Evidence of Skin Barrier Damage by Cyclic Siloxanes (Silicones)—Using Digital Holographic Microscopy
Abstract
1. Introduction
2. Results and Discussion
Statistical Data Evaluation
3. Methods
3.1. Test Substances
3.2. Research Methodology
3.3. Preparation of Ex Vivo Skin Samples
3.4. Preparation of the Samples to Microscopic Investigation
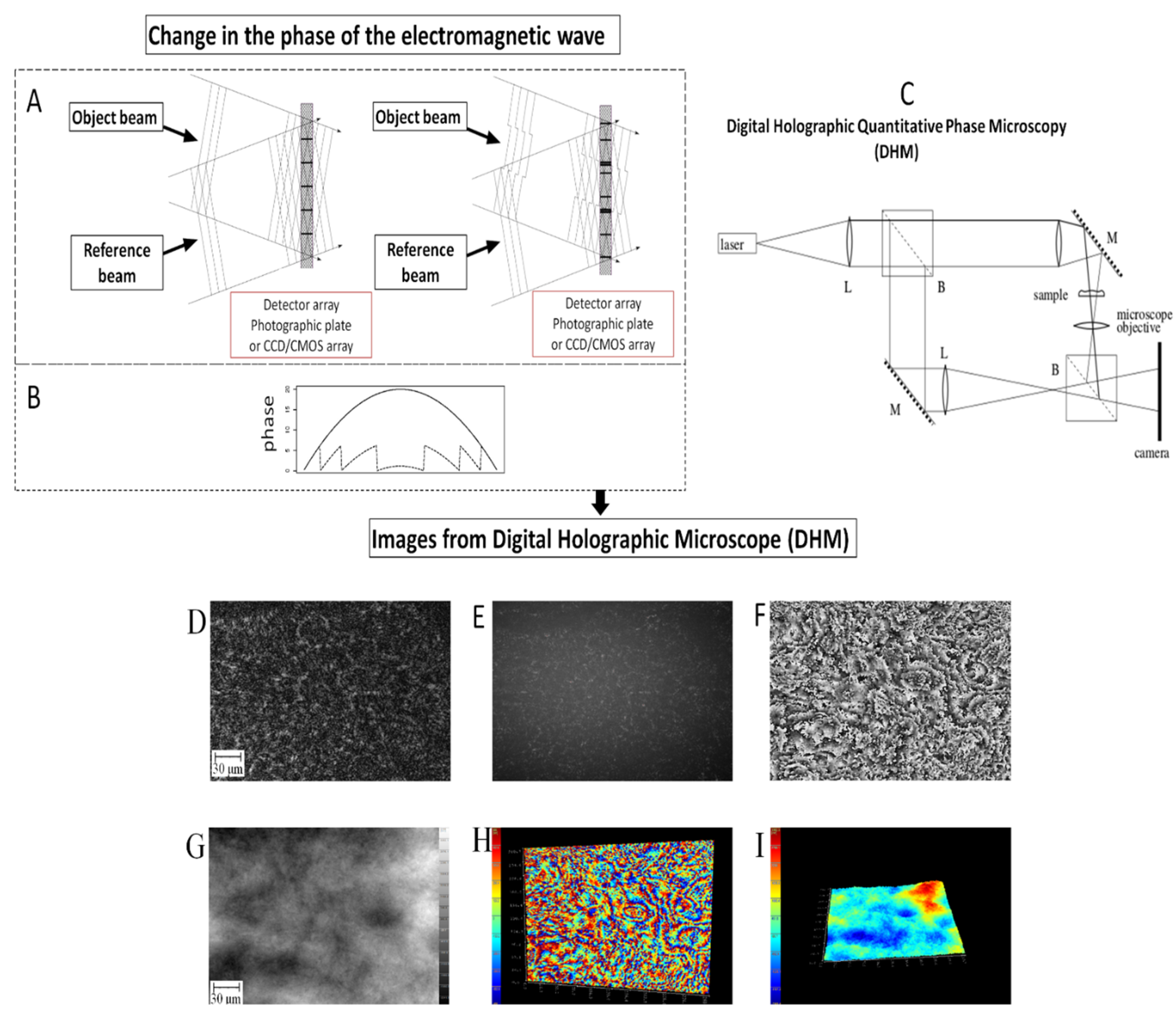
3.5. Digital Holographic Microscopy
3.6. Equipment and Settings
3.7. Statistical Analysis
4. Conclusions
- (a)
- the first level of the barrier—destabilization of the lipid bilayer resulting in the destruction of the corneocyte structure, observed as a change in geometry with an axial resolution of nanometers and even collapse in space. We can conclude that these compounds have affinity for amphiphilic structures of the lipid bilayer due to the lipophilic properties of cyclic siloxanes ((logPo/w 5.10—about 9), resulting in a change in their conformation, e.g., orthorhombic (most regular and densely packed), responsible for the largest barrier, and hexagonal (slightly relaxed), to liquid crystal (relaxed conformation responsible for the reduction of the barrier), and even irreversible lipid extraction;
- (b)
- the second level of the barrier—destruction of the structure of the lipid bilayer causing the collapse of not only corneocytes, but also a significant part of the clusters, which leads to the loss of the SC integrity and lacunae formation. The lacunae occurring might cause transepidermal drug delivery or enhanced penetration of undesirable substances. Lipophilic siloxanes can also interact with lipid canyons. Obtaining further knowledge is required.
