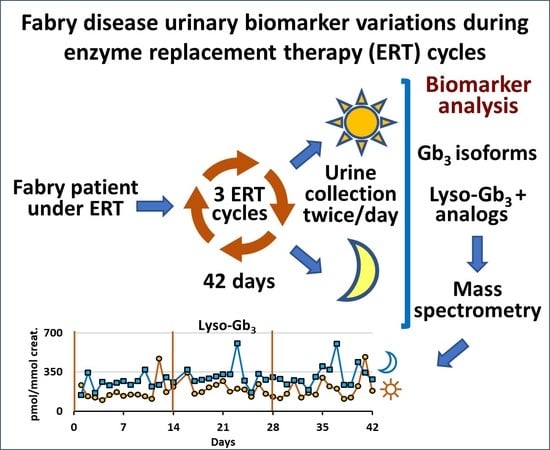
Diurnal Variation of Urinary Fabry Disease Biomarkers during Enzyme Replacement Therapy Cycles
Abstract
1. Introduction
2. Results
2.1. Biomarker Measures according to the Collection Time
2.1.1. Mean Measured Biomarker Levels
2.1.2. Variance of Biomarker Measures
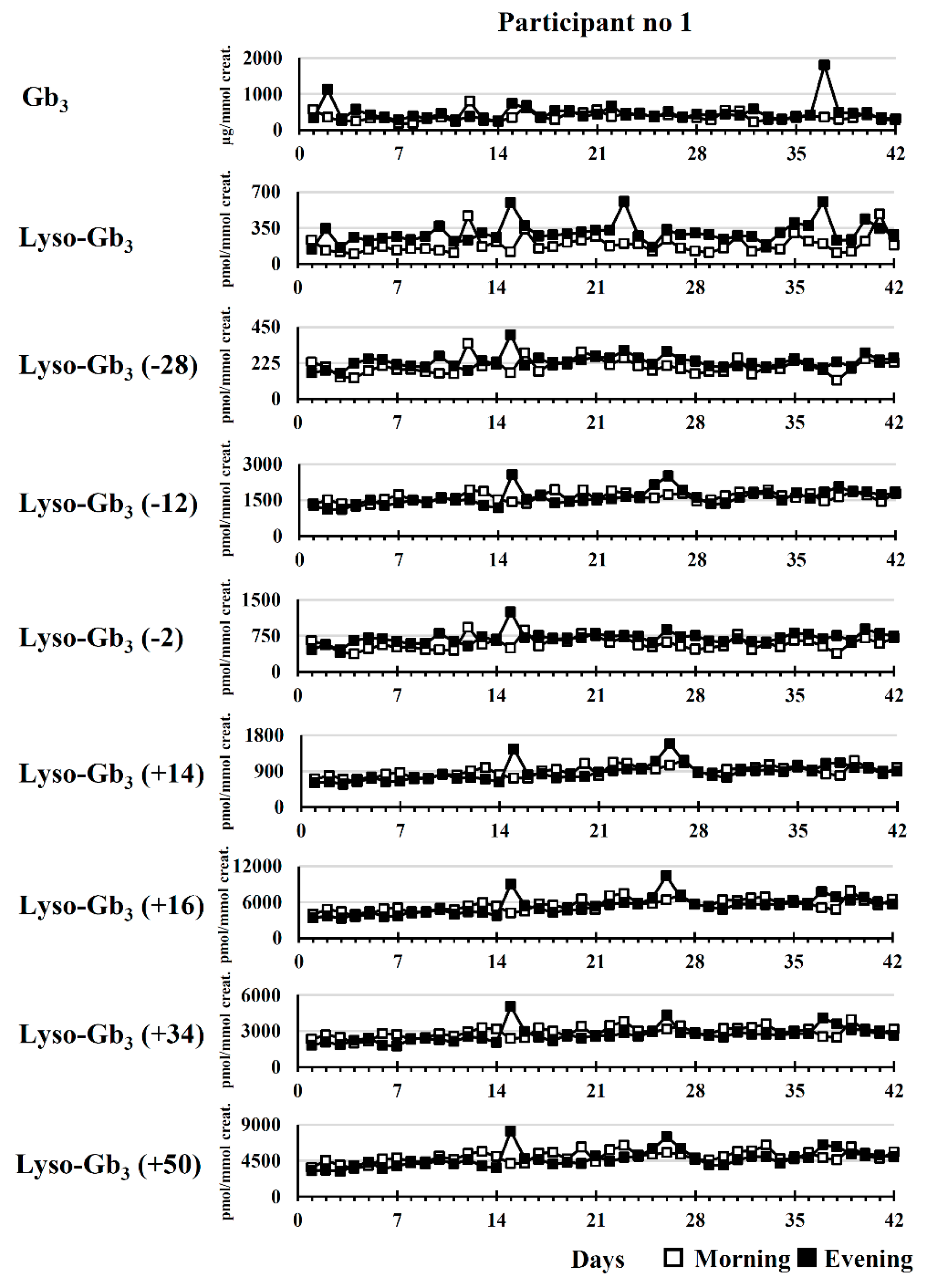
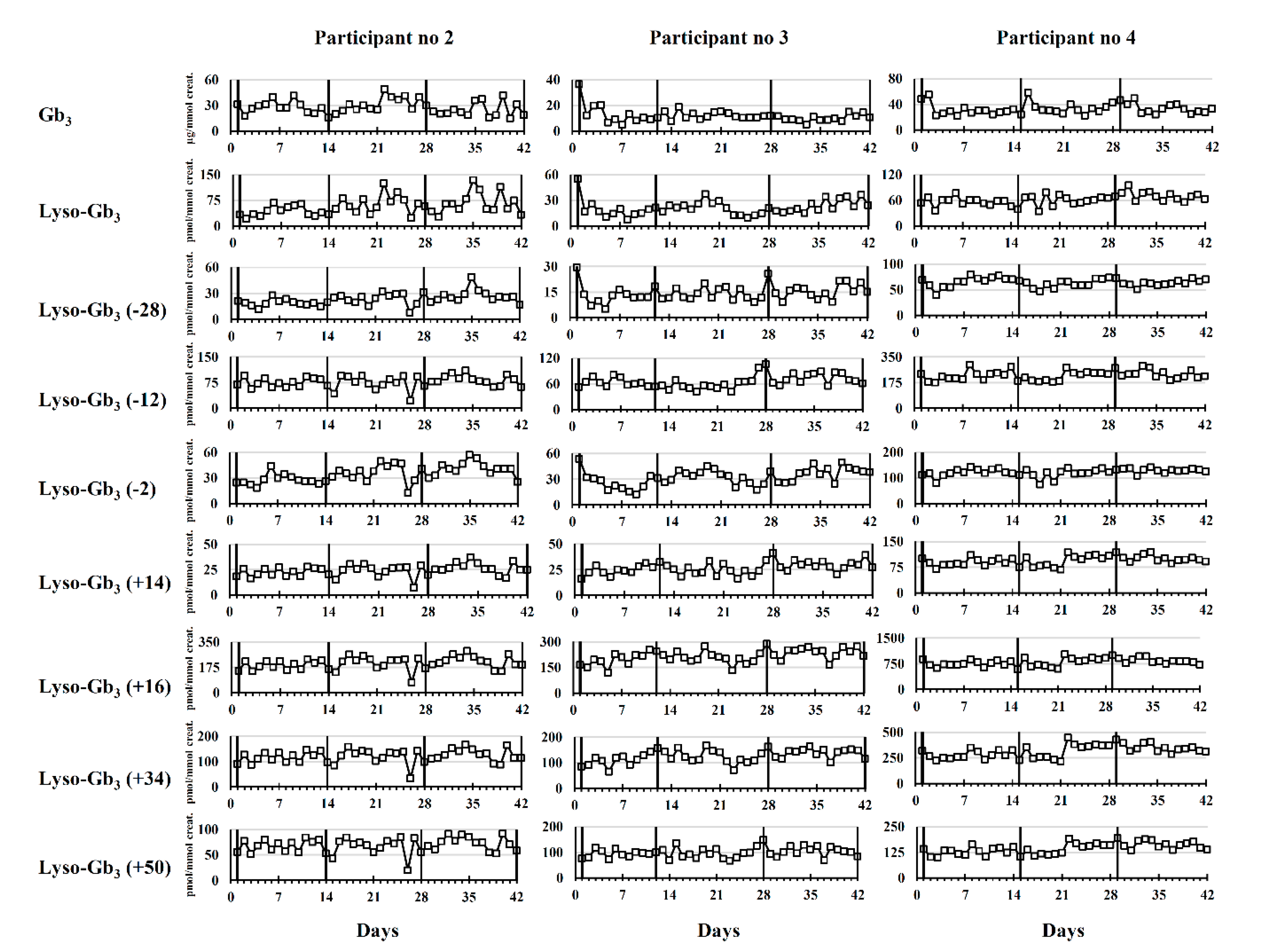
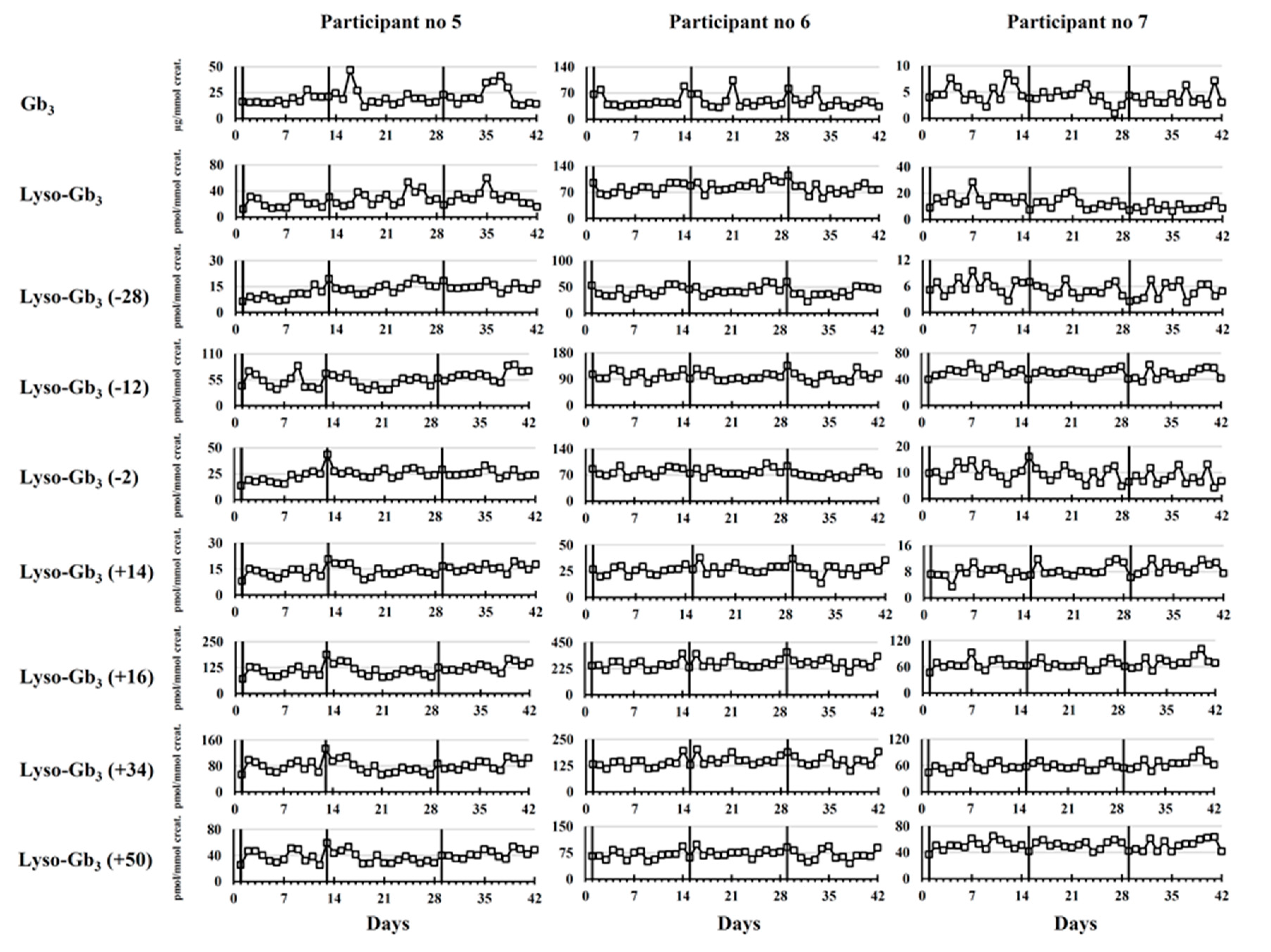
2.2. Longitudinal Follow-Up
2.2.1. Untreated Fabry Disease Participant
2.2.2. Participants Treated with ERT
Male Fabry Disease Participants
Female Fabry Disease Participants
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Approval
5.2. Recruitment of Participants
5.3. Urine Specimen Collection
5.4. Analysis of Fabry Disease Biomarkers
5.4.1. Gb3
5.4.2. Lyso-Gb3 and Related Analogues
5.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CIUSSSE-CHUS | Centre Intégré Universitaire de Santé et de Services Sociaux de l’Estrie-Centre Hospitalier Universitaire de Sherbrooke |
| DUS | Dried urine spots |
| ERT | Enzyme replacement therapy |
| FD | Fabry disease |
| Ga2 | Galabiosylceramide |
| Gb3 | Globotriaosylceramide |
| HPLC | High-performance liquid chromatography |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| Lyso-Gb3 | Globotriaosylsphingosine |
| MCX | Mixed-mode strong cation exchange |
| MS/MS | Tandem mass spectrometry |
| REB | Research Ethics Board |
| RSD | Relative standard deviation |
| TIC | Total ion count |
| UPLC | Ultra-performance liquid chromatography |
References
- Juchniewicz, P.; Kloska, A.; Tylki-Szymanska, A.; Jakobkiewicz-Banecka, J.; Wegrzyn, G.; Moskot, M.; Gabig Ciminska, M.; Piotrowska, E. Female fabry disease patients and X-chromosome inactivation. Gene 2018, 641, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.T.R. Narrative review: Fabry disease. Ann. Intern. Med. 2007, 146, 425–433. [Google Scholar] [CrossRef]
