Targeted Therapies in Early Stage NSCLC: Hype or Hope?
Abstract
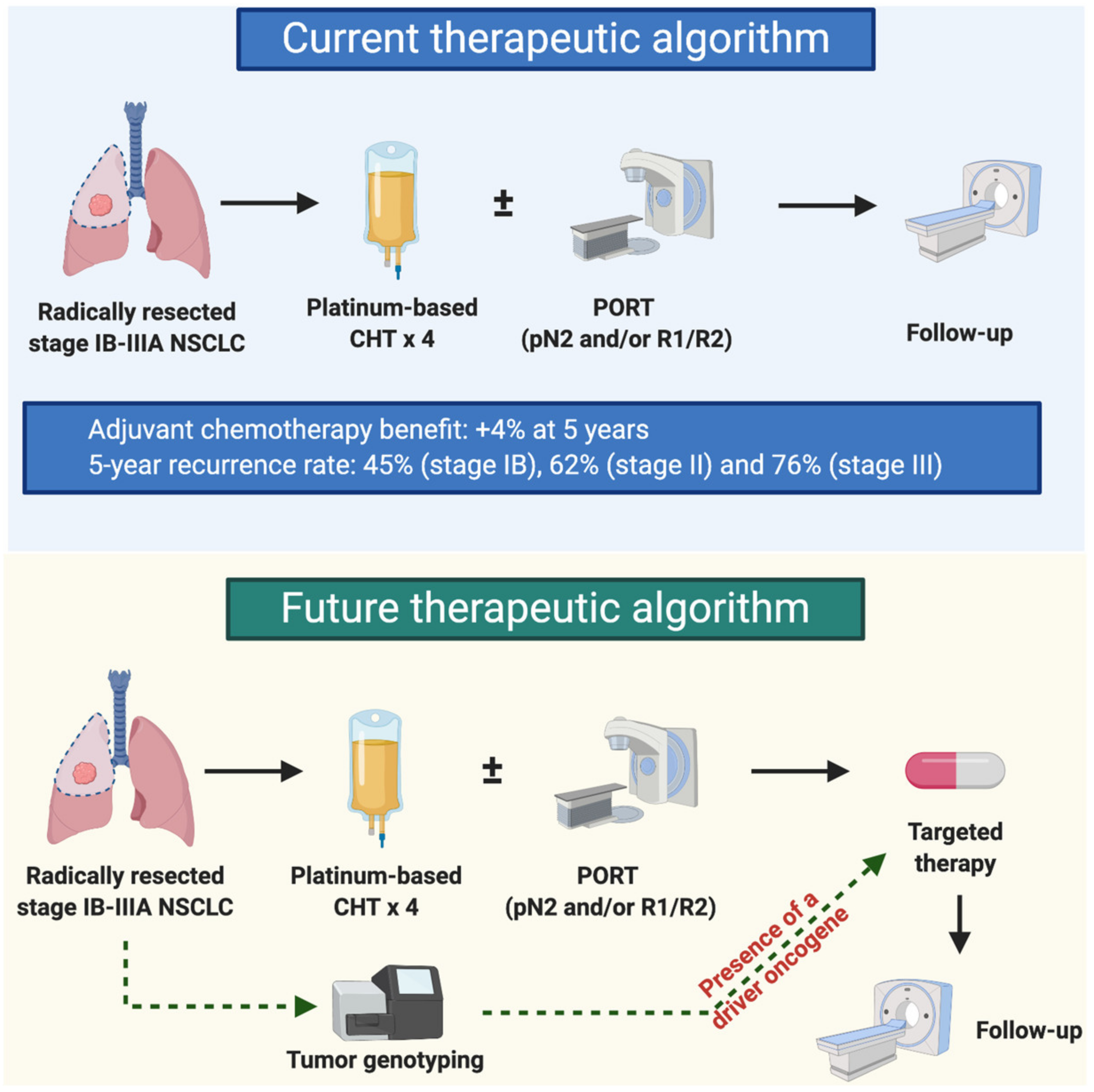
1. Introduction
2. Epidermal Growth Factor Receptor (EGFR) mutations
3. Anaplastic Lymphoma Kinase (ALK) Gene Fusions
4. Others Oncogenic Drivers
5. Limitations of TKIs in the Early Stage Setting
6. Future Directions and Challenges
Funding
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Gloeckler Ries, L.A.; Reichman, M.E.; Lewis, D.R.; Hankey, B.F.; Edwards, B.K. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist 2003, 8, 541–552. [Google Scholar] [CrossRef]
- Pisters, K.M.W.; Chevalier, T.L. Adjuvant Chemotherapy in Completely Resected Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 3270–3278. [Google Scholar] [CrossRef] [PubMed]
- Group, N.M.-a.C.; Arriagada, R.; Auperin, A.; Burdett, S.; Higgins, J.P.; Johnson, D.H.; Le Chevalier, T.; Le Pechoux, C.; Parmar, M.K.B.; Pignon, J.P.; et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 2014, 383, 1561–1571. [CrossRef]
- Kelly, K.; Altorki, N.K.; Eberhardt, W.E.; O’Brien, M.E.; Spigel, D.R.; Crinò, L.; Tsai, C.M.; Kim, J.H.; Cho, E.K.; Hoffman, P.C.; et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2015, 33, 4007–4014. [Google Scholar] [CrossRef]
- Goss, G.D.; O’Callaghan, C.; Lorimer, I.; Tsao, M.-S.; Masters, G.A.; Jett, J.; Edelman, M.J.; Lilenbaum, R.; Choy, H.; Khuri, F.; et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: Results of the NCIC CTG BR19 study. J. Clin. Oncol. 2013, 31, 3320–3326. [Google Scholar] [CrossRef]
- Pennell, N.A.; Neal, J.W.; Chaft, J.E.; Azzoli, C.G.; Jänne, P.A.; Govindan, R.; Evans, T.L.; Costa, D.B.; Wakelee, H.A.; Heist, R.S.; et al. SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients With Resected Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Zhong, W.; Wang, Q.; Mao, W.; Xu, S.-T.; Wu, L.; Chen, C.; Cheng, Y.; Xu, L.; Wang, J. CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation—Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J. Clin. Oncol. 2020, 38, 9005-9005. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Gray, J.; Ohe, Y.; Cho, B.; Vansteenkiste, J.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; Shah, R. Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): Final overall survival analysis. Ann. Oncol. 2019, 30, v914–v915. [Google Scholar] [CrossRef]
- Fukuoka, M.; Wu, Y.-L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.-S.; Sriuranpong, V.; Chao, T.-Y.; Nakagawa, K.; Chu, D.-T.; Saijo, N. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 2011, 29, 2866–2874. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Schuler, M.H.; Yamamoto, N.; O’Byrne, K.J.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.V.; Tsai, C.-M.; Boyer, M.J.; et al. LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J. Clin Oncol. 2012, 30, LBA7500-LBA7500. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhou, C.; Hu, C.-P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Chevallier, M.; Tsantoulis, P.; Addeo, A.; Friedlaender, A. Influence of Concurrent Mutations on Overall Survival in EGFR-mutated Non-small Cell Lung Cancer. Cancer Genom.Proteom. 2020, 17, 597–603. [Google Scholar] [CrossRef]