- (c)
- the third level of the barrier—changing the topography of the SC surface and interrupting the barrier continuity of this skin layer, measured with a lateral resolution of micrometers. On the basis of the results obtained, we found that of the cyclic siloxanes tested, siloxane D6 disturbs the integrity of the SC, and thus reduces the skin barrier less then D5 and especially D4. Additional additional research to increase our knowledge is required.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. In vivo confocal Raman microscopic determination of depth profiles of the stratum corneum lipid organization influenced by application of various oils. J. Dermatol. Sci. 2017, 87, 183–191. [Google Scholar] [CrossRef]
- Danby, S.G.; Chalmers, J.; Brown, K.; Williams, H.C.; Cork, M.J. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br. J. Dermatol. 2016, 175, 1011–1019. [Google Scholar] [CrossRef]
- Yanase, K.; Hatta, I. Disruption of human stratum corneum lipid structure by sodium dodecyl sulphate. Int. J. Cosmet. Sci. 2018, 40, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Leskur, D.; Bukić, J.; Petrić, A.; Zekan, L.; Rušić, D.; Šešelja Perišin, A.; Petrić, I.; Stipić, M.; Puizina-Ivić, N.; Modun, D. Anatomical site differences of sodium lauryl sulfate-induced irritation: Randomized controlled trial. Br. J. Dermatol. 2019, 181, 175–185. [Google Scholar] [CrossRef]
- Lodén, M. Interactions between the stratum corneum and topically applied products: Regulatory, instrumental and formulation issues with focus on moisturizers. Br. J. Dermatol. 2014, 171, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Greenfield, D.A.; Hermsmeier, M.; Yamamoto, A.; Chen, X.; Chan, K.F.; Evans, C.L. Time-resolved fluorescence microscopy with phasor analysis for visualizing multicomponent topical drug distribution within human skin. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Lin, K.H.; Chuang, H.C.; Tsai, C.H.; Lin, Y.C.; Wang, C.H.; Shih, C.P.; Liu, H.L. Low-frequency dual-frequency ultrasound-mediated microbubble cavitation for transdermal minoxidil delivery and hair growth enhancement. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sjövall, P.; Skedung, L.; Gregoire, S.; Biganska, O.; Clément, F.; Luengo, G.S. Imaging the distribution of skin lipids and topically applied compounds in human skin using mass spectrometry. Sci. Rep. 2018, 8, 16683. [Google Scholar] [CrossRef]