- Michaud, L.; Auray-Blais, C. Improved ways to screen for patients with fabry disease, involving optometry in a multidisciplinary approach. Can. J. Optom. 2012, 74, 25–32. [Google Scholar] [CrossRef]
- Lenders, M.; Stappers, F.; Niemietz, C.; Schmitz, B.; Boutin, M.; Ballmaier, P.J.; Zibert, A.; Schmidt, H.; Brand, S.M.; Auray-Blais, C.; et al. Mutation-specific fabry disease patient-derived cell model to evaluate the amenability to chaperone therapy. J. Med. Genet. 2019, 56, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Provençal, P.; Boutin, M.; Dworski, S.; Au, B.; Medin, J.A.; Auray-Blais, C. Relative distribution of Gb3 isoforms/analogs in NOD/SCID/fabry mice tissues determined by tandem mass spectrometry. Bioanalysis 2016, 8, 1793–1807. [Google Scholar] [CrossRef]
- Boutin, M.; Menkovic, I.; Martineau, T.; Vaillancourt-Lavigueur, V.; Toupin, A.; Auray-Blais, C. Separation and analysis of Lactosylceramide, Galabiosylceramide, and Globotriaosylceramide by LC-MS/MS in urine of fabry disease patients. Anal. Chem. 2017, 89, 13382–13390. [Google Scholar] [CrossRef] [PubMed]
- Abaoui, M.; Boutin, M.; Lavoie, P.; Auray-Blais, C. tandem mass spectrometry multiplex analysis of methylated and non-methylated urinary Gb3 isoforms in fabry disease patients. Clin. Chim. Acta 2016, 452, 191–198. [Google Scholar] [CrossRef]
- Boutin, M.; Auray-Blais, C. Multiplex tandem mass spectrometry analysis of novel plasma lyso-Gb3-related analogues in fabry disease. Anal. Chem. 2014, 86, 3476–3483. [Google Scholar] [CrossRef]
