High Numbers and Densities of PD1+ T-Follicular Helper Cells in Triple-Negative Breast Cancer Draining Lymph Nodes Are Associated with Lower Survival
Abstract
1. Introduction
2. Results
2.1. Study Cohort
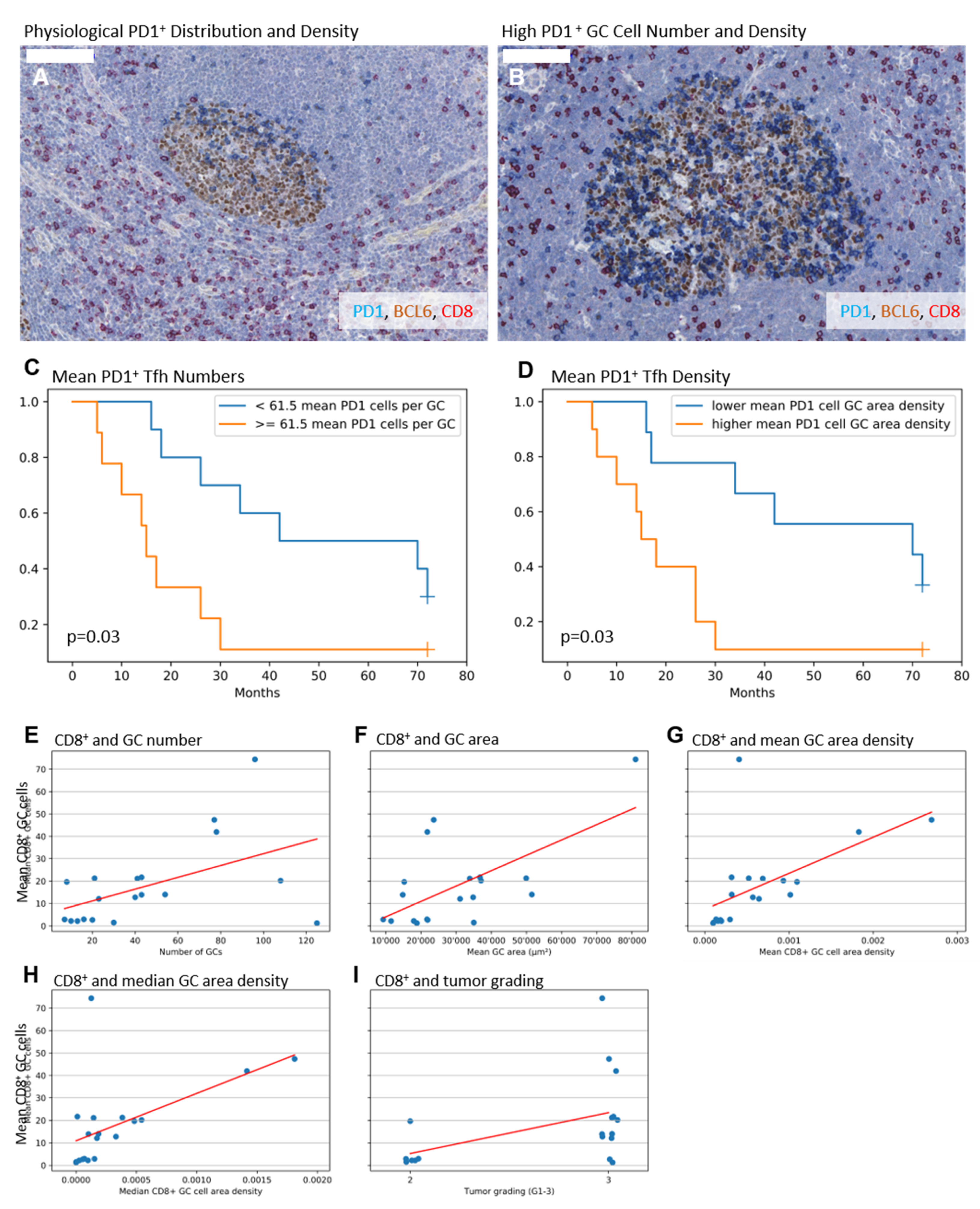
2.2. Higher Numbers and Higher Densities of PD1+ Tfh Are Associated with Shorter Survival
2.3. Higher Numbers of CD8+ GC Cells Are Associated with Elevated GC Amount, Enlarged GCs, and a Higher Tumor Grade
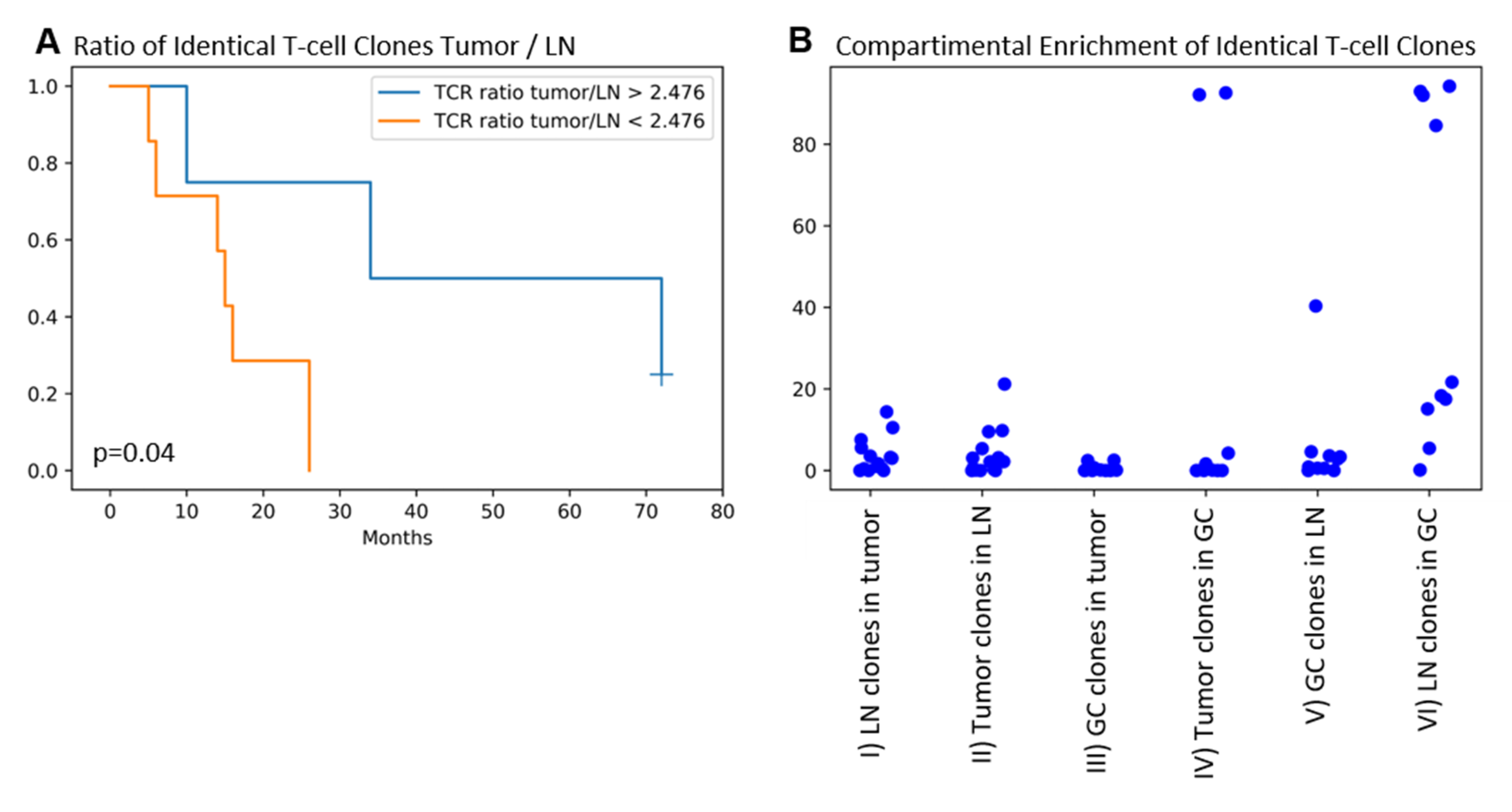
2.4. Identical TRG/T-Cell Clones Are Present in the Tumor, the Draining LN, and the GC in the Majority of Patients. Higher T-cell Clone Tumor/LN Ratios Are Associated with Improved Patients’ Overall Survival
3. Discussion
4. Materials and Methods
4.1. Patients and Cohort
4.2. Multiple Immunohistochemical Staining
4.3. Slide Scanning and Evaluation of LNs
4.4. Laser-Capture Microdissection, DNA Extraction, and NGS Analysis of TCR Gamma Clonality
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Germinal center |
| LN | Lymph node |
| ROI | Region of interest |
| TDLN | Tumor draining lymph node |
| Tfh | T-follicular helper cell |
| TNBC | Triple-negative breast cancer |
| TRG | T-cell receptor gamma chain |
References
