Investigation of a Staphylococcus argenteus Strain Involved in a Chronic Prosthetic-Joint Infection
Abstract
1. Introduction
2. Results
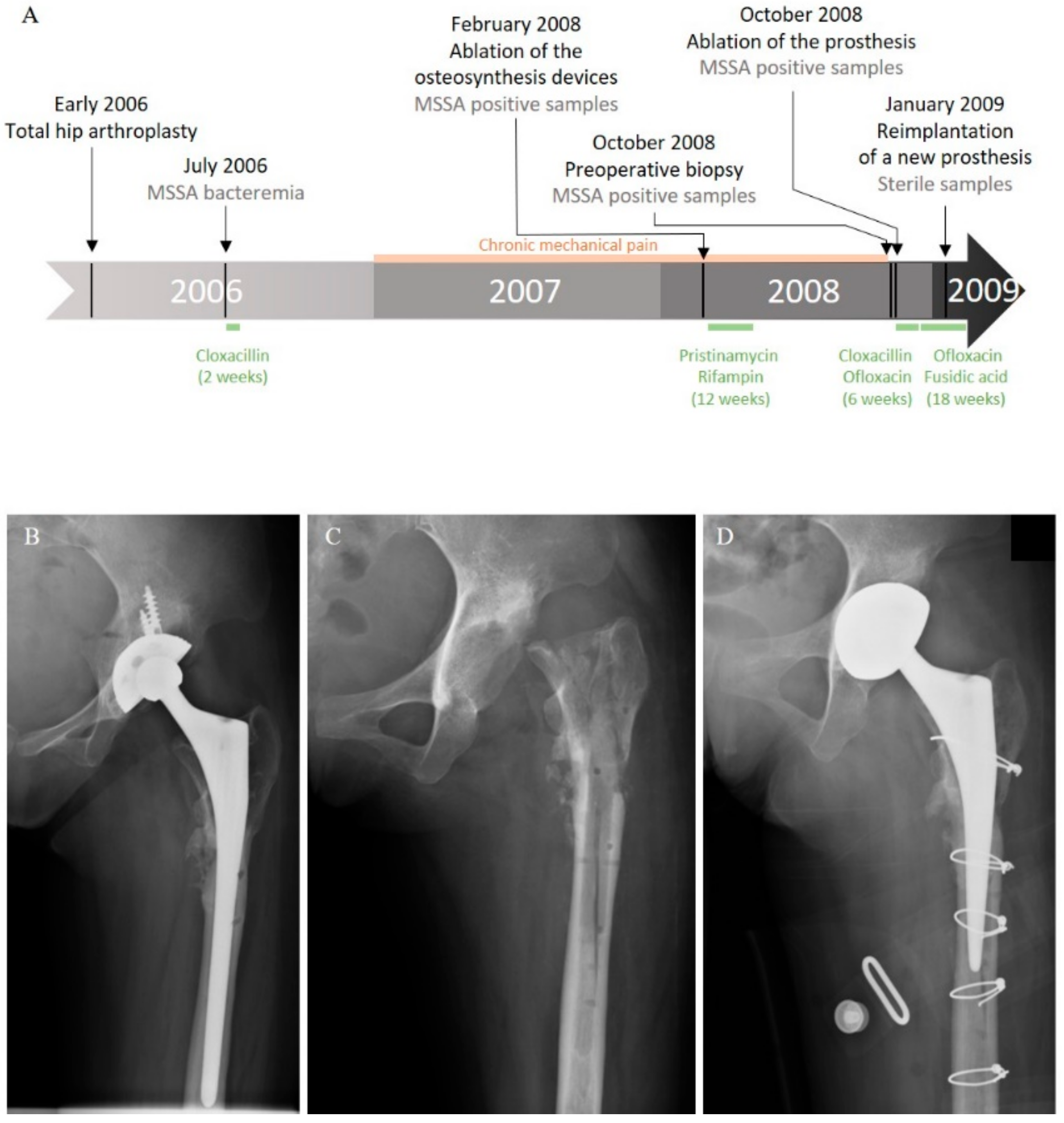
2.1. Clinical History
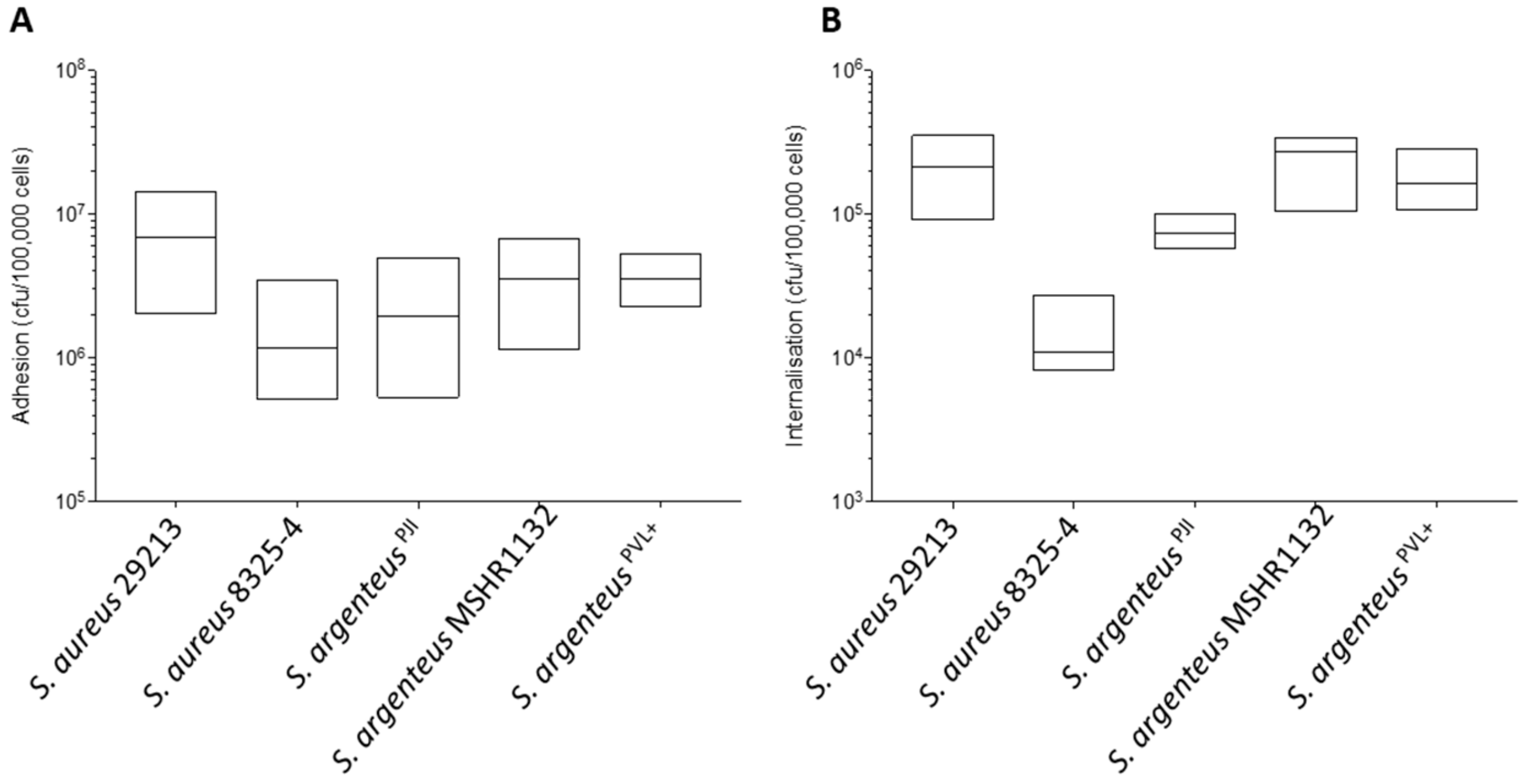
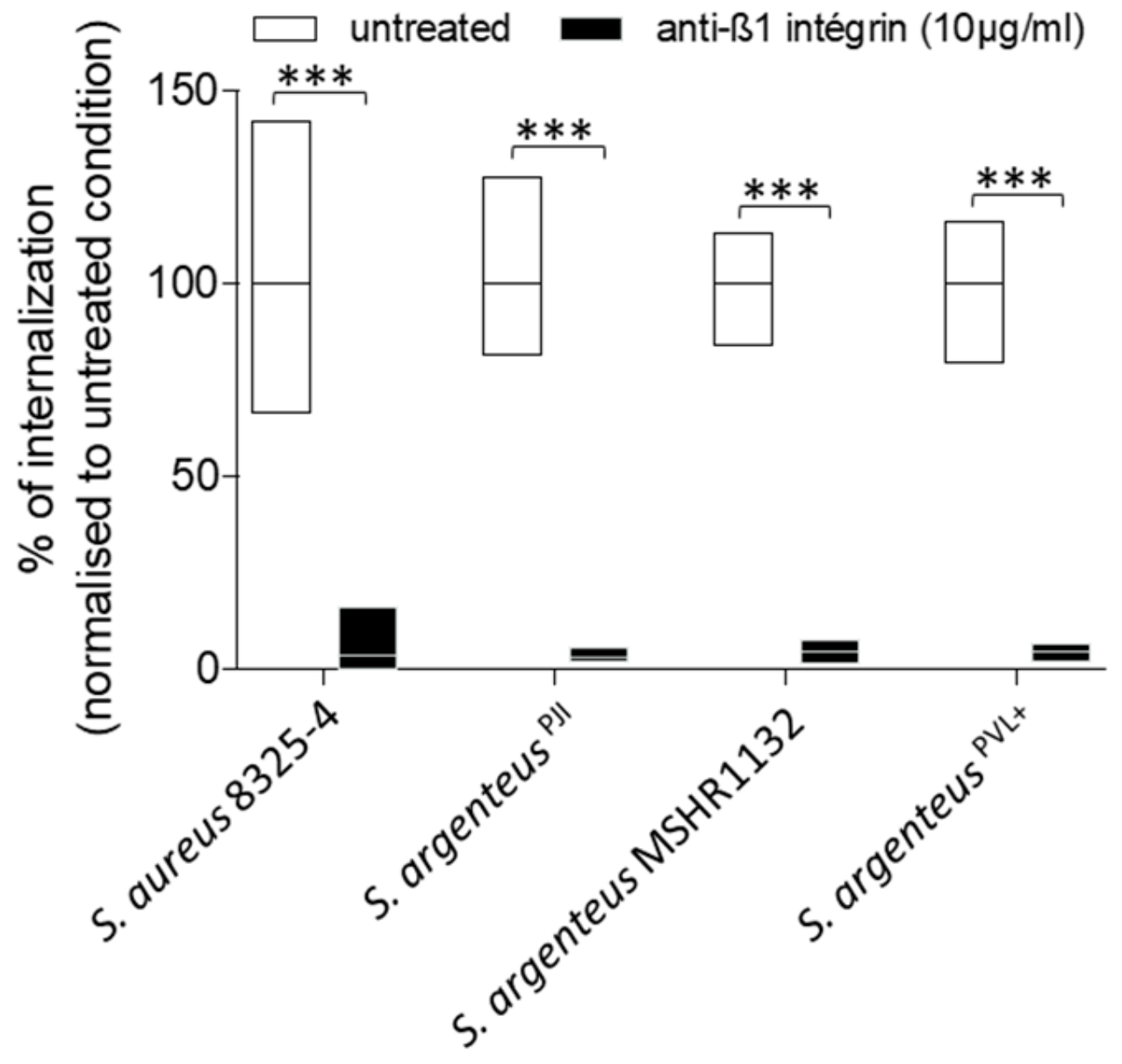
2.2. Cellular Invasion Properties
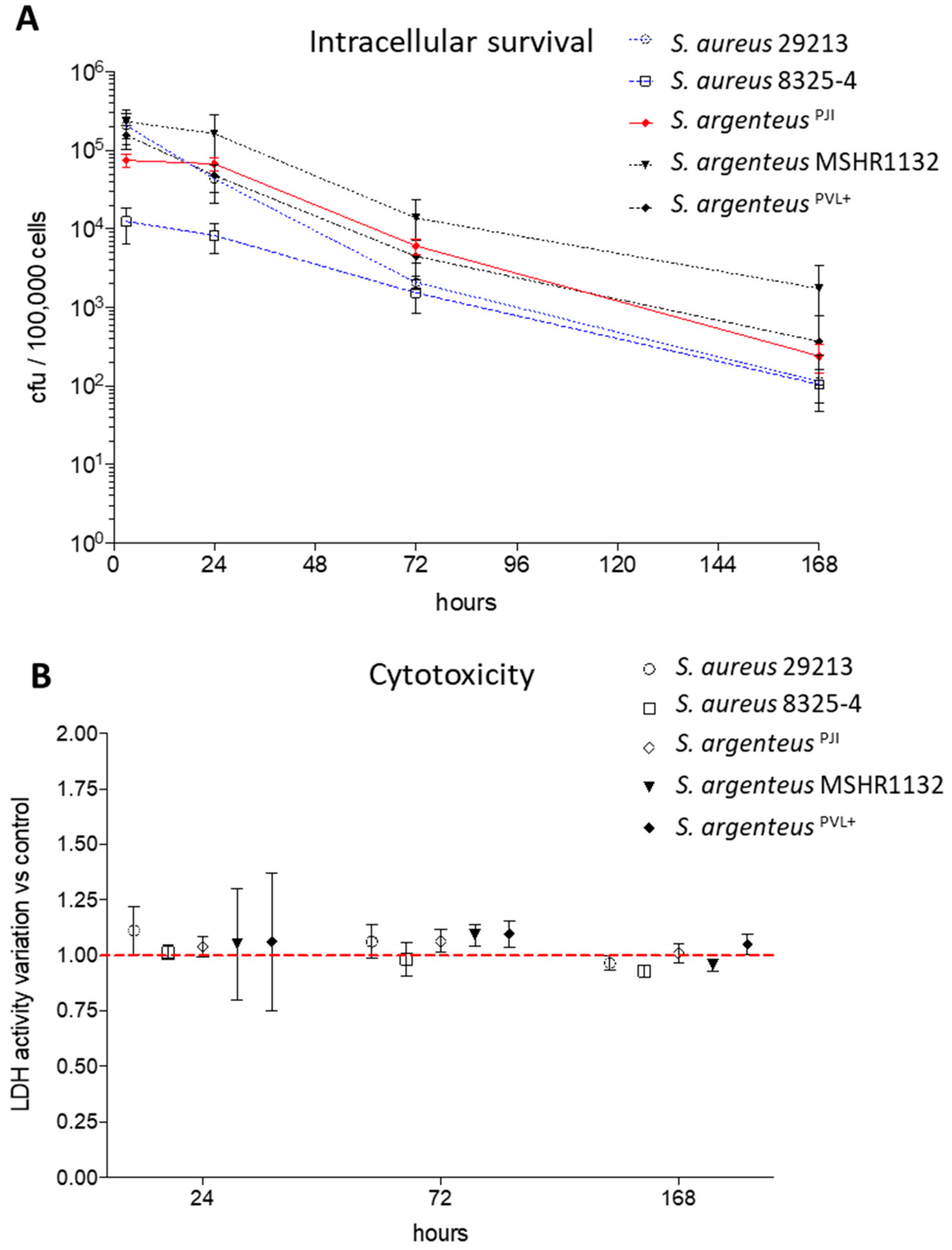
2.3. Intracellular Survival
2.4. Cell Death Induction by Intracellular Staphylococci
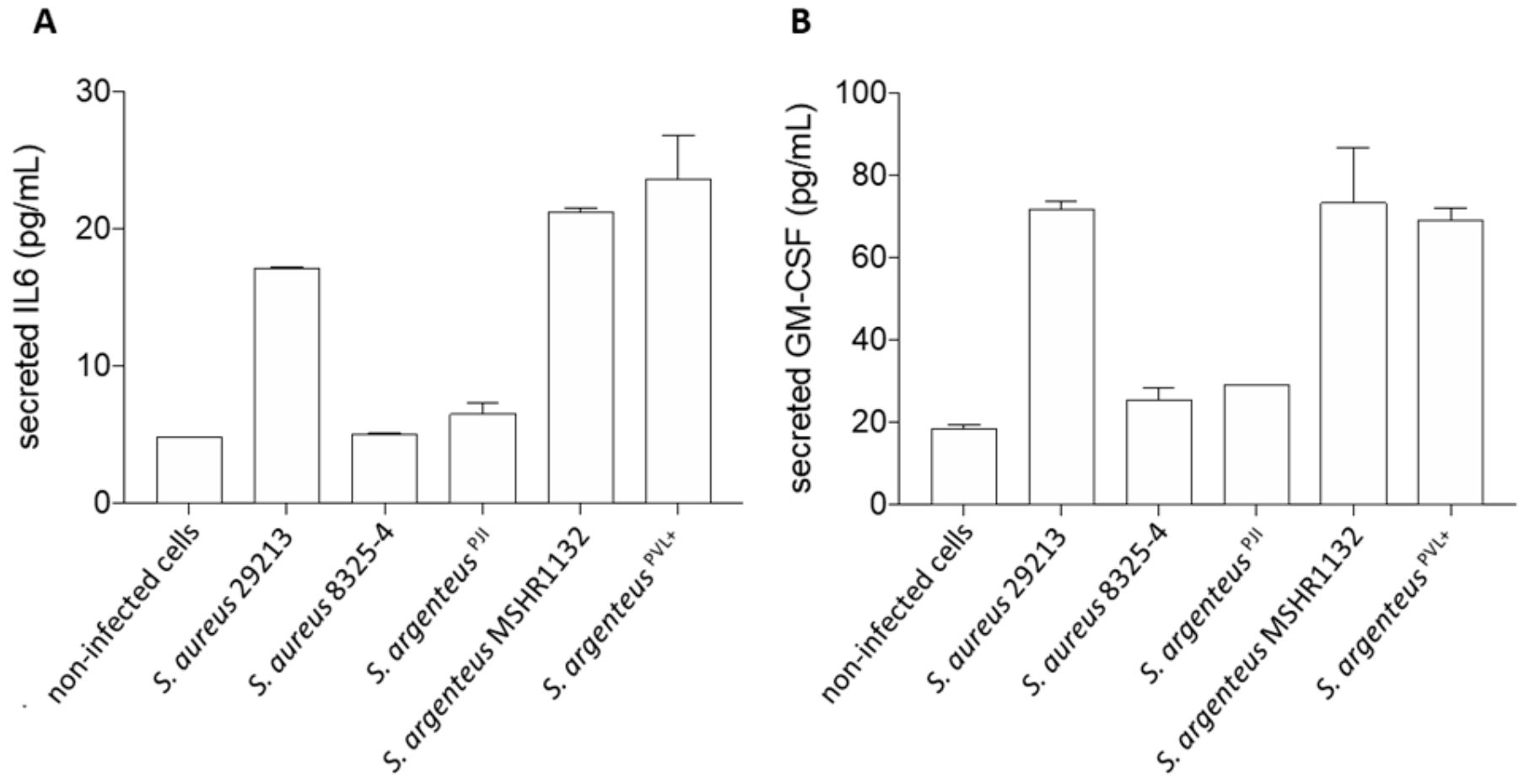
2.5. Induction of a Cytokinic Response
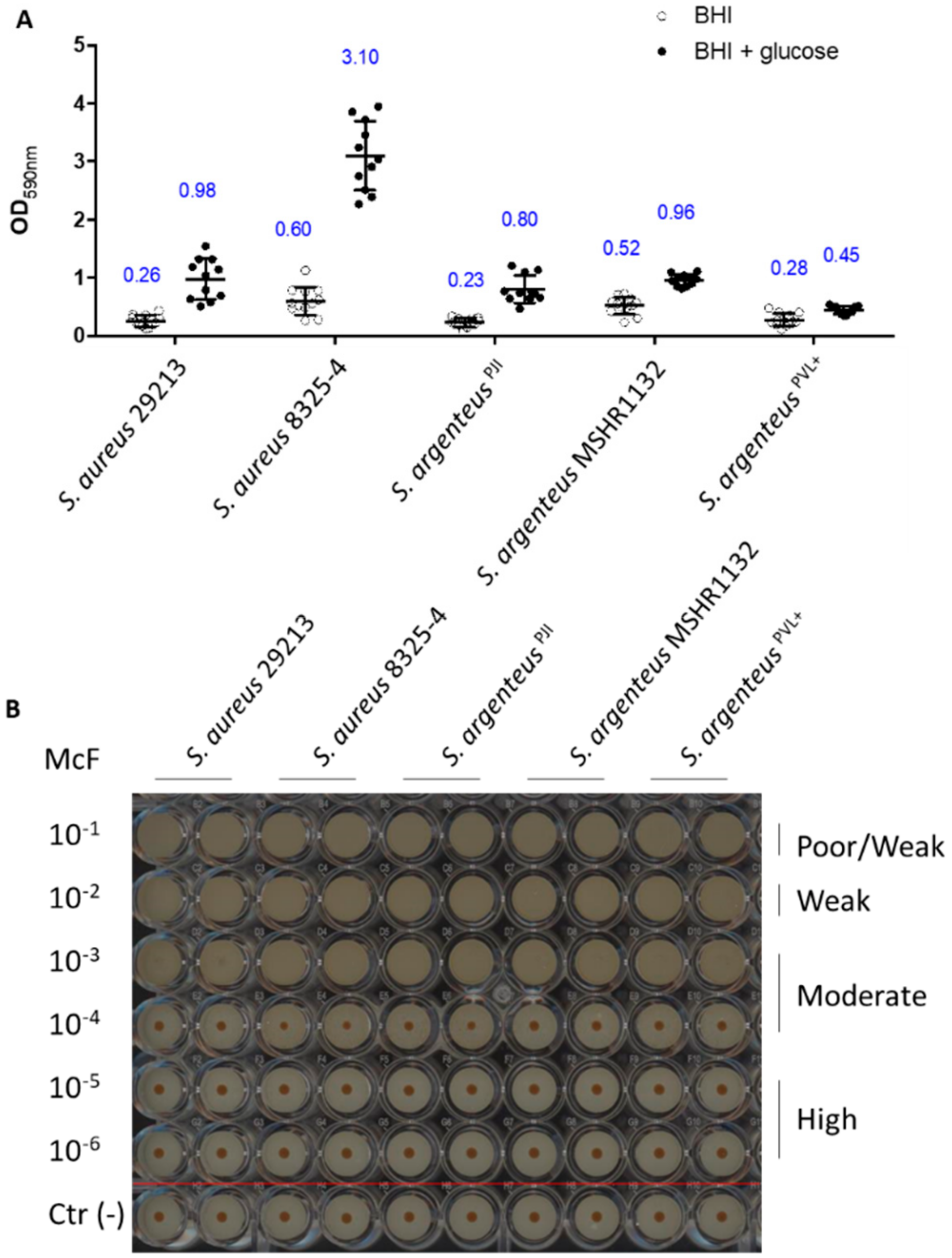
2.6. Assessment of the Ability to Form Biofilm
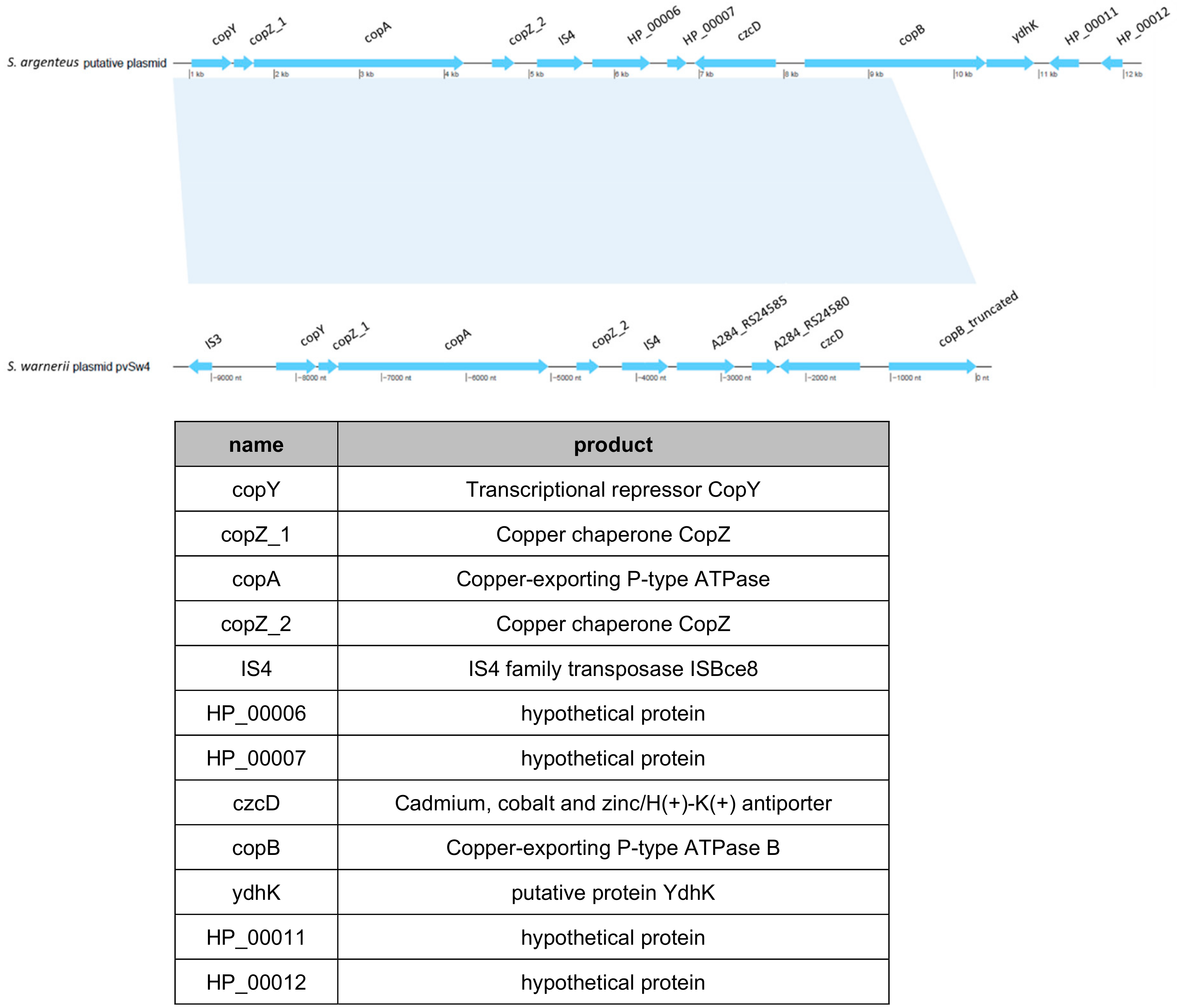
2.7. Genomic Analysis of Staphylococcus argenteusPJI
3. Discussion
4. Materials and Methods
4.1. Cell Line and Bacteria Strains
4.2. Cell Culture Conditions
4.3. Infection and Gentamicin Protection Assay
4.4. Cell Death Assessment
4.5. Cytokines Secretion by Infected Osteoblasts
4.6. Biofilm Formation
4.7. Whole Genome Sequencing
4.8. Study Protocol and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BJI | Bone and Joint Infection |
| CFU | Colony Forming Unit |
| FnBP | Fibronectin Binding Protein |
| GM-CSF | Granulocyte Macrophage Colony Stimulating Factor |
| LDH | Lactate Deshydrogenase |
| MRSA | Meticillin Resistant Staphylococcus aureus |
| MSCRAMM | microbial surface components recognizing adhesive matrix molecule |
