Arachidonic Acid Metabolites of CYP450 Enzymes and HIF-1α Modulate Endothelium-Dependent Vasorelaxation in Sprague-Dawley Rats under Acute and Intermittent Hyperbaric Oxygenation
Abstract
1. Introduction
2. Results
2.1. Body Mass and Blood Pressure of Studied Groups
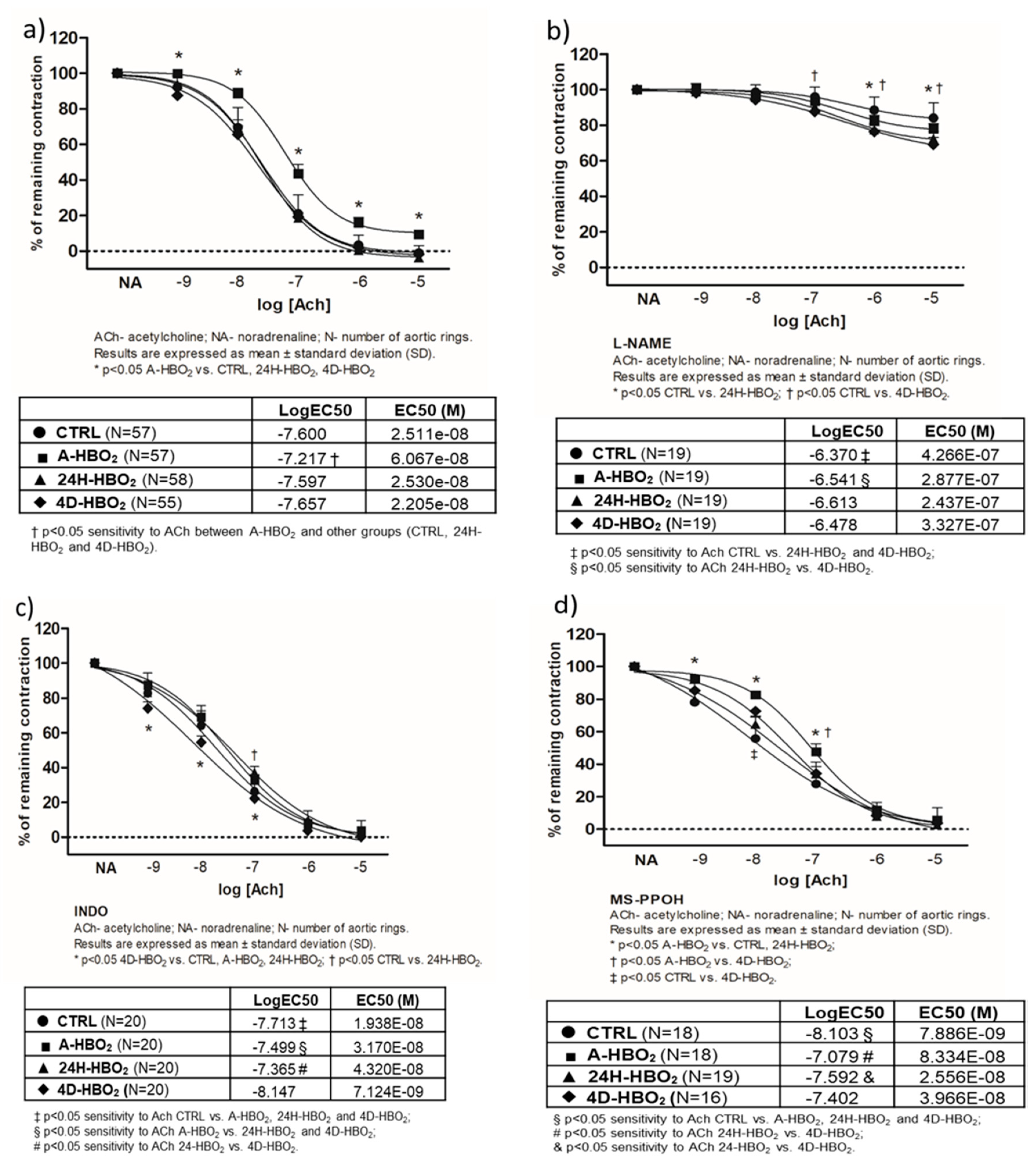
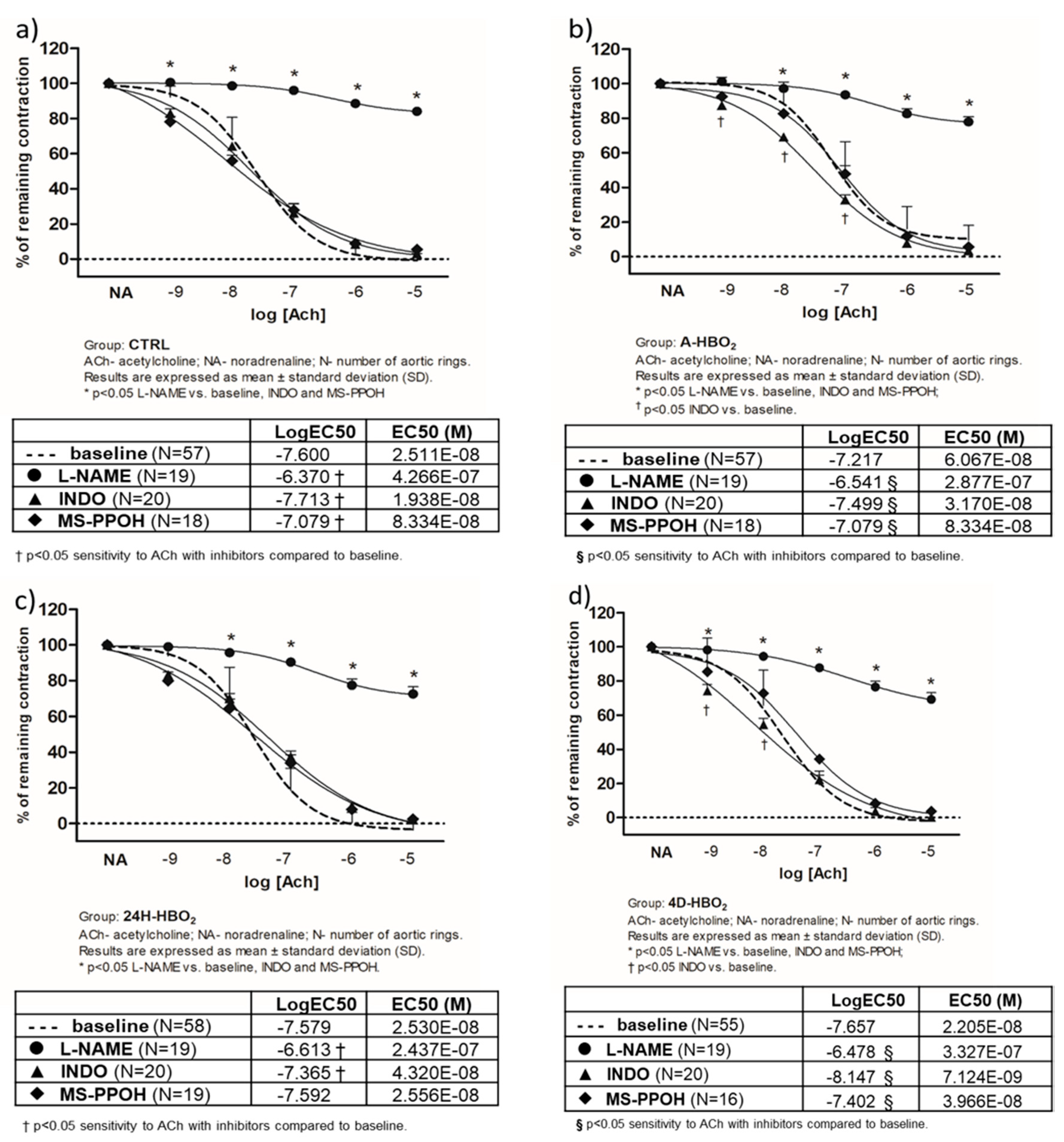
2.2. Acetylcholine-Induced Vasorelaxation (AChIR) of Isolated Rat Aortic Rings
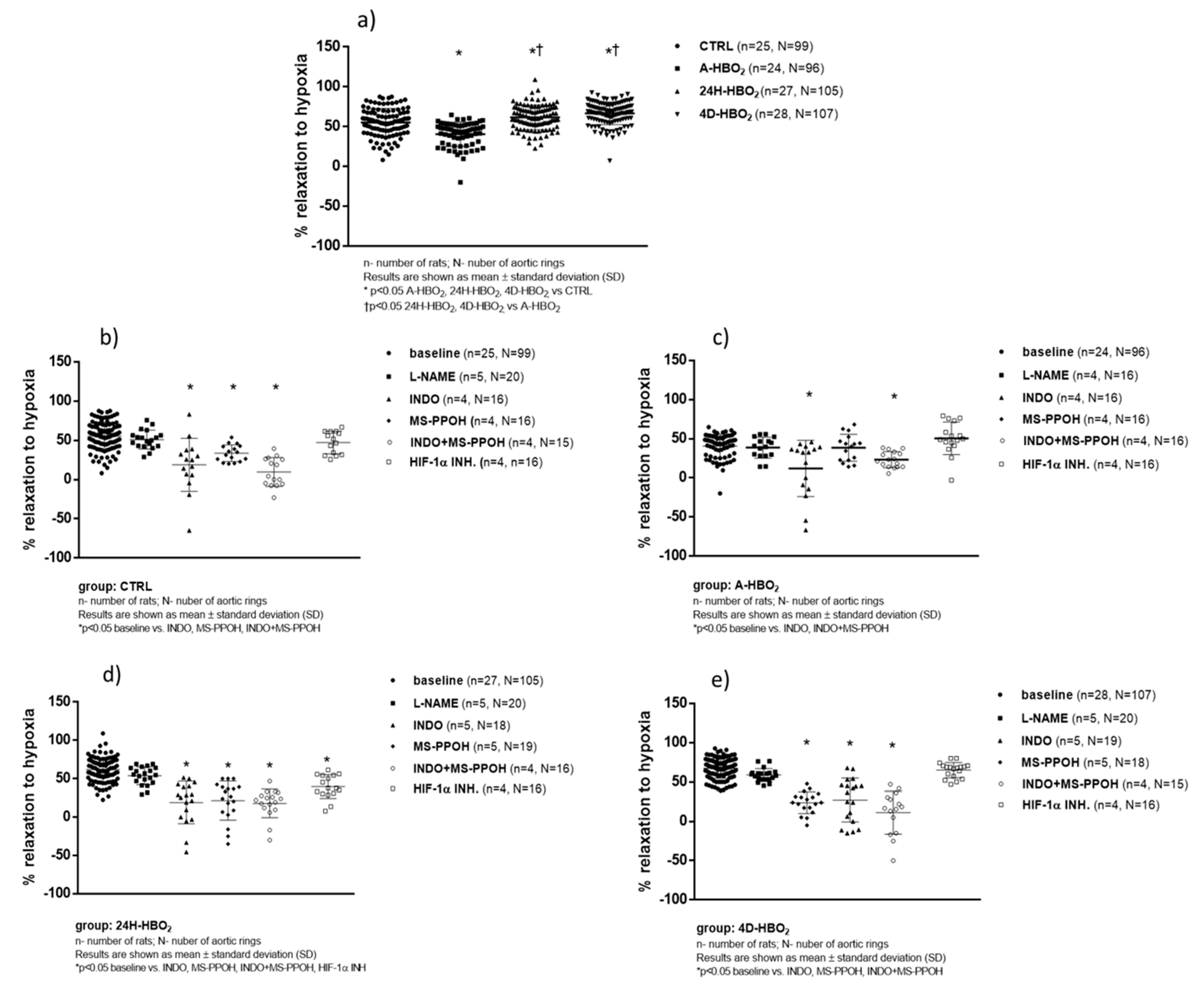
2.3. Hypoxia-Induced Vasorelaxation of Isolated Rat Aortic Rings
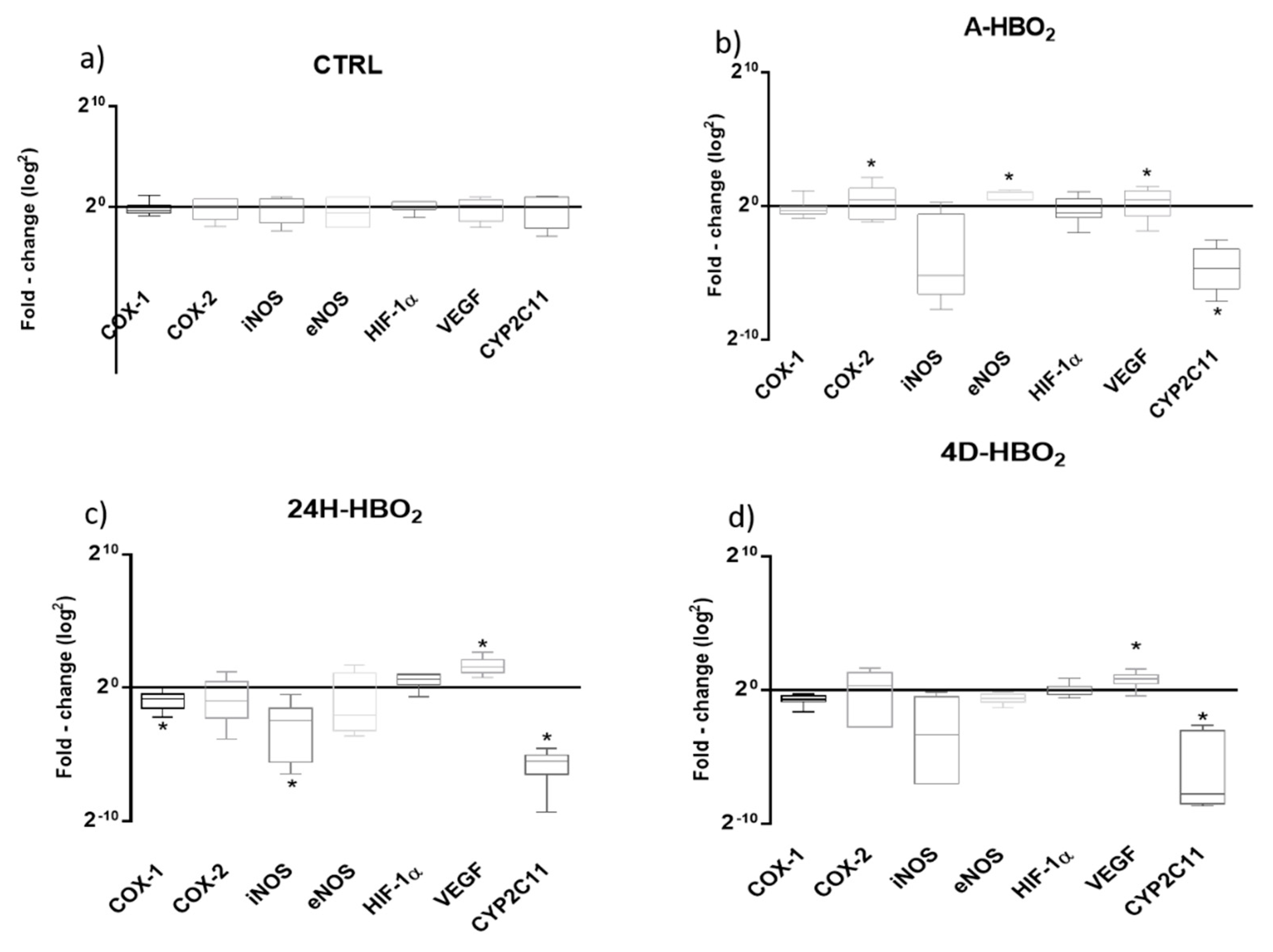
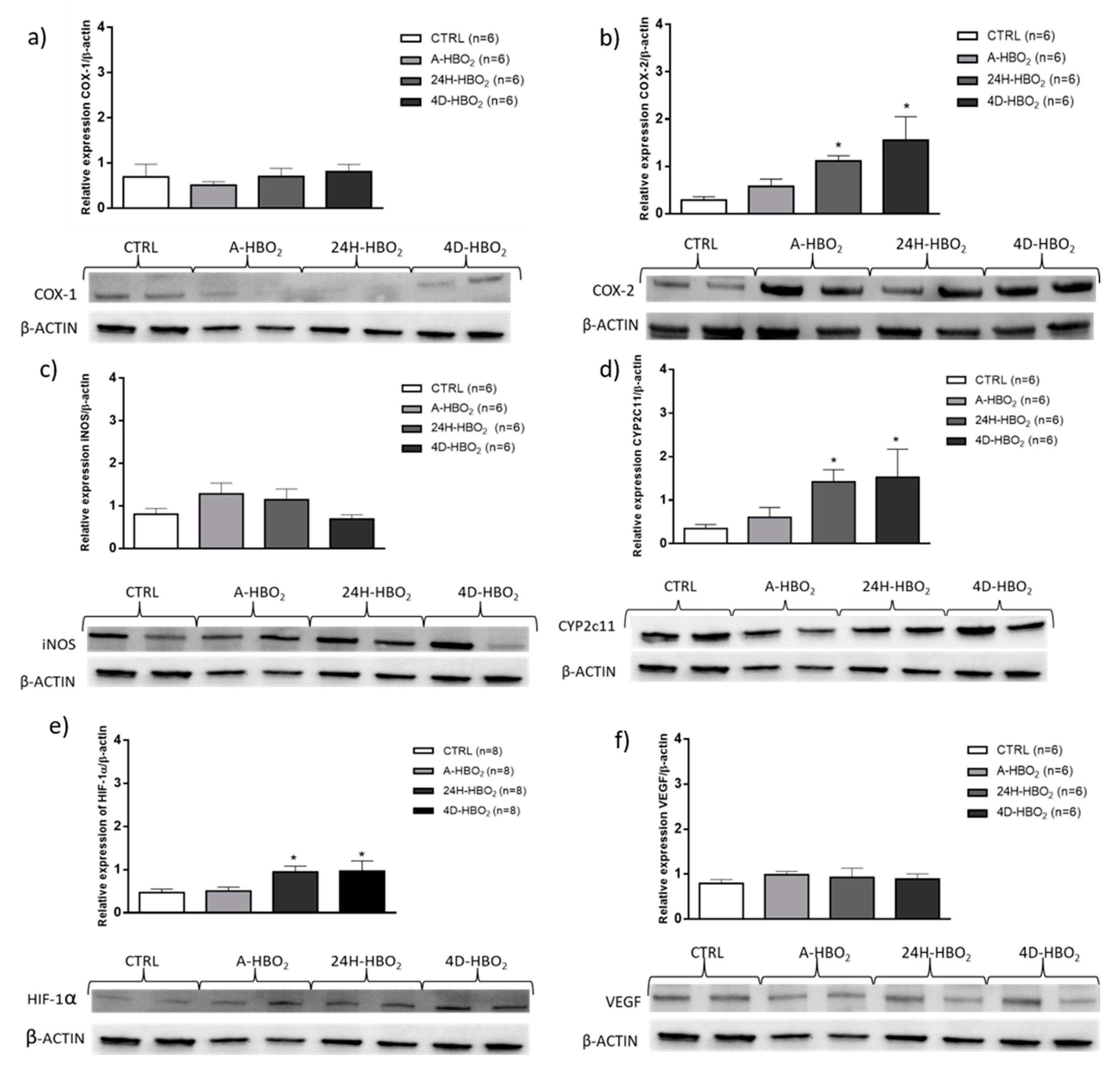
2.4. Relative mRNA and Protein Expression of Enzymes in Rat Aorta
2.5. HIF-1α mRNA and Protein Expression
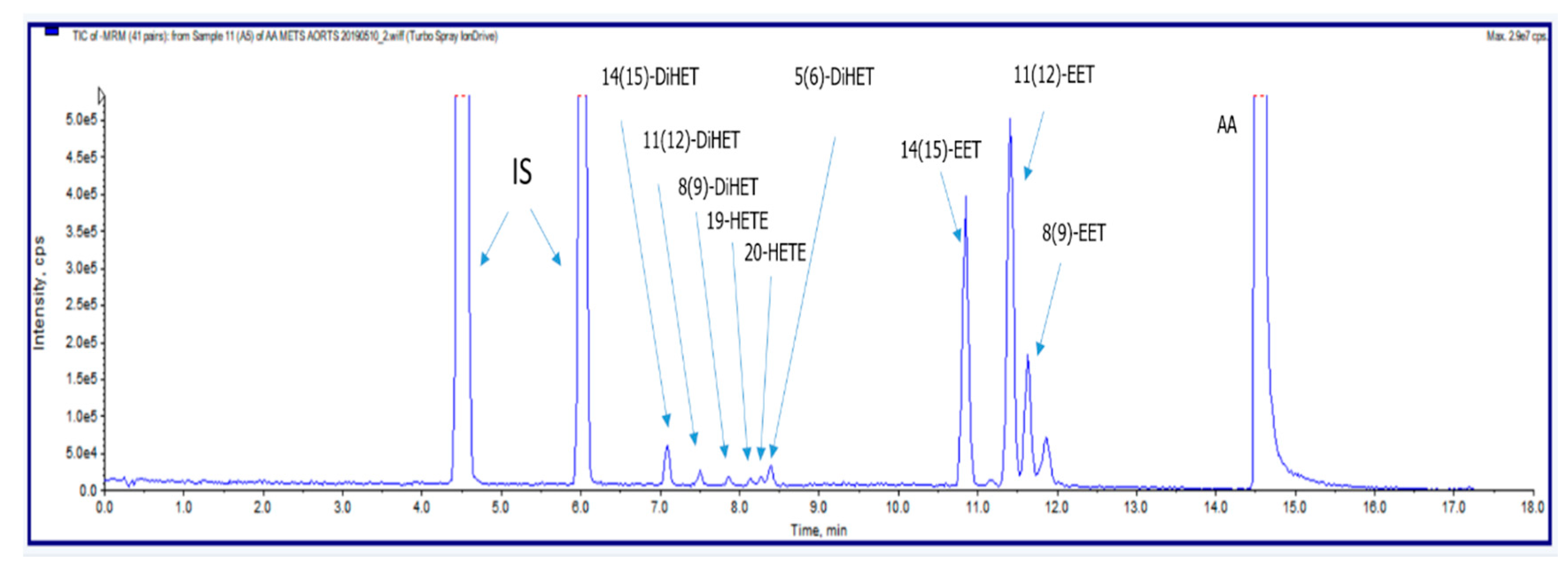
2.6. Liquid Chromatography-Tandem Mass Spectrometry Determination of CYP450 Enzymatic Pathway Metabolites of Arachidonic Acid
3. Discussion
3.1. Body Mass and Blood Pressure of Studied Groups
3.2. The Mechanisms of Acetylcholine-Induced Vasorelaxation
3.3. The Mechanisms of Hypoxia-Induced Vasorelaxation
3.4. The Role of HIF-1α in Vasorelaxation in Response to ACh and Hypoxia
4. Materials and Methods
4.1. Hyperbaric Oxygenation Exposure Protocols
4.2. Measurement of Blood Pressure
4.3. Experiments on Isolated Aortic Rings
4.3.1. Acetylcholine-Mediated Relaxation (AChIR)
4.3.2. Hypoxia-Induced Relaxation (HIR)
4.4. Relative Gene Expression Determined by RT-qPCR Method
4.5. Relative Protein Expression Determined by Western Blot
4.6. Determination of CYP450 Enzymatic Pathway Metabolites of Arachidonic Acid
Sample Preparation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CYP450 | Cytochrome P450 |
| HIF-1α | Hypoxia inducible factor 1 alpha |
| AChIR | Acetylcholine-induced relaxation |
| HIR | Hypoxia-induced relaxation |
| HBO2 | Hyperbaric oxygen |
| EET | Epoxyeicosatrienoic acid |
| HETE | Hydroxyeicosatetraenoic acid |
| COX-1 | Cyclooxygenase 1 |
| COX-2 | Cyclooxygenase 2 |
| AA | Arachidonic acid |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| NO | Nitric oxide |
| ANGII/ANG(1-7) | Angiotensin II/Angiotensin (1-7) |
| PGI2 | Prostacyclin |
| cAMP | Cyclic adenosine monophosphate |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| MS-PPOH | N-(methyl sulfonyl)-2-(2-propynyloxy)-benzenehexanamide |
| INDO | Indomethacin |
| L-NAME | Nω-nitro-L-arginine methyl ester |
| iNOS | Inducible nitric oxide synthase |
| eNOS | Endothelial nitric oxide synthase |
| VEGF | Vascular endothelial growth factor |
| mRNA | Messenger ribonucleic acid |
| EDHFs | Endothelium-derived hyperpolarizing factors |
| ACh | Acetylcholine |
| SD | Sprague-Dawley rats |
| RT qPCR | Quantitative reverse transcription polymerase chain reaction |
