The Metabolic Reprogramming Induced by Sub-Optimal Nutritional and Light Inputs in Soilless Cultivated Green and Red Butterhead Lettuce
Abstract
1. Introduction
2. Results
2.1. Leaf Gas Exchange Analysis
2.2. Metabolomics
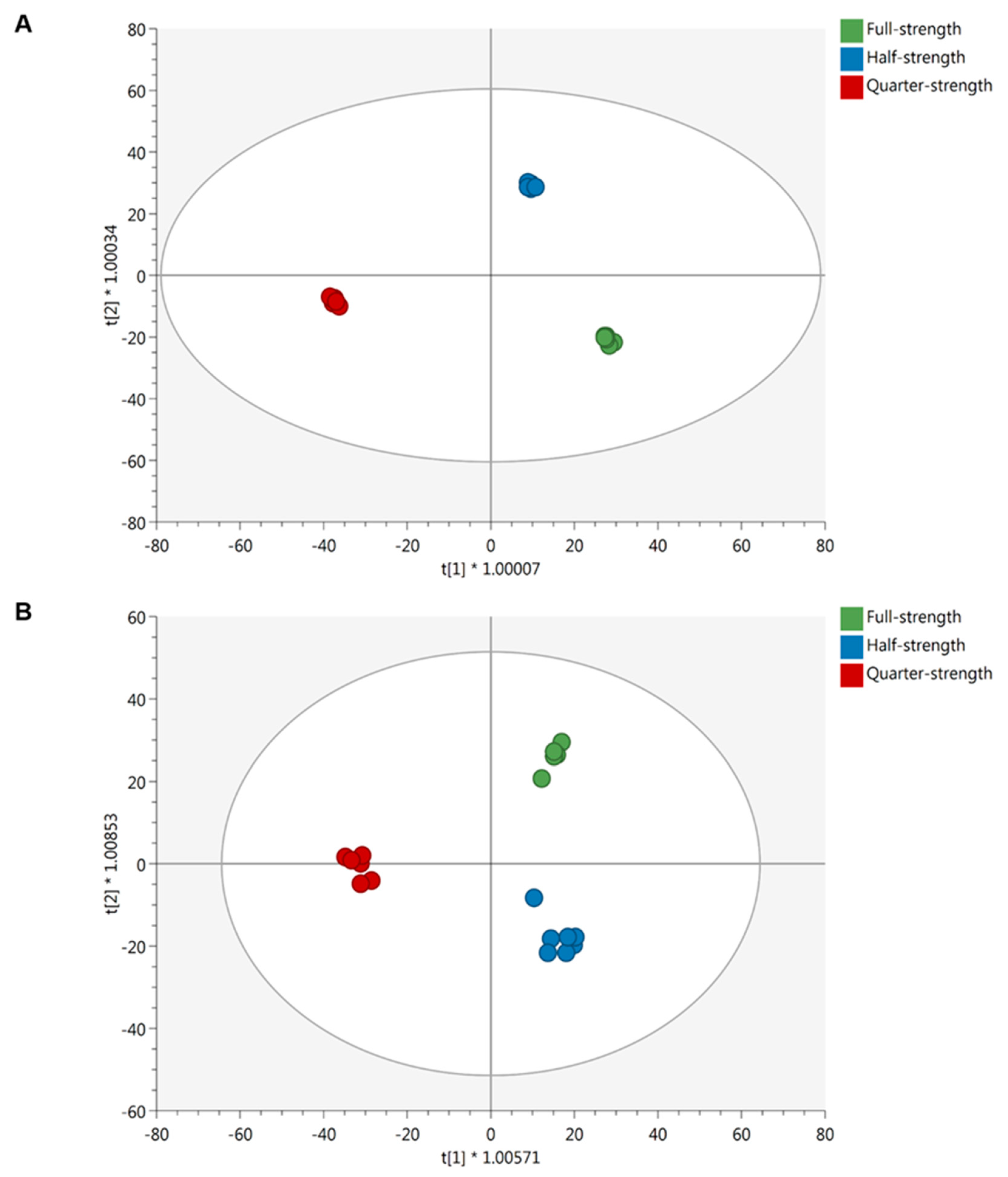
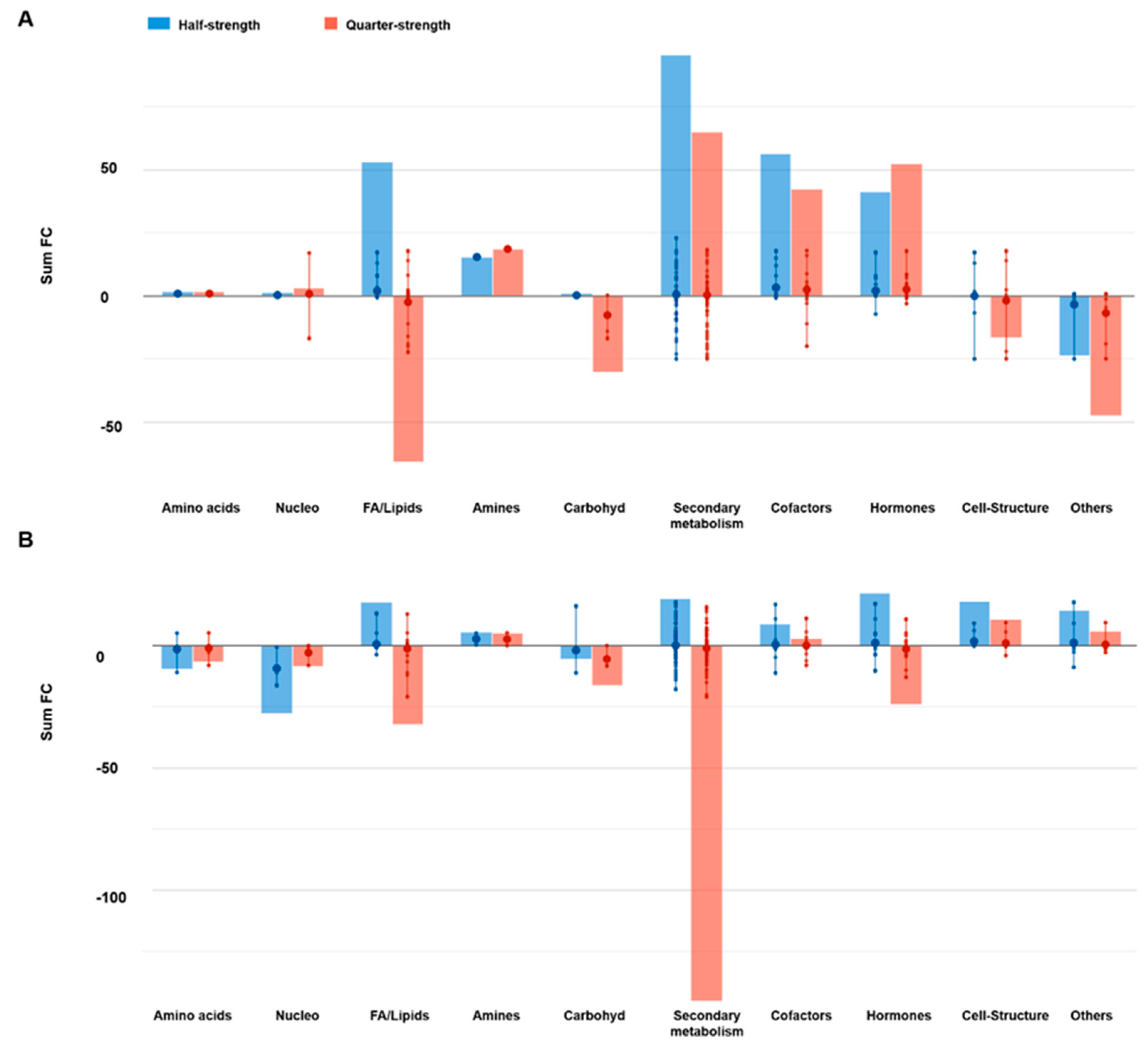
2.2.1. Effect of the Nutrient Solution Strength on Metabolic Profiles
2.2.2. Effect of Light Intensity on Metabolic Profiles
3. Discussion
4. Materials and Methods
4.1. Growth Chamber Conditions, Plant Material, and Treatments
4.2. Physiological Parameters
4.3. Untargeted Metabolomics
4.4. Chemometric Interpretation of Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCA | Hierarchical cluster analysis |
| OPLS-DA | Orthogonal projection to latent structures discriminant analysis |
| FC | Fold change |
| VIP | Variable importance in projection |
| PC | Phosphatidylcholine |
| PL | Phospholipases |
| ROS | Reactive oxygen species |
| MGDG | Monogalactosyldiacylglycerol |
| DGDG | Digalactosyldiacylglycerol |
| JA | Jasmonic acids |
| ABA | Abscisic acid |
| BR | Brassinosteroids |
References
- Chang, C.; Bowman, J.L.; Meyerowitz, E.M. Field guide to plant model systems. Cell 2016, 167, 325–339. [Google Scholar] [CrossRef]
- De Vries, I.M. Origin and domestication of Lactuca sativa L. Genet. Resour. Crop Evol. 1997, 44, 165–174. [Google Scholar] [CrossRef]
- Reyes-Chin-Wo, S.; Wang, Z.; Yang, X.; Kozik, A.; Arikit, S.; Song, C.; Xia, L.; Froenicke, L.; Lavelle, D.O.; Truco, M.J.; et al. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat. Commun. 2017. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Almeida Gadelha, F.D.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. Conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879. [Google Scholar] [CrossRef]
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004. [Google Scholar] [CrossRef]
- Mulabagal, V.; Ngouajio, M.; Nair, A.; Zhang, Y.; Gottumukkala, A.L.; Nair, M.G. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food Chem. 2010. [Google Scholar] [CrossRef]
- Damerum, A.; Chapman, M.A.; Taylor, G. Innovative breeding technologies in lettuce for improved post-harvest quality. Postharvest Biol. Technol. 2020, 168, 111266. [Google Scholar] [CrossRef]