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of Chemical Permeation Enhancers on Skin Permeability: In silico screening using Molecular Dynamics simulations. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Van Reeth, I. The Beauty of Silicone in Skin Care Applications; No. 27-1624-01 C; Dow Corning: Midland, MI, USA, 2017; pp. 1–8. [Google Scholar]
- Krenczkowska, D.; Mojsiewicz-Pieńkowska, K.; Wielgomas, B.; Cal, K.; Bartoszewski, R.; Bartoszewska, S.; Jankowski, Z. The consequences of overcoming the human skin barrier by siloxanes (silicones) Part 1. Penetration and permeation depth study of cyclic methyl siloxanes. Chemosphere 2019, 231, 607–623. [Google Scholar] [CrossRef]
- Biesterbos, J.W.H.; Beckmann, G.; van Wel, L.; Anzion, R.B.M.; von Goetz, N.; Dudzina, T.; Roeleveld, N.; Ragas, A.M.J.; Russel, F.G.M.; Scheepers, P.T.J. Aggregate dermal exposure to cyclic siloxanes in personal care products: Implications for risk assessment. Environ. Int. 2015, 74, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mackay, D.; Whelan, M.J. Predicted persistence and response times of linear and cyclic volatile methylsiloxanes in global and local environments. Chemosphere 2018, 195, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Mojsiewicz-Pieńkowska, K. Size exclusion chromatography with evaporative light scattering detection as a method for speciation analysis of polydimethylsiloxanes. III. Identification and determination of dimeticone and simeticone in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2012, 25, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Mojsiewicz-Pieńkowska, K.; Krenczkowska, D. Evolution of consciousness of exposure to siloxanes—Review of publications. Chemosphere 2018, 191, 204–271. [Google Scholar] [CrossRef]
- Krenczkowska, D.; Mojsiewicz-Pieńkowska, K.; Wielgomas, B.; Bazar, D.; Jankowski, Z. Ex Vivo Human Skin is not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure. Pharmaceutics 2020, 12, 586. [Google Scholar] [CrossRef]
- Alm, K.; Cirenajwis, H.; Gisselsson, L.; Gjorloff, A.; Janicke, B.; Molder, A.; Oredsson, S.; Persso, J. Digital Holography and Cell Studies. In Holography, Research and Technologies; InTech: London, UK, 2011. [Google Scholar]
- Daloglu, M.U.; Ozcan, A. Computational imaging of sperm locomotion. Biol. Reprod. 2017, 97, 182. [Google Scholar] [CrossRef]
- Miniotis, M.F.; Mukwaya, A.; Wingren, A.G. Digital holographic microscopy for non-invasive monitoring of cell cycle arrest in L929 cells. PLoS ONE 2014, 9, e106546. [Google Scholar]
