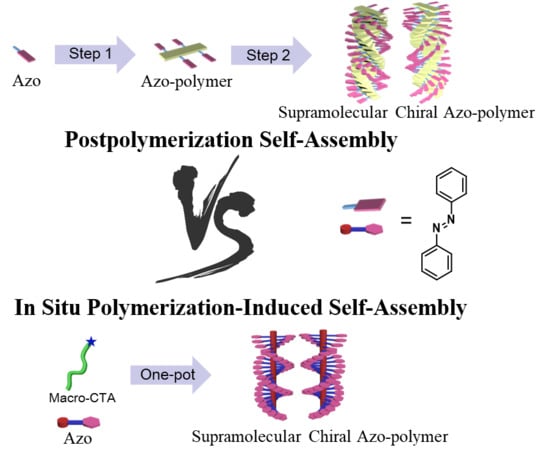
Supramolecular Chirality in Azobenzene-Containing Polymer System: Traditional Postpolymerization Self-Assembly Versus In Situ Supramolecular Self-Assembly Strategy
Abstract
1. Introduction
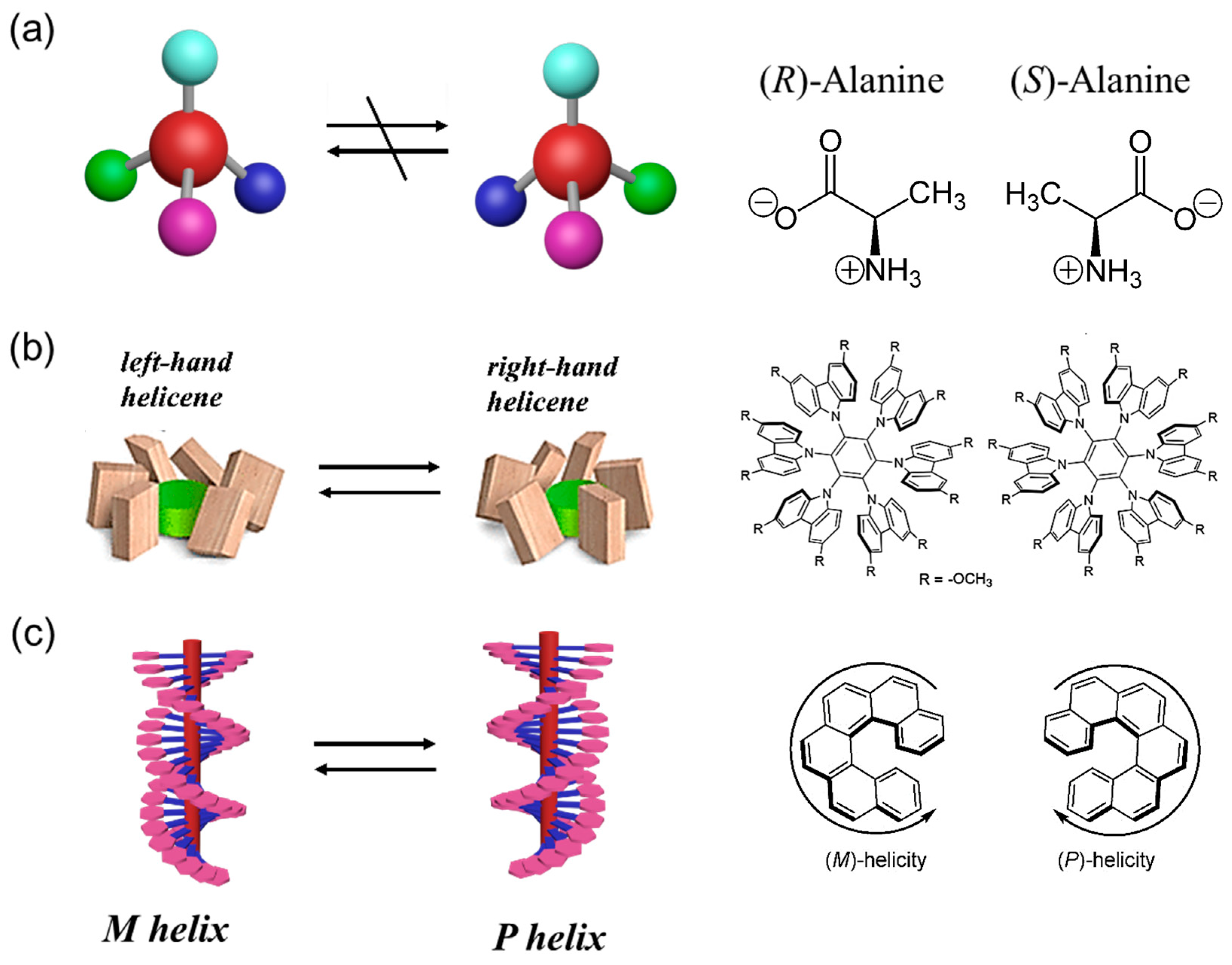
2. Basic Concepts Related to Supramolecular Chirality
2.1. Configurational, Conformational and Helical Chirality
2.2. Supramolecular Chirality
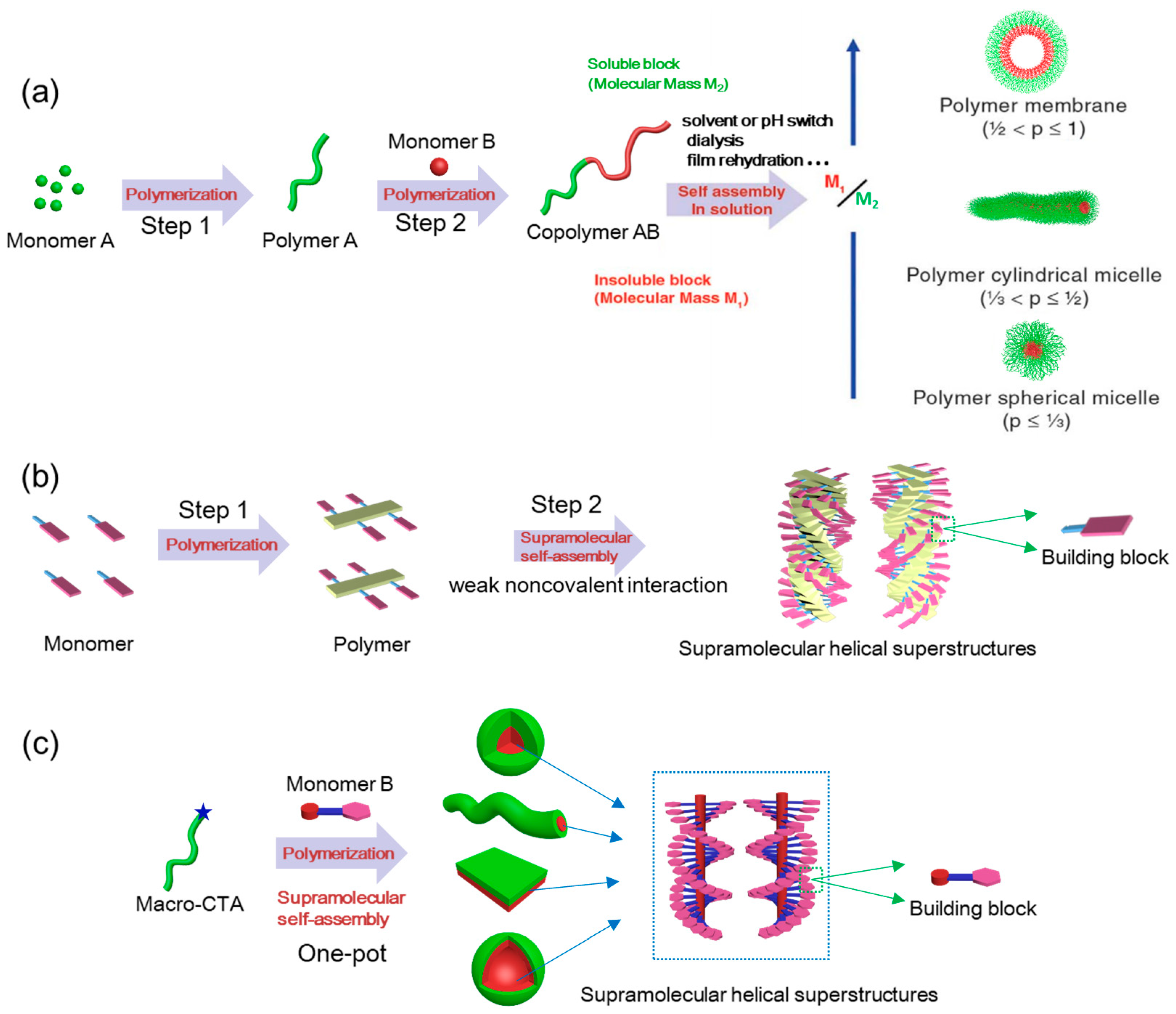
2.3. Traditional Postpolymerization Self-Assembly versus In situ Supramolecular Self-Assembly
3. Supramolecular Chirality of Azo-Polymers Constructed by Postpolymerization Self-Assembly
3.1. Supramolecular Chirality of Chiral Azo-Polymers in Solution
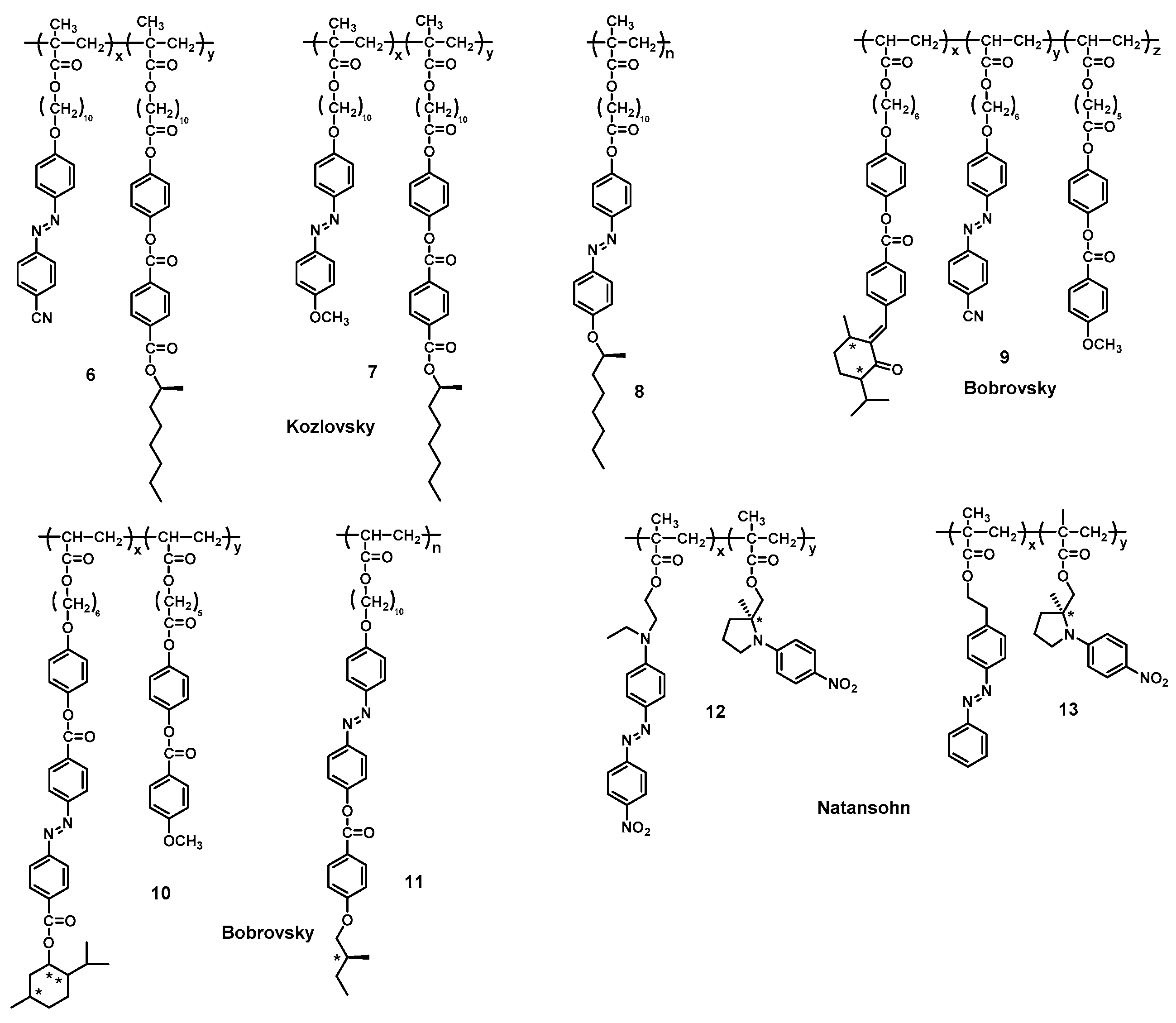
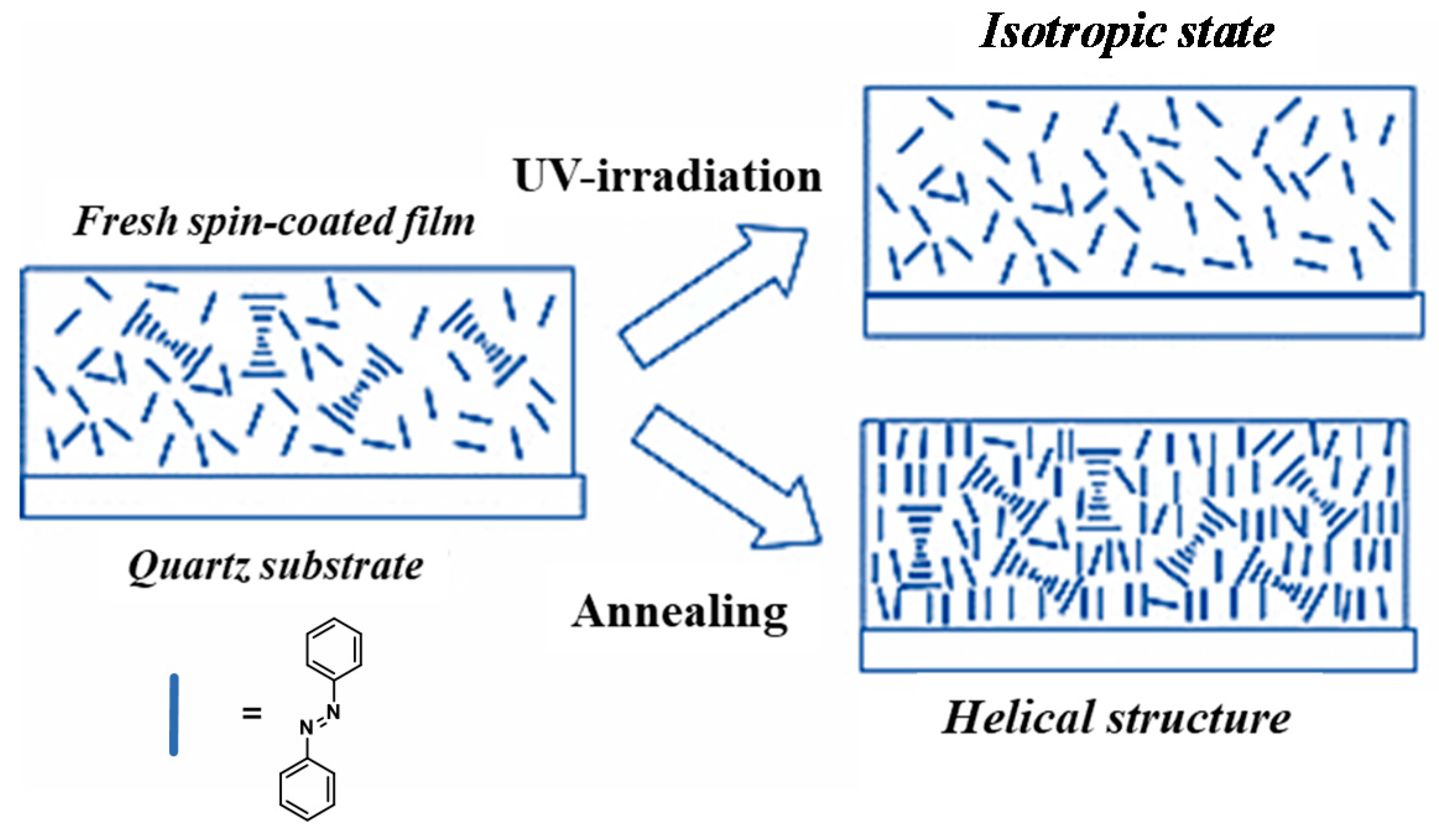
3.2. Supramolecular Chirality of Chiral Azo-Polymers in Film
3.3. Supramolecular Chirality of Achiral Azo-Polymers
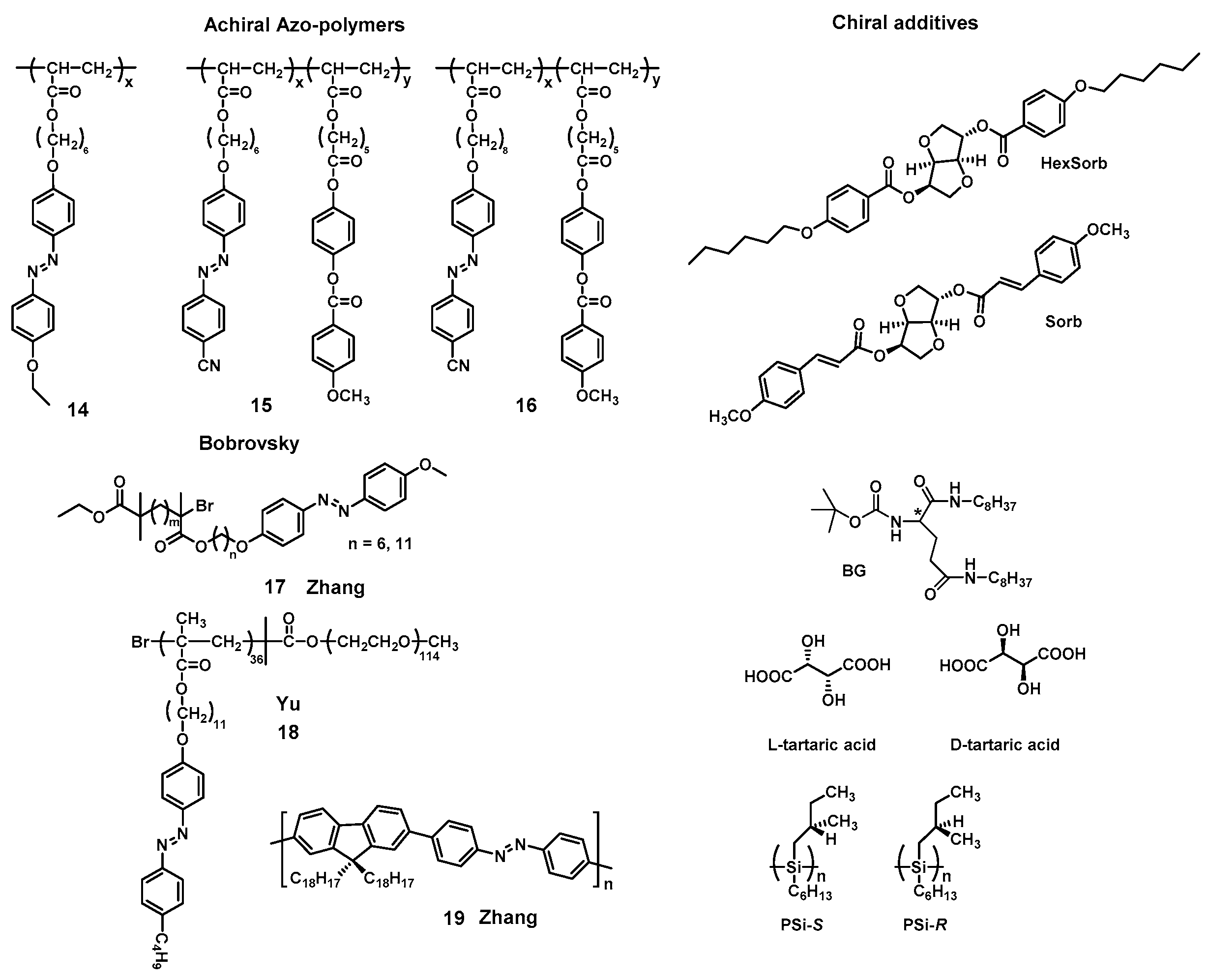
3.3.1. Supramolecular Chirality Induced by Chiral Additives
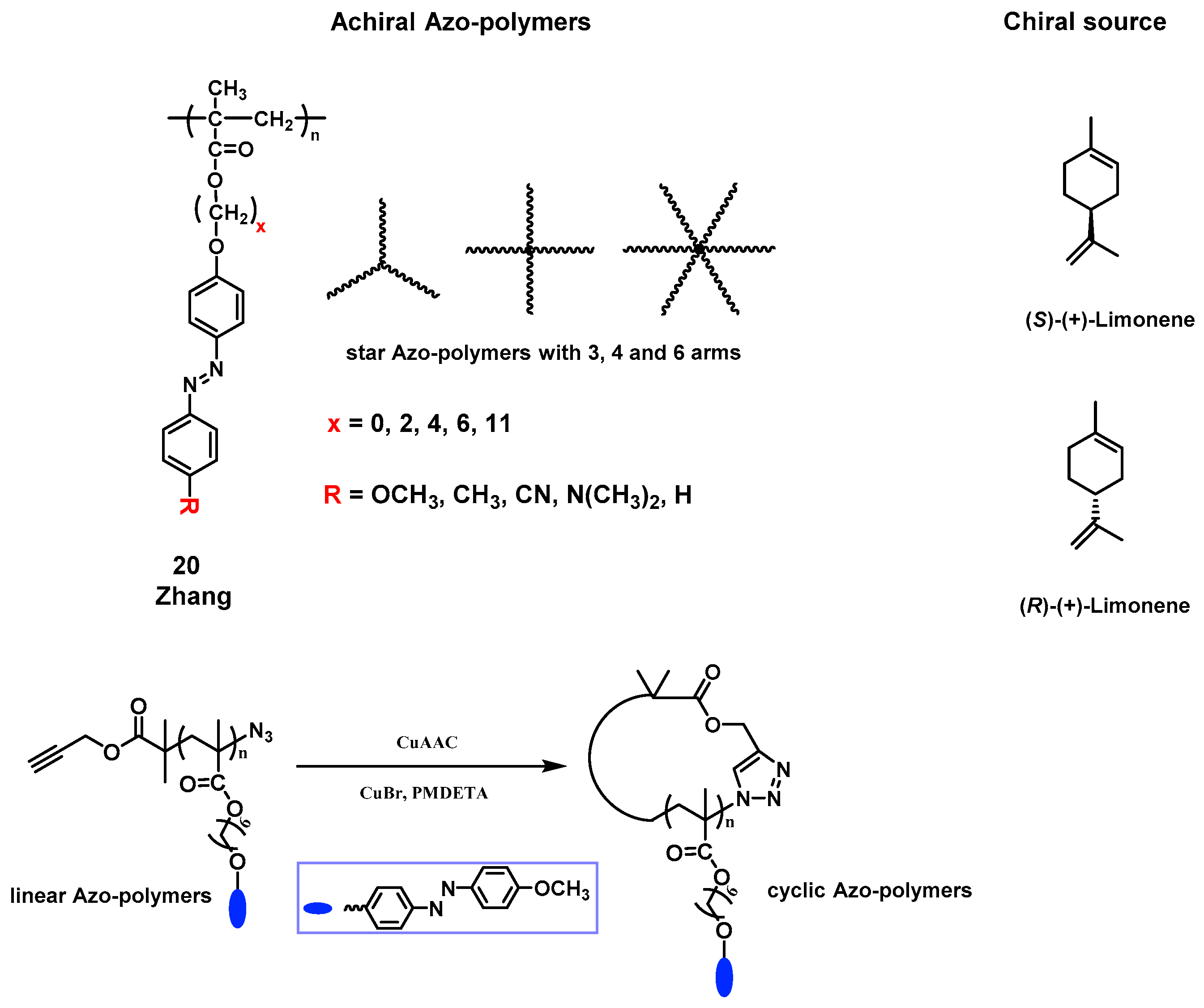
3.3.2. Supramolecular Chirality Induced by Chiral Solvent
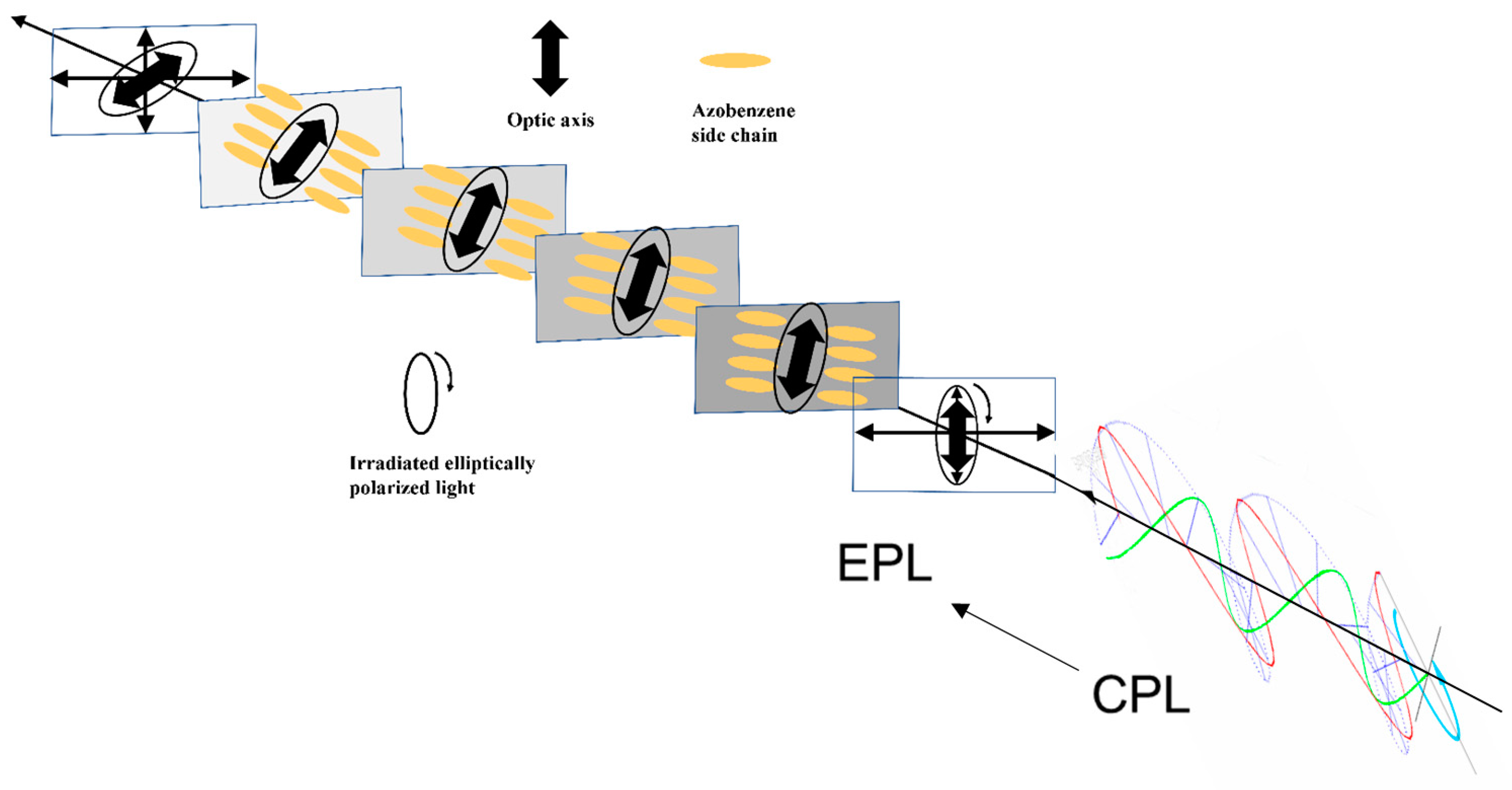
3.3.3. Supramolecular Chirality Induced by CPL
4. Supramolecular Chirality of Azo-Polymers Constructed by In situ Supramolecular Self-Assembly
5. Theoretical Calculations and Simulations
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Chiral architectures from macromolecular building blocks. Chem. Rev. 2001, 101, 4039–4070. [Google Scholar] [CrossRef] [PubMed]
- Hembury, G.A.; Borovkov, V.V.; Inoue, Y. Chirality-sensing supramolecular systems. Chem. Rev. 2008, 108, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, L.; Wang, T.Y. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Nakano, T.; Okamoto, Y. Synthetic helical polymers: Conformation and function. Chem. Rev. 2001, 101, 4013–4038. [Google Scholar] [CrossRef]
- Xiong, J.B.; Feng, H.T.; Sun, J.P.; Xie, W.Z.; Yang, D.; Liu, M.H.; Zheng, Y.S. The fixed propeller-like conformation of tetraphenylethylene that reveals aggregation-induced emission effect, chiral recognition, and enhanced chiroptical property. J. Am. Chem. Soc. 2016, 138, 11469–11472. [Google Scholar] [CrossRef]
- Feringa, B.L.; van Delden, R.A.; Koumura, N.; Geertsema, E.M. Chiroptical molecular switches. Chem. Rev. 2000, 100, 1789–1816. [Google Scholar] [CrossRef]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef]
- Crassous, J. Chiral transfer in coordination complexes: Towards molecular materials. Chem. Soc. Rev. 2009, 38, 830–845. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Wang, Y.W.; Chen, H.Y. Emerging chirality in nanoscience. Chem. Soc. Rev. 2013, 42, 2930–2962. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.G.; de Moura, A.F.; Wu, X.L.; Kuang, H.; Xu, C.L.; Kotov, N.A. Chiral inorganic nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Liu, M. From chemistry to nanoscience: Not just a matter of size. Angew. Chem. Int. Ed. 2013, 52, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Timoneda, M.A.; Crego-Calama, M.; Reinhoudt, D.N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004, 33, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Mao, C. Bio-inspired supramolecular self-assembly towards soft nanomaterials. Front. Mater. Sci. 2011, 5, 247–265. [Google Scholar] [CrossRef]
- De Greef, T.F.; Smulders, M.M.; Wolffs, M.; Schenning, A.P.; Sijbesma, R.P.; Meijer, E.W. Supramolecular polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef]
- Palmans, A.R.A.; Meijer, E.W. Amplification of chirality in dynamic supramolecular aggregates. Angew. Chem. Int. Ed. 2007, 46, 8948–8968. [Google Scholar] [CrossRef]
- Moriuchi, T.; Nishiyama, M.; Yoshida, K.; Ishikawa, T.; Hirao, T. Chiral helicity induced by hydrogen bonding and chirality of podand histidyl moieties. Org. Lett. 2001, 3, 1459–1461. [Google Scholar] [CrossRef]
- Bai, S.; Debnath, S.; Javid, N.; Frederix, P.W.J.M.; Fleming, S.; Pappas, C.; Ulijn, R.V. Differential self-assembly and tunable emission of aromatic peptide bola-amphiphiles containing perylene bisimide in polar solvents including water. Langmuir 2014, 30, 7576–7584. [Google Scholar] [CrossRef]
- Xing, P.; Zhao, Y. Controlling supramolecular chirality in multicomponent self-assembled systems. Acc. Chem. Res. 2018, 51, 2324–2334. [Google Scholar] [CrossRef]
- Marinelli, F.; Sorrenti, A.; Corvaglia, V.; Leone, V.; Mancini, G. Molecular description of the propagation of chirality from molecules to complex systems: Different mechanisms controlled by hydrophobic interactions. Chem. Eur. J. 2012, 18, 14680–14688. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Iida, H.; Akagi, K. Circularly polarized blue luminescent spherulites consisting of hierarchically assembled ionic conjugated polymers with a helically π-stacked structure. Adv. Mater. 2012, 24, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.L.; Yang, Z.M.; Zhang, R.J.; Li, L.H.; Fan, Y.J.; Kuang, Y.; Gao, Y.; Wang, T.; Lu, W.W.; Xu, B. Supramolecular hydrogel of a D-amino acid dipeptide for controlled drug release in vivo. Langmuir 2009, 25, 8419–8422. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.A.; Cecchini, M.; Samorì, P. Predicting self-assembly: From empirism to determinism. Chem. Soc. Rev. 2012, 41, 3713–3730. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hao, J. Ordered patterns and structures via interfacial self-assembly: Superlattices, honeycomb structures and coffee rings. Chem. Soc. Rev. 2011, 40, 5457–5471. [Google Scholar] [CrossRef] [PubMed]
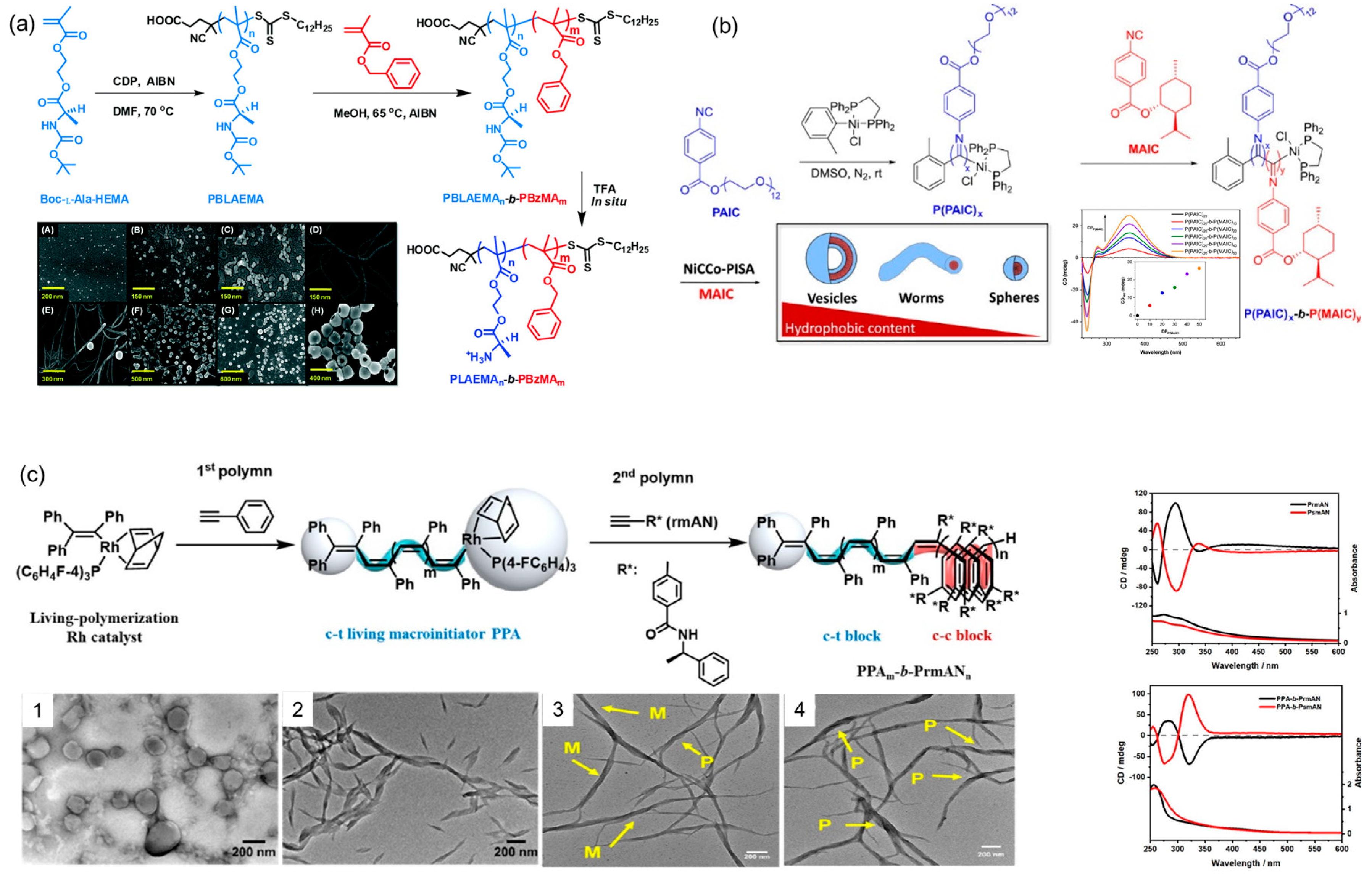
- Zhou, H.; Liu, C.G.; Qu, Y.Q.; Gao, C.Q.; Shi, K.Y.; Zhang, W.Q. How the polymerization procedures affect the morphology of the block copolymer nanoassemblies: Comparison between dispersion raft polymerization and seeded RAFT polymerization. Macromolecules 2016, 49, 8167–8176. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Zhang, L.F.; Eisenberg, A. Morphogenic effect of added ions on crew-cut aggregates of polystyrene-b-poly(acrylic acid) block copolymers in solutions. Macromolecules 1996, 29, 8805–8815. [Google Scholar] [CrossRef]
- Lawrence, D.S.; Jiang, T.; Levett, M. Self-Assembling supramolecular complexes. Chem. Rev. 1995, 95, 2229–2260. [Google Scholar] [CrossRef]
- Yagai, S.; Kitamura, A. Recent advances in photoresponsive supramolecular self-assemblies. Chem. Soc. Rev. 2008, 37, 1520–1529. [Google Scholar] [CrossRef]
- Mellot, G.; Guigner, J.M.; Bouteiller, L.; Stoffelbach, F.; Rieger, J. Templated PISA: Driving polymerization-induced self-assembly towards fibre morphology. Angew. Chem. Int. Ed. 2019, 58, 3173–3177. [Google Scholar] [CrossRef] [PubMed]
- Warren, N.J.; Mykhaylyk, O.O.; Ryan, A.J.; Williams, M.; Doussineau, T.; Dugourd, P.; Antoine, R.; Portale, G.; Armes, S.P. Testing the vesicular morphology to destruction: Birth and death of diblock copolymer vesicles prepared via polymerization-induced self-assembly. J. Am. Chem. Soc. 2015, 137, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Nakano, M.; Ikeda, T. Directed bending of a polymer film by light-miniaturizing a simple photomechanical system could expand its range of applications. Nature 2003, 425, 145. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kobayashi, T.; Yang, H. Liquid-crystalline ordering helps block copolymer self-assembly. Adv. Mater. 2011, 23, 3337–3344. [Google Scholar] [CrossRef]