- Yue, D.; Xu, S.; Wang, Q.; Li, X.; Shen, Y.; Zhao, H.; Chen, C.; Mao, W.; Liu, W.; Liu, J.; et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): A randomised, open-label, phase 2 trial. Lancet. Respir. Med. 2018, 6, 863–873. [Google Scholar] [CrossRef]
- Herbst, R.S.; Tsuboi, M.; John, T.; Grohé, C.; Majem, M.; Goldman, J.W.; Kim, S.-W.; Marmol, D.; Rukazenkov, Y.; Wu, Y.-L. Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Lara-Guerra, H.; Waddell, T.K.; Salvarrey, M.A.; Joshua, A.M.; Chung, C.T.; Paul, N.; Boerner, S.; Sakurada, A.; Ludkovski, O.; Ma, C.; et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 6229–6236. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guerra, H.; Chung, C.T.; Schwock, J.; Pintilie, M.; Hwang, D.M.; Leighl, N.B.; Waddell, T.K.; Tsao, M.S. Histopathological and immunohistochemical features associated with clinical response to neoadjuvant gefitinib therapy in early stage non-small cell lung cancer. Lung Cancer 2012, 76, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yang, X.; Yan, H.; Zhang, X.; Su, J.; Chen, Z.; Liao, R.; Nie, Q.; Dong, S.; Zhou, Q.; et al. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol. Oncol. 2015, 8, 54. [Google Scholar] [CrossRef]
- Xiong, L.; Li, R.; Sun, J.; Lou, Y.; Zhang, W.; Bai, H.; Wang, H.; Shen, J.; Jing, B.; Shi, C.; et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2) EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study. Oncologist 2019, 24, e157–e164. [Google Scholar] [CrossRef]
- Xiong, L.; Lou, Y.; Bai, H.; Li, R.; Xia, J.; Fang, W.; Zhang, J.; Han-Zhang, H.; Lizaso, A.; Li, B.; et al. Efficacy of erlotinib as neoadjuvant regimen in EGFR-mutant locally advanced non-small cell lung cancer patients. J. Int. Med. Res. 2019. [Google Scholar] [CrossRef]
- Chaft, J.E.; Oxnard, G.R.; Sima, C.S.; Kris, M.G.; Miller, V.A.; Riely, G.J. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin. Cancer Res. 2011, 17, 6298–6303. [Google Scholar] [CrossRef]
- Addeo, A.; Tabbò, F.; Robinson, T.; Buffoni, L.; Novello, S. Precision medicine in ALK rearranged NSCLC: A rapidly evolving scenario. Crit. Rev. Oncol. Hematol. 2018, 122, 150–156. [Google Scholar] [CrossRef]
- Friedlaender, A.; Banna, G.; Patel, S.; Addeo, A. Diagnosis and Treatment of ALK Aberrations in Metastatic NSCLC. Curr. Treat. Opt. Oncol. 2019, 20, 79. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Massó-Vallés, D.; Beaulieu, M.-E.; Soucek, L. MYC, MYCL, and MYCN as therapeutic targets in lung cancer. Expert Opin. Ther. Targets 2020, 24, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Frisone, D.; Friedlaender, A.; Malapelle, U.; Banna, G.; Addeo, A. A BRAF new world. Crit. Rev. Oncol. Hematol. 2020, 152, 103008. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; Soo, R.A.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef]