- Funamizu, H.; Aizu, Y. Three-dimensional quantitative phase imaging of blood coagulation structures by optical projection tomography in flow cytometry using digital holographic microscopy. J. Biomed. Opt. 2018, 24, 1. [Google Scholar] [CrossRef]
- Jung, J.; Kim, K.; Yu, H.; Lee, K.; Lee, S.; Nahm, S.; Park, H.; Park, Y. Biomedical applications of holographic microspectroscopy. Appl. Opt. 2014, 53, 111–122. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Jung, J.; Heo, J.; Cho, S.; Lee, S.; Chang, G.; Jo, Y.; Park, H.; Park, Y. Quantitative phase imaging techniques for the study of cell pathophysiology: From principles to applications. Sensors (Basel) 2013, 13, 4170–4191. [Google Scholar] [CrossRef]
- Lévesque, S.A.; Mugnes, J.-M.; Bélanger, E.; Marquet, P. Sample and substrate preparation for exploring living neurons in culture with quantitative-phase imaging. Methods 2018, 136, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Midtvedt, D.; Olsén, E.; Höök, F.; Jeffries, G.D.M. Label-free spatio-temporal monitoring of cytosolic mass, osmolarity, and volume in living cells. Nat. Commun. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Robic, J.; Nkengne, A.; Yan, H.; Zhang, X.; Soo, X.Y. Optical Phantom Development for Skin Measurement. In Proceedings of the 19th International Congress of Metrology (CIM2019), Pairs, France, 24–26 September 2019. [Google Scholar]
- Drutis, D.M.; Hancewicz, T.M.; Pashkovski, E.; Feng, L.; Mihalov, D.; Holtom, G.; Ananthapadmanabhan, K.P.; Xie, X.S.; Misra, M. Three-dimensional chemical imaging of skin using stimulated Raman scattering microscopy. J. Biomed. Opt. 2014, 19, 111604. [Google Scholar] [CrossRef]
- Peralta, M.F.; Guzmán, M.L.; Pérez, A.P.; Apezteguia, G.A.; Fórmica, M.L.; Romero, E.L.; Olivera, M.E.; Carrer, D.C. Liposomes can both enhance or reduce drugs penetration through the skin. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carrer, D.C.; Vermehren, C.; Bagatolli, L.A. Pig skin structure and transdermal delivery of liposomes: A two photon microscopy study. J. Control. Release 2008, 132, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Higa, L.H.; Arnal, L.; Vermeulen, M.; Perez, A.P.; Schilrreff, P.; Mundiña-Weilenmann, C.; Yantorno, O.; Vela, M.E.; Morilla, M.J.; Romero, E.L. Ultradeformable archaeosomes for needle free nanovaccination with leishmania braziliensis antigens. PLoS ONE 2016, 11, e0150185. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Santer, V.; Mondon, K.; Patmanidis, I.; Chiriano, G.; Scapozza, L.; Gurny, R.; Möller, M.; Kalia, Y.N. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J. Control. Release 2014, 196, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Schätzlein, A.; Cevc, G. Non-uniform cellular packing of the stratum corneum and permeability barrier function of intact skin: A high-resolution confocal laser scanning microscopy study using highly deformable vesicles (Transfersomes). Br. J. Dermatol. 1998, 138, 583–592. [Google Scholar] [CrossRef]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Diembeck, W.; Beck, H.; Benech-Kieffer, F.; Courtellemont, P.; Dupuis, J.; Lovell, W.; Paye, M.; Spengler, J.; Steiling, W. Test Guidelines for In Vitro Assessment of Dermal Absorption and Percutaneous Penetration of Cosmetic Ingredients. Food Chem. Toxicol. 1999, 37, 191–205. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for Thesting of Chemicals. In Skin Absorption: In Vitro METHOD; OECD: Pairs, France, 2004. [Google Scholar]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; No 28; OECD: Pairs, France, 2004. [Google Scholar]
- Davies, D.J.; Heylings, J.R.; McCarthy, T.J.; Correa, C.M. Development of an in vitro model for studying the penetration of chemicals through compromised skin. Toxicol. Vitr. 2015, 29, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol. Vitr. 2015, 29, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Picart, P.; Montresor, S. Digital Holography. In Optical Holography-Materials, Theory and Applications; Elsevier, St. Louis: Maryland Heights, MO, USA, 2020; pp. 83–120. [Google Scholar]
- Leith, E.N.; Upatnieks, J. Reconstructed Wavefronts and Communication Theory. J. Opt. Soc. Am. 1962, 52, 1123. [Google Scholar] [CrossRef]
- Schnars, U.; Jüptner, W.P.O. Digital recording and numerical reconstruction of holograms. Meas. Sci. Technol. 2002, 13, 85. [Google Scholar] [CrossRef]
- Ghiglia, D.C.; Pritt, M.D. Two-Dimensional Phase Unwrapping: Theory, Algorithms, and Software; Wiley-Interscience: Hoboken, NJ, USA, 1998; Volume 120. [Google Scholar]
- LynceeTec—Digital Holographic Microscopy. Available online: https://www.lynceetec.com/ (accessed on 10 March 2020).
- LynceeTec. Software Koala V4 User Operating Manual; Lyncee: Lausanne, Switzerland, 2014. [Google Scholar]