| MSSA | Meticillin Susceptible Staphylococcus aureus |
| NPPC | Non Professional Phagocytic Cell |
| PJI | Prosthetic Joint Infection |
| PSM | Phenol Soluble Modulin |
| PVL | Panton Valentine Leukocidin |
| SCV | Small Colony Variant |
References
- Holt, D.C.; Holden, M.T.G.; Tong, S.Y.C.; Castillo-Ramirez, S.; Clarke, L.; Quail, M.A.; Currie, B.J.; Parkhill, J.; Bentley, S.D.; Feil, E.J.; et al. A Very Early-Branching Staphylococcus aureus Lineage Lacking the Carotenoid Pigment Staphyloxanthin. Genome Biol. Evol. 2011, 3, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Moradigaravand, D.; Jamrozy, D.; Mostowy, R.; Anderson, A.; Nickerson, E.K.; Thaipadungpanit, J.; Wuthiekanun, V.; Limmathurotsakul, D.; Tandhavanant, S.; Wikraiphat, C.; et al. Evolution of the Staphylococcus argenteus ST2250 Clone in Northeastern Thailand Is Linked with the Acquisition of Livestock-Associated Staphylococcal Genes. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.R.; Thomas, M.G.; Rainey, P.B. The genetic structure of Staphylococcus aureus populations from the Southwest Pacific. PLoS ONE 2014, 9, e100300. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Dodémont, M.; Vandendriessche, S.; Rottiers, S.; Tribes, C.; Roisin, S.; de Mendonça, R.; Nonhoff, C.; Deplano, A.; Denis, O. Low occurrence of the new species Staphylococcus argenteus in a Staphylococcus aureus collection of human isolates from Belgium. Eur. J. Clin. Microbiol. Infect