- Groen, H.J.; Akerley, W.L.; Souquet, P.J.; Laack, E.; Han, J.-Y.; Smit, E.F.; Mansfield, A.S.; Garon, E.B.; Wolf, J.; Tan, D.S.-W. Capmatinib in patients with METex14-mutated or high-level MET-amplified advanced non–small-cell lung cancer (NSCLC): Results from cohort 6 of the phase 2 GEOMETRY mono-1 study. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Friedlaender, A.; Drilon, A.; Banna, G.L.; Peters, S.; Addeo, A. The METeoric rise of MET in lung cancer. Cancer 2020. [Google Scholar]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Lee, D.H.; Subbiah, V.; Gainor, J.F.; Taylor, M.H.; Zhu, V.W.; Doebele, R.C.; Lopes, G.; Baik, C.; Garralda, E.; Gadgeel, S.M.; et al. Treatment with pralsetinib (formerly BLU-667), a potent and selective RET inhibitor, provides rapid clearance of ctDNA in patients with RET-altered non-small cell lung cancer (NSCLC) and medullary thyroid cancer (MTC). Ann. Oncol. 2019, 30, ix122. [Google Scholar] [CrossRef]
- Friedlaender, A.; Drilon, A.; Weiss, G.J.; Banna, G.L.; Addeo, A. KRAS as a druggable target in NSCLC: Rising like a phoenix after decades of development failures. Cancer Treat. Rev. 2020, 85, 101978. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.F.; Nakagawa, K.; Nagasaka, M.; Felip, E.; Goto, Y.; Li, B.T.; Pacheco, J.M.; Murakami, H.; Barlesi, F.; Saltos, A.N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J. Clin. Oncol. 2020, 38, 9504-9504. [Google Scholar] [CrossRef]
- Vallette, F.M.; Olivier, C.; Lézot, F.; Oliver, L.; Cochonneau, D.; Lalier, L.; Cartron, P.F.; Heymann, D. Dormant, quiescent, tolerant and persister cells: Four synonyms for the same target in cancer. Biochem. Pharmacol. 2019, 162, 169–176. [Google Scholar] [CrossRef]
- Recasens, A.; Munoz, L. Targeting Cancer Cell Dormancy. Trends Pharmacol. Sci. 2019, 40, 128–141. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Brannon, A.R.; Ferris, L.A.; Campbell, C.D.; Lin, J.J.; Schultz, K.R.; Ackil, J.; Stevens, S.; Dardaei, L.; Yoda, S.; et al. Tracking the Evolution of Resistance to ALK Tyrosine Kinase Inhibitors through Longitudinal Analysis of Circulating Tumor DNA. JCO Precis Oncol. 2018, 2018. [Google Scholar] [CrossRef]
- Chabon, J.J.; Simmons, A.D.; Lovejoy, A.F.; Esfahani, M.S.; Newman, A.M.; Haringsma, H.J.; Kurtz, D.M.; Stehr, H.; Scherer, F.; Karlovich, C.A.; et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat. Commun. 2016, 7, 11815. [Google Scholar] [CrossRef]
| Study | Phase | Population | n | Arm(s) | Patients Receiving Adjuvant Chemotherapy (%) | Median DFS (mos) | 2-Year DFS | 3-Year DFS | Median OS (mos) |
|---|---|---|---|---|---|---|---|---|---|
| RADIANT [9] | III | IB-IIIA NSCLCs, EGFR-positive by IHC and/or FISH | 623 vs. 250 | Erlotinib for 2 years vs. placebo | 50.6% vs. 57.1% | 50.5 vs. 48.2 (HR 0.90) | 75% vs. 54% | N.R. | Not reached vs. Not reached (HR 1.09) |
| BR19 [10] | III | IB-IIIA NSCLCs | 251 vs. 252 | Gefitinib for 2 years vs. placebo | 17% vs. 17% | 4.2 years vs. Not reached (HR 1.22) | N.R. | N.R. | 5.1 years vs. Not reached (HR 1.24) |
| SELECT [11] | II | IA-IIIA EGFR-mutated NSCLC | 100 | Erlotinib for 2 years | N.R. | Not reached | 88% | N.R. | Not reached |