- Wang, R.; Wu, Z.; Yang, S.; Guo, S.; Dai, X.; Qiao, Y.; Shi, X. A molecular interpretation on the different penetration enhancement effect of borneol and menthol towards 5-Fluorouracil. Int. J. Mol. Sci. 2017, 18, 2747. [Google Scholar] [CrossRef]
- Volz, P.; Boreham, A.; Wolf, A.; Kim, T.-Y.; Balke, J.; Frombach, J.; Hadam, S.; Afraz, Z.; Rancan, F.; Blume-Peytavi, U.; et al. Application of single molecule fluorescence microscopy to characterize the penetration of a large amphiphilic molecule in the stratum corneum of human skin. Int. J. Mol. Sci. 2015, 16, 6960–6977. [Google Scholar] [CrossRef]
- Carrer, D.C.; Higa, L.H.; Tesoriero, M.V.D.; Morilla, M.J.; Roncaglia, D.I.; Romero, E.L. Structural features of ultradeformable archaeosomes for topical delivery of ovalbumin. Colloids Surf. B Biointerfaces 2014, 121, 281–289. [Google Scholar] [CrossRef]
- Jacobi, U.; Schanzer, S.; Weigmann, H.-J.; Patzelt, A.; Vergou, T.; Sterry, W.; Lademann, J. Pathways of Lateral Spreading. Skin Pharmacol. Physiol. 2011, 24, 231–237. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Nikolovski, J.; Luedtke, M.A.; Kollias, N.; Wiegand, B.C. Infant Skin Microstructure Assessed In Vivo Differs from Adult Skin in Organization and at the Cellular Level. Pediatr. Dermatol. 2010, 27, 125–131. [Google Scholar] [CrossRef]
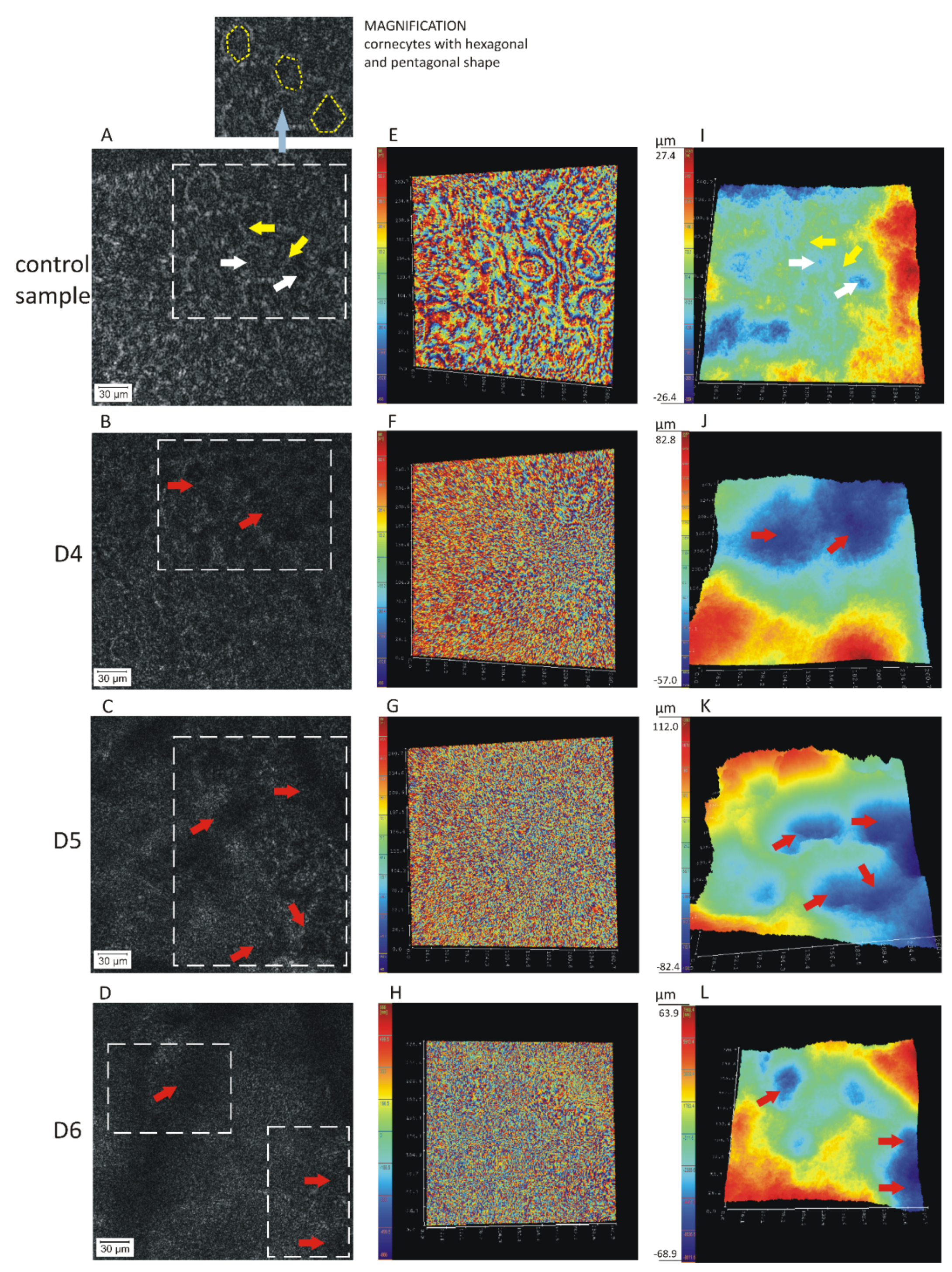
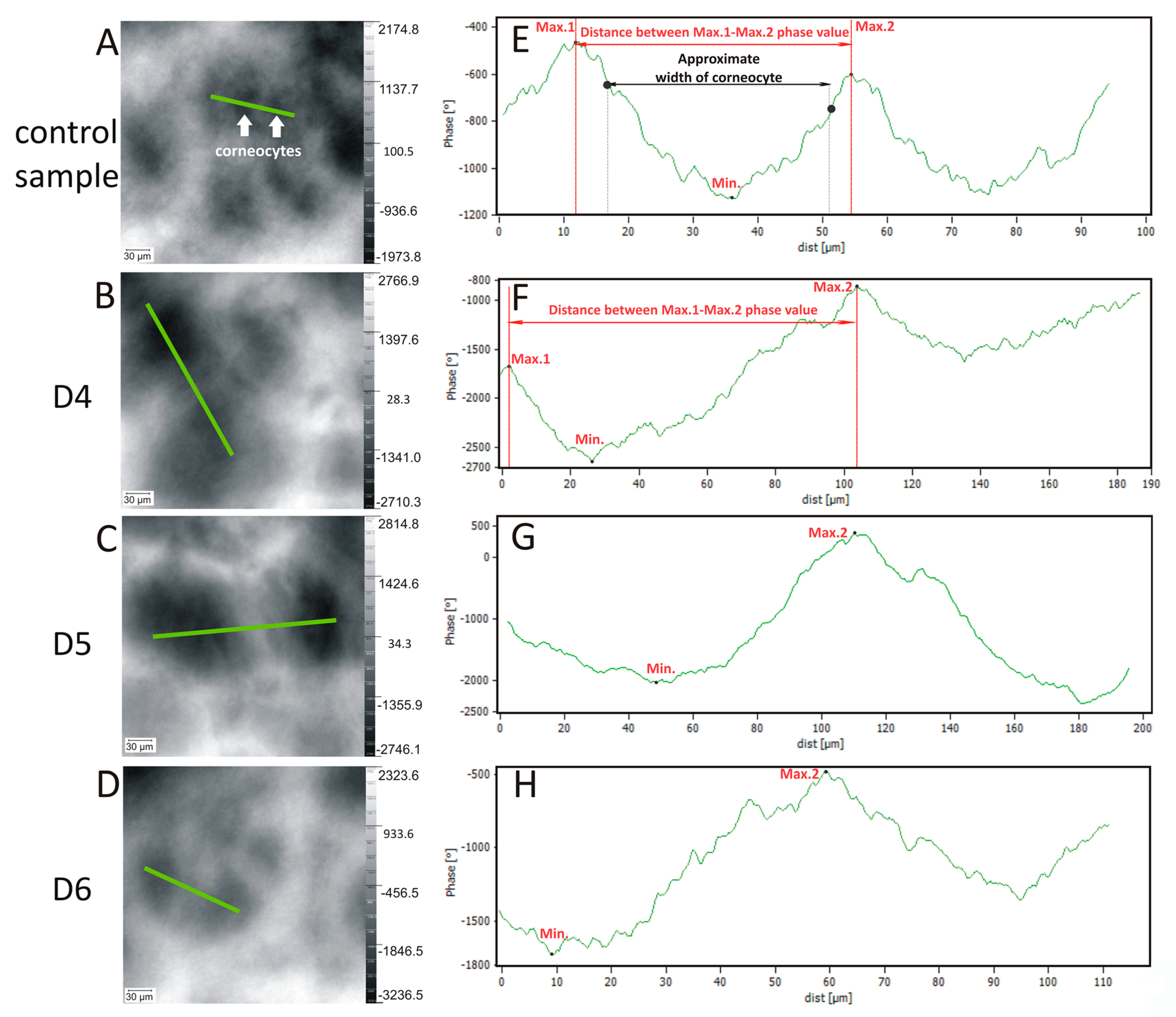
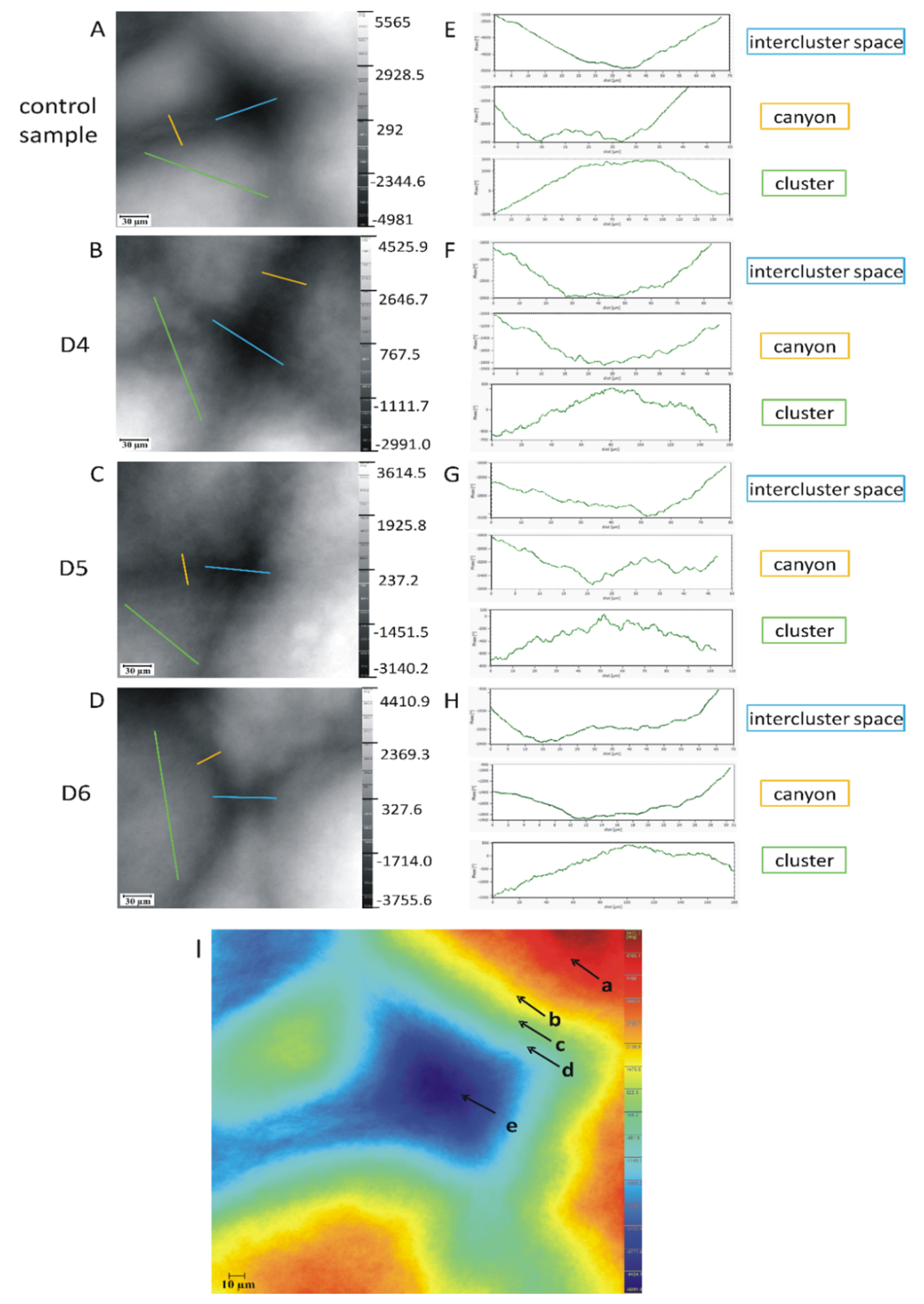

| Imaging | Feature | Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean | SD | RSD (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Figure 1I–L Figure 4A | Width of the corneocyte/lacunae (μm) | Control sample | 38 | 30 | 31 | 28 | 35 | 35 | 34 | 33 | 4 | 11 |
| D4 | 100 | 130 | 104 | 110 | 104 | 104 | 90 | 106 | 12 | 12 | ||
| D5 | 76 | 104 | 126 | 117 | 100 | 122 | 104 | 107 | 17 | 16 | ||
| D6 | 104 | 100 | 130 | 91 | 117 | 104 | 100 | 107 | 13 | 12 | ||
| Figure 1I–L Figure 4B | Depth alteration in skin topography (μm) | Control sample | 54 | 72 | 64 | 48 | 72 | 48 | 48 | 58 | 11 | 19 |
| D4 | 140 | 160 | 120 | 104 | 96 | 160 | 92 | 125 | 29 | 23 | ||
| D5 | 194 | 184 | 160 | 120 | 144 | 112 | 102 | 145 | 36 | 25 | ||
| D6 | 133 | 136 | 112 | 157 | 120 | 144 | 136 | 134 | 15 | 11 | ||
| Figure 2A–D Figure 4C | Phase change in skin topography (°) | Control sample | 4149 | 4443 | 3977 | 3310 | 2927 | 2397 | 5464 | 3810 | 1025 | 27 |
| D4 | 5477 | 7610 | 6272 | 10,561 | 7964 | 7349 | 8365 | 7657 | 1623 | 21 | ||
| D5 | 5561 | 4869 | 5819 | 4681 | 9509 | 7059 | 6268 | 6252 | 1649 | 26 | ||