| EDTA | Ethylenediaminetetraacetic acid |
| RNA | Ribonucleic acid |
| HPRT | Hypoxanthine-guanine phosphoribosyl transferase |
| SIM | Single (Selected) Ion Monitoring |
| MRM | Multiple Reaction Monitoring |
| SDS | Sodium dodecyl sulfate |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| DMSO | Dimethyl sulfoxide |
| HPLC-ESI/MS-MS | High-performance liquid chromatography/electrospray ionization tandem mass spectrometry |
References
- Zenere, B.M.; Arcaro, G.; Saggiani, F.; Rossi, L.; Muggeo, M.; Lechi, A. Noninvasive Detection of Functional Alterations of the Arterial Wall in IDDM Patients with and without Microalbuminuria. Diabetes Care 1995, 18, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Gazis, A.; White, D.J.; Page, S.R.; Cockcroft, J.R. Effect of oral vitamin E (α-tocopherol) supplementation on vascular endothelial function in Type 2 diabetes mellitus. Diabet. Med. 1999, 16, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Katusic, Z.S. Vascular endothelial dysfunction: Does tetrahydrobiopterin play a role? Am. J. Physiol. Circ. Physiol. 2001, 281, H981–H986. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.K.; Warnholtz, A.; et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001, 88, 14–22. [Google Scholar] [CrossRef]
- Bagi, Z.; Koller, A. Lack of Nitric Oxide Mediation of Flow-Dependent Arteriolar Dilation in Type I Diabetes Is Restored by Sepiapterin. J. Vasc. Res. 2003, 40, 47–57. [Google Scholar] [CrossRef]
- Drenjancevic-Peric, I.; Phillips, S.A.; Falck, J.R.; Lombard, J.H. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS.13BN rats. Am. J. Physiol. Circ. Physiol. 2005, 289, H188–H195. [Google Scholar] [CrossRef]
- Unfirer, S.; Mihalj, M.; Novak, S.; Kibel, A.; Cavka, A.; Mijalevic, Z.; Gros, M.; Brizic, I.; Budimir, D.; Cosic, A.; et al. Hyperbaric oxygenation affects the mechanisms of acetylcholine-induced relaxation in diabetic rats. Undersea Hyperb. Med. 2017, 43, 787–803. [Google Scholar]
- Kibel, A.; Cavka, A.; Cosic, A.; Falck, J.R.; Drenjancevic, I. Effects of hyperbaric oxygenation on vascular reactivity to angiotensin II and angiotensin-(1-7) in rats. Undersea Hyperb. Med. 2012, 39, 1053–1066. [Google Scholar]
- Kibel, A.; Novak, S.; Cosic, A.; Mihaljević, Z.; Falck, J.R.; Drenjancevic-Peric, I. Hyperbaric oxygenation modulates vascular reactivity to angiotensin-(1-7) in diabetic rats: Potential role of epoxyeicosatrienoic acids. Diabetes Vasc. Dis. Res. 2014, 12, 33–45. [Google Scholar] [CrossRef]
- Drenjančević, I.; Jukić, I.; Mihaljević, Z.; Ćosić, A.; Kibel, A. The Metabolites of Arachidonic Acid in Microvascular Function // Microcirculation Revisited–From Molecules to Clinical Practice; Lenasi, H., Ed.; IntechOpen: Rijeka, Croatia, 2016; pp. 101–133. [Google Scholar] [CrossRef]