- Walters, K.J.; Behe, B.K.; Currey, C.J.; Lopez, R.G. Historical, Current, and Future Perspectives for Controlled Environment Hydroponic Food Crop Production in the United States. HortScience 2020. [Google Scholar] [CrossRef]
- Johnson, G.E.; Buzby, K.M.; Semmens, K.J.; Waterland, N.L. Comparison of Two Harvest Methods for Lettuce Production in an Aquaponic System. J. Agric. Sci. 2016, 9. [Google Scholar] [CrossRef]
- Mou, B. Genetic variation of beta-carotene and lutein contents in lettuce. J. Am. Soc. Hortic. Sci. 2005. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Rock, E.; Michel, H.; Amoureux, P.; Remesy, C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J. Sci. Food Agric. 2004. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008. [Google Scholar] [CrossRef] [PubMed]
- Hohl, U.; Neubert, B.; Pforte, H.; Schonhof, I.; Böhm, H. Flavonoid concentrations in the inner leaves of head lettuce genotypes. Eur. Food Res. Technol. 2001. [Google Scholar] [CrossRef]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015. [Google Scholar] [CrossRef]
- Coria-Cayupán, Y.S.; De Pinto, M.I.S.; Nazareno, M.A. Variations in bioactive substance contents and crop yields of lettuce (Lactuca sativa L.) cultivated in soils with different fertilization treatments. J. Agric. Food Chem. 2009. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Carillo, P.; Pizzolongo, F.; Romano, R.; Sifola, M.I. Chemical Eustress Elicits Tailored Responses and Enhances the Functional Quality of Novel Food Perilla frutescens. Molecules 2019, 24, 185. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013. [Google Scholar] [CrossRef]
- Kleinhenz, M.D.; French, D.G.; Gazula, A.; Scheerens, J.C. Variety, shading, and growth stage effects on pigment concentrations in lettuce grown under contrasting temperature regimens. HortTechnology 2003, 13, 677–683. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar]