- Polk, A.; Svane, I.-M.; Andersson, M.; Nielsen, D. Checkpoint inhibitors in breast cancer–Current status. Cancer Treat. Rev. 2018, 63, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Omar, H.A. Immunotherapy, an evolving approach for the management of triple-negative breast cancer: Converting non-responders to responders. Crit. Rev. Oncol. Hematol. 2018, 122, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, W.; van den Berg, M.; van der Niet, S.; Limpens, J.; Luiten, R.M. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: A systematic review. Melanoma Res. 2019, 29, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Krieg, C.; Nowicka, M.; Guglietta, S.; Schindler, S.; Hartmann, F.J.; Weber, L.M.; Dummer, R.; Robinson, M.D.; Levesque, M.P.; Becher, B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 2018, 24, 144–153. [Google Scholar] [CrossRef]
- Spitzer, M.H.; Carmi, Y.; Reticker-Flynn, N.E.; Kwek, S.S.; Madhireddy, D.; Martins, M.M.; Gherardini, P.F.; Prestwood, T.R.; Chabon, J.; Bendall, S.C.; et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017, 168, 487–502. [Google Scholar] [CrossRef]
- Fransen, M.F.; Schoonderwoerd, M.; Knopf, P.; Camps, M.G.; Hawinkels, L.J.; Kneilling, M.; van Hall, T.; Ossendorp, F. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Gasteiger, G.; Ataide, M.; Kastenmüller, W. Lymph node–an organ for T-cell activation and pathogen defense. Immunol. Rev. 2016, 271, 200–220. [Google Scholar] [CrossRef]