- Lv, J.A.; Liu, Y.Y.; Wei, J.; Chen, E.Q.; Qin, L.; Yu, Y.L. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, U.; Pagliusi, P.; Provenzano, C.; Shibaev, V.P.; Cipparrone, G. Supramolecular chiral structures: Smart polymer organization guided by 2D polarization light patterns. Adv. Funct. Mater. 2012, 22, 2964–2970. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry: Where from? Where to? Chem. Soc. Rev. 2017, 46, 2378–2379. [Google Scholar] [CrossRef]
- Epstein, E.D.; Singh, L.; Sternklar, S.; Gorodetski, Y. The role of plasmonic excitations in the optical activity of periodic structures with configurational chirality. Appl. Phys. Lett. 2020, 116, 131106. [Google Scholar] [CrossRef]
- Chong, K.C.W.; Scheffer, J.R. Thermal and photochemical transformation of conformational chirality into configurational chirality in the crystalline state. J. Am. Chem. Soc. 2003, 125, 4040–4041. [Google Scholar] [CrossRef]
- Feng, H.-T.; Liu, C.C.; Li, Q.Y.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Structure, assembly, and function of (latent)-chiral AIEgens. ACS Materials Lett. 2019, 1, 192–202. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K. Chirality-responsive helical polymers. Macromolecules 2008, 41, 3–12. [Google Scholar] [CrossRef]
- Green, M.M.; Reidy, M.P.; Johnson, R.J.; Darling, G.; Oleary, D.J.; Willson, G. Macromolecular Stereochemistry: The out-of-proportion influence of optically-active co-monomers on the conformational characteristics of polyisocyanates. The Sergeants and Soldiers Experiment. J. Am. Chem. Soc. 1989, 111, 6452–6454. [Google Scholar] [CrossRef]
- Green, M.M.; Peterson, N.C.; Sato, T.; Teramoto, A.; Cook, R.; Lifson, S. A Helical polymer with a cooperative response to chiral information. Science 1995, 268, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Murguly, E.; McDonald, R.; Branda, N.R. Chiral discrimination in hydrogen-bonded [7]helicenes. Org. Lett. 2000, 2, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.E.; Thompson, A. Advances in the chemistry of dipyrrins and their complexes. Chem. Rev. 2007, 107, 1831–1861. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, X.; Liu, D.; Ren, C.L.; Yang, W.T.; Deng, J.P. Optically active particles of chiral polymers. Macromol. Rapid Commun. 2013, 34, 1426–1445. [Google Scholar] [CrossRef] [PubMed]
- Miyake, G.M.; Mariott, W.R.; Chen, E.Y.X. Asymmetric coordination polymerization of acrylamides by enantiomeric metallocenium ester enolate catalysts. J. Am. Chem. Soc. 2007, 129, 6724–6725. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Furusho, Y. Single- and double-stranded helical polymers: Synthesis, structures, and functions. Acc. Chem. Res. 2008, 41, 1166–1180. [Google Scholar] [CrossRef]
- Akagi, K. Helical polyacetylene: Asymmetric polymerization in a chiral liquid-crystal field. Chem. Rev. 2009, 109, 5354–5401. [Google Scholar] [CrossRef]
- Okamoto, Y.; Nakano, T. Asymmetric polymerization. Chem. Rev. 1994, 94, 349–372. [Google Scholar] [CrossRef]
- Zhang, D.; Song, C.; Deng, J.; Yang, W. Chiral microspheres consisting purely of optically active helical substituted polyacetylene: The first preparation via precipitation polymerization and application in enantioselective crystallization. Macromolecules 2012, 45, 7329–7338. [Google Scholar] [CrossRef]
- Okamoto, Y.; Suzuki, K.; Ohta, K.; Hatada, K.; Yuki, H. Optically-active poly(triphenylmethyl methacrylate) with one-handed helical conformation. J. Am. Chem. Soc. 1979, 101, 4763–4765. [Google Scholar] [CrossRef]
- Cui, J.X.; Lu, X.C.; Liu, A.H.; Wan, X.H.; Zhou, Q.F. Long-range chirality transfer in free radical polymerization of bulky vinyl monomers containing laterally attached p-terphenyl groups. Macromolecules 2009, 42, 7678–7688. [Google Scholar] [CrossRef]
- Chen, J.L.; Yang, L.; Wang, Q.; Jiang, Z.Q.; Liu, N.; Yin, J.; Ding, Y.S.; Wu, Z.Q. Helix-sense-selective and enantiomer-selective living polymerization of phenyl isocyanide induced by reusable chiral lactide using achiral palladium initiator. Macromolecules 2015, 48, 7737–7746. [Google Scholar] [CrossRef]
- Cheng, X.X.; Miao, T.F.; Ma, H.T.; Yin, L.; Zhang, W.; Zhang, Z.B.; Zhu, X.L. The construction of photoresponsive polymer particles with supramolecular helicity from achiral monomers by helix-sense-selective polymerization. Poly. Chem. 2020, 11, 2089–2097. [Google Scholar] [CrossRef]
- Cheng, X.X.; Miao, T.F.; Yin, L.; Chen, H.L.; Liu, M.; Zhang, W.; Zhu, X.L. Advances in chiral assembly of achiral substance induced by chiral oligomer/polymer. J. Funct. Polym. 2019, 32, 647–659. [Google Scholar]
- Yin, L.; Miao, T.F.; Cheng, X.X.; Zhao, Y.; Li, J.A.; Zhang, W.; Zhu, X.L. Research advance in photoinduced chirality of achiral polymers. J. Funct. Polym. 2018, 31, 387–401. [Google Scholar]
- Zhang, S.S.; Liu, J.F.; Zhang, J.; Wang, L.B.; Zhang, W.; Zhu, X.L. Preparation of chiral and fluorescent nanoparticles of hyperbranched conjugated polymers by the solvent chirality transfer technology. ACTA POLYM. SIN. 2013, 4, 426–435. [Google Scholar]
- Zhao, Y.; Yin, L.; Liu, J.J.; Zhang, W.; Zhu, X.L. Advance in chirality of achiral substance induced by chiral solvent. J. Funct. Polym. 2016, 29, 20–28. [Google Scholar] [CrossRef]
- Yue, B.B.; Zhu, L.L. Dynamic modulation of supramolecular chirality driven by factors from internal to external levels. Chem. Asian J. 2019, 14, 2172–2180. [Google Scholar] [CrossRef]
- Duan, P.F.; Li, Y.G.; Li, L.C.; Deng, J.G.; Liu, M.H. Multiresponsive chiroptical switch of an azobenzene-containing lipid: Solvent, temperature, and photoregulated supramolecular chirality. J. Phys. Chem. B 2011, 115, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Nakashima, T.; Kawai, T. Inversion of supramolecular chirality in bichromophoric perylene bisimides: Influence of temperature and ultrasound. Langmuir 2014, 30, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Qin, M.G.; Peng, T.; Tang, X.H.; Dang-i, A.Y.; Feng, C.L. Modulating supramolecular chirality in alanine derived assemblies by multiple external stimuli. Langmuir 2018, 34, 7869–7876. [Google Scholar] [CrossRef] [PubMed]
- Ashida, Y.; Sato, T.; Morino, K.; Maeda, K.; Okamoto, Y.; Yashima, E. Helical structural change in poly((4-carboxyphenyl)acetylene) by acid-base complexation with an optically active amine. Macromolecules 2003, 36, 3345–3350. [Google Scholar] [CrossRef]
- Nishimura, T.; Tsuchiya, K.; Ohsawa, S.; Maeda, K.; Yashima, E.; Nakamura, Y.; Nishimura, J. Macromolecular helicity induction on a poly(phenylacetylene) with C2-Symmetric chiral [60]fullerene-bisadducts. J. Am. Chem. Soc. 2004, 126, 11711–11717. [Google Scholar] [CrossRef]
- Onouchi, H.; Kashiwagi, D.; Hayashi, K.; Maeda, K.; Yashima, E. Helicity induction on poly(phenylacetylene)s bearing phosphonic acid pendants with chiral amines and memory of the macromolecular helicity assisted by interaction with achiral amines in dimethyl sulfoxide. Macromolecules 2004, 37, 5495–5503. [Google Scholar] [CrossRef]
- Yashima, E.; Matsushima, T.; Okamoto, Y. Poly((4-carboxyphenyl)acetylene) as a probe for chirality assignment of amines by circular dichroism. J. Am. Chem. Soc. 1995, 117, 11596–11597. [Google Scholar] [CrossRef]
- Nakashima, H.; Koe, J.R.; Torimitsu, K.; Fujiki, M. Transfer and amplification of chiral molecular information to polysilylene aggregates. J. Am. Chem. Soc. 2001, 123, 4847–4848. [Google Scholar] [CrossRef]