- Melandri, G.; AbdElgawad, H.; Riewe, D.; Hageman, J.A.; Asard, H.; Beemster, G.T.; Kadam, N.; Jagadish, K.; Altmann, T.; Ruyter-Spira, C.; et al. Biomarkers for grain yield stability in rice under drought stress. J. Exp. Bot. 2020, 71, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Sublett, W.L.; Barickman, T.C.; Sams, C.E. The effect of environment and nutrients on Hydroponic Lettuce yield, quality, and Phytonutrients. Horticulturae 2018, 4, 48. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Bernardo, L.; Kane, D.; Trevisan, M.; Lucini, L. Zinc excess triggered polyamines accumulation in lettuce root metabolome, as compared to osmotic stress under high salinity. Front. Plant Sci. 2016. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Petropoulos, S.A.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Colla, G.; Troise, A.D.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. The bioactive profile of lettuce produced in a closed soilless system as configured by combinatorial effects of genotype and macrocation supply composition. Food Chem. 2020. [Google Scholar] [CrossRef]
- Spalholz, H.; Perkins-Veazie, P.; Hernández, R. Impact of sun-simulated white light and varied blue:red spectrums on the growth, morphology, development, and phytochemical content of green- and red-leaf lettuce at different growth stages. Sci. Hortic. 2020. [Google Scholar] [CrossRef]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under stress: Involvement of auxin and cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar]
- Hong, Y.; Zheng, S.; Wang, X. Dual functions of phospholipase Dα1 in plant response to drought. Mol. Plant 2008. [Google Scholar] [CrossRef]
- Gaude, N.; Bréhélin, C.; Tischendorf, G.; Kessler, F.; Dörmann, P. Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J. 2007. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 2014. [Google Scholar] [CrossRef]
- Majumdar, R.; Shao, L.; Minocha, R.; Long, S.; Minocha, S.C. Ornithine: The overlooked molecule in the regulation of polyamine metabolism3. Plant Cell Physiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Fernandez, C. Effect of Soil Nutrient on Production and Diversity of Volatile Terpenoids from Plants. Curr. Bioact. Compd. 2012. [Google Scholar] [CrossRef]
- Siwko, M.E.; Marrink, S.J.; de Vries, A.H.; Kozubek, A.; Schoot Uiterkamp, A.J.M.; Mark, A.E. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochim. Biophys. Acta-Biomembr. 2007. [Google Scholar] [CrossRef] [PubMed]