- Lavoie, P.; Boutin, M.; Auray-Blais, C. Multiplex analysis of novel urinary lyso-Gb3-related biomarkers for fabry disease by tandem mass spectrometry. Anal. Chem. 2013, 85, 1743–1752. [Google Scholar] [CrossRef]
- Auray-Blais, C.; Boutin, M.; Gagnon, R.; Dupont, F.O.; Lavoie, P.; Clarke, J.T.R. Urinary globotriaosylsphingosine-related biomarkers for fabry disease targeted by metabolomics. Anal. Chem. 2012, 84, 2745–2753. [Google Scholar] [CrossRef]
- Dupont, F.O.; Gagnon, R.; Boutin, M.; Auray-Blais, C. A Metabolomic study reveals novel plasma lyso-gb3 analogs as fabry disease biomarkers. Curr. Med. Chem. 2012, 20, 280–288. [Google Scholar] [CrossRef]
- Boutin, M.; Auray-Blais, C. Metabolomic discovery of novel urinary Galabiosylceramide analogs as fabry disease biomarkers. J. Am. Soc. Mass Spectrom. 2015, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Manwaring, V.; Boutin, M.; Auray-Blais, C. A metabolomic study to identify new Globotriaosylceramide-related biomarkers in the plasma of fabry disease patients. Anal. Chem. 2013, 85, 9039–9048. [Google Scholar] [CrossRef]
- Auray-Blais, C.; Blais, C.M.; Ramaswami, U.; Boutin, M.; Germain, D.P.; Dyack, S.; Bodamer, O.; Pintos-Morell, G.; Clarke, J.T.; Bichet, D.G.; et al. Urinary biomarker investigation in children with fabry disease using tandem mass spectrometry. Clin. Chim. Acta 2015, 438, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Lavoie, P.; Boutin, M.; Ntwari, A.; Hsu, T.R.; Huang, C.K.; Niu, D.M. Biomarkers associated with clinical manifestations in fabry disease patients with a late-onset cardiac variant mutation. Clin. Chim. Acta 2017, 466, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Huynh-Do, U.; Krayenbuehl, P.A.; Beuschlein, F.; Schiffmann, R.; Barbey, F. Fabry disease genotype, phenotype, and migalastat amenability: Insights from a national cohort. J. Inherit. Metab. Dis. 2020, 43, 326–333. [Google Scholar] [CrossRef]