- Oltman, C.L.; Weintraub, N.L.; VanRollins, M.; Dellsperger, K.C. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ. Res. 1998, 83, 932–939. [Google Scholar] [CrossRef]
- Bellien, J.; Joannides, R. Epoxyeicosatrienoic Acid Pathway in Human Health and Diseases. J. Cardiovasc. Pharmacol. 2013, 61, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, J.; Xu, X.; Yan, S.; Pan, S.; Pan, X.; Zhang, C.; Leung, G.P.H. Vasodilatory effect of 14,15-epoxyeicosatrienoic acid on mesenteric arteries in hypertensive and aged rats. Prostaglandins Other Lipid Mediat. 2014, 112, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bihzad, S.M.; Yousif, M.H.M. 11,12-Epoxyeicosatrienoic acid induces vasodilator response in the rat perfused mesenteric vasculature. Auton. Autacoid Pharmacol. 2017, 37, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jukić, I.; Mišir, M.; Mihalj, M.; Mihaljević, Z.; Unfirer, S.; Kibel, D.; Kibel, A. Mechanisms of HBO-Induced Vascular Functional Changes in Diabetic Animal Models. In Hyperbaric Oxygen Treatment in Research and Clinical Practice; Drenjančević, I., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 87–108. [Google Scholar] [CrossRef]
- Capdevila, J.; Wang, W. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr. Opin. Nephrol. Hypertens. 2013, 22, 163–169. [Google Scholar] [CrossRef]
- Harder, D.R.; Narayanan, J.; Birks, E.K.; Liard, J.F.; Imig, J.D.; Lombard, J.H.; Lange, A.R.; Roman, R.J. Identification of a Putative Microvascular Oxygen Sensor. Circ. Res. 1996, 79, 54–61. [Google Scholar] [CrossRef]
- Mihaljević, Z.; Matic, A.; Stupin, A.; Rasic, L.; Jukic, I.; Drenjancevic-Peric, I. Acute Hyperbaric Oxygenation, Contrary to Intermittent Hyperbaric Oxygenation, Adversely Affects Vasorelaxation in Healthy Sprague-Dawley Rats due to Increased Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 1–15. [Google Scholar] [CrossRef]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarkis, S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3’ to the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; Bunn, H.F. Activation of hypoxia-inducible transcription factor depends primarly upon redox-sensitive stabilization of its α subunit. J. Biol. Chem. 1996, 271, 32253–32259. [Google Scholar] [CrossRef]
- Sunkari, V.G.; Lind, F.; Botusan, I.R.; Kashif, A.; Liu, Z.-J.; Ylä-Herttuala, S.; Brismar, K.; Velazquez, O.; Catrina, S.-B. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015, 23, 98–103. [Google Scholar] [CrossRef]
- Wenger, R.H.; Gassmann, M. Oxygen(es) and the hypoxia – inducible factor-1. Biol. Chem. 1997, 378, 609–616. [Google Scholar]