- Harley, P.C.; Litvak, M.E.; Sharkey, T.D.; Monson, R.K. Isoprene emission from velvet bean leaves. Interactions among nitrogen availability, growth photon flux density, and leaf development. Plant Physiol. 1994, 105, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Tokaji, Y.; Nagano, A.J.; Hara-Nishimura, I.; Hayashi, M.; Nishimura, M.; Ohta, H.; Masuda, S. Arabidopsis mutants affecting oxylipin signaling in photo-oxidative stress responses. Plant Physiol. Biochem. 2014. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegoń-Putze, I.; Bosch, N.; Ibanḛs, M.; Cano-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146. [Google Scholar] [CrossRef]
- Dobisova, T.; Hrdinova, V.; Cuesta, C.; Michlickova, S.; Urbankova, I.; Hejatkova, R.; Zadnikova, P.; Pernisova, M.; Benkova, E.; Hejatko, J. Light controls cytokinin signaling via transcriptional regulation of constitutively active sensor histidine kinase CKI1. Plant Physiol. 2017. [Google Scholar] [CrossRef]
- Geigenberger, P. Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol. 2011. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Troise, A.D.; Vitaglione, P.; De Pascale, S. Reducing Energy Requirements in Future Bioregenerative Life Support Systems (BLSSs): Performance and Bioactive Composition of Diverse Lettuce Genotypes Grown under Optimal and Suboptimal Light Conditions. Front. Plant Sci. 2019. [Google Scholar] [CrossRef]
- Corrado, G.; Lucini, L.; Miras-Moreno, B.; Chiaiese, P.; Colla, G.; De Pascale, S.; Rouphael, Y. Metabolic insights into the anion-anion antagonism in sweet basil: Effects of different nitrate/chloride ratios in the nutrient solution. Int. J. Mol. Sci. 2020, 21, 2482. [Google Scholar] [CrossRef]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic Responses of Maize Shoots and Roots Elicited by Combinatorial Seed Treatments With Microbial and Non-microbial Biostimulants. Front. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Neumann, S.; Schober, D.; Hummel, J.; Billiau, K.; Kopka, J.; Correa, E.; Reijmers, T.; Rosato, A.; Tenori, L.; et al. COordination of Standards in MetabOlomicS (COSMOS): Facilitating integrated metabolomics data access. Metabolomics 2015. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vandergheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Dreher, K.; Karp, P.D. The challenge of constructing, classifying, and representing metabolic pathways. FEMS Microbiol. Lett. 2013, 345, 85–93. [Google Scholar] [CrossRef]


| Source of Variance | ACO2 | rs | E | WUEi | ||||
|---|---|---|---|---|---|---|---|---|
| (μmol CO2 m−2 s−1) | (m2 s−1 mol−1) | (mol H2O m−2 s−1) | (μmol CO2 mol−1 H2O) | |||||
| Cultivar (C) | ||||||||
| ‘Green Salanova’ | 7.86 | ±0.39 b | 4.15 | ±0.23 b | 2.74 | ±0.06 a | 2.89 | ±0.15 b |
| ‘Red Salanova’ | 9.88 | ±0.43 a | 5.82 | ±0.32 a | 2.42 | ±0.06 b | 4.14 | ±0.20 a |
| t-test | *** | *** | *** | *** | ||||
| Nutrient solution concentration (S) | ||||||||
| FS (EC = 1.50 dS m−1) | 10.36 | ±0.46 a | 4.69 | ±0.41 | 2.70 | ±0.10 | 3.96 | ±0.28 a |
| HS (EC = 0.75 dS m−1) | 9.00 | ±0.42 b | 5.10 | ±0.33 | 2.53 | ±0.06 | 3.61 | ±0.22 a |
| QS (EC = 0.50 dS m−1) | 7.26 | ±0.52 c | 5.16 | ±0.46 | 2.51 | ±0.09 | 2.97 | ±0.25 b |
| *** | ns | ns | ** | |||||
| C × S | ||||||||
| ‘Green Salanova’ × FS | 9.29 | ±0.69 | 3.68 | ±0.38 | 2.92 | ±0.11 | 3.23 | ±0.30 |
| ‘Green Salanova’ × HS | 7.83 | ±0.49 | 4.51 | ±0.30 | 2.61 | ±0.08 | 3.01 | ±0.20 |
| ‘Green Salanova’’ × QS | 6.46 | ±0.48 | 4.27 | ±0.48 | 2.69 | ±0.12 | 2.43 | ±0.21 |
| ‘Red Salanova’ × FS | 11.43 | ±0.30 | 5.71 | ±0.54 | 2.48 | ±0.12 | 4.70 | ±0.31 |
| ‘Red Salanova’ × HS | 10.17 | ±0.35 | 5.70 | ±0.51 | 2.44 | ±0.09 | 4.22 | ±0.24 |
| ‘Red Salanova’ × QS | 8.05 | ±0.86 | 6.05 | ±0.68 | 2.33 | ±0.11 | 3.50 | ±0.38 |
| ns | ns | ns | ns | |||||
| Source of Variance | ACO2 | rs | E | WUEi |
|---|---|---|---|---|
| (μmol CO2 m−2 s−1) | (m2 s−1 mol−1) | (mol H2O m−2 s−1) | (μmol CO2 mol−1 H2O) | |
| Cultivar (C) | ||||
| ‘Green Salanova’ | 7.53 ± 0.52 b | 5.07 ± 0.49 | 2.54 ± 0.12 | 2.94 ± 0.13 b |
| ‘Red Salanova’ | 10.60 ± 0.83 a | 5.54 ± 0.76 | 2.84 ± 0.17 | 3.67 ± 0.11 a |
| t-test | ** | ns | ns | *** |
| Light intensity (L) | ||||
| OL (420 μmol m−2 s−1) | 11.61 ± 0.59 a | 3.00 ± 0.19 b | 3.20 ± 0.11 a | 3.63 ± 0.13 a |