| D6 | 5560 | 6986 | 6655 | 6031 | 4806 | 4476 | 6565 | 5868 | 961 | 16 | ||
| Figure 2E–H Figure 4D | Distance between max./min. phase value (μm) | Control sample | 45 | 47 | 45 | 42 | 48 | 45 | 43 | 45 | 2 | 5 |
| D4 | 120 | 100 | 130 | 130 | 120 | 120 | 120 | 120 | 10 | 8 | ||
| D5 | 130 | 140 | 130 | 130 | 110 | 150 | 120 | 130 | 13 | 10 | ||
| D6 | 110 | 80 | 80 | 90 | 80 | 100 | 90 | 90 | 12 | 13 | ||
| Figure 2E–H Figure 4E | Phase change in skin topography—profile line (°) | Control sample | 800 | 1000 | 1400 | 1000 | 800 | 800 | 1200 | 1000 | 231 | 24 |
| D4 | 1900 | 1600 | 1800 | 2000 | 3100 | 1700 | 2200 | 2043 | 506 | 23 | ||
| D5 | 3000 | 1100 | 1100 | 1400 | 2000 | 1700 | 1600 | 1700 | 658 | 25 | ||
| D6 | 1300 | 2300 | 1200 | 1400 | 1400 | 1600 | 1800 | 1571 | 377 | 24 |
| Level of Organisation | Stratum Corneum Component | Structural Characteristics | Impact of the Skin Barrier Function |
|---|---|---|---|
| First | Corneocyte (contribution—70%) | -a single, dead, flattened cell, with regular shapes, e.g., hexagonal, pentagonal and diameter approx. 10–40 µm; -the building blocks of the internal structure are:
-The corneocytes are connected by corneodesmosomes | 1. the smallest structurally level of skin barrier 2. maintenance of the mechanical stability |
| lipid matrix (contribution—20%) | -multilayer structure composed of lipid bilayers—width 12 nm- a thermodynamically stable self-assembly system, maintained by van der Waals bonds, hydrogen and electrostatic bonds; these bilayers form regions of semicrystalline, gel and liquid crystals domains; most molecules penetrate through the skin via this intercellular microroute and therefore many enhancing techniques aim to disrupt or bypass its highly organized structure; -The building block of the structure is a mixture of:
-the skin barrier function is determined by:
| 1.guarantee skin barrier (limits permeability of substances, allergens and microorganisms) | |
| Second | Clusters | -specific organization approx. 15–30 corneocytes (that range from 100–250 µm in width across the surface), and 150–300 cells close to the basal layer separated by canyons—intercluster spaces, intercluster region | 1. strengthening mechanical stability |