- Wang, Y.; Sakamoto, T.; Nakano, T. Molecular chirality induction to an achiral π-conjugated polymer by circularly polarized light. Chem. Commun. 2012, 48, 1871–1873. [Google Scholar] [CrossRef]
- Zou, G.; Jiang, H.; Kohn, H.; Manaka, T.; Iwamoto, M. Control and modulation of chirality for azobenzene-substituted polydiacetylene LB films with circularly polarized light. Chem. Commun. 2009, 5627–5629. [Google Scholar] [CrossRef]
- Liu, G.F.; Zhu, L.Y.; Ji, W.; Feng, C.L.; Wei, Z.X. Inversion of the supramolecular chirality of nanofibrous structures through co-assembly with achiral molecules. Angew. Chem. Int. Ed. 2016, 55, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Yuan, F.; Ma, X.Y.; Auphedeous, D.I.Y.; Zhao, C.L.; Liu, C.T.; Shen, C.Y.; Feng, C.L. The cooperative effect of both molecular and supramolecular chirality on cell adhesion. Angew. Chem. Int. Ed. 2018, 57, 6475–6479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, L.; Wang, X.F.; Cao, H.; Liu, M.H. Supramolecular chirality in self-assembled soft materials: Regulation of chiral nanostructures and chiral functions. Adv. Mater. 2014, 26, 6959–6964. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Nagai, K.; Iida, H.; Maeda, K.; Ochi, N.; Sawabe, K.; Sakajiri, K.; Okoshi, K.; Yashima, E. Mechanism of helix induction in poly(4-carboxyphenyl isocyanide) with chiral amines and memory of the macromolecular helicity and its helical structures. J. Am. Chem. Soc. 2009, 131, 10719–10732. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhang, J.; Zhang, S.S.; Suzuki, N.; Fujiki, M.; Wang, L.B.; Li, L.; Zhang, W.; Zhou, N.C.; Zhu, X.L. Chiroptical generation and amplification of hyperbranched π-conjugated polymers in aggregation states driven by limonene chirality. Poly. Chem. 2014, 5, 784–791. [Google Scholar] [CrossRef]
- Nakano, Y.; Ichiyanagi, F.; Naito, M.; Yang, Y.G.; Fujiki, M. Chiroptical generation and inversion during the mirror-symmetry-breaking aggregation of dialkylpolysilanes due to limonene chirality. Chem. Commun. 2012, 48, 6636–6638. [Google Scholar] [CrossRef]
- Li, S.; Miao, T.F.; Cheng, X.X.; Zhao, Y.; Zhang, W.; Zhu, X.L. Different phase-dominated chiral assembly of polyfluorenes induced by chiral solvation: Axial and supramolecular chirality. RSC Adv. 2019, 9, 38257–38264. [Google Scholar] [CrossRef]
- Zhao, Y.; Abdul Rahim, N.A.; Xia, Y.; Fujiki, M.; Song, B.; Zhang, Z.; Zhang, W.; Zhu, X. Supramolecular chirality in achiral polyfluorene: Chiral gelation, memory of chirality, and chiral sensing property. Macromolecules 2016, 49, 3214–3221. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, Y.; Lv, K.; Wang, X.; Zhang, W.; Zhang, L.; Liu, M. A strategy for tuning achiral main-chain polymers into helical assemblies and chiral memory systems. Soft Matter 2016, 12, 1170–1175. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Nair, V.S.; Nair, V.C.; Ajayaghosh, A. Formation of coaxial nanocables with amplified supramolecular chirality through an interaction between carbon nanotubes and a chiral π-gelator. Angew. Chem. Int. Ed. 2016, 55, 10345–10349. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhang, L.; Yang, D.; Li, T.S.; Liu, M.H. Chiral signs of TPPS co-assemblies with chiral gelators: Role of molecular and supramolecular chirality. Chem. Commun. 2016, 52, 12434–12437. [Google Scholar] [CrossRef] [PubMed]
- Fossépré, M.; Trévisan, M.E.; Cyriaque, V.; Wattiez, R.; Beljonne, D.; Richeter, S.; Clément, S.; Surin, M. Detection of the Enzymatic Cleavage of DNA through Supramolecular Chiral Induction to a Cationic Polythiophene. ACS Appl. Bio Mater. 2019, 2, 2125–2136. [Google Scholar] [CrossRef]
- Chen, H.; Yin, L.; Liu, M.; Wang, L.; Fujiki, M.; Zhang, W.; Zhu, X. Aggregation-induced chiroptical generation and photoinduced switching of achiral azobenzene-alt-fluorene copolymer endowed with left- and right-handed helical polysilanes. RSC Adv. 2019, 9, 4849–4856. [Google Scholar] [CrossRef]
- Fujiki, M. Switching handedness in optically active polysilanes. J. Organomet. Chem. 2003, 685, 15–34. [Google Scholar] [CrossRef]
- Li, C.; Numata, M.; Bae, A.H.; Sakurai, K.; Shinkai, S. Self-assembly of supramolecular chiral insulated molecular wire. J. Am. Chem. Soc. 2005, 127, 4548–4549. [Google Scholar] [CrossRef] [PubMed]
- Rahim, N.A.A.; Fujiki, M. Aggregation-induced scaffolding: Photoscissable helical polysilane generates circularly polarized luminescent polyfluorene. Poly. Chem. 2016, 7, 4618–4629. [Google Scholar] [CrossRef]
- Gonzalez, V.; Cotte, M.; Vanmeert, F.; de Nolf, W.; Janssens, K. X-ray diffraction mapping for cultural heritage science: A review of experimental configurations and applications. Chem. Eur. J. 2020, 26, 1703–1719. [Google Scholar] [CrossRef]
- Louzao, I.; Seco, J.M.; Quinoa, E.; Riguera, R. 13C NMR as a general tool for the assignment of absolute configuration. Chem. Commun. 2010, 46, 7903–7905. [Google Scholar] [CrossRef]
- Shiozaki, M.; Hiraoka, T. Stereo-controlled synthesis of intermediates of (+/-)-thienamycin. Tetrahedron Lett. 1980, 21, 4473–4476. [Google Scholar] [CrossRef]
- Deschamps, J.R. X-ray crystallography of chemical compounds. Life Sci. 2010, 86, 585–589. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Seco, J.M.; Quinoa, E.; Riguera, R. Assignment of the absolute configuration of polyfunctional compounds by NMR using chiral derivatizing agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Di Bari, L.; Berova, N. Conformational aspects in the studies of organic compounds by electronic circular dichroism. Chem. Soc. Rev. 2011, 40, 4603–4625. [Google Scholar] [CrossRef]
- Miles, A.J.; Wallace, B.A. Circular dichroism spectroscopy of membrane proteins. Chem. Soc. Rev. 2016, 45, 4859–4872. [Google Scholar] [CrossRef] [PubMed]
- Sadlej, J.; Dobrowolski, J.C.; Rode, J.E. VCD spectroscopy as a novel probe for chirality transfer in molecular interactions. Chem. Soc. Rev. 2010, 39, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, S.; De Schryver, F.C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 2003, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.S.; Luo, Q.; Liu, J.Q. Protein self-assembly via supramolecular strategies. Chem. Soc. Rev. 2016, 45, 2756–2767. [Google Scholar] [CrossRef]
- Wang, Z.P.; van Oers, M.C.M.; Rutjes, F.P.J.T.; van Hest, J.C.M. Polymersome colloidosomes for enzyme catalysis in a biphasic system. Angew. Chem. Int. Ed. 2012, 51, 10746–10750. [Google Scholar] [CrossRef]
- Ning, Y.; Fielding, L.A.; Andrews, T.S.; Growney, D.J.; Armes, S.P. Sulfate-based anionic diblock copolymer nanoparticles for efficient occlusion within zinc oxide. Nanoscale 2015, 7, 6691–6702. [Google Scholar] [CrossRef]
- Liu, T.; Hu, J.M.; Jin, Z.Y.; Jin, F.; Liu, S.Y. Two-photon ratiometric fluorescent mapping of intracellular transport pathways of pH-responsive block copolymer micellar nanocarriers. Adv. Healthcare Mater. 2013, 2, 1576–1581. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.C.; Pochan, D.J. Tailored assemblies of block copolymers in solution: It is all about the process. Macromolecules 2010, 43, 3577–3584. [Google Scholar] [CrossRef]