| LL (210 μmol m−2 s−1) | 6.53 ± 0.27 b | 7.61 ± 0.27 a | 2.19 ± 0.02 b | 2.99 ± 0.13 b |
| t-test | *** | *** | *** | *** |
| C × L | ||||
| ‘Green Salanova’ × OL | 9.45 ± 0.30 b | 3.34 ± 0.31 c | 2.90 ± 0.15 b | 3.30 ± 0.16 |
| ‘Green Salanova’ × LL | 5.62 ± 0.11 d | 6.81 ± 0.23 b | 2.18 ± 0.05 c | 2.59 ± 0.10 |
| ‘Red Salanova’ × OL | 13.76 ± 0.25 a | 2.67 ± 0.17 c | 3.49 ± 0.06 a | 3.96 ± 0.12 |
| ‘Red Salanova’ × LL | 7.43 ± 0.23 c | 8.41 ± 0.25 a | 2.20 ± 0.02 c | 3.39 ± 0.12 |
| *** | *** | ** | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miras-Moreno, B.; Corrado, G.; Zhang, L.; Senizza, B.; Righetti, L.; Bruni, R.; El-Nakhel, C.; Sifola, M.I.; Pannico, A.; Pascale, S.D.; et al. The Metabolic Reprogramming Induced by Sub-Optimal Nutritional and Light Inputs in Soilless Cultivated Green and Red Butterhead Lettuce. Int. J. Mol. Sci. 2020, 21, 6381. https://doi.org/10.3390/ijms21176381
Miras-Moreno B, Corrado G, Zhang L, Senizza B, Righetti L, Bruni R, El-Nakhel C, Sifola MI, Pannico A, Pascale SD, et al. The Metabolic Reprogramming Induced by Sub-Optimal Nutritional and Light Inputs in Soilless Cultivated Green and Red Butterhead Lettuce. International Journal of Molecular Sciences. 2020; 21(17):6381. https://doi.org/10.3390/ijms21176381
Chicago/Turabian StyleMiras-Moreno, Begoña, Giandomenico Corrado, Leilei Zhang, Biancamaria Senizza, Laura Righetti, Renato Bruni, Christophe El-Nakhel, Maria Isabella Sifola, Antonio Pannico, Stefania De Pascale, and et al. 2020. "The Metabolic Reprogramming Induced by Sub-Optimal Nutritional and Light Inputs in Soilless Cultivated Green and Red Butterhead Lettuce" International Journal of Molecular Sciences 21, no. 17: 6381. https://doi.org/10.3390/ijms21176381
APA StyleMiras-Moreno, B., Corrado, G., Zhang, L., Senizza, B., Righetti, L., Bruni, R., El-Nakhel, C., Sifola, M. I., Pannico, A., Pascale, S. D., Rouphael, Y., & Lucini, L. (2020). The Metabolic Reprogramming Induced by Sub-Optimal Nutritional and Light Inputs in Soilless Cultivated Green and Red Butterhead Lettuce. International Journal of Molecular Sciences, 21(17), 6381. https://doi.org/10.3390/ijms21176381