| CTONG1104 ADJUVANT [12] | III | II-IIIA EGFR-mutated NSCLC | 111 vs. 111 | Gefitinib for 2 years vs. vinorelbine/cisplatin | 0% vs. 100% | 30.8 vs. 19.8 (HR 0.56) | N.R. | 39.6% vs. 32.5% | 75.5 vs. 62.8 (HR 0.92) |
| EVAN [20] | II | IIIA EGFR-mutated NSCLC | 51 vs. 51 | Erlotinib for 2 years vs. vinorelbine/cisplatin | 0% vs. 100% | 42.4 vs. 21.0 (HR 0.268) | 81.4% vs. 44.6% | 54.2% vs. 19.8% | Not reached vs. Not reached (HR 0.165) |
| ADAURA [21] | III | IB-IIIA EGFR-mutated NSCLC | 339 vs. 343 | Osimertinib for 3 years vs. placebo | 55% vs. 56% | Not reached vs. 20.4 (HR 0.17) * | 90% * vs. 44% * | 80% * vs. 28% * | Not reached vs. Not reached (HR 0.40) * |
| Trial | Phase | Design | Population | Arm(s) | Primary Outcome | Clinical Trial Identification |
|---|---|---|---|---|---|---|
| ALCHEMIST | III | adjuvant | IB-IIIA NSCLCs, EGFR-mutated NSCLC | erlotinib for 2 years vs. placebo | OS | NCT02194738 |
| ALCHEMIST | III | adjuvant | IB-IIIA NSCLCs, ALK-rearranged NSCLC | crizotinib for 2 years vs. placebo | OS | NCT02194738 |
| ALINA | III | adjuvant | IB-IIIA NSCLCs, ALK-rearranged NSCLC | alectinib for 2 years vs. chemotherapy | DFS | NCT03456076 |
| EMERGING | II | Neoadjuvant + adjuvant | IIIA EGFR-mutated NSCLCs | erlotinib for 6 weeks then 1 year post-op vs. cisplatin-gemcitabine | ORR | NCT01407822 |
| NCT03203590 | III | neoadjuvant | II-IIIA EGFR-mutated NSCLC | gefinitib for 8 weeks vs. carboplatin-vinorelbine | 2 year DFS | NCT03203590 |
| NeoADAURA | III | neoadjuvant | II-IIIA EGFR-mutated NSCLC | osimertinib +/− platinum-pemetrexed vs. platinum-pemetrexed | MPR | NCT04351555 |
| NCT04302025 | II | Neoadjuvant +/− adjuvant | IB-IIIB NSCLC with altered ALK, ROS1, NTRK or BRAF | 8 weeks neoadjuvant +/− adjuvant with alectinib, entrectinib or vemurafenib+cobimetinib | MPR | NCT04302025 |
| NCT03088930 | II | neoadjuvant | IA-IIIA NSCLC with altered MET, ROS1 or ALK | crizotinib for 6 weeks | ORR | NCT03088930 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedlaender, A.; Addeo, A.; Russo, A.; Gregorc, V.; Cortinovis, D.; Rolfo, C.D. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int. J. Mol. Sci. 2020, 21, 6329. https://doi.org/10.3390/ijms21176329
Friedlaender A, Addeo A, Russo A, Gregorc V, Cortinovis D, Rolfo CD. Targeted Therapies in Early Stage NSCLC: Hype or Hope? International Journal of Molecular Sciences. 2020; 21(17):6329. https://doi.org/10.3390/ijms21176329
Chicago/Turabian StyleFriedlaender, Alex, Alfredo Addeo, Alessandro Russo, Vanesa Gregorc, Diego Cortinovis, and Christian D. Rolfo. 2020. "Targeted Therapies in Early Stage NSCLC: Hype or Hope?" International Journal of Molecular Sciences 21, no. 17: 6329. https://doi.org/10.3390/ijms21176329
APA StyleFriedlaender, A., Addeo, A., Russo, A., Gregorc, V., Cortinovis, D., & Rolfo, C. D. (2020). Targeted Therapies in Early Stage NSCLC: Hype or Hope? International Journal of Molecular Sciences, 21(17), 6329. https://doi.org/10.3390/ijms21176329








