regulatory T cells. J. Toxicol. 2013. [Google Scholar] [CrossRef]
- Lv, Q.; Shi, C.; Qiao, S.; Cao, N.; Guan, C.; Dai, Y.; Wei, Z. Alpinetin exerts anti-colitis efficacy by activating AhR, regulating miR-302/DNMT-1/CREB signals, and therefore promoting Treg differentiation. Cell Death Dis. 2018, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Aluru, N.; Kuo, E.; Helfrich, L.W.; Karchner, S.I.; Linney, E.A.; Pais, J.E.; Franks, D.G. Developmental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters DNA methyltransferase (dnmt) expression in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2015. [Google Scholar] [CrossRef]
- Amenya, H.Z.; Tohyama, C.; Ohsako, S. Dioxin induces Ahr-dependent robust DNA demethylation of the Cyp1a1 promoter via Tdg in the mouse liver. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Tóth, D.M.; Ocskó, T.; Balog, A.; Markovics, A.; Mikecz, K.; Kovács, L.; Jolly, M.; Bukiej, A.A.; Ruthberg, A.D.; Vida, A.; et al. Amelioration of Autoimmune Arthritis in Mice Treated With the DNA Methyltransferase Inhibitor 5′-Azacytidine. Arthritis Rheumatol. 2019. [Google Scholar] [CrossRef]
- Anquetil, F.; Clavel, C.; Offer, G.; Serre, G.; Sebbag, M. IgM and IgA Rheumatoid Factors Purified from Rheumatoid Arthritis Sera Boost the Fc Receptor– and Complement-Dependent Effector Functions of the Disease-Specific Anti–Citrullinated Protein Autoantibodies. J. Immunol. 2015. [Google Scholar] [CrossRef]
- Quddus, J.; Johnson, K.J.; Gavalchin, J.; Amento, E.P.; Chrisp, C.E.; Yung, R.L.; Richardson, B.C. Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J. Clin. Investig. 1993. [Google Scholar] [CrossRef]
- Yung, R.L.; Quddus, J.; Chrisp, C.E.; Johnson, K.J.; Richardson, B.C. Mechanism of drug-induced lupus. I. Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J. Immunol. 1995, 154, 3025–3035. [Google Scholar]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Skene, P.J.; Henikoff, S. Histone variants in pluripotency and disease. Dev. 2013, 140, 2513–2524. [Google Scholar] [CrossRef]
- Perosa, F.; Prete, M.; Di Lernia, G.; Ostuni, C.; Favoino, E.; Valentini, G. Anti-centromere protein A antibodies in systemic sclerosis: Significance and origin. Autoimmun. Rev. 2016, 15, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wen, B.; Liang, Y.; Yu, W.; Li, H. Histone Modifications and their Role in Colorectal Cancer (Review). Pathol. Oncol. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chory, E.J.; Calarco, J.P.; Hathaway, N.A.; Bell, O.; Neel, D.S.; Crabtree, G.R. Nucleosome Turnover Regulates Histone Methylation Patterns over the Genome. Mol. Cell 2019. [Google Scholar] [CrossRef] [PubMed]
- Zylicz, J.J.; Dietmann, S.; Günesdogan, U.; Hackett, J.A.; Cougot, D.; Lee, C.; Surani, M.A. Chromatin dynamics and the role of G9a in gene regulation and enhancer silencing during early mouse development. Elife 2015. [Google Scholar] [CrossRef]
- Sun, X.J.; Man, N.; Tan, Y.; Nimer, S.D.; Wang, L. The role of histone acetyltransferases in normal and malignant hematopoiesis. Front. Oncol. 2015, 5, 108. [Google Scholar] [CrossRef]
- Joshi, A.D.; Mustafa, M.G.; Lichti, C.F.; Elferink, C.J. Homocitrullination is a novel histone H1 epigenetic mark dependent on aryl hydrocarbon receptor recruitment of carbamoyl phosphate synthase. J. Biol. Chem. 2015. [Google Scholar] [CrossRef]
- Bright, R.; Proudman, S.M.; Rosenstein, E.D.; Bartold, P.M. Is there a link between carbamylation and citrullination in periodontal disease and rheumatoid arthritis? Med. Hypotheses 2015, 84, 570–576. [Google Scholar] [CrossRef]
- Bergum, B.; Koro, C.; Delaleu, N.; Solheim, M.; Hellvard, A.; Binder, V.; Jonsson, R.; Valim, V.; Hammenfors, D.S.; Jonsson, M.V.; et al. Antibodies against carbamylated proteins are present in primary Sjögren’s syndrome and are associated with disease severity. Ann. Rheum. Dis. 2016. [Google Scholar] [CrossRef]
- Mydel, P.; Wang, Z.; Brisslert, M.; Hellvard, A.; Dahlberg, L.E.; Hazen, S.L.; Bokarewa, M. Carbamylation-Dependent Activation of T Cells: A Novel Mechanism in the Pathogenesis of Autoimmune Arthritis. J. Immunol. 2010, 184, 6882–6890. [Google Scholar] [CrossRef]