- Fransen, M.F.; Arens, R.; Melief, C.J.M. Local targets for immune therapy to cancer: Tumor draining lymph nodes and tumor microenvironment. Int. J. cancer. 2013, 132, 1971–1976. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.L.; Lake, R.A.; Lo, D.; Sherman, L.; McWilliam, A.; Nelson, D.; Robinson, B.W.; Scott, B. Tumor antigens are constitutively presented in the draining lymph nodes. J. Immunol. 1999, 162, 5838–5845. [Google Scholar] [PubMed]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Seidl, M.; Bader, M.; Vaihinger, A.; Wellner, U.F.; Todorova, R.; Herde, B.; Schrenk, K.; Maurer, J.; Schilling, O.; Erbes, T.; et al. Morphology of Immunomodulation in Breast Cancer Tumor Draining Lymph Nodes Depends on Stage and Intrinsic Subtype. Sci. Rep. 2018, 8, 5321. [Google Scholar] [CrossRef]
- Sun, S.; Fei, X.; Mao, Y.; Wang, X.; Garfield, D.H.; Huang, O.; Wang, J.; Yuan, F.; Sun, L.; Yu, Q.; et al. PD-1+ immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol. Immunother. 2014, 63, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular Helper CD4 T Cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Eivazi, S.; Bagheri, S.; Hashemzadeh, M.S.; Ghalavand, M.; Qamsari, E.