- Mai, Y.Y.; Eisenberg, A. Selective localization of preformed nanoparticles in morphologically controllable block copolymer aggregates in solution. Acc. Chem. Res. 2012, 45, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Eisenberg, A. Proton diffusion across membranes of vesicles of poly(styrene-b-acrylic acid) diblock copolymers. J. Am. Chem. Soc. 2006, 128, 2880–2884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Eisenberg, A. Thermodynamic vs kinetic aspects in the formation and morphological transitions of crew-cut aggregates produced by self-assembly of polystyrene-b-poly(acrylic acid) block copolymers in dilute solution. Macromolecules 1999, 32, 2239–2249. [Google Scholar] [CrossRef]
- Prins, L.J.; Reinhoudt, D.N.; Timmerman, P. Noncovalent synthesis using hydrogen bonding. Angew. Chem. Int. Ed. 2001, 40, 2382–2460. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry-A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef]
- Foster, J.C.; Varlas, S.; Couturaud, B.; Jones, J.R.; Keogh, R.; Mathers, R.T.; O’Reilly, R.K. Predicting monomers for use in polymerization-induced self-assembly. Angew. Chem. Int. Ed. 2018, 57, 15733–15737. [Google Scholar] [CrossRef]
- Bauri, K.; Narayanan, A.; Haldar, U.; De, P. Polymerization-induced self-assembly driving chiral nanostructured materials. Poly. Chem. 2015, 6, 6152–6162. [Google Scholar] [CrossRef]
- Ratcliffe, L.P.D.; Derry, M.J.; Ianiro, A.; Tuinier, R.; Armes, S.P. A single thermoresponsive diblock copolymer can form spheres, worms or vesicles in aqueous solution. Angew. Chem. Int. Ed. 2019, 58, 18964–18970. [Google Scholar] [CrossRef]
- Yeow, J.; Boyer, C. Photoinitiated polymerization-induced self-assembly (Photo-PISA): New insights and opportunities. Adv. Sci. 2017, 4, 1700137. [Google Scholar] [CrossRef] [PubMed]
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-induced self-assembly: From soluble macromolecules to block copolymer nano-objects in one step. Macromolecules 2012, 45, 6753–6765. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hong, C.Y.; Pan, C.Y. Fabrication of spaced concentric vesicles and polymerizations in raft dispersion polymerization. Macromolecules 2014, 47, 1664–1671. [Google Scholar] [CrossRef]
- Lv, F.; An, Z.S.; Wu, P.Y. Scalable preparation of alternating block copolymer particles with inverse bicontinuous mesophases. Nat. Commun. 2019, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Q.; Zhou, H.; Qu, Y.Q.; Wang, W.; Khan, H.; Zhang, W.Q. In situ synthesis of block copolymer nanoassemblies via polymerization-induced self-assembly in poly(ethylene glycol). Macromolecules 2016, 49, 3789–3798. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Zeng, M.; Liu, L.; Wei, Y.; Yuan, J. Morphology evolution of polymeric assemblies regulated with fluoro-containing mesogen in polymerization-induced self-assembly. Macromolecules 2017, 50, 8192–8201. [Google Scholar] [CrossRef]
- Tan, J.B.; Liu, D.D.; Bai, Y.H.; Huang, C.D.; Li, X.L.; He, J.; Xu, Q.; Zhang, L. Enzyme-assisted photoinitiated polymerization-induced self-assembly: An oxygen-tolerant method for preparing block copolymer nano-objects in open vessels and multiwell plates. Macromolecules 2017, 50, 5798–5806. [Google Scholar] [CrossRef]
- Xu, Q.S.; Huang, T.; Li, S.L.; Li, K.; Li, C.L.; Liu, Y.N.; Wang, Y.L.; Yu, C.Y.; Zhou, Y.F. Emulsion-assisted polymerization-induced hierarchical self-assembly of giant sea urchin-like aggregates on a large scale. Angew. Chem. Int. Ed. 2018, 57, 8043–8047. [Google Scholar] [CrossRef]
- Huo, M.; Wan, Z.; Zeng, M.; Wei, Y.; Yuan, J. Polymerization-induced self-assembly of liquid crystalline ABC triblock copolymers with long solvophilic chains. Poly. Chem. 2018, 9, 3944–3951. [Google Scholar] [CrossRef]
- Ding, Y.; Cai, M.; Cui, Z.; Huang, L.; Wang, L.; Lu, X.; Cai, Y. Synthesis of low-dimensional polyion complex nanomaterials via polymerization-induced electrostatic self-assembly. Angew. Chem. Int. Ed. 2018, 57, 1053–1056. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huo, M.; Zeng, M.; Peng, L.; Feng, A.C.; Wang, X.S.; Yuan, J.Y. Direct Synthesis of Polymer Nanotubes by Aqueous Dispersion Polymerization of a Cyclodextrin/Styrene Complex. Angew. Chem. Int. Ed. 2017, 56, 16541–16545. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.T.; Shi, Y.; Fu, Z.F.; Yang, W.T. In situ synthesis of diblock copolymer nanoassemblies via dispersion RAFT polymerization induced self-assembly and Ag/copolymer composite nanoparticles thereof. Poly. Chem. 2018, 9, 1082–1094. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.B.; Pan, X.Q.; Wu, J.A.; Zhang, W.; Zhang, Z.B.; Zhu, X.L. Establishment of a molecular design to obtain visible-light-activated Azoxy polymer actuators. Poly. Chem. 2018, 9, 2438–2445. [Google Scholar] [CrossRef]
- Wang, K.; Yin, L.; Miu, T.F.; Liu, M.; Zhao, Y.; Chen, Y.; Zhou, N.C.; Zhang, W.; Zhu, X.L. Design and synthesis of a novel azobenzene-containing polymer both in the main- and side-chain toward unique photocontrolled isomerization properties. Mater. Chem. Front. 2018, 2, 1112–1118. [Google Scholar] [CrossRef]
- Wang, L.B.; Chen, Y.; Yin, L.; Zhang, S.S.; Zhou, N.C.; Zhang, W.; Zhu, X.L. Synthesis and characterization of visible-light-activated Azo hyperbranched polymers. Poly. Chem. 2016, 7, 5407–5413. [Google Scholar] [CrossRef]
- Wang, L.B.; Pan, X.Q.; Zhao, Y.; Chen, Y.; Zhang, W.; Tu, Y.F.; Zhang, Z.B.; Zhu, J.; Zhou, N.C.; Zhu, X.L. A Straightforward protocol for the highly efficient preparation of main-chain Azo polymers directly from bisnitroaromatic compounds by the photocatalytic process. Macromolecules 2015, 48, 1289–1295. [Google Scholar] [CrossRef]
- Yin, L.; Han, L.; Ge, F.; Tong, X.; Zhang, W.; Soldera, A.; Zhao, Y. A novel side-chain liquid crystal elastomer exhibiting anomalous reversible shape change. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Merino, E. Synthesis of azobenzenes: The coloured pieces of molecular materials. Chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef]
- Royes, J.; Polo, V.; Uriel, S.; Oriol, L.; Piñol, M.; Tejedor, R.M. Chiral supramolecular organization from a sheet-like achiral gel: A study of chiral photoinduction. Phys. Chem. Chem. Phys. 2017, 19, 13622–13628. [Google Scholar] [CrossRef]
- Tejedor, R.M.; Oriol, L.; Serrano, J.L.; Ureña, F.P.; González, J.J.L. Photoinduced chiral nematic organization in an achiral glassy nematic Azopolymer. Adv. Funct. Mater. 2007, 17, 3486–3492. [Google Scholar] [CrossRef]
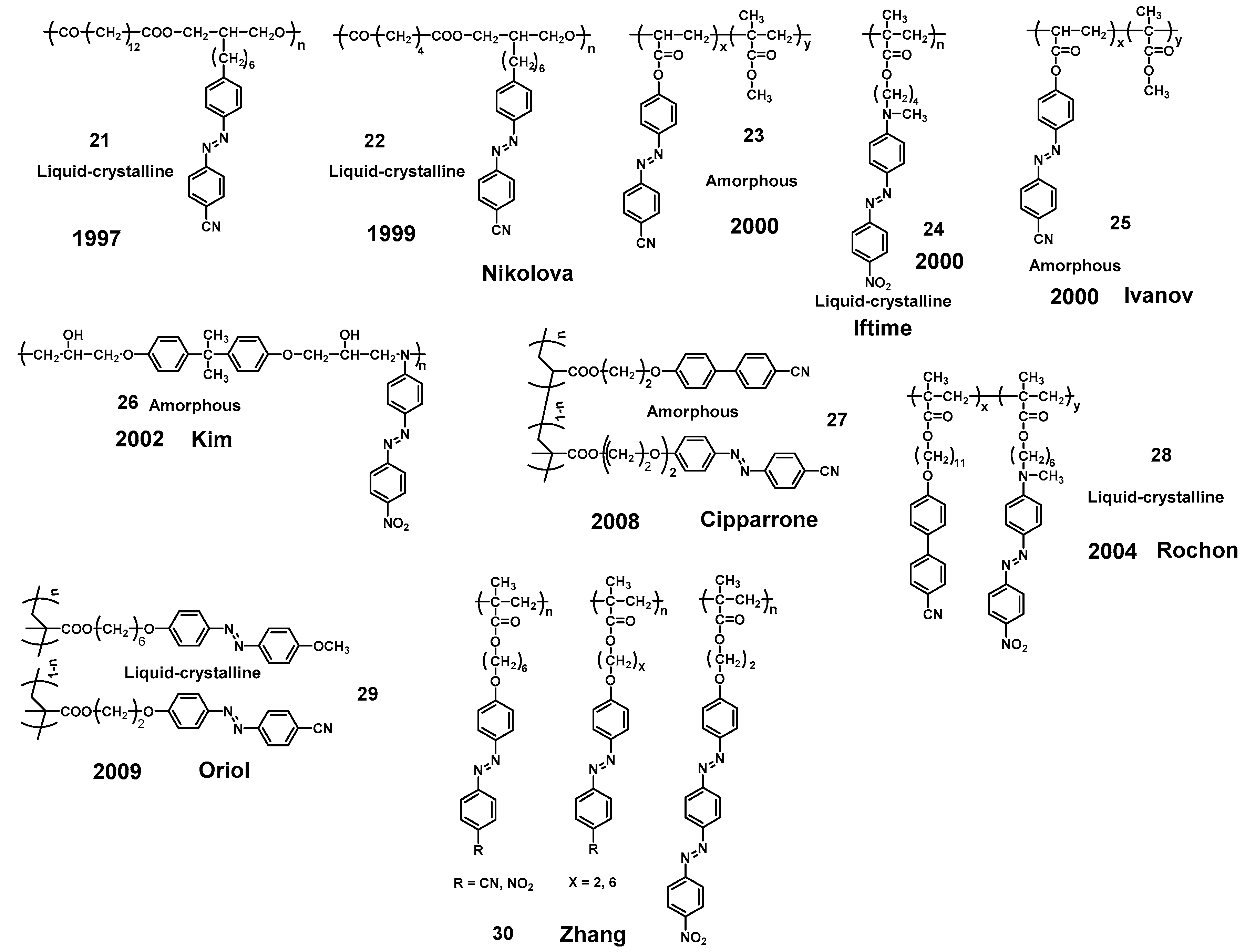
- Iftime, G.; Natansohn, A.; Rochon, P. Synthesis and characterization of two chiral azobenzene-containing copolymers. Macromolecules 2002, 35, 365–369. [Google Scholar] [CrossRef]
- Yin, L.; Liu, M.; Zhao, Y.; Zhang, S.S.; Zhang, W.; Zhang, Z.B.; Zhu, X.L. Supramolecular chirality induced by chiral solvation in achiral cyclic Azo-containing polymers: Topological effects on chiral aggregation. Poly. Chem. 2018, 9, 769–776. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, Y.; Liu, M.; Zhou, N.; Zhang, W.; Zhu, X. Induction of supramolecular chirality by chiral solvation in achiral Azo polymers with different spacer lengths and push–pull electronic substituents: Where will chiral induction appear? Poly. Chem. 2017, 8, 1906–1913. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, Y.; Wang, L.; Yin, L.; Zhang, Z.; Zhu, J.; Zhang, W.; Zhu, X. Photocontrollable induction of supramolecular chirality in achiral side chain Azo-containing polymers through preferential chiral solvation. Poly. Chem. 2015, 6, 4230–4239. [Google Scholar] [CrossRef]