- Song, S.; Yu, Y. Progression on citrullination of proteins in gastrointestinal cancers. Front. Oncol. 2019, 9, 15. [Google Scholar] [CrossRef]
- Corsiero, E.; Marrelli, A. An update on research advances in rheumatoid arthritis: From clinic to basic science. J. Lab. Precis. Med. 2018, 3, 54. [Google Scholar] [CrossRef]
- Garcia, B.A.; Busby, S.A.; Shabanowitz, J.; Hunt, D.F.; Mishra, N. Resetting the epigenetic histone code in the MRL-lpr/lpr mouse model of lupus by histone deacetylase inhibition. J. Proteome Res. 2005, 4, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Devendra, D.; Eisenbarth, G.S. Interferon alpha - A potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunity. Clin. Immunol. 2004, 111, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.B. Interferon alpha as a primary pathogenic factor in human lupus. J. Interf. Cytokine Res. 2011, 31, 887–892. [Google Scholar] [CrossRef]
- Stefan, M.; Wei, C.; Lombardi, A.; Li, C.W.; Concepcion, E.S.; Inabnet, W.B.; Owen, R.; Zhang, W.; Tomer, Y. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proc. Natl. Acad. Sci. USA 2014, 111, 12562–12567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, Y.; Shi, Y.; Luo, Y.; Li, Y.P.; Zhao, M.; Lu, Q.; Xiao, R. Overexpression of JMJD3 may contribute to demethylation of H3K27me3 in CD4+ T cells from patients with systemic sclerosis. Clin. Immunol. 2015, 161, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Tenbrock, K.; Juang, Y.-T.; Leukert, N.; Roth, J.; Tsokos, G.C. The Transcriptional Repressor cAMP Response Element Modulator α Interacts with Histone Deacetylase 1 to Repress Promoter Activity. J. Immunol. 2006. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, R.; Shao, M.; Zhao, X.; Miao, M.; Chen, J.; Liu, J.; Zhang, X.; Zhang, X.; Jin, Y.; et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: A randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu. Rev. Immunol. 2014. [Google Scholar] [CrossRef]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Beedanagari, S.R.; Taylor, R.T.; Bui, P.; Wang, F.; Nickerson, D.W.; Hankinson, O. Role of epigenetic mechanisms in differential regulation of the dioxin-inducible human CYP1A1 and CYP1B1 genes. Mol. Pharmacol. 2010, 78, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.T.; Wang, F.; Hsu, E.L.; Hankinson, O. Roles of coactivator proteins in dioxin induction of CYP1A1 and CYP1B1 in human breast cancer cells. Toxicol. Sci. 2009, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.M.; Thomas, M.; Gogal, R.; Olgun, S.; Santo, A.; Sodhi, R.; Samy, E.T.; Peng, S.L.; Gilkeson, G.S.; Mishra, N. The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. J. Autoimmun. 2008, 31, 123–130. [Google Scholar] [CrossRef]
- Gillespie, J.; Savic, S.; Wong, C.; Hempshall, A.; Inman, M.; Emery, P.; Grigg, R.; McDermott, M.F. Histone deacetylases are dysregulated in rheumatoid arthritis and a novel histone deacetylase 3-selective inhibitor reduces interleukin-6 production by peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Rheum. 2012. [Google Scholar] [CrossRef]
- Ahmed, S.; Riegsecker, S.; Beamer, M.; Rahman, A.; Bellini, J.V.; Bhansali, P.; Tillekeratne, L.M.V. Largazole, a class I histone deacetylase inhibitor, enhances TNF-α-induced ICAM-1 and VCAM-1 expression in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2013. [Google Scholar] [CrossRef]
- Vojinovic, J.; Damjanov, N.; D’Urzo, C.; Furlan, A.; Susic, G.; Pasic, S.; Iagaru, N.; Stefan, M.; Dinarello, C.A. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2011. [Google Scholar] [CrossRef]
- Huber, L.C.; Distler, J.H.W.; Moritz, F.; Hemmatazad, H.; Hauser, T.; Michel, B.A.; Gay, R.E.; Matucci-Cerinic, M.; Gay, S.; Distler, O.; et al. Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum. 2007. [Google Scholar] [CrossRef]