- Rothfuss, A.; Dennog, C.; Speit, G. Adaptive protection against the induction of oxidative DNA damage after hyperbaric oxygen treatment. Carcinogenesis 1998, 19, 1913–1917. [Google Scholar] [CrossRef]
- Rothfuss, A.; Speit, G. Investigations on the mechanism of hyperbaric oxygen (HBO)-induced adaptive protection against oxidative stress. Mutat. Res. Mol. Mech. Mutagen. 2002, 508, 157–165. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Zhang, L.; Wang, B.; Xiong, L. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 2007, 80, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Ostrowski, R.; Tong, W.; Matus, B.; Jesunathadas, R.; Zhang, J.H. Cyclo-oxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke 2009, 40, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Capobianco, S.; Gao, X.; Falck, J.R.; Dellsperger, K.C.; Zhang, C. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am. J. Physiol. Circ. Physiol. 2008, 295, H1982–H1988. [Google Scholar] [CrossRef] [PubMed]
- Gesell, L.B. (Ed.) Hyperbaric Oxygen Therapy Indications, 12th ed.; Undersea and Hyperbaric Medical Society: Durham, NC, USA, 2008. [Google Scholar]
- Drenjancevic-Peric, I.; Kibel, A.; Kibel, D.; Seric, V.; Cosic, A. Blood pressure, acid-base and blood gas status and indicators of oxidative stress in healthy male rats exposed to acute hyperbaric oxygenation. Undersea Hyperb. Med. 2013, 40, 319–328. [Google Scholar]
- Fredricks, K.T.; Liu, Y.; Rusch, N.J.; Lombard, J.H. Role of endothelium and arterial K+ channels in mediating hypoxic dilation of middle cerebral arteries. Am. J. Physiol. Circ. Physiol. 1994, 267, H580–H586. [Google Scholar] [CrossRef]
- Frisbee, J.C.; Roman, R.J.; Krishna, U.M.; Falck, J.R.; Lombard, J.H. Relative contributions of cyclooxygenase and cytochrome P450 ω-hydroxylase-dependent pathways to hypoxic dilation of skeletal muscle resistance arteries. J. Vasc. Res. 2001, 38, 305–314. [Google Scholar] [CrossRef]
- Feletou, M.; Vanhoutte, P. EDHF and the Physiological Control of Blood Flow in EDHF: The Complete Story; CRC Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 1–298. [Google Scholar]
- Metzen, E.; Berchner-Pfannschmidt, U.; Stengel, P.; Marxsen, J.H.; Stolze, I.; Klinger, M.; Huang, W.Q.; Wotzlaw, C.; Hellwig-Bürgel, T.; Jelkmann, W.; et al. Intracellular localisation of human HIF-1alpha hydroxylases: Implications for oxygen sensing. J. Cell Sci. 2003, 116, 1319–1326. [Google Scholar] [CrossRef]
- Shih, S.C.; Claffey, K.P. Hypoxia-mediated regulation of gene expression in mammalian cells. Int. J. Exp. Pathol. 1998, 79, 347–357. [Google Scholar] [CrossRef]