Dis. 2016, 35, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Rigaill, J.; Grattard, F.; Grange, S.; Forest, F.; Haddad, E.; Carricajo, A.; Tristan, A.; Laurent, F.; Botelho-Nevers, E.; Verhoeven, P.O. Community-Acquired Staphylococcus argenteus Sequence Type 2250 Bone and Joint Infection, France, 2017. Emerg. Infect Dis. 2018, 24, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef]
- Löffler, B.; Tuchscherr, L.; Niemann, S.; Peters, G. Staphylococcus aureus persistence in non-professional phagocytes. Int. J. Med. Microbiol. 2014, 304, 170–176. [Google Scholar] [CrossRef]
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet. Med. 2018. [Google Scholar] [CrossRef]
- Abel, J.; Goldmann, O.; Ziegler, C.; Höltje, C.; Smeltzer, M.S.; Cheung, A.L.; Bruhn, D.; Rohde, M.; Medina, E. Staphylococcus aureus Evades the Extracellular Antimicrobial Activity of Mast Cells by Promoting Its Own Uptake. JIN 2011, 3, 495–507. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Medina, E.; Hussain, M.; Völker, W.; Heitmann, V.; Niemann, S.; Holzinger, D.; Roth, J.; Proctor, R.A.; Becker, K.; et al. Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 2011, 3, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Tran Van Nhieu, G.; Isberg, R.R. Bacterial internalization mediated by beta 1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 1993, 12, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; François, P.P.; Nüße, O.; Foti, M.; Hartford, O.M.; Vaudaux, P.; Foster, T.J.; Lew, D.P.; Herrmann, M.; Krause, K.