- Germain, D.P.; Elliott, P.M.; Falissard, B.; Fomin, V.V.; Hilz, M.J.; Jovanovic, A.; Kantola, I.; Linhart, A.; Mignani, R.; Namdar, M.; et al. The effect of enzyme replacement therapy on clinical outcomes in male patients with fabry disease: A systematic literature review by a European panel of experts. Mol. Genet. Metab. Rep. 2019, 19, 100454. [Google Scholar] [CrossRef]
- Medin, J.A. FACTS fabry gene therapy clinical trial: Two year data. Mol. Genet. Metab. 2019, 126, S7–S16. [Google Scholar] [CrossRef]
- Wagner, B.D.; Accurso, F.J.; Laguna, T.A. The applicability of urinary creatinine as a method of specimen normalization in the cystic fibrosis population. J. Cyst. Fibros. 2010, 9, 212–216. [Google Scholar] [CrossRef]
- Schiffmann, R.; Waldek, S.; Benigni, A.; Auray-Blais, C. Biomarkers of fabry disease nephropathy. Clin. J. Am. Soc. Nephrol. 2010, 5, 360–364. [Google Scholar] [CrossRef]
- Mills, K.; Morris, P.; Lee, P.; Vellodi, A.; Waldek, S.; Young, E.; Winchester, B. Measurement of urinary CDH and CTH by tandem mass spectrometry in patients hemizygous and heterozygous for Fabry disease. J. Inherit. Metab. Dis. 2005, 28, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.D.; Calvin, J.; Hogg, S.; O’Driscoll, E.; Halsall, D.; Burling, K.; Maguire, G.; Wright, N.; Cox, T.M.; Meikle, P.J.; et al. Monitoring enzyme replacement therapy in Fabry disease—Role of urine globotriaosylceramide. J. Inherit. Metab. Dis. 2005, 28, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Lavoie, P.; Boutin, M.; Abaoui, M. High-risk screening for fabry disease: Analysis by tandem mass spectrometry of Globotriaosylceramide (Gb3) in urine collected on filter paper. Curr. Protoc. Hum. Genet. 