- Salceda, S.; Caro, J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 1997, 272, 22642–22647. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Schroedl, C.; McClintock, D.S.; Budinger, G.R.S.; Chandel, N.S. Hypoxic but not anoxic stabilization of HIF-1α requires mitochondrial reactive oxygen species. Am. J. Physiol. Cell. Mol. Physiol. 2002, 283, L922–L931. [Google Scholar] [CrossRef] [PubMed]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.N.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Oguro, A.; Osada-Oka, M.; Funae, Y.; Imaoka, S. Epoxyeicosatrienoic acids and/or their metabolites promote hypoxic response of cells. J. Pharmacol. Sci. 2008, 108, 79–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Ming, J.; Li, T.; Yang, G.; Xu, J.; Chen, W.; Lin, L. Regulatory effects of hypoxia-inducible factor 1 alpha on vascular reactivity and its mechanisms following hemorragic shock in rats. Shock 2008, 30, 557–562. [Google Scholar] [CrossRef]
- Kerkhof, C.J.M.; Bakker, E.N.T.P.; Sipkema, P. Role of cytochrome P-450 4A in oxygen sensing and NO production in rat cremaster resistance arteries. Am. J. Physiol. Content 1999, 277, H1546–H1552. [Google Scholar] [CrossRef]
- Gu, G.-J.; Li, Y.-P.; Peng, Z.-Y.; Xu, J.-J.; Kang, Z.-M.; Xu, W.-G.; Tao, H.-Y.; Ostrowski, R.P.; Zhang, J.H.; Sun, X.-J. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor - 1α and erithropoietin in rats. J. Appl. Physiol. 2008, 104, 1185–1191. [Google Scholar] [CrossRef]
- Peng, Z.; Ren, P.; Kang, Z.; Du, J.; Lian, Q.; Liu, Y.; Zhang, J.H.; Sun, X. Up-regulated HIF-1α is involved in the hypoxic tolerance induced by hyperbaric oxygen preconditioning. Brain Res. 2008, 1212, 71–78. [Google Scholar] [CrossRef]
- Bai, X.; Sun, B.; Pan, S.; Jiang, H.-C.; Wang, F.; Krissansen, G.W.; Sun, X. Down-Regulation of Hypoxia-Inducible Factor-1α by Hyperbaric Oxygen Attenuates the Severity of Acute Pancreatitis in Rats. Pancreas 2009, 38, 515–522. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.-H.; Qu, S.-D.; Yang, J.; Wang, Z.; Gao, C.-J.; Su, Q.-J. Hyperbaric oxygen intervention on expression of hypoxia-inducible factor-1α and vascular endothelial growth factor in spinal cord injury models in rats. Chin. Med. J. 2013, 126, 3897–3903. [Google Scholar] [PubMed]
- Kaidi, A.; Qualtrough, D.; Williams, A.C.; Paraskeva, C. Direct Transcriptional Up-regulation of Cyclooxygenase-2 by Hypoxia-Inducible Factor (HIF)-1 Promotes Colorectal Tumor Cell Survival and Enhances HIF-1 Transcriptional Activity during Hypoxia. Cancer Res. 2006, 66, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Goldstein, J.A. The transcriptional regulation of the human CYP2C genes. Curr. Drug Metab. 2009, 10, 567–578. [Google Scholar] [CrossRef]