- del Barrio, J.; Tejedor, R.M.; Oriol, L. Thermal and light control of the chiral order of azopolymers. Eur. Polym. J. 2012, 48, 384–390. [Google Scholar] [CrossRef]
- Miao, T.; Yin, L.; Cheng, X.; Zhao, Y.; Hou, W.; Zhang, W.; Zhu, X. Chirality construction from preferred π-π stacks of achiral azobenzene units in polymer: Chiral induction, transfer and memory. Polymers 2018, 10, 612. [Google Scholar] [CrossRef]
- Van der veen, G.; Hoguet, R.; Prins, W. Photoregulation of polymer conformation by photochromic moieties-II. Cationic and neutral moieties on an anionic polymer. Photochem. Photobiol. 1974, 19, 197–204. [Google Scholar] [CrossRef]
- Altomare, A.; Carlini, C.; Ciardelli, F.; Solaro, R. Photochromism of 4-acryloxyazobenzene/(-)-menthyl acrylate copolymers. J. Polym. Sci. Part A Polym. Chem. 1984, 22, 1267–1280. [Google Scholar] [CrossRef]
- Altomare, A.; Ciardelli, F.; Lima, R.; Solaro, R. Chiroptical and photochromic properties of stereoregular copolymers of 4-methacryloxyethylenoxyazobenzene with (-)-menthyl methacrylate. Chirality 1991, 3, 292–298. [Google Scholar] [CrossRef]
- Angiolini, L.; Caretti, D.; Giorgini, L.; Salatelli, E.; Altomare, A.; Carlini, C.; Solaro, R. Synthesis, chiroptical properties and photoresponsive behaviour of optically active poly[(S)-4-(2-methacryloyloxy-propanoyloxy)azobenzene]. Polymer 1998, 39, 6621–6629. [Google Scholar] [CrossRef]
- Angiolini, L.; Caretti, D.; Carlini, C.; Giorgini, L.; Salatelli, E. Synthesis and chiroptical properties of optically active photochromic polymers with side-chain L-lactic residues connected to trans-azobenzene moieties bearing a reactive formyl electron-withdrawing group. Macromol. Chem. Phys. 1999, 200, 390–398. [Google Scholar] [CrossRef]
- Angiolini, L.; Caretti, D.; Giorgini, L.; Salatelli, E. Synthesis and chiroptical properties of optically active photochromic methacrylic polymers bearing in the side chain the (S)-3-hydroxypyrrolidinyl group conjugated with the trans-azoaromatic chromophore. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3257–3268. [Google Scholar] [CrossRef]
- Angiolini, L.; Caretti, D.; Giorgini, L.; Salatelli, E.; Altomare, A.; Carlini, C.; Solaro, R. Optically active polymethacrylates with side-chain L-lactic acid residues connected to push-pull azobenzene chromophores. Polymer 2000, 41, 4767–4780. [Google Scholar] [CrossRef]
- Angiolini, L.; Giorgini, L.; Salatelli, E. Methacrylic polymers bearing in the side-chain an optically active moiety linked to the trans-4-azobenzene chromophore: Chiroptical properties of poly[(S)-(+)-N-methyl-(2-methacryloyloxypropanoyl)-4-aminoazobenzene]. Macromol. Symp. 2004, 218, 165–172. [Google Scholar]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Salatelli, E.; Bozio, R.; Daurù, A.; Pedron, D. Synthesis, chiroptical properties and photoinduced linear birefringence of the homopolymer of (R)-3-methacryloyloxy-1-(4/-cyano-4-azobenzene) pyrrolidine and of the copolymers with the enantiomeric monomer. Eur. Polym. J. 2005, 41, 2045–2054. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Salatelli, E. Synthesis of optically active methacrylic oligomeric models and polymers bearing the side-chain Azo-aromatic moiety and dependence of their chiroptical properties on the polymerization degree. Polymer 2006, 47, 1875–1885. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Salatelli, E.; Bozio, R.; Daurù, A.; Pedron, D. Improvement of photoinduced birefringence properties of optically active methacrylic polymers through copolymerization of monomers bearing azoaromatic moieties. Macromolecules 2006, 39, 489–497. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Mauriello, F.; Salatelli, E. Chiroptical and optical thermoplastic acid sensors based on chiral methacrylic polymers containing azoaromatic moieties. Sensor. Actuat. B-Chem. 2007, 126, 56–61. [Google Scholar] [CrossRef]
- Angiolini, L.; Caretti, D.; Giorgini, L.; Salatelli, E. Methacrylic polymers bearing side-chain permanent dipole azobenzene chromophores spaced from the main chain by chiral moieties: Synthesis and characterization. Polymer 2001, 42, 4005–4016. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Mauriello, F.; Salatelli, E. Optically active methacrylic copolymers bearing side-chain bisazoaromatic and bulky achiral moieties. Macromol. Chem. Phys. 2007, 208, 1548–1559. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Mauriello, F.; Salatelli, E. New optically active methacrylic polymers bearing side-chain bisazoaromatic moieties. Macromol. Chem. Phys. 2007, 208, 207–217. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Mauriello, F.; Salatelli, E.; Bozio, R.; Daurù, A.; Pedron, D. Synthesis, chiroptical properties and photoinduced birefringence of optically active methacrylic copolymers bearing side-chain bisazoaromatic moieties. Eur. Polym. J. 2007, 43, 3550–3561. [Google Scholar] [CrossRef]
- Kosaka, N.; Oda, T.; Hiyama, T.; Nozaki, K. Synthesis and photoisomerization of optically active 1,4-polyketones substituted by azobenzene side chains. Macromolecules 2004, 37, 3159–3164. [Google Scholar] [CrossRef]
- Mayer, S.; Zentel, R. A new chiral polyisocyanate: An optical switch triggered by a small amount of photochromic side groups. Macromol. Chem. Phys. 1998, 199, 1675–1682. [Google Scholar] [CrossRef]
- Pomarico, S.K.; Wang, C.Y.; Weck, M. Synthesis and light-mediated structural disruption of an azobenzene-containing helical poly(isocyanide). Macromol. Rapid Commun. 2020, 41, 1900324. [Google Scholar] [CrossRef]
- del Barrio, J.; Tejedor, R.M.; Chinelatto, L.S.; Sánchez, C.; Piñol, M.; Oriol, L. Bistable mesomorphism and supramolecular stereomutation in chiral liquid crystal Azopolymers. J. Mater. Chem. 2009, 19, 4922–4930. [Google Scholar] [CrossRef]
- Todorov, T.; Nikolova, L.; Tomova, N. Polarization holography. 1: A new high-efficiency organic material with reversible photoinduced birefringence. Appl. Opt. 1984, 23, 4309–4312. [Google Scholar] [CrossRef]
- Bilinov, L.; Kozlovsky, M.V.; Ozaki, M.; Skarp, K.; Yoshino, K. Photoinduced dichroism and optical anisotropy in a liquid-crystalline azobenzene side chain polymer caused by anisotropic angular distribution of trans and cis isomers. J. Appl. Phys. 1998, 84, 3860–3866. [Google Scholar] [CrossRef]
- Kozlovsky, M.V.; Shibaev, V.P.; Stakhanov, A.I.; Weyrauch, T.; Haase, W. A new approach to photorecording based on hindering a phase transition in photochromic chiral liquid crystalline polymers. Liq. Cryst. 1998, 24, 759–767. [Google Scholar] [CrossRef]
- Kilian, D.; Kozlovsky, M.; Haase, W. Dielectric measurements on the ‘isotropic smectic phase’ of dyed side group polymers. Liq. Cryst. 1999, 26, 705–708. [Google Scholar] [CrossRef]
- Blinov, L.M.; Cipparrone, G.; Kozlovsky, M.V.; Lazarev, V.V.; Scaramuzza, N. Holographic ‘development’ of a hidden UV image recorded on a liquid crystalline polymer. Opt. Commun. 2000, 173, 137–144. [Google Scholar] [CrossRef]
- Kozlovsky, M.; Jungnickel, B.; Ehrenberg, H. Bistable phase behavior and kinetics of nonisothermal mesophase formation in a chiral side chain polymethacrylate. Macromolecules 2005, 38, 2729–2738. [Google Scholar] [CrossRef]
- Kozlovsky, M.; Lazarev, V.V. Effect of molecular weight on photoinduced birefringence in a chiral liquid crystalline Azodye polymer. Macromol. Rapid Commun. 2006, 27, 361–365. [Google Scholar] [CrossRef]