2017, 93, 17.26.1–17.26.12. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Cyr, D.; Ntwari, A.; West, M.L.; Cox-Brinkman, J.; Bichet, D.G.; Germain, D.P.; Laframboise, R.; Melançon, S.B.; Stockley, T.; et al. Urinary Globotriaosylceramide excretion correlates with the genotype in children and adults with fabry disease. Mol. Genet. Metab. 2008, 93, 331–340. [Google Scholar] [CrossRef]
- Lavoie, P.; Boutin, M.; Abaoui, M.; Auray-Blais, C. Fabry disease biomarkers: Analysis of urinary Lyso-Gb3 and seven related analogs using tandem mass spectrometry. Curr. Protoc. Hum. Genet. 2016, 90, 17.22.1–17.22.12. [Google Scholar] [CrossRef]
- Aickin, M.; Gensler, H. Adjusting for multiple testing when reporting research results: The bonferroni vs holm methods. Am. J. Public Health 1996, 86, 726–728. [Google Scholar] [CrossRef]
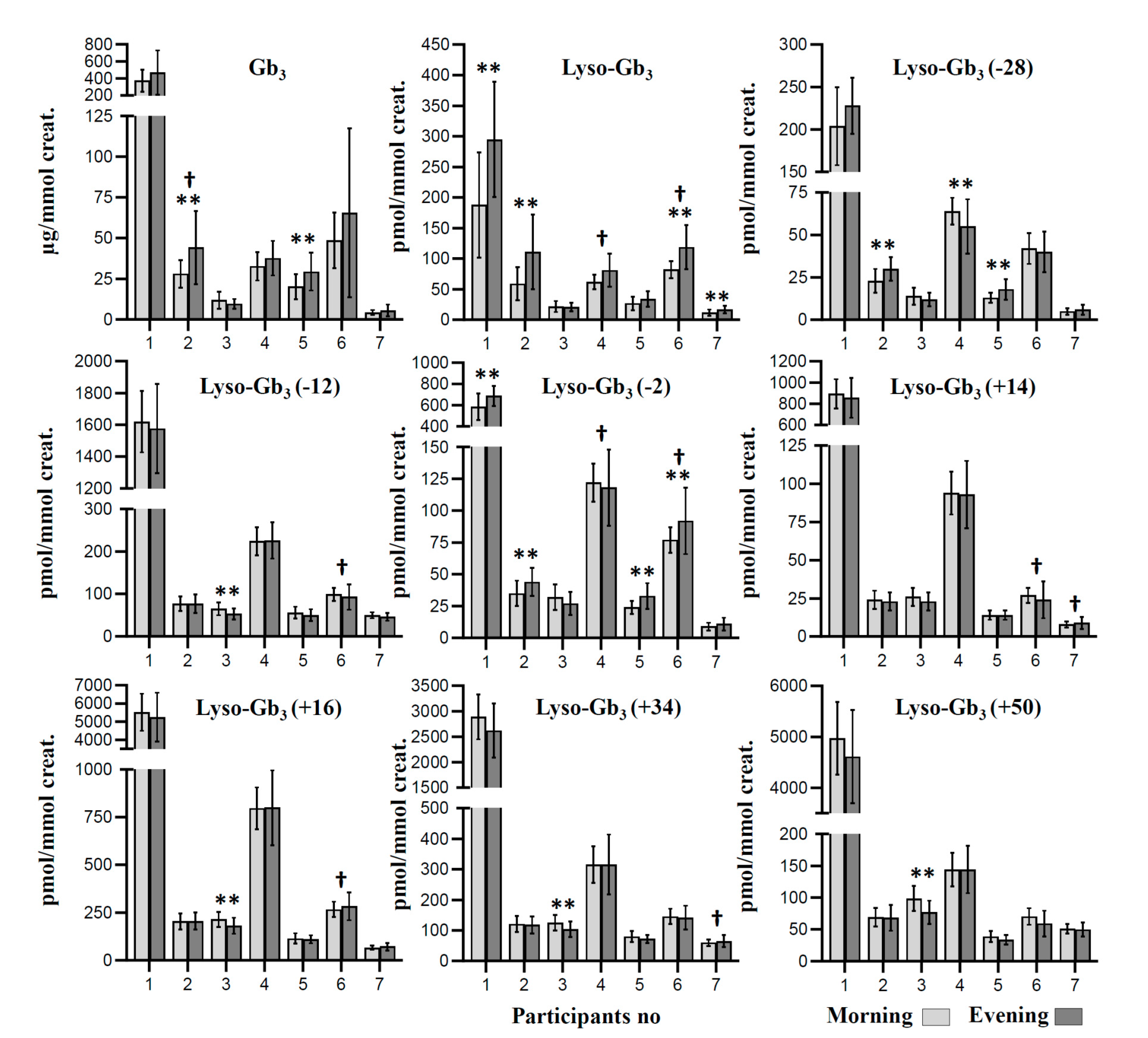
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | Participant 6 | Participant 7 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | |
| Gb3 | 373 ± 128 | 468 ± 261 | <0.050 | 28 ± 8 | 44 ± 23 | <0.001 † | 12 ± 5 | 10 ± 3 | <0.050 | 33 ± 9 | 38 ± 11 | <0.050 | 20 ± 8 | 29 ± 12 | <0.001 † | 49 ± 17 | 66 ± 52 | 0.059 | 4 ± 2 | 6 ± 4 | 0.060 |
| Lyso-Gb3 | 188 ± 86 | 295 ± 94 | <0.001 † | 59 ± 27 | 111 ± 61 | <0.001 † | 22 ± 9 | 21 ± 7 | 0.525 | 62 ± 12 | 81 ± 27 | <0.050 | 27 ± 11 | 34 ± 13 | <0.050 | 82 ± 14 | 119 ± 36 | <0.001 † | 12 ± 5 | 17 ± 6 | <0.001 † |
| Lyso-Gb3 (−28) | 204 ± 46 | 228 ± 33 | <0.050 | 23 ± 7 | 30 ± 7 | <0.001 † | 14 ± 5 | 12 ± 4 | <0.050 | 64 ± 8 | 55 ± 16 | 0.001 † | 13 ± 3 | 18 ± 6 | <0.001 † | 42 ± 9 | 40 ± 12 | 0.224 | 5 ± 2 | 6 ± 3 | 0.208 |