- Carroll, M.A.; Garcia, M.P.; Falck, J.R.; McGiff, J.C. 5,6-epoxyeicosatrienoic acid, a novel arachidonate metabolite. Mechanism of vasoactivity in the rat. Circ. Res. 1990, 67, 1082–1088. [Google Scholar] [CrossRef]
- Takahashi, K.; Capdevila, J.; Karara, A.; Falck, J.R.; Jacobson, H.R.; Badr, K.F. Cytochrome P-450 arachidonate metabolites in rat kidney: Characterization and hemodynamic responses. Am. J. Physiol. Physiol. 1990, 258, F781–F789. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Navar, L.G.; Roman, R.J.; Reddy, K.K.; Falck, J.R. Actions of epoxygenase metabolites on the preglomerular vasculature. J. Am. Soc. Nephrol. 1996, 7, 2364–2370. [Google Scholar] [PubMed]
- Roman, R.J. P-450 Metabolites of Arachidonic Acid in the Control of Cardiovascular Function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef]
- Mišir, M.; Renić, M.; Novak, S.; Mihalj, M.; Ćosić, A.; Vesel, M.; Drenjancevic-Peric, I. Hyperbaric oxygenation and 20-hydroxyeicosatetreanoic acid inhibition reduce stroke volume in female diabetic Sprague-Dawley rats. Exp. Physiol. 2017, 102, 1596–1606. [Google Scholar] [CrossRef][Green Version]
- Mišir, M.; Renić, M.; Mihalj, M.; Novak, S.; Drenjancevic-Peric, I. Is shorter transient middle cerebral artery occlusion (t-MCAO) duration better in stroke experiments on diabetic female Sprague Dawely rats? Brain Inj. 2016, 30, 1390–1396. [Google Scholar] [CrossRef]
- Cosic, A.; Jukic, I.; Stupin, A.; Mihalj, M.; Mihaljević, Z.; Novak, S.; Vukovic, R.; Drenjancevic-Peric, I. Attenuated flow-induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short-term high salt diet. J. Physiol. 2016, 594, 4917–4931. [Google Scholar] [CrossRef]
- Matić, A.; Jukić, I.; Stupin, A.; Baric, L.; Mihaljevic, Z.; Unfirer, S.; Bujak, I.T.; Mihaljević, B.; Lombard, J.H.; Drenjancevic-Peric, I. High salt intake shifts the mechanisms of flow-induced dilation in the middle cerebral arteries of Sprague-Dawley rats. Am. J. Physiol. Circ. Physiol. 2018, 315, H718–H730. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol—Chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Jansen, S.A.; Strauss, K.I.; Borenstein, M.R.; Barbe, M.F.; Rossi, L.J.; Murphy, E. A liquid chromatography/mass spectrometric method for simultaneous analysis of arachidonic acid and its endogenous eicosanoid metabolites prostaglandins, dihydroxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and epoxyeicosatrienoic acids in rat brain tissue. J. Pharm. Biomed. Anal. 2007, 43, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Edin, M.L.; Hoopes, S.L.; Li, H.; Bradbury, J.A.; Graves, J.P.; DeGraff, L.M.; Lih, F.B.; Garcia, V.; Shaik, J.S.B.; et al. Vascular characterization of mice with endothelial expression of cytochrome P450 4F2. FASEB J. 2014, 28, 2915–2931. [Google Scholar] [CrossRef]