-H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1999, 1, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Grundmeier, M.; Hussain, M.; Becker, P.; Heilmann, C.; Peters, G.; Sinha, B. Truncation of Fibronectin-Binding Proteins in Staphylococcus aureus Strain Newman Leads to Deficient Adherence and Host Cell Invasion Due to Loss of the Cell Wall Anchor Function. Infect Immun. 2004, 72, 7155–7163. [Google Scholar] [CrossRef] [PubMed]
- Suci, P.A.; Mittelman, M.W.; Yu, F.P.; Geesey, G.G. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 1994, 38, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J.; Brown, M.R.; Allison, D.G.; Gilbert, P. Susceptibility of bacterial biofilms to tobramycin: Role of specific growth rate and phase in the division cycle. J. Antimicrob. Chemother. 1990, 25, 585–591. [Google Scholar] [CrossRef]
- Zhang, L.; Mah, T.-F. Involvement of a Novel Efflux System in Biofilm-Specific Resistance to Antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef]
- Dupieux, C.; Blondé, R.; Bouchiat, C.; Meugnier, H.; Bes, M.; Laurent, S.; Vandenesch, F.; Laurent, F.; Tristan, A. Community-acquired infections due to Staphylococcus argenteus lineage isolates harbouring the Panton-Valentine leucocidin, France, 2014. Eurosurveillance 2015, 20, 21154. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Umeda, K.; Yonogi, S.; Nakamura, H.; Yamamoto, K.; Kumeda, Y.; Kawatsu, K. Staphylococcal food poisoning caused by Staphylococcus argenteus harboring staphylococcal enterotoxin genes. Int. J. Food Microbiol. 2018, 265, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; San, T.; Aye, M.M.; Mya, S.; Maw, W.W.; Zan, K.N.; Htut, W.H.W.; Kawaguchiya, M.; Urushibara, N.; Kobayashi, N. Prevalence and Genetic Characteristics of Staphylococcus aureus and Staphylococcus argenteus Isolates Harboring Panton-Valentine Leukocidin, Enterotoxins, and TSST-1 Genes from Food Handlers in Myanmar. Toxins 2017, 9, 241. [Google Scholar] [CrossRef]
- Jiang, B.; You, B.; Tan, L.; Yu, S.; Li, H.; Bai, G.; Li, S.; Rao, X.; Xie, Z.; Shi, X.; et al. Clinical Staphylococcus argenteus Develops to Small Colony Variants to Promote Persistent Infection. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Jatzwauk, L.; Weber, S.; Slickers, P.; Ehricht, R. DNA microarray-based genotyping of methicillin-resistant Staphylococcus aureus strains from Eastern Saxony. Clin. Microbiol. Infect. 2008, 14, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Slickers, P.; Ehricht, R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol. Med. Microbiol. 2008, 53, 237–251. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T.; et al. Development of an in vitro Assay, Based on the BioFilm Ring Test®, for Rapid Profiling of Biofilm-Growing Bacteria. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Waldrop, R.; McLaren, A.; Calara, F.; McLemore, R. Biofilm Growth Has a Threshold Response to Glucose in Vitro. Clin. Orthop. Relat. Res. 2014, 472, 3305–3310. [Google Scholar] [CrossRef]
- Cheng, V.W.T.; Zhang, G.; Oyedotun, K.S.; Ridgway, D.; Ellison, M.J.; Weiner, J.H. Complete Genome of the Solvent-Tolerant Staphylococcus warneri Strain SG1. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Anton, A.; Große, C.; Reißmann, J.; Pribyl, T.; Nies, D.H. CzcD Is a Heavy Metal Ion Transporter Involved in Regulation of Heavy Metal Resistance in Ralstonia sp. Strain CH34. J. Bacteriol. 1999, 181, 6876–6881. [Google Scholar] [CrossRef]
- Ding, C.; Festa, R.A.; Chen, Y.-L.; Espart, A.; Palacios, Ò.; Espín, J.; Capdevila, M.; Atrian, S.; Heitman, J.; Thiele, D.J. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host. Microbe 2013, 13, 265–276. [Google Scholar] [CrossRef]
- Wiemann, P.; Perevitsky, A.; Lim, F.Y.; Shadkchan, Y.; Knox, B.P.; Landero Figueora, J.A.; Choera, T.; Niu, M.; Steinberger, A.J.; Wüthrich, M.; et al. Host copper-mediated oxidative antimicrobial offense is countered by Aspergillus fumigatus copper export machinery and reactive oxygen intermediate defense. Cell Rep. 2017, 19, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Wolschendorf, F.; Ackart, D.; Shrestha, T.B.; Hascall-Dove, L.; Nolan, S.; Lamichhane, G.; Wang, Y.; Bossmann, S.H.; Basaraba, R.J.; Niederweis, M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2011, 108, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Gleason, J.E.; Zhang, S.X.; Bruno, V.M.; Cormack, B.P.; Culotta, V.C. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc. Natl. Acad. Sci. USA 2015, 112, E5336–E5342. [Google Scholar] [CrossRef] [PubMed]
- Zapotoczna, M.; Riboldi, G.P.; Moustafa, A.M.; Dickson, E.; Narechania, A.; Morrissey, J.A.; Planet, P.J.; Holden, M.T.G.; Waldron, K.J.; Geoghegan, J.A. Mobile-Genetic-Element-Encoded Hypertolerance to Copper Protects Staphylococcus aureus from Killing by Host Phagocytes. MBio 2018, 9. [Google Scholar] [CrossRef]
- Tajima, A.; Iwase, T.; Shinji, H.; Seki, K.; Mizunoe, Y. Inhibition of endothelial interleukin-8 production and neutrophil transmigration by Staphylococcus aureus beta-hemolysin. Infect. Immun. 2009, 77, 327–334. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: The non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 15–22. [Google Scholar] [CrossRef]
- Tasse, J.; Cara, A.; Saglio, M.; Villet, R.; Laurent, F. A steam-based method to investigate biofilm. Sci. Rep. 2018, 8, 13040. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Abricate, Github. Available online: https://github.com/tseemann/abricate (accessed on 28 August 2020).