| Lyso-Gb3 (−12) | 1620 ± 193 | 1576 ± 281 | 0.282 | 77 ± 17 | 77 ± 22 | 0.894 | 65 ± 15 | 53 ± 13 | <0.001 † | 224 ± 33 | 226 ± 43 | 0.831 | 56 ± 14 | 50 ± 15 | <0.050 | 99 ± 15 | 93 ± 30 | 0.224 | 50 ± 7 | 46 ± 9 | <0.050 |
| Lyso-Gb3 (−2) | 585 ± 126 | 689 ± 95 | <0.001 † | 35 ± 10 | 44 ± 11 | <0.001 † | 32 ± 10 | 27 ± 9 | <0.050 | 122 ± 15 | 118 ± 30 | 0.393 | 24 ± 5 | 33 ± 10 | <0.001 † | 77 ± 10 | 92 ± 26 | 0.001 † | 9 ± 3 | 11 ± 5 | 0.135 |
| Lyso-Gb3 (+14) | 894 ± 138 | 855 ± 188 | <0.050 | 24 ± 6 | 23 ± 6 | 0.105 | 26 ± 6 | 23 ± 6 | <0.050 | 94 ± 14 | 93 ± 22 | 0.457 | 14 ± 3 | 14 ± 3 | 0.768 | 27 ± 5 | 24 ± 12 | 0.280 | 8 ± 2 | 9 ± 4 | 0.457 |
| Lyso-Gb3 (+16) | 5524 ± 1017 | 5246 ± 1351 | <0.050 | 204 ± 43 | 206 ± 45 | 0.760 | 215 ± 39 | 182 ± 42 | <0.001 † | 797 ± 109 | 800 ± 196 | 0.927 | 115 ± 27 | 110 ± 21 | 0.279 | 267 ± 40 | 283 ± 73 | 0.234 | 67 ± 11 | 72 ± 20 | 0.094 |
| Lyso-Gb3 (+34) | 2892 ± 440 | 2622 ± 532 | 0.001 | 121 ± 26 | 118 ± 28 | 0.527 | 125 ± 25 | 104 ± 26 | <0.001 † | 315 ± 60 | 316 ± 98 | 0.797 | 80 ± 18 | 72 ± 13 | <0.050 | 146 ± 25 | 142 ± 39 | 0.609 | 60 ± 11 | 65 ± 20 | 0.137 |
| Lyso-Gb3 (+50) | 4973 ± 711 | 4613 ± 913 | <0.050 | 69 ± 15 | 68 ± 20 | 0.779 | 99 ± 20 | 77 ± 18 | <0.001 † | 144 ± 27 | 144 ± 37 | 0.847 | 39 ± 9 | 34 ± 8 | <0.050 | 70 ± 13 | 59 ± 20 | <0.050 | 51 ± 7 | 50 ± 11 | 0.583 |
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | Participant 6 | Participant 7 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %RSD | %RSD | %RSD | %RSD | %RSD | %RSD | %RSD | |||||||||||||||
| M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | |
| Gb3 | 34 | 56 | 0.190 | 30 | 51 | <0.001 † | 44 | 31 | 0.101 | 27 | 28 | 0.089 | 38 | 40 | 0.495 | 35 | 79 | <0.050 | 37 | 65 | <0.050 |
| Lyso-Gb3 | 46 | 32 | 0.813 | 46 | 55 | <0.050 | 41 | 32 | 0.247 | 20 | 33 | <0.001 † | 39 | 37 | 0.448 | 17 | 30 | <0.001 † | 38 | 37 | 0.118 |
| Lyso-Gb3 (−28) | 23 | 15 | 0.126 | 30 | 25 | 0.614 | 33 | 32 | 0.170 | 13 | 29 | <0.050 | 26 | 33 | <0.050 | 21 | 31 | 0.137 | 33 | 53 | <0.050 |
| Lyso-Gb3 (−12) | 12 | 18 | 0.156 | 22 | 29 | 0.227 | 23 | 24 | 0.562 | 15 | 19 | 0.538 | 26 | 29 | 0.889 | 15 | 32 | <0.001 † | 14 | 19 | 0.738 |
| Lyso-Gb3 (−2) | 22 | 14 | 0.079 | 28 | 24 | 0.790 | 30 | 32 | 0.426 | 12 | 25 | <0.001 † | 22 | 30 | <0.050 | 14 | 28 | <0.001 † | 32 | 48 | <0.050 |
| Lyso-Gb3 (+14) | 15 | 22 | 0.277 | 23 | 26 | 0.696 | 22 | 25 | 0.708 | 14 | 24 | 0.189 | 20 | 24 | 0.903 | 18 | 49 | <0.001 † | 22 | 42 | <0.001 † |
| Lyso-Gb3 (+16) | 18 | 26 | 0.300 | 21 | 22 | 0.691 | 18 | 23 | 0.881 | 14 | 24 | 0.183 | 23 | 20 | 0.317 | 15 | 26 | <0.001 † | 17 | 28 | <0.050 |
| Lyso-Gb3 (+34) | 15 | 20 | 0.783 | 21 | 24 | 0.917 | 20 | 25 | 0.776 | 19 | 31 | 0.170 | 23 | 18 | <0.050 | 17 | 27 | <0.050 | 18 | 31 | <0.001 † |
| Lyso-Gb3 (+50) | 14 | 20 | 0.285 | 21 | 30 | 0.150 | 20 | 24 | 0.308 | 18 | 26 | 0.536 | 23 | 22 | 0.056 | 18 | 34 | 0.063 | 14 | 22 | <0.050 |
| Patient No | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | F | F | F |
| Age (years) | 30 | 28 | 40 | 36 | 49 | 57 | 67 |
| Treatment status | No treatment | Replagal | Fabrazyme | Fabrazyme | Replagal | Replagal | Fabrazyme |
| Mutation | c.17_327del | c.612G > A | c.35_47del13 | c.1241T > C | c.1042G > C | c.1241T > C | c.877C > A |
| ERT start date | n/a | 2005-07-26 | 2008-09-30 | 2003-05-31 | 2009-01-14 | 2011-02-02 | 2003-04-15 |