- Hemmatazad, H.; Rodrigues, H.M.; Maurer, B.; Brentano, F.; Pileckyte, M.; Distler, J.H.W.; Gay, R.E.; Michel, B.A.; Gay, S.; Huber, L.C.; et al. Histone deacetylase 7, a potential target for the antifibrotic treatment of systemic sclerosis. Arthritis Rheum. 2009. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Huang, W.; Cai, C.; Xia, W.; Peng, Y.; Zheng, S.; Li, G.; Xu, Y.; Wang, J.; et al. Stanniocalcin-2 contributes to mesenchymal stromal cells attenuating murine contact hypersensitivity mainly via reducing CD8+ Tc1 cells article. Cell Death Dis. 2018. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Sun, Y.; Yi, X.; Wei, X.; Liu, W.; Zhang, Q.; Yi, H.; Chen, G. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann. Transl. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Coope, A.; Pascoal, L.B.; Botezelli, J.D.; Da Silva, F.A.R.; De Lourdes Setsuko Ayrizono, M.; Rodrigues, B.L.; Milanski, M.; Carvalho, R.B.; Fagundes, J.J.; Velloso, L.A.; et al. ER stress activation in the intestinal mucosa but not in mesenteric adipose tissue is associated with inflammation in Crohn’s disease patients. PLoS ONE 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiao, Y.; Song, Y.; Liu, J.; Li, X.; Zhang, H.; Yang, J.; Lu, Y. Stanniocalcin 2 ameliorates hepatosteatosis through activation of STAT3 signaling. Front. Physiol. 2018. [Google Scholar] [CrossRef]
- Kanellopoulou, C.; Muljo, S.A.; Kung, A.L.; Ganesan, S.; Drapkin, R.; Jenuwein, T.; Livingston, D.M.; Rajewsky, K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.W.; Wang, G. The Mediator complex: A master coordinator of transcription and cell lineage development. Development 2014. [Google Scholar] [CrossRef]
- Wang, S.; Ge, K.; Roeder, R.G.; Hankinson, O. Role of Mediator in Transcriptional Activation by the Aryl Hydrocarbon Receptor. J. Biol. Chem. 2004. [Google Scholar] [CrossRef]
- Chowdhary, V.; Teng, K.Y.; Thakral, S.; Zhang, B.; Lin, C.H.; Wani, N.; Bruschweiler-Li, L.; Zhang, X.; James, L.; Yang, D.; et al. miRNA-122 Protects Mice and Human Hepatocytes from Acetaminophen Toxicity by Regulating Cytochrome P450 Family 1 Subfamily A Member 2 and Family 2 Subfamily E Member 1 Expression. Am. J. Pathol. 2017. [Google Scholar] [CrossRef]
- Wang, S.; Hankinson, O. Functional involvement of the Brahma/SWI2-related gene 1 protein in cytochrome P4501A1 transcription mediated by the aryl hydrocarbon receptor complex. J. Biol. Chem. 2002, 277, 11821–11827. [Google Scholar] [CrossRef]
- Georgopoulos, K.; Moore, D.D.; Derfler, B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science (80-) 1992. [Google Scholar] [CrossRef]
- Georgopoulos, K.; Bigby, M.; Wang, J.H.; Molnar, A.; Wu, P.; Winandy, S.; Sharpe, A. The ikaros gene is required for the development of all lymphoid lineages. Cell 1994. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Boisson, B.; Cunningham-Rundles, C.; Reichenbach, J.; Stray-Pedersen, A.; Gelfand, E.W.; Maffucci, P.; Pierce, K.R.; Abbott, J.K.; Voelkerding, K.V.; et al. Loss of B cells in patients with heterozygous mutations in IKAROS. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.Y.; Hatfield, J.K.; Brown, M.A. Ikaros sets the potential for Th17 lineage gene expression through effects on chromatin state in early T Cell Development. J. Biol. Chem. 2013. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bostick, J.W.; Ye, J.; Qiu, J.; Zhang, B.; Urban, J.F.; Avram, D.; Zhou, L. Aryl Hydrocarbon Receptor Signaling Cell Intrinsically Inhibits Intestinal Group 2 Innate Lymphoid Cell Function. Immunity 2018, 49, 915–928.e5. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.L.; Jeong, K.W. Regulation of aryl hydrocarbon receptor-mediated transcription in human retinal pigmented epithelial cells. Biochem. Biophys. Res. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Schroeder, J.C.; Perdew, G.H. Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Mol. Carcinog. 2011, 50, 173–183. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Therapeutic targeting of the interleukin-6 receptor. Annu. Rev. Pharmacol. Toxicol. 2012. [Google Scholar] [CrossRef]
- Möröy, T.; Khandanpour, C. Role of GFI1 in epigenetic regulation of MDS and AML pathogenesis: Mechanisms and therapeutic implications. Front. Oncol. 2019, 9, 824. [Google Scholar] [CrossRef]
- Ye, J.; Qiu, J.; Bostick, J.W.; Ueda, A.; Schjerven, H.; Li, S.; Jobin, C.; Chen, Z.; Ming, E.; Zhou, L. The Aryl Hydrocarbon Receptor Preferentially Marks and Promotes Gut Regulatory T Cells. Cell Rep. 2017, 21. [Google Scholar] [CrossRef]