- Puppolo, M.; Varma, D.; Jansen, S.A. A review of analytical methods for eicosanoids in brain tissue. J. Chromatogr. B 2014, 964, 50–64. [Google Scholar] [CrossRef]
- Mukai, Y.; Toda, T.; Takeuchi, S.; Senda, A.; Yamashita, M.; Eliasson, E.; Rane, A.; Inotsume, N. Simultaneous Determination Method of Epoxyeicosatrienoic Acids and Dihydroxyeicosatrienoic Acids by LC-MS/MS System. Biol. Pharm. Bull. 2015, 38, 1673–1679. [Google Scholar] [CrossRef]
- Wang, W.; Qin, S.; Li, L.; Chen, X.; Wang, Q.; Wei, J. An Optimized High Throughput Clean-Up Method Using Mixed-Mode SPE Plate for the Analysis of Free Arachidonic Acid in Plasma by LC-MS/MS. Int. J. Anal. Chem. 2015, 1–6. [Google Scholar] [CrossRef]
- Rago, B.; Fu, C. Development of a high-throughput ultra performance liquid chromatography–mass spectrometry assay to profile 18 eicosanoids as exploratory biomarkers for atherosclerotic diseases. J. Chromatogr. B 2013, 936, 25–32. [Google Scholar] [CrossRef]
| CTRL | A-HBO2 | 24H-HBO2 | 4D-HBO2 | |
|---|---|---|---|---|
| Body mass [g] | 329.4 ± 18.25 | 332.4 ± 21.53 | 318.6 ± 29.62 | 323.20 ± 39.49 |
| Mean arterial pressure [mmHg] | 107 ± 4 | 82 ± 5 * | 107 ± 9 | 105 ± 3 |
| CTRL | A-HBO2 | 24H-HBO2 | 4D-HBO2 | |
|---|---|---|---|---|
| COX-1 | 4.20 ± 1.49 | 4.42 ± 1.87 | 1.74 ± 0.59 *†‡ | 3.53 ± 0.88 |
| COX-2 | 1.18 ± 0.78 | 1.45 ± 0.88 | 0.47 ± 0.33 † | 0.33 ± 0.37 *† |
| eNOS | 0.13 ± 0.12 | 0.03 ± 0.03 | 0.13 ± 0.13 | 0.04 ± 0.03 |
| iNOS | 0.38 ± 0.21 | 0.11 ± 0.04 * | 0.12 ± 0.08 * | 0.21 ± 0.11 |
| HIF-1α | 0.38 ± 0.31 | 0.48 ± 0.33 | 0.82 ± 0.28 *† | 0.75 ± 0.18 *† |
| VEGF | 0.19 ± 0.40 | 0.30 ± 0.26 | 0.53 ± 0.24 *† | 0.58 ± 0.62 *† |
| CYP2c11 | 1.11 ± 0.45 | 0.69 ± 0.26 | 0.55 ± 0.29 | 2.21 ± 1.09 *†‡ |
| CTRL | A-HBO2 | 24H-HBO2 | 4D-HBO2 | |
|---|---|---|---|---|
| AA-259 [μg g−1] | 60.4 ± 20.39 | 48.7 ± 23.72 | 89.3 ± 34.19 † | 45.4 ± 17.23 |
| 11 (12)-EET [ng g−1] | 0.57 ± 0.17 | 0.63 ± 0.26 | 0.46 ± 0.25 | 0.39 ± 0.16 |
| 14 (15)-EET [ng g−1] | 0.49 ± 0.11 | 0.49 ± 0.23 | 0.47 ± 0.26 | 0.35 ± 0.22 |
| 20-HETE [ng g−1] | 3.94 ± 1.59 | 6.43 ± 1.65 *† | 4.64 ± 2.68 | 2.94 ± 1.51 |
| 14 (15)-DiHET [ng g−1] | 6.87 ± 0.92 | 9.84 ± 2.45 * | 9.83 ± 2.11 * | 7.51 ± 1.10 |
| 11 (12)-DiHET [ng g−1] | 2.62 ± 0.29 | 4.92 ± 1.74 * | 4.13 ± 1.30 * | 3.08 ± 0.53 |
| 5 (6)-DiHET [ng g−1] | 1.23 ± 0.41 | 1.12 ± 0.51 | 1.39 ± 0.23 † | 1.08 ± 0.13 |
| 8(9)-EET [ng g−1] | 0.98 ± 0.17 | 1.41 ± 0.35 *† | 1.50 ± 0.20 *† | 0.88 ± 0.36 |
| 8 (9)-DiHET [ng g−1] | 1.67 ± 0.32 | 5.14 ± 0.94 * | 4.75 ± 1.62 * | 3.45 ± 1.77 |
| 11 (12)-EET/20 HETE | 14 (15)-EET/20 HETE | 8 (9)-EET/20 HETE | 11 (12)-DiHET/20HETE | |
|---|---|---|---|---|
| CTRL | 0.158 ± 0.107 | 0.149 ± 0.078 | 0.317 ± 0.099 | 0.885 ± 0.391 |