- Polushin, S.; Rogozin, V.; Beloborodov, I.; Rjumtsev, E.; Kozlovsky, M. Existence of two different isotropic phases as a reason for bistable phase behavior of an LC side-chain polymethacrylate. Macromol. Rapid Commun. 2008, 29, 224–228. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, J.; Sun, Y.Y.; Zhou, J.L.; Chen, B.; Zhang, Q.J.; Wang, K.Y. Synthesis and chiroptical properties of optically active polymer liquid crystals containing azobenzene chromophores. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3210–3219. [Google Scholar] [CrossRef]
- Zheng, Z.; Sun, Y.Y.; Xu, J.; Chen, B.; Su, Z.Q.; Zhang, Q.J. Synthesis and chiroptical properties of liquid crystalline copolymers containing azobenzene chromophores. Polym. Int. 2007, 56, 699–706. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Boiko, N.; Shibaev, V. A new type of multifunctional material based on dual photochromism of ternary chiral photochromic liquid crystalline copolymers for optical data recording and storage. J. Mater. Chem. 2000, 10, 1075–1081. [Google Scholar] [CrossRef]
- Bobrovsky, A.Y.; Boiko, N.I.; Shibaev, V.P. Photosensitive cholesteric polymers with azobenzene-containing chiral groups and mixtures of cholesteric copolymer with chiral-photochromic dopants. Liq. Cryst. 2001, 28, 919–931. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Boiko, N.; Shibaev, V.; Stumpe, J. Photo-orientation phenomena in photosensitive chiral nematic copolymers. Liq. Cryst. 2002, 29, 1469–1476. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V. Photo-optical behaviour of a photosensitive chiral nematic copolymer and mixtures containing non-chiral photoactive azobenzene groups. Liq. Cryst. 2003, 30, 671–680. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Boiko, N.; Shibaev, V.; Wendorff, J. Photoinduced textural and optical changes in a cholesteric copolymer with azobenzene-containing side groups. Liq. Cryst. 2004, 31, 351–359. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V. Thermo-, chiro- and photo-optical properties of cholesteric azobenzene-containing copolymer in thin films. J. Photochem. Photobiol. A Chem. 2005, 172, 140–145. [Google Scholar] [CrossRef]
- Bobrovsky, A.Y.; Boiko, N.I.; Shibaev, V.P.; Springer, J. New chiral nematic materials with photovariable helical supramolecular structure for reversible optical data recording. Adv. Mater. 2000, 12, 1180–1183. [Google Scholar] [CrossRef]
- Bobrovsky, A.Y.; Boiko, N.I.; Shibaev, V.P. Chiral nematic copolymers with photoreversible and irreversible changing of helical supramolecular structure pitch. Chem. Mater. 2001, 13, 1998–2001. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Hamplová, V.; Kašpar, M.; Glogarová, M. Chirooptical and photooptical properties of a novel side-chain azobenzene-containing LC polymer. Monatsh. Chem. 2009, 140, 789–799. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Bubnov, A.; Hamplová, V.; Kašpar, M.; Pociecha, D.; Glogarová, M. Effect of molecular structure and thermal treatment on photo-optical properties of photochromic azobenzene-containing polymer films. Macromol. Chem. Phys. 2011, 212, 342–352. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Cigl, M.; Hamplová, V.; Pociecha, D.; Bubnov, A. Azobenzene-containing LC polymethacrylates highly photosensitive in broad spectral range. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2962–2970. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Piryazev, A.; Anokhin, D.V.; Ivanov, D.A.; Sinitsyna, O.; Hamplová, V.; Kašpar, M.; Bubnov, A. Photo-orientation phenomena in photochromic liquid crystalline azobenzene-containing polymethacrylates with different spacer length. Macromol. Chem. Phys. 2017, 218, 1700127. [Google Scholar] [CrossRef]
- Sulyanov, S.N.; Dorovatovskii, P.V.; Bobrovsky, A.Y.; Shibaev, V.P.; Cigl, M.; Hamplová, V.; Bubnov, A.; Ostrovskii, B.I. Mesomorphic and structural properties of liquid crystalline side-chain polymethacrylates: From smectic C* to columnar phases. Liq. Cryst. 2019, 46, 825–834. [Google Scholar] [CrossRef]
- Angiolini, L.; Bozio, R.; Giorgini, L.; Pedron, D.; Turco, G.; Daurù, A. Photomodulation of the chiroptical properties of new chiral methacrylic polymers with side chain azobenzene moieties. Chem. Eur. J. 2002, 8, 4241–4247. [Google Scholar] [CrossRef]
- del Barrio, J.; Tejedor, R.M.; Chinelatto, L.S.; Sánchez, C.; Piñol, M.; Oriol, L. Photocontrol of the supramolecular chirality imposed by stereocenters in liquid crystalline azodendrimers. Chem. Mater. 2010, 22, 1714–1723. [Google Scholar] [CrossRef]
- Mayer, S.; Zentel, R. Switching of the helical polymer conformation in a solid polymer film. Macromol. Rapid Commun. 2000, 21, 927–930. [Google Scholar] [CrossRef]
- He, X.Z.; Gao, Y.F.; Zheng, J.J.; Li, X.Y.; Meng, F.B.; Hu, J.S. Chiral photosensitive side-chain liquid crystalline polymers-synthesis and characterization. Colloid Polym. Sci. 2016, 294, 1823–1832. [Google Scholar] [CrossRef]
- Zhao, H.W.; He, X.Z.; Zheng, J.J.; Meng, F.B.; Hu, J.S.; Song, W.L. Synthesis and properties of chiral Azo-liquid crystalline terpolymer containing cyano mesogenic units. Liq. Cryst. 2017, 44, 2379–2390. [Google Scholar] [CrossRef]
- Hassan, F.; Sassa, T.; Hirose, T.; Ito, Y.; Kawamoto, M. Light-driven molecular switching of atropisomeric polymers containing Azo-binaphthyl groups in their side chains. Polym. J. (Tokyo, Jpn.) 2018, 50, 455–465. [Google Scholar] [CrossRef]
- Liu, Z.P.; Cong, Y.H.; He, X.Z.; Zhang, B.Y.; Zheng, J.J.; Meng, F.B. Synthesis and characterization of chiral Azo LCPs with optical properties. Liq. Cryst. 2019, 46, 1696–1706. [Google Scholar] [CrossRef]
- Farooq, M.A.; Wei, W.; Xiong, H. Chiral photonic liquid crystalline polyethers with widely tunable helical superstructures. Langmuir 2020, 3072–3079. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V. A study of photooptical processes in photosensitive cholesteric azobenzene-containing polymer mixture under an action of the polarized and nonpolarized light. Polymer 2006, 47, 4310–4317. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Stumpe, J. Cholesteric polymer guest-host mixture with circularly polarized fluorescence: Two ways for phototuning of polarization and its intensity. J. Phys. Chem. A 2006, 110, 2331–2336. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Ryabchun, A.; Medvedev, A.; Shibaev, V. Ordering phenomena and photoorientation processes in photochromic thin films of LC chiral azobenzene-containing polymer systems. J. Photochem. Photobiol. A Chem. 2009, 206, 46–52. [Google Scholar] [CrossRef]
- Ryabchun, A.; Bobrovsky, A.; Sobolewska, A.; Shibaev, V.; Stumpe, J. Dual photorecording on cholesteric azobenzene-containing LC polymer films using helix pitch phototuning and holographic grating recording. J. Mater. Chem. 2012, 22, 6245–6250. [Google Scholar] [CrossRef]
- Ryabchun, A.; Sobolewska, A.; Bobrovsky, A.; Shibaev, V.; Stumpe, J. Polarization holographic grating recording in the cholesteric azobenzene-containing films with the phototunable helix pitch. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 773–781. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.; Yin, L.; Zhao, Y.; Zhang, W.; Liu, M.H. Fabrication of chiroptically switchable films via co-gelation of a small chiral gelator with an achiral azobenzene-containing polymer. Soft Matter 2017, 13, 6129–6136. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, Y.; Ma, S.; Yu, H. Hierarchical self-assembly in liquid-crystalline block copolymers enabled by chirality transfer. Angew. Chem. Int. Ed. 2018, 57, 12524–12528. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Le Gac, S.; Cornelissen, J.J.L.M.; Nolte, R.J.M.; Rowan, A.E. Macromolecular multi-chromophoric scaffolding. Chem. Soc. Rev. 2010, 39, 1576–1599. [Google Scholar] [CrossRef]