| Serum creatinine (µmol/L) | 97 | 78 | 97 | 96 | 57 | 76 | 92 |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 91 | 116 | 83 | 87 | 105 | 76 | 56 |
| Urinary proteins (g/24 h) | 1.07 | 0.14 | 1.19 | 1.38 | 0.25 | 3.21 | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutin, M.; Lavoie, P.; Menkovic, I.; Toupin, A.; Abaoui, M.; Elidrissi-Elawad, M.; Arthus, M.-F.; Fortier, C.; Ménard, C.; Maranda, B.; et al. Diurnal Variation of Urinary Fabry Disease Biomarkers during Enzyme Replacement Therapy Cycles. Int. J. Mol. Sci. 2020, 21, 6114. https://doi.org/10.3390/ijms21176114
Boutin M, Lavoie P, Menkovic I, Toupin A, Abaoui M, Elidrissi-Elawad M, Arthus M-F, Fortier C, Ménard C, Maranda B, et al. Diurnal Variation of Urinary Fabry Disease Biomarkers during Enzyme Replacement Therapy Cycles. International Journal of Molecular Sciences. 2020; 21(17):6114. https://doi.org/10.3390/ijms21176114
Chicago/Turabian StyleBoutin, Michel, Pamela Lavoie, Iskren Menkovic, Amanda Toupin, Mona Abaoui, Maha Elidrissi-Elawad, Marie-Françoise Arthus, Carole Fortier, Claudia Ménard, Bruno Maranda, and et al. 2020. "Diurnal Variation of Urinary Fabry Disease Biomarkers during Enzyme Replacement Therapy Cycles" International Journal of Molecular Sciences 21, no. 17: 6114. https://doi.org/10.3390/ijms21176114
APA StyleBoutin, M., Lavoie, P., Menkovic, I., Toupin, A., Abaoui, M., Elidrissi-Elawad, M., Arthus, M.-F., Fortier, C., Ménard, C., Maranda, B., Bichet, D. G., & Auray-Blais, C. (2020). Diurnal Variation of Urinary Fabry Disease Biomarkers during Enzyme Replacement Therapy Cycles. International Journal of Molecular Sciences, 21(17), 6114. https://doi.org/10.3390/ijms21176114