| A-HBO2 | 0.098 ± 0.035 | 0.076 ± 0.033 *†‡ | 0.173 ± 0.055 *†‡ | 0.760 ± 0.179 *†‡ |
| 24H-HBO2 | 0.146 ± 0.084 | 0.131 ± 0.076 | 0.323 ± 0.173 | 1.228 ± 0.595 |
| 4D-HBO2 | 0.166 ± 0.107 | 0.151 ± 0.095 | 0.312 ± 0.187 | 1.313 ± 0.758 * |
| Analyte | Units | RT (min) | MRM Transition |
|---|---|---|---|
| 14(15)-DiHET | ng mL−1 | 7.09 | 337.100/207.100 Da |
| 11(12)-DiHET | ng mL−1 | 7.50 | 337.100/167.100 Da |
| 8(9)-DiHET | ng mL−1 | 7.87 | 337.100/185.100 Da |
| 19-HETE | ng mL−1 | 8.10 | 319.095/231.300 Da |
| 20-HETE | ng mL−1 | 8.27 | 319.095/245.300 Da |
| 5(6)-DiHET | ng mL−1 | 8.39 | 337.100/145.100 Da |
| 14(15)-EET | ng mL−1 | 10.8 | 319.143/219.100 Da |
| 11(12)-EET | ng mL−1 | 11.4 | 319.113/167.100 Da |
| 8(9)-EET | ng mL−1 | 11.6 | 319.113/127.100 Da |
| AA | μg mL−1 | 14.5 | 303.300/259.300 Da |
| Time [min] | %B |
|---|---|
| 0.10 | 25 |
| 7.5 | 50 |
| 12.5 | 60 |
| 16 | 95 |
| 18 | 95 |
| 18.01 | 25 |
| 20 | 25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaljević, Z.; Matić, A.; Stupin, A.; Frkanec, R.; Tavčar, B.; Kelava, V.; Tartaro Bujak, I.; Kolobarić, N.; Kibel, A.; Drenjančević, I. Arachidonic Acid Metabolites of CYP450 Enzymes and HIF-1α Modulate Endothelium-Dependent Vasorelaxation in Sprague-Dawley Rats under Acute and Intermittent Hyperbaric Oxygenation. Int. J. Mol. Sci. 2020, 21, 6353. https://doi.org/10.3390/ijms21176353
Mihaljević Z, Matić A, Stupin A, Frkanec R, Tavčar B, Kelava V, Tartaro Bujak I, Kolobarić N, Kibel A, Drenjančević I. Arachidonic Acid Metabolites of CYP450 Enzymes and HIF-1α Modulate Endothelium-Dependent Vasorelaxation in Sprague-Dawley Rats under Acute and Intermittent Hyperbaric Oxygenation. International Journal of Molecular Sciences. 2020; 21(17):6353. https://doi.org/10.3390/ijms21176353
Chicago/Turabian StyleMihaljević, Zrinka, Anita Matić, Ana Stupin, Ruža Frkanec, Branka Tavčar, Vanja Kelava, Ivana Tartaro Bujak, Nikolina Kolobarić, Aleksandar Kibel, and Ines Drenjančević. 2020. "Arachidonic Acid Metabolites of CYP450 Enzymes and HIF-1α Modulate Endothelium-Dependent Vasorelaxation in Sprague-Dawley Rats under Acute and Intermittent Hyperbaric Oxygenation" International Journal of Molecular Sciences 21, no. 17: 6353. https://doi.org/10.3390/ijms21176353
APA StyleMihaljević, Z., Matić, A., Stupin, A., Frkanec, R., Tavčar, B., Kelava, V., Tartaro Bujak, I., Kolobarić, N., Kibel, A., & Drenjančević, I. (2020). Arachidonic Acid Metabolites of CYP450 Enzymes and HIF-1α Modulate Endothelium-Dependent Vasorelaxation in Sprague-Dawley Rats under Acute and Intermittent Hyperbaric Oxygenation. International Journal of Molecular Sciences, 21(17), 6353. https://doi.org/10.3390/ijms21176353