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Kaya, H.; Hasman, H.; Larsen, J.; Stegger, M.; Johannesen, T.B.; Allesøe, R.L.; Lemvigh, C.K.; Aarestrup, F.M.; Lund, O.; Larsen, A.R. SCCmecFinder, a Web-Based Tool for Typing of Staphylococcal Cassette Chromosome mec in Staphylococcus aureus Using Whole-Genome Sequence Data. mSphere 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Tritt, A.; Eisen, J.A.; Facciotti, M.T.; Darling, A.E. An Integrated Pipeline for de Novo Assembly of Microbial Genomes. PLoS ONE 2012, 7, e42304. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
| Antibiotic | Susceptibility | Antibiotic | Susceptibility | Antibiotic | Susceptibility |
|---|---|---|---|---|---|
| Peni-G | R | Erythromycin | R | Vancomycin | S |
| Peni-M | S | Lincomycin | R | Teicoplanin | S |
| Gentamicin | S | Synergistins | S | Fosfomycin | S |
| Tobramycin | S | Rifampicin | S | Fusidic acid | S |
| Kanamycin | S | Nitrofurantoin | S | Linezolid | S |
| Tetracyclin | S | Cotrimoxazol | S | ||
| Minocyclin | S | Ofloxacin | S |
| S. argenteusPJI | S. argenteus MSHR1132 | S. argenteusPVL+ | |
|---|---|---|---|
| PSM genes | |||
| PSM_alpha1 | + | + | + |
| PSM_alpha2 | + | + | + |
| PSM_alpha3 | + | + | + |
| PSM_alpha4 | + | + | - |
| PSM_beta1 | + | + | + |
| Biofilm formation | |||
| ebpS | + | + | + |
| icaA | + | + | + |
| icaB | + | + | + |
| icaC | + | + | + |
| fib | + | + | + |
| eno | + | + | + |
| bbp | + | + | + |
| clfA | + | + | - |
| clfB | + | + | - |
| Internalisation | |||
| fnbpA | + | + | + |
| fnbpB | + | + | + |
| atl | + | + | + |
| clfA | + | + | + |
| sdrD | + | + | - |
| tet38 | + | + | + |
| sraP | + | + | - |
| eap | + | + | + |
| gapC | + | + | + |
| Persistence | |||
| sdhA | + | + | + |
| sdhB | + | + | + |
| ureG | + | + | + |
| mnhG | + | + | + |
| fbaA | + | + | + |
| ctaB | + | + | + |
| mazF | + | + | + |
| glpX | + | + | + |
| clpX | + | + | + |
| parE | + | + | + |
| Leukocidins | |||
| hglA | + | + | + |
| hglB | + | + | + |
| hglC | + | + | + |
| Virulence | |||
| sigB | + | + | + |
| agrA | + | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diot, A.; Dyon-Tafani, V.; Bergot, M.; Tasse, J.; Martins-Simões, P.; Josse, J.; Valour, F.; Laurent, F. Investigation of a Staphylococcus argenteus Strain Involved in a Chronic Prosthetic-Joint Infection. Int. J. Mol. Sci. 2020, 21, 6245. https://doi.org/10.3390/ijms21176245
Diot A, Dyon-Tafani V, Bergot M, Tasse J, Martins-Simões P, Josse J, Valour F, Laurent F. Investigation of a Staphylococcus argenteus Strain Involved in a Chronic Prosthetic-Joint Infection. International Journal of Molecular Sciences. 2020; 21(17):6245. https://doi.org/10.3390/ijms21176245
Chicago/Turabian StyleDiot, Alan, Virginie Dyon-Tafani, Marine Bergot, Jason Tasse, Patricia Martins-Simões, Jérôme Josse, Florent Valour, and Frédéric Laurent. 2020. "Investigation of a Staphylococcus argenteus Strain Involved in a Chronic Prosthetic-Joint Infection" International Journal of Molecular Sciences 21, no. 17: 6245. https://doi.org/10.3390/ijms21176245
APA StyleDiot, A., Dyon-Tafani, V., Bergot, M., Tasse, J., Martins-Simões, P., Josse, J., Valour, F., & Laurent, F. (2020). Investigation of a Staphylococcus argenteus Strain Involved in a Chronic Prosthetic-Joint Infection. International Journal of Molecular Sciences, 21(17), 6245. https://doi.org/10.3390/ijms21176245