- Zhu, J.; Davidson, T.S.; Wei, G.; Jankovic, D.; Cui, K.; Schones, D.E.; Guo, L.; Zhao, K.; Shevach, E.M.; Paul, W.E. Down-regulation of gfi-1 expression by TGF-β is important for differentiation of Th17 and CD103 + inducible regulatory T cells. J. Exp. Med. 2009. [Google Scholar] [CrossRef]
- Rathinam, C.; Lassmann, H.; Mengel, M.; Klein, C. Transcription Factor Gfi1 Restricts B Cell-Mediated Autoimmunity. J. Immunol. 2008, 181, 6222–6229. [Google Scholar] [CrossRef]
- Desnues, B.; Macedo, A.B.; Ordoñez-Rueda, D.; Roussel-Queval, A.; Malissen, B.; Bruhns, P.; Malissen, M.; Alexopoulou, L. The transcriptional repressor Gfi1 prevents lupus autoimmunity by restraining TLR7 signaling. Eur. J. Immunol. 2016, 46, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.S.; Sharp, P.A. Roles for MicroRNAs in conferring robustness to biological processes. Cell 2012, 149, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Ansel, K.M. RNA regulation of the immune system. Immunol. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Gallo, A.; Jang, S.I.; Illei, G.G.; Alevizos, I. Deep sequencing of short RNAs reveals novel microRNAs in minor salivary glands of patients with Sjögren’s syndrome. Oral Dis. 2012. [Google Scholar] [CrossRef]
- Gourzi, V.C.; Kapsogeorgou, E.K.; Kyriakidis, N.C.; Tzioufas, A.G. Study of microRNAs (miRNAs) that are predicted to target the autoantigens Ro/SSA and La/SSB in primary Sjögren’s Syndrome. Clin. Exp. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Li, W.; Fu, B. MicroRNAs in autoimmune diseases. Biomed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, D.; Baccarelli, A. Environmental chemicals and microRNAs. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2011, 714, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, X.; Wang, D.; Baccarelli, A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 2012. [Google Scholar] [CrossRef]
- Al-Ghezi, Z.Z.; Singh, N.; Mehrpouya-Bahrami, P.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Ahr activation by tcdd (2,3,7,8-tetrachlorodibenzo-p-dioxin) attenuates pertussis toxin-induced inflammatory responses by differential regulation of tregs and th17 cells through specific targeting by microrna. Front. Microbiol. 2019. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, T.; Chen, W.; Chen, Y.; Li, M.; Ren, L.; Chen, J.; Cao, R.; Feng, Y.; Zhang, H.; et al. MicroRNA-124 promotes intestinal inflammation by targeting aryl hydrocarbon receptor in Crohn’s disease. J. Crohn’s Colitis 2016. [Google Scholar] [CrossRef] [PubMed]
- Vautrin, A.; Manchon, L.; Garcel, A.; Campos, N.; Lapasset, L.; Laaref, A.M.; Bruno, R.; Gislard, M.; Dubois, E.; Scherrer, D.; et al. Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Singh, U.P.; Rouse, M.; Zhang, J.; Chatterjee, S.; Nagarkatti, P.S.; Nagarkatti, M. Dietary Indoles Suppress Delayed-Type Hypersensitivity by Inducing a Switch from Proinflammatory Th17 Cells to Anti-Inflammatory Regulatory T Cells through Regulation of MicroRNA. J. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, J.; Liu, X.; Huang, E.; Yang, J.; Qian, J.; Zhang, D.; Liu, R.; Chu, Y. MicroRNA 15a/16-1 suppresses aryl hydrocarbon receptor–dependent interleukin-22 secretion in CD4+ T cells and contributes to immune-mediated organ injury. Hepatology 2018, 67, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nakahama, T.; Nguyen, C.H.; Tran, T.T.; Le, V.S.; Chu, H.H.; Kishimoto, T. Aryl hydrocarbon receptor antagonism and its role in rheumatoid arthritis. J. Exp. Pharmacol. 2015, 7, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ogando, J.; Tardáguila, M.; Díaz-Alderete, A.; Usategui, A.; Miranda-Ramos, V.; Martínez-Herrera, D.J.; De La Fuente, L.; García-León, M.J.; Moreno, M.C.; Escudero, S.; et al. Notch-regulated MIR-223 targets the aryl hydrocarbon receptor pathway and increases cytokine production in macrophages from rheumatoid arthritis patients. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, S.Y.; Wang, C.R.; Liu, M.F.; Lin, C.C.; Jou, I.M.; Shiau, A.L.; Wu, C.L. Amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum. 2012. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, J.; Li, W.; Bao, C.; Fu, Q. Targeting miR-155 to Treat Experimental Scleroderma. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Xin, Q.; Li, J.; Dang, J.; Bian, X.; Shan, S.; Yuan, J.; Qian, Y.; Liu, Z.; Liu, G.; Yuan, Q.; et al. miR-155 Deficiency Ameliorates Autoimmune Inflammation of Systemic Lupus Erythematosus by Targeting S1pr1 in Fas lpr/lpr Mice. J. Immunol. 2015, 194, 5437–5445. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Volinia, S.; Costinean, S.; Galasso, M.; Neinast, R.; Santhanam, R.; Parthun, M.R.; Perrotti, D.; Marcucci, G.; Garzon, R.; et al. MiR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Eμ-miR-155 transgenic mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, 20047–20052. [Google Scholar] [CrossRef]
- Eissa, M.G.; Artlett, C.M. The microRNA miR-155 is essential in fibrosis. Non-coding RNA 2019, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Nakahama, T.; Hanieh, H.; Nguyen, N.T.; Chinen, I.; Ripley, B.; Millrine, D.; Lee, S.; Nyati, K.K.; Dubey, P.K.; Chowdhury, K.; et al. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef] [PubMed]
- Chinen, I.; Nakahama, T.; Kimura, A.; Nguyen, N.T.; Takemori, H.; Kumagai, A.; Kayama, H.; Takeda, K.; Lee, S.; Hanieh, H.; et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.M.; Hanieh, H.; Ibrahim, H.M.; Mohafez, O.; Shehata, T.; Bani Ismail, M.; Alfwuaires, M. Enhancing miR-132 expression by aryl hydrocarbon receptor attenuates tumorigenesis associated with chronic colitis. Int. Immunopharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H.; Alzahrani, A. MicroRNA-132 suppresses autoimmune encephalomyelitis by inducing cholinergic anti-inflammation: A new Ahr-based exploration. Eur. J. Immunol. 2013. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, J.; Pei, G. Activation of aryl hydrocarbon receptor (AhR) by tranilast, an anti-allergy drug, promotes miR-302 expression and cell reprogramming. J. Biol. Chem. 2013. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Fang, Q.; Li, Y. Transcription alterations of microRNAs, cytochrome P4501A1 and 3A65, and AhR and PXR in the liver of zebrafish exposed to crude microcystins. Toxicon 2013, 73, 17–22. [Google Scholar] [CrossRef]
- Ushakov, D.S.; Dorozhkova, A.S.; Babayants, E.V.; Ovchinnikov, V.Y.; Kushlinskii, D.N.; Adamyan, L.V.; Gulyaeva, L.F.; Kushlinskii, N.E. Expression of microRNA Potentially Regulated by AhR and CAR in Malignant Tumors of the Endometrium. Bull. Exp. Biol. Med. 2018, 165, 688–691. [Google Scholar] [CrossRef]
- Honardoost, M.A.; Naghavian, R.; Ahmadinejad, F.; Hosseini, A.; Ghaedi, K. Integrative computational mRNA-miRNA interaction analyses of the autoimmune-deregulated miRNAs and well-known Th17 differentiation regulators: An attempt to discover new potential miRNAs involved in Th17 differentiation. Gene 2015, 572, 153–162. [Google Scholar] [CrossRef]
- Wang-Renault, S.F.; Boudaoud, S.; Nocturne, G.; Roche, E.; Sigrist, N.; Daviaud, C.; Tinggaard, A.B.; Renault, V.; Deleuze, J.F.; Mariette, X.; et al. Deregulation of microRNA expression in purified T and B lymphocytes from patients with primary Sjögren’s syndrome. Ann. Rheum. Dis. 2018. [Google Scholar] [CrossRef]
- Ghadiri, N.; Emamnia, N.; Ganjalikhani-Hakemi, M.; Ghaedi, K.; Etemadifar, M.; Salehi, M.; Shirzad, H.; Nasr-Esfahani, M.H. Analysis of the expression of mir-34a, mir-199a, mir-30c and mir-19a in peripheral blood CD4+T lymphocytes of relapsing-remitting multiple sclerosis patients. Gene 2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yeon, Y.; Lee, W.J.; Shin, Y.U.; Cho, H.; Sung, Y.K.; Kim, D.R.; Lim, H.W.; Kang, M.H. Comparison of microRNA expression in tears of normal subjects and Sjögren syndrome patients. Investig. Ophthalmol. Vis. Sci. 2019. [Google Scholar] [CrossRef]
- Chen, W.-X.; Ren, L.-H.; Shi, R.-H. Implication of miRNAs for inflammatory bowel disease treatment: Systematic review. World J. Gastrointest. Pathophysiol. 2014. [Google Scholar] [CrossRef]
- Fiorillo, A.A.; Heier, C.R.; Huang, Y.F.; Tully, C.B.; Punga, T.; Punga, A.R. Estrogen Receptor, Inflammatory, and FOXO Transcription Factors Regulate Expression of Myasthenia Gravis-Associated Circulating microRNAs. Front. Immunol. 2020. [Google Scholar] [CrossRef]