| Canyons | -canyons (intercluster region)—the invaginations or microfolds of the stratum corneum cell layers, the intercluster spaces (width ranging from 10–30 µm); -structurally built of lipids; hydrophobic and lipophilic properties; -in the surface the intercluster regions start as small wrinkles and deeper into the skin, these wrinkles close and are replaced by canyons; -a cross-section perpendicular to the skin surface, the canyons appear as invaginations of the SC into the tissue -the canyons can be observed up to 58 μm depth from the surface of the tissue, 6 μm away from the dermis | 1. structure can even extend in depth to dermoepidermal junction, which allows xenobiotics to diffuse even directly into blood or lymph vessels, omitting stratum corneum lipids | |
| Third | Compact surface | -skin surface with regular cells -specific and compact structural organization composed of tightly adhering corneocytes, surrounded by an extracellular lipid matrix (lipid—enriched extracellular matrix). -layered construction—15–20 layers with a total thickness of 10–20 μm (thick) -the integrity of the layer is also maintained by the corneodesmosomes—intercellular proteins that combine with the cohesion forces with adjacent corneocytes, both in the plane of a single layer of stratum corneum and with a deeper neighboring layer; directly related to the exfoliation process. | 1. maintenance of tightness and flexibility 2. barrier function |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojsiewicz-Pieńkowska, K.; Stachowska, E.; Krenczkowska, D.; Bazar, D.; Meijer, F. Evidence of Skin Barrier Damage by Cyclic Siloxanes (Silicones)—Using Digital Holographic Microscopy. Int. J. Mol. Sci. 2020, 21, 6375. https://doi.org/10.3390/ijms21176375
Mojsiewicz-Pieńkowska K, Stachowska E, Krenczkowska D, Bazar D, Meijer F. Evidence of Skin Barrier Damage by Cyclic Siloxanes (Silicones)—Using Digital Holographic Microscopy. International Journal of Molecular Sciences. 2020; 21(17):6375. https://doi.org/10.3390/ijms21176375
Chicago/Turabian StyleMojsiewicz-Pieńkowska, Krystyna, Ewa Stachowska, Dominika Krenczkowska, Dagmara Bazar, and Frans Meijer. 2020. "Evidence of Skin Barrier Damage by Cyclic Siloxanes (Silicones)—Using Digital Holographic Microscopy" International Journal of Molecular Sciences 21, no. 17: 6375. https://doi.org/10.3390/ijms21176375
APA StyleMojsiewicz-Pieńkowska, K., Stachowska, E., Krenczkowska, D., Bazar, D., & Meijer, F. (2020). Evidence of Skin Barrier Damage by Cyclic Siloxanes (Silicones)—Using Digital Holographic Microscopy. International Journal of Molecular Sciences, 21(17), 6375. https://doi.org/10.3390/ijms21176375