- Fujiki, M. Supramolecular chirality: Solvent chirality transfer in molecular chemistry and polymer chemistry. Symmetry 2014, 6, 677–703. [Google Scholar] [CrossRef]
- Kim, H.; Jin, Y.J.; Kim, B.S.I.; Aoki, T.; Kwak, G. Optically active conjugated polymer nanoparticles from chiral solvent annealing and nanoprecipitation. Macromolecules 2015, 48, 4754–4757. [Google Scholar] [CrossRef]
- Green, M.M.; Khatri, C.; Peterson, N.C. A macromolecular conformational change driven by a minute chiral solvation energy. J. Am. Chem. Soc. 1993, 115, 4941–4942. [Google Scholar] [CrossRef]
- Wang, L.B.; Suzuki, N.; Liu, J.F.; Matsuda, T.; Rahim, N.A.A.; Zhang, W.; Fujiki, M.; Zhang, Z.B.; Zhou, N.C.; Zhu, X.L. Limonene induced chiroptical generation and inversion during aggregation of achiral polyfluorene analogs: Structure-dependence and mechanism. Poly. Chem. 2014, 5, 5920–5927. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, Y.; Jiang, S.; Wang, L.; Zhang, Z.; Zhu, J.; Zhang, W.; Zhu, X. Preferential chiral solvation induced supramolecular chirality in optically inactive star Azo polymers: Photocontrollability, chiral amplification and topological effects. Poly. Chem. 2015, 6, 7045–7052. [Google Scholar] [CrossRef]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Menard, F.; Tamura, M. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, L.; Todorov, T.; Ivanov, M.; Andruzzi, F.; Hvilsted, S.; Ramanujam, P.S. Photoinduced circular anisotropy in side-chain azobenzene polyesters. Opt. Mater. 1997, 8, 255–258. [Google Scholar] [CrossRef]
- Naydenova, I.; Nikolova, L.; Ramanujam, P.S.; Hvilsted, S. Light-induced circular birefringence in cyanoazobenzene side-chain liquid-crystalline polyester films. J. Opt. A Pure Appl. Opt. 1999, 1, 438–441. [Google Scholar] [CrossRef]
- Nikolova, L.; Nedelchev, L.; Todorov, T.; Petrova, T.; Tomova, N.; Dragostinova, V.; Ramanujam, P.S.; Hvilsted, S. Self-induced light polarization rotation in azobenzene-containing polymers. Appl. Phys. Lett. 2000, 77, 657–659. [Google Scholar] [CrossRef]
- Choi, S.W.; Kawauchi, S.; Ha, N.Y.; Takezoe, H. Photoinduced Chirality in Azobenzene-containing polymer systems. Phys. Chem. Chem. Phys. 2007, 9, 3671–3681. [Google Scholar] [CrossRef]
- Iftime, G.; Labarthet, F.L.; Natansohn, A.; Rochon, P. Control of chirality of an azobenzene liquid crystalline polymer with circularly polarized light. J. Am. Chem. Soc. 2000, 122, 12646–12650. [Google Scholar] [CrossRef]
- Ivanov, M.; Naydenova, I.; Todorov, T.; Nikolova, L.; Petrova, T.; Tomova, N.; Dragostinova, V. Light-induced optical activity in optically ordered amorphous side-chain azobenzene containing polymer. J. Mod. Opt. 2000, 47, 861–867. [Google Scholar] [CrossRef]
- Nedelchev, L.; Nikolova, L.; Todorov, T.; Petrova, T.; Tomova, N.; Dragostinova, V.; Ramanujam, P.S.; Hvilsted, S. Light propagation through photoinduced chiral structures in azobenzene-containing polymers. J. Opt. A Pure Appl. Opt. 2001, 3, 304–310. [Google Scholar] [CrossRef]
- Nedelchev, L.; Matharu, A.; Nikolova, L.; Hvilsted, S.; Ramanujam, P.S. Propagation of polarized light through azobenzene polyester films. Mol. Cryst. Liq. Cryst. 2002, 375, 563–575. [Google Scholar] [CrossRef]
- Nedelchev, L.; Nikolova, L.; Matharu, A.; Ramanujam, P.S. Photoinduced macroscopic chiral structures in a series of azobenzene copolyesters. Appl. Phys. B 2002, 75, 671–676. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, B.G.; Kim, J.J.; Kim, D.Y. Photoinduced supramolecular chirality in amorphous azobenzene polymer films. J. Am. Chem. Soc. 2002, 124, 3504–3505. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yoo, S.J.; Kim, D.Y. A supramolecular chiroptical switch using an amorphous azobenzene polymer. Adv. Funct. Mater. 2006, 16, 2089–2094. [Google Scholar] [CrossRef]
- Kim, M.J.; Kumar, A.; Kim, D.Y. Photofabricatoin of superhelix like patterns on azobenzene polymer films. Adv. Mater. 2003, 15, 2005–2008. [Google Scholar] [CrossRef]
- Cipparrone, G.; Pagliusi, P.; Provenzano, C.; Shibaev, V.P. Reversible photoinduced chiral structure in amorphous polymer for light polarization control. Macromolecules 2008, 41, 5992–5996. [Google Scholar] [CrossRef]
- Cipparrone, G.; Pagliusi, P.; Provenzano, C.; Shibaev, V.P. Polarization holographic recording in amorphous polymer with photoinduced linear and circular birefringence. J. Phys. Chem. B 2010, 114, 8900–8904. [Google Scholar] [CrossRef]
- Royes, J.; Provenzano, C.; Pagliusi, P.; Tejedor, R.M.; Piñol, M.; Oriol, L. A bifunctional amorphous polymer exhibiting equal linear and circular photoinduced birefringences. Macromol. Rapid Commun. 2014, 35, 1890–1895. [Google Scholar] [CrossRef]
- Hore, D.K.; Natansohn, A.L.; Rochon, P.L. Optical anisotropy as a probe of structural order by Stokes polarimetry. J. Phys. Chem. B 2002, 106, 9004–9012. [Google Scholar] [CrossRef]
- Hore, D.; Wu, Y.L.; Natansohn, A.; Rochon, P. Investigation of circular Bragg reflection in an Azo polymer with photoinduced chirality. J. Appl. Phys. 2003, 94, 2162–2166. [Google Scholar] [CrossRef]
- Hore, D.K.; Natansohn, A.L.; Rochon, P.L. The characterization of photoinduced chirality in a liquid-crystalline Azo polymer on irradiation with circularly polarized light. J. Phys. Chem. B 2003, 107, 2506–2518. [Google Scholar] [CrossRef]
- Wu, Y.L.; Natansohn, A.; Rochon, P. Photoinduced chirality in thin films of achiral polymer liquid crystals containing azobenzene chromophores. Macromolecules 2004, 37, 6801–6805. [Google Scholar] [CrossRef]
- Tejedor, R.M.; Millaruelo, M.; Oriol, L.; Serrano, J.L.; Alcalá, R.; Rodríguez, F.J.; Villacampa, B. Photoinduced supramolecular chirality in side-chain liquid crystalline azopolymers. J. Mater. Chem. 2006, 16, 1674–1680. [Google Scholar] [CrossRef]
- Tejedor, R.M.; Oriol, L.; Serrano, J.L.; Sierra, T. Chiral photochemical induction in liquid crystals. J. Mater. Chem. 2008, 18, 2899–2908. [Google Scholar] [CrossRef]
- Tejedor, R.M.; Vera, F.; Oriol, L.; Sierra, T.; Serrano, J.L. Photoinduction and control of the supramolecular chirality in liquid crystalline azomaterials. Mol. Cryst. Liq. Cryst. 2008, 489, 105–118. [Google Scholar] [CrossRef]
- Tejedor, R.M.; Serrano, J.L.; Oriol, L. Photocontrol of supramolecular architecture in azopolymers: Achiral and chiral aggregation. Eur. Polym. J. 2009, 45, 2564–2571. [Google Scholar] [CrossRef]
- Royes, J.; Rebolé, J.; Custardoy, L.; Gimeno, N.; Oriol, L.; Tejedor, R.M.; Piñol, M. Preparation of side-chain liquid crystalline Azopolymers by CuAAC postfunctionalization using bifunctional azides: Induction of chirality using circularly polarized light. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1579–1590. [Google Scholar] [CrossRef]
- Royes, J.; Nogales, A.; Ezquerra, T.A.; Oriol, L.; Tejedor, R.M.; Piñol, M. Effect of the polymer architecture on the photoinduction of stable chiral organizations. Polymer 2018, 143, 58–68. [Google Scholar] [CrossRef]