- Sabre, L.; Punga, T.; Punga, A.R. Circulating miRNAs as Potential Biomarkers in Myasthenia Gravis: Tools for Personalized Medicine. Front. Immunol. 2020. [Google Scholar] [CrossRef]
- Lai, N.S.; Yu, H.C.; Tung, C.H.; Huang, K.Y.; Huang, H.B.; Lu, M.C. The role of aberrant expression of T cell miRNAs affected by TNF-α in the immunopathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2017. [Google Scholar] [CrossRef]
- Navarro-Quiroz, E.; Pacheco-Lugo, L.; Navarro-Quiroz, R.; Lorenzi, H.; España-Puccini, P.; Díaz-Olmos, Y.; Almendrales, L.; Olave, V.; Gonzalez-Torres, H.; Diaz-Perez, A.; et al. Profiling analysis of circulating microRNA in peripheral blood of patients with class IV lupus nephritis. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Asquith, M.; Pasala, S.; Engelmann, F.; Haberthur, K.; Meyer, C.; Park, B.; Grant, K.A.; Messaoudi, I. Chronic Ethanol Consumption Modulates Growth Factor Release, Mucosal Cytokine Production, and MicroRNA Expression in Nonhuman Primates. Alcohol. Clin. Exp. Res. 2014, 38, 980–993. [Google Scholar] [CrossRef]
- Li, H.S.; Ning, Y.; Li, S.B.; Shao, P.Y.; Chen, S.J.; Ye, Q.; Heng, X. Expression and clinical significance of miR-181a and miR-203 in systemic lupus erythematosus patients. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4790–4796. [Google Scholar]
- Peng, L.; Ma, W.; Yi, F.; Yang, Y.J.; Lin, W.; Chen, H.; Zhang, X.; Zhang, L.H.; Zhang, F.; Du, Q. MicroRNA profiling in Chinese patients with primary sjögren syndrome reveals elevated miRNA-181a in peripheral blood mononuclear cells. J. Rheumatol. 2014, 41, 2208–2213. [Google Scholar] [CrossRef]
- Kanaan, Z.; Rai, S.N.; Eichenberger, M.R.; Barnes, C.; Dworkin, A.M.; Weller, C.; Cohen, E.; Roberts, H.; Keskey, B.; Petras, R.E.; et al. Differential MicroRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Hum. Mutat. 2012, 33, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Fukami, T.; Gotoh, S.; Takamiya, M.; Aoki, Y.; Nakajima, M. RNA editing modulates human hepatic aryl hydrocarbon receptor expression by creating MicroRNA recognition sequence. J. Biol. Chem. 2016, 291, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, C.L.; Fannin, E.E.; Toth, C.L.; Pearson, D.S.; Vickers, K.C.; Sethupathy, P. Inhibition of miR-29 has a significant lipid-lowering benefit through suppression of lipogenic programs in liver. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Shi, X.; Ye, L.; Xu, S.; Guo, G.; Zuo, Z.; Ye, M.; Zhu, L.; Li, B.; Xue, X.; Lin, Q.; et al. Downregulated miR-29a promotes B cell overactivation by upregulating Crk-like protein in systemic lupus erythematosus. Mol. Med. Rep. 2020, 22, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fei, D.; Xing, J.; Du, J. MicroRNA-29a inhibits proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes by repressing STAT3. Biomed. Pharmacother. 2017, 96, 173–181. [Google Scholar] [CrossRef]
- Lv, B.; Liu, Z.; Wang, S.; Liu, F.; Yang, X.; Hou, J.; Hou, Z.; Chen, B. miR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int. J. Clin. Exp. Pathol. 2014, 7, 8542–8552. [Google Scholar]
- Hillen, M.R.; Chouri, E.; Wang, M.; Blokland, S.L.M.; Hartgring, S.A.Y.; Concepcion, A.N.; Kruize, A.A.; Burgering, B.M.T.; Rossato, M.; Van Roon, J.A.G.; et al. Dysregulated miRNome of plasmacytoid dendritic cells from patients with Sjögren’s syndrome is associated with processes at the centre of their function. Rheumatology (UK) 2019, 58, 2305–2314. [Google Scholar] [CrossRef]
- Scoville, S.D.; Nalin, A.P.; Chen, L.; Chen, L.; Zhang, M.H.; McConnell, K.; Casas, S.B.; Ernst, G.; Al-Rahman Traboulsi, A.; Hashi, N.; et al. Human AML activates the aryl hydrocarbon receptor pathway to impair NK cell development and function. Blood 2018. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Wu, Z.; Chen, Y.; Shi, W. CircIBTK inhibits DNA demethylation and activation of AKT signaling pathway via miR-29b in peripheral blood mononuclear cells in systemic lupus erythematosus. Arthritis Res. Ther. 2018, 20, 118. [Google Scholar] [CrossRef]
- Smith, K.M.; Guerau-de-Arellano, M.; Costinean, S.; Williams, J.L.; Bottoni, A.; Mavrikis Cox, G.; Satoskar, A.R.; Croce, C.M.; Racke, M.K.; Lovett-Racke, A.E.; et al. miR-29ab1 Deficiency Identifies a Negative Feedback Loop Controlling Th1 Bias That Is Dysregulated in Multiple Sclerosis. J. Immunol. 2012, 189, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Camacho, K.; Diaz-Jimenez, D.; De la Fuente, M.; Quera, R.; Simian, D.; Martínez, M.; Landskron, G.; Olivares-Morales, M.; Cidlowski, J.A.; Xu, X.; et al. Inhibition of miR-378a-3p by Inflammation Enhances IL-33 Levels: A Novel Mechanism of Alarmin Modulation in Ulcerative Colitis. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, X.; Jin, C.; Li, L.; Cui, Q.; Guo, Y.; Dong, Y.; Yang, X.; Guo, L.; Zhang, M. Identification and verification of differentially expressed microRNAs and their target genes for the diagnosis of esophageal cancer. Oncol. Lett. 2018, 16, 3642–3650. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, J.; Ospelt, C.; Karouzakis, E.; Filer, A.; Raza, K.; Kolling, C.; Gay, R.; Buckley, C.D.; Tak, P.P.; Gay, S.; et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011, 63, 373–381. [Google Scholar] [CrossRef]
- Sonkoly, E.; Wei, T.; Janson, P.C.J.; Sääf, A.; Lundeberg, L.; Tengvall-Linder, M.; Norstedt, G.; Alenius, H.; Homey, B.; Scheynius, A.; et al. MicroRNAs: Novel Regulators Involved in the Pathogenesis of Psoriasis? PLoS ONE 2007, 2. [Google Scholar] [CrossRef]
- Coskun, M.; Bjerrum, J.T.; Seidelin, J.B.; Troelsen, J.T.; Olsen, J.; Nielsen, O.H. miR-20b, miR-98, miR-125b-1*, and let-7e* as new potential diagnostic biomarkers in ulcerative colitis. World J. Gastroenterol. 2013, 19, 4289–4299. [Google Scholar] [CrossRef]
- Hecht, E.; Zago, M.; Sarill, M.; Rico de Souza, A.; Gomez, A.; Matthews, J.; Hamid, Q.; Eidelman, D.H.; Baglole, C.J. Aryl hydrocarbon receptor-dependent regulation of miR-196a expression controls lung fibroblast apoptosis but not proliferation. Toxicol. Appl. Pharmacol. 2014, 280, 511–525. [Google Scholar] [CrossRef]
- Honda, N.; Jinnin, M.; Kajihara, I.; Makino, T.; Makino, K.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; Fujimoto, M.; et al. TGF-β–Mediated Downregulation of MicroRNA-196a Contributes to the Constitutive Upregulated Type I Collagen Expression in Scleroderma Dermal Fibroblasts. J. Immunol. 2012, 188, 3323–3331. [Google Scholar] [CrossRef]
- Wang, Z.; Jinnin, M.; Kudo, H.; Inoue, K.; Nakayama, W.; Honda, N.; Makino, K.; Kajihara, I.; Fukushima, S.; Inoue, Y.; et al. Detection of hair-microRNAs as the novel potent biomarker: Evaluation of the usefulness for the diagnosis of scleroderma. J. Dermatol. Sci. 2013, 72, 134–141. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chang, W.A.; Hsu, Y.L.; Chen, C.H.; Kuo, P.L. Deduction of Novel Genes Potentially Involved in Osteoblasts of Rheumatoid Arthritis Using Next-Generation Sequencing and Bioinformatic Approaches. Int. J. Mol. Sci. 2017, 18, 2396. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Papp, G.; Póliska, S.; Szabó, K.; Tarr, T.; Bálint, B.L.; Szodoray, P.; Zeher, M. MicroRNA expression profiles identify disease-specific alterations in systemic lupus erythematosus and primary Sjögren’s syndrome. PLoS ONE 2017, 12, e0174585. [Google Scholar] [CrossRef] [PubMed]
- Haftmann, C.; Stittrich, A.B.; Zimmermann, J.; Fang, Z.; Hradilkova, K.; Bardua, M.; Westendorf, K.; Heinz, G.A.; Riedel, R.; Siede, J.; et al. MiR-148a is upregulated by Twist1 and T-bet and promotes Th1-cell survival by regulating the proapoptotic gene Bim. Eur. J. Immunol. 2015, 45, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liang, D.; Tang, Y.; Qu, B.; Cui, H.; Luo, X.; Huang, X.; Chen, S.; Higgs, B.W.; Jallal, B.; et al. Identification of microRNA-31 as a novel regulator contributing to impaired interleukin-2 production in T cells from patients with systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Joyce, C.E.; Zhou, X.; Xia, J.; Ryan, C.; Thrash, B.; Menter, A.; Zhang, W.; Bowcock, A.M. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum. Mol. Genet. 2011, 20, 4025–4040. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Nyberg, W.A.; Sjöstrand, M.; Moruzzi, N.; Bergman, P.; Khademi, M.; Andersson, M.; Piehl, F.; Berggren, P.; Covacu, R.; et al. miR-31 regulates energy metabolism and is suppressed in T cells from patients with Sjögren’s syndrome. Eur. J. Immunol. 2019, 49, 313–322. [Google Scholar] [CrossRef]
- Jin, U.H.; Kim, S.B.; Safe, S. Omeprazole Inhibits Pancreatic Cancer Cell Invasion through a Nongenomic Aryl Hydrocarbon Receptor Pathway. Chem. Res. Toxicol. 2015. [Google Scholar] [CrossRef]
- Ishida, M.; Mikami, S.; Shinojima, T.; Kosaka, T.; Mizuno, R.; Kikuchi, E.; Miyajima, A.; Okada, Y.; Oya, M. Activation of aryl hydrocarbon receptor promotes invasion of clear cell renal cell carcinoma and is associated with poor prognosis and cigarette smoke. Int. J. Cancer 2015. [Google Scholar] [CrossRef]
- Ishida, M.; Mikami, S.; Kikuchi, E.; Kosaka, T.; Miyajima, A.; Nakagawa, K.; Mukai, M.; Okada, Y.; Oya, M. Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis 2010. [Google Scholar] [CrossRef]
- Safe, S.; Cheng, Y.; Jin, U.H. The aryl hydrocarbon receptor (AhR) as a drug target for cancer chemotherapy. Curr. Opin. Toxicol. 2017, 2, 24–29. [Google Scholar] [CrossRef]
- Clinical Trial on Advanced Solid Tumors: BAY2416964, BAY2416964 - Clinical Trials Registry - ICH GCP. Available online: https://ichgcp.net/clinical-trials-registry/NCT04069026 (accessed on 25 August 2020).
- Clinical Trial on Urothelial Carcinoma: IK-175 - Clinical Trials Registry-ICH GCP. Available online: https://ichgcp.net/clinical-trials-registry/NCT04200963 (accessed on 25 August 2020).
- Zhang, J.; Zong, H.; Li, S.; Zhang, D.; Zhang, L.; Xia, Q. Activation of Aryl Hydrocarbon Receptor Suppresses Invasion of Esophageal Squamous Cell Carcinoma Cell Lines. Tumori J. 2012. [Google Scholar] [CrossRef]
- Gluschnaider, U.; Hidas, G.; Cojocaru, G.; Yutkin, V.; Ben-Neriah, Y.; Pikarsky, E. β-TrCP inhibition reduces prostate cancer cell growth via upregulation of the aryl hydrocarbon receptor. PLoS ONE 2010. [Google Scholar] [CrossRef] [PubMed]
- Koliopanos, A.; Kleeff, J.; Xiao, Y.; Safe, S.; Zimmermann, A.; Büchler, M.W.; Friess, H. Increased arylhydrocarbon receptor expression offers a potential therapeutic target for pancreatic cancer. Oncogene 2002. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bogaard, E.H.; Bergboer, J.G.M.; Vonk-Bergers, M.; Van Vlijmen-Willems, I.M.J.J.; Hato, S.V.; Van Der Valk, P.G.M.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.J.M.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Invest. 2013, 123, 917–927. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Invest. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef]
- Peppers, J.; Paller, A.S.; Maeda-Chubachi, T.; Wu, S.; Robbins, K.; Gallagher, K.; Kraus, J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef]
- Robbins, K.; Bissonnette, R.; Maeda-Chubachi, T.; Ye, L.; Peppers, J.; Gallagher, K.; Kraus, J.E. Phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef]
- Murai, M.; Yamamura, K.; Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M.; Mitoma, C. Tryptophan photo-product FICZ upregulates AHR/MEK/ERK-mediated MMP1 expression: Implications in anti-fibrotic phototherapy. J. Dermatol. Sci. 2018, 91, 97–103. [Google Scholar] [CrossRef]
- Tauchi, M.; Hida, A.; Negishi, T.; Katsuoka, F.; Noda, S.; Mimura, J.; Hosoya, T.; Yanaka, A.; Aburatani, H.; Fujii-Kuriyama, Y.; et al. Constitutive Expression of Aryl Hydrocarbon Receptor in Keratinocytes Causes Inflammatory Skin Lesions. Mol. Cell. Biol. 2005, 25, 9360–9368. [Google Scholar] [CrossRef]
- Merches, K.; Schiavi, A.; Weighardt, H.; Steinwachs, S.; Teichweyde, N.; Förster, I.; Hochrath, K.; Schumak, B.; Ventura, N.; Petzsch, P.; et al. AHR signaling dampens inflammatory signature in neonatal skin γδ T cells. Int. J. Mol. Sci. 2020, 21, 2249. [Google Scholar] [CrossRef]
- O’Donnell, E.F.; Saili, K.S.; Koch, D.C.; Kopparapu, P.R.; Farrer, D.; Bisson, W.H.; Mathew, L.K.; Sengupta, S.; Kerkvliet, N.I.; Tanguay, R.L.; et al. The Anti-Inflammatory Drug Leflunomide Is an Agonist of the Aryl Hydrocarbon Receptor. PLoS ONE 2010, 5, e13128. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).