- Royes, J.; Oriol, L.; Tejedor, R.M.; Piñol, M. Strategies to stabilize the photoinduced supramolecular chirality in azobenzene liquid crystalline polymers. Polymers 2019, 11, 885. [Google Scholar] [CrossRef]
- Zheng, Z.; Su, Z.Q.; Wang, L.L.; Xu, J.; Zhang, Q.J.; Yang, J.J. Photoinduced chirality in achiral liquid crystalline azobenzene polymers containing different polar side groups. Eur. Polym. J. 2007, 43, 2738–2744. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, L.L.; Su, Z.Q.; Xu, J.; Yang, J.J.; Zhang, Q.J. Photoinduced chirality in achiral liquid crystalline polymethacrylates containing bisazobenzene and azobenzene chromophores. J. Photochem. Photobiol. A Chem. 2007, 185, 338–344. [Google Scholar] [CrossRef]
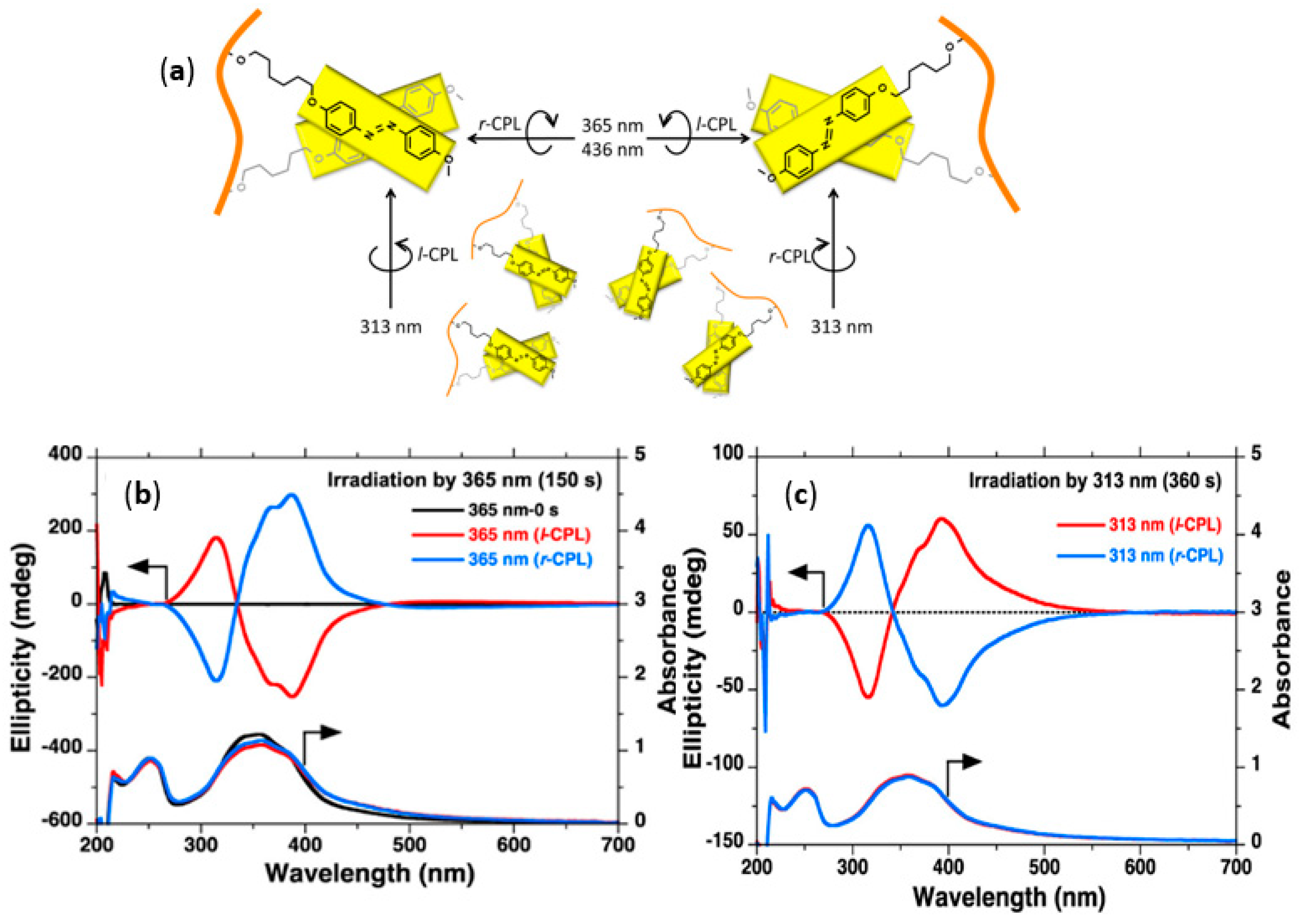
- Wang, L.; Yin, L.; Zhang, W.; Zhu, X.; Fujiki, M. Circularly polarized light with sense and wavelengths to regulate azobenzene supramolecular chirality in optofluidic medium. J. Am. Chem. Soc. 2017, 139, 13218–13226. [Google Scholar] [CrossRef] [PubMed]
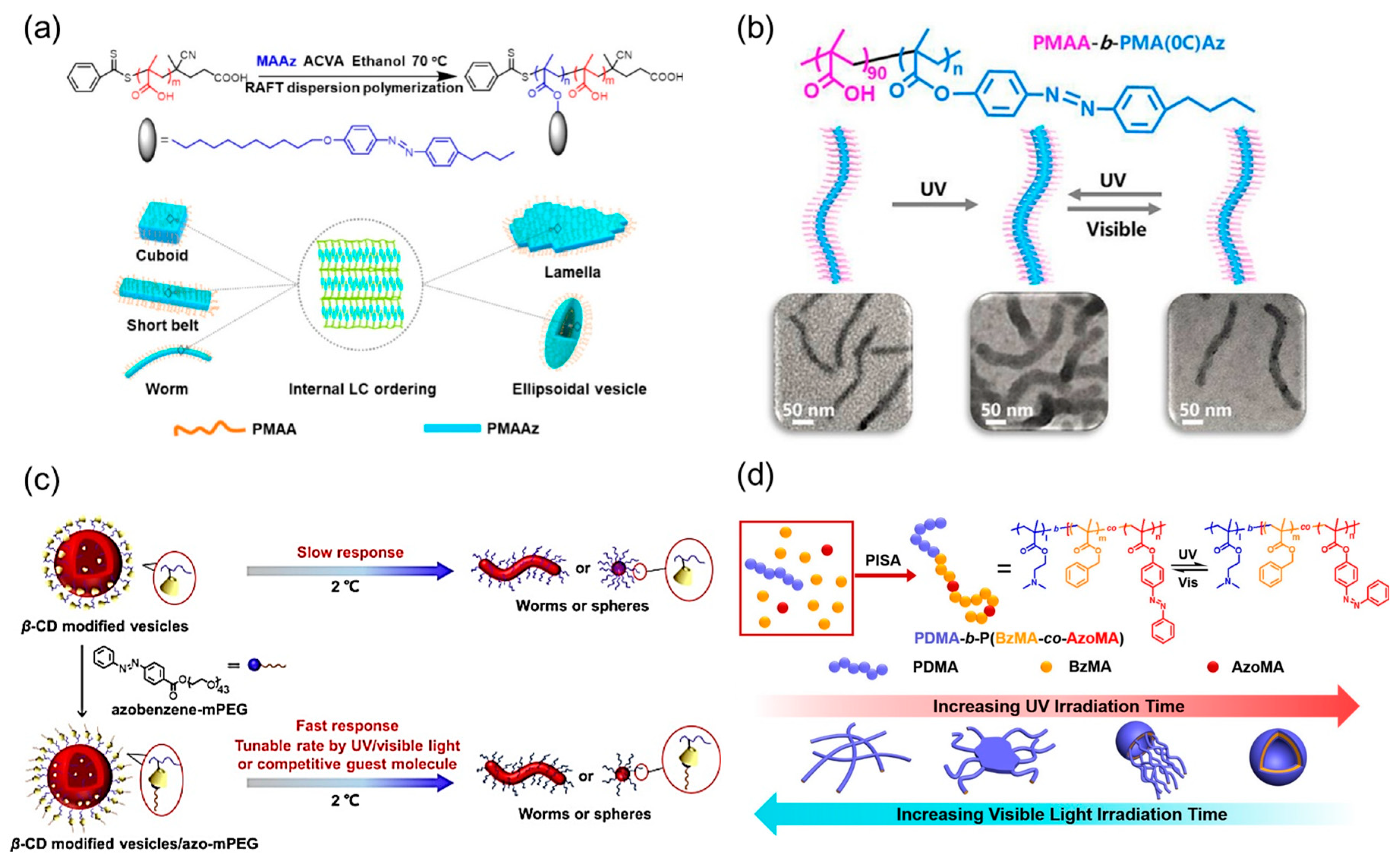
- Guan, S.; Zhang, C.; Wen, W.; Qu, T.; Zheng, X.; Zhao, Y.; Chen, A. Formation of anisotropic liquid crystalline nanoparticles via polymerization-induced hierarchical self-assembly. ACS Macro Lett. 2018, 7, 358–363. [Google Scholar] [CrossRef]
- Guan, S.; Deng, Z.; Huang, T.; Wen, W.; Zhao, Y.; Chen, A. Light-triggered reversible slimming of azobenzene-containing wormlike nanoparticles synthesized by polymerization-induced self-assembly for nanofiltration switches. ACS Macro Lett. 2019, 8, 460–465. [Google Scholar] [CrossRef]
- Yao, H.; Ning, Y.; Jesson, C.P.; He, J.; Deng, R.; Tian, W.; Armes, S.P. Using host-guest chemistry to tune the kinetics of morphological transitions undertaken by block copolymer vesicles. ACS Macro Lett. 2017, 6, 1379–1385. [Google Scholar] [CrossRef]
- Ye, Q.; Huo, M.; Zeng, M.; Liu, L.; Peng, L.; Wang, X.; Yuan, J. Photoinduced reversible worm-to-vesicle transformation of Azo-containing block copolymer assemblies prepared by polymerization-induced self-assembly. Macromolecules 2018, 51, 3308–3314. [Google Scholar] [CrossRef]
- Liu, X.; Sun, M.; Sun, J.; Hu, J.; Wang, Z.; Guo, J.; Gao, W. Polymerization induced self-assembly of a site-specific interferon alpha-block copolymer conjugate into micelles with remarkably enhanced pharmacology. J. Am. Chem. Soc. 2018, 140, 10435–10438. [Google Scholar] [CrossRef]
- Ebeling, B.; Belal, K.; Stoffelbach, F.; Woisel, P.; Lansalot, M.; D’Agosto, F. Polymer nanospheres with hydrophobic surface groups as supramolecular building blocks produced by aqueous PISA. Macromol. Rapid Commun. 2019, 40, 1800455. [Google Scholar] [CrossRef]
- Jimaja, S.; Varlas, S.; Xie, Y.; Foster, J.C.; Taton, D.; Dove, A.P.; O’Reilly, R.K. Nickel-catalyzed coordination polymerization-induced self-assembly of helical poly(aryl isocyanide)s. ACS Macro Lett. 2020, 9, 226–232. [Google Scholar] [CrossRef]
- Chen, J.; Cai, S.; Wang, R.; Wang, S.; Zhang, J.; Wan, X. Polymerization-induced self-assembly of conjugated block copoly(phenylacetylene)s. Macromolecules 2020, 53, 1638–1644. [Google Scholar] [CrossRef]
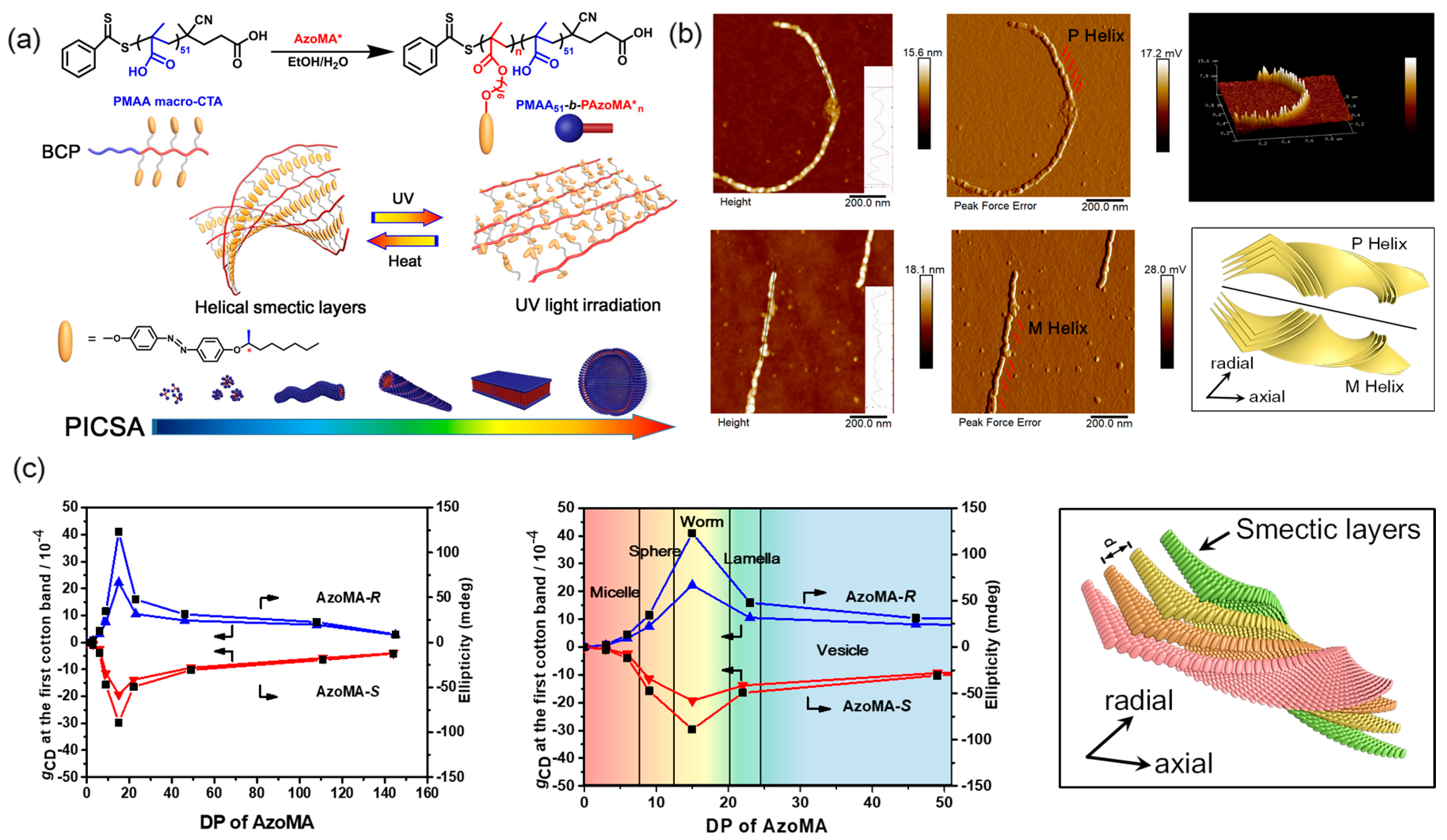
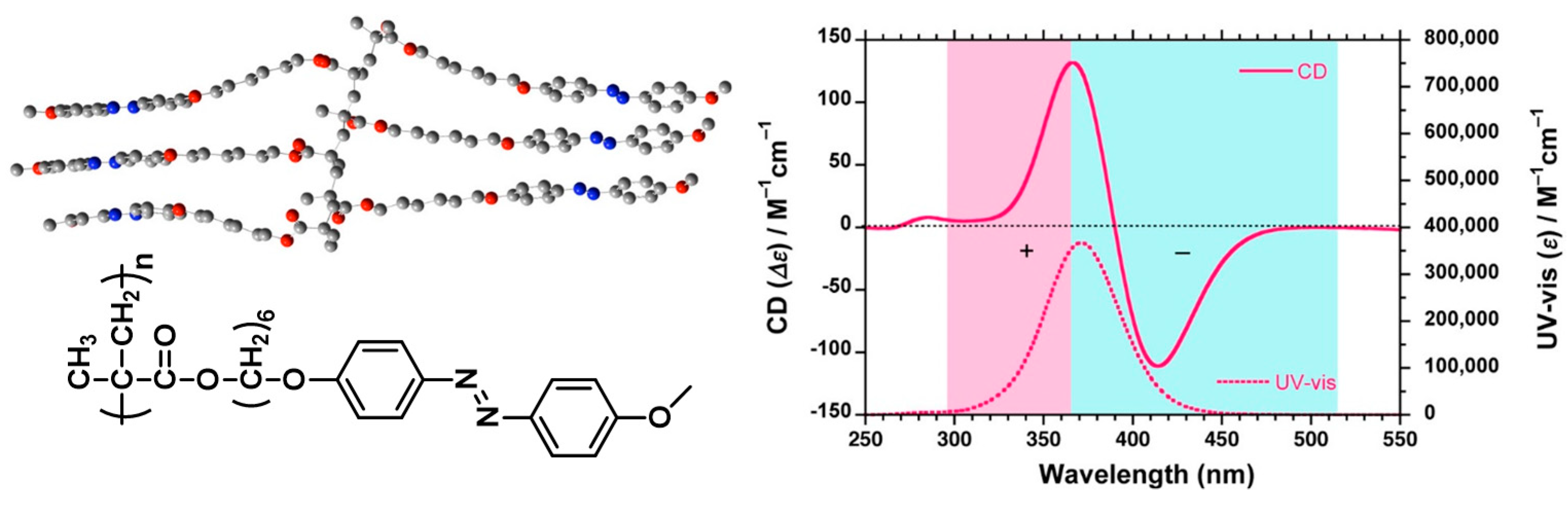
- Cheng, X.X.; Miao, T.F.; Yin, L.; Ji, Y.J.; Li, Y.Y.; Zhang, Z.B.; Zhang, W.; Zhu, X.L. In situ controlled construction of a hierarchical supramolecular chiral liquid-crystalline polymer assembly. Angew. Chem. Int. Ed. 2020, 59, 9669–9677. [Google Scholar] [CrossRef]
- Bochicchio, D.; Pavan, G.M. Molecular modelling of supramolecular polymers. Adv. Phy. X 2018, 3, 1436408. [Google Scholar] [CrossRef]
- Chami, F.; Wilson, M.R. Molecular order in a chromonic liquid crystal: A molecular simulation study of the anionic Azo dye sunset yellow. J. Am. Chem. Soc. 2010, 132, 7794–7802. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S.; Lee, J.; Malpani, Y.; Jung, Y.S.; Kang, B.; Lee, J.Y.; Ozasa, K.; Isoshima, T.; Lee, S.Y.; et al. Stimulus-responsive azobenzene supramolecules: Fibers, gels, and hollow spheres. Langmuir 2013, 29, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Larson, R.G. A coarse-grained implicit solvent model for poly(ethylene oxide), CnEm surfactants, and hydrophobically end-capped poly(ethylene oxide) and its application to micelle self-assembly and phase behavior. Macromolecules 2015, 48, 7709–7718. [Google Scholar] [CrossRef]
- Bedrov, D.; Hooper, J.B.; Glaser, M.A.; Clark, N.A. Photoinduced and thermal relaxation in surface-grafted azobenzene-based monolayers: A molecular dynamics simulation study. Langmuir 2016, 32, 4004–4015. [Google Scholar] [CrossRef]
- Cantatore, V.; Granucci, G.; Rousseau, G.; Padula, G.; Persico, M. Photoisomerization of self-assembled monolayers of azobiphenyls: Simulations highlight the role of packing and defects. J. Phys. Chem. Lett. 2016, 7, 4027–4031. [Google Scholar] [CrossRef]
- Zhao, H.; Sen, S.; Udayabhaskararao, T.; Sawczyk, M.; Kučanda, K.; Manna, D.; Kundu, P.K.; Lee, J.W.; Král, P.; Klajn, R. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nat. Nanotechnol. 2016, 11, 82–88. [Google Scholar]
- Moon, J.; Kim, B.; Choi, J.; Cho, M. Multiscale study of the relationship between photoisomerization and mechanical behavior of Azo-polymer based on the coarse-grained molecular dynamics simulation. Macromolecules 2019, 52, 2033–2049. [Google Scholar] [CrossRef]
- Savchenko, V.; Koch, M.; Pavlov, A.S.; Saphiannikova, M.; Guskova, O. Stacks of azobenzene stars: Self-assembly scenario and stabilising forces quantified in computer modelling. Molecules 2019, 24, 4387. [Google Scholar] [CrossRef]
- Koch, M.; Saphiannikova, M.; Guskova, O. Do Columns of azobenzene stars disassemble under light illumination? Langmuir 2019, 35, 14659–14669. [Google Scholar] [CrossRef]
- Fredy, J.W.; Mendez-Ardoy, A.; Kwangmettatam, S.; Bochicchio, D.; Matt, B.; Stuart, M.C.A.; Huskens, J.; Katsonis, N.; Pavan, G.M.; Kudernac, T. Molecular photoswitches mediating the strain-driven disassembly of supramolecular tubules. Proc. Natl. Acad. Sci. USA 2017, 114, 11850–11855. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Miao, T.; Qian, Y.; Zhang, Z.; Zhang, W.; Zhu, X. Supramolecular Chirality in Azobenzene-Containing Polymer System: Traditional Postpolymerization Self-Assembly Versus In Situ Supramolecular Self-Assembly Strategy. Int. J. Mol. Sci. 2020, 21, 6186. https://doi.org/10.3390/ijms21176186
Cheng X, Miao T, Qian Y, Zhang Z, Zhang W, Zhu X. Supramolecular Chirality in Azobenzene-Containing Polymer System: Traditional Postpolymerization Self-Assembly Versus In Situ Supramolecular Self-Assembly Strategy. International Journal of Molecular Sciences. 2020; 21(17):6186. https://doi.org/10.3390/ijms21176186
Chicago/Turabian StyleCheng, Xiaoxiao, Tengfei Miao, Yilin Qian, Zhengbiao Zhang, Wei Zhang, and Xiulin Zhu. 2020. "Supramolecular Chirality in Azobenzene-Containing Polymer System: Traditional Postpolymerization Self-Assembly Versus In Situ Supramolecular Self-Assembly Strategy" International Journal of Molecular Sciences 21, no. 17: 6186. https://doi.org/10.3390/ijms21176186
APA StyleCheng, X., Miao, T., Qian, Y., Zhang, Z., Zhang, W., & Zhu, X. (2020). Supramolecular Chirality in Azobenzene-Containing Polymer System: Traditional Postpolymerization Self-Assembly Versus In Situ Supramolecular Self-Assembly Strategy. International Journal of Molecular Sciences, 21(17), 6186. https://doi.org/10.3390/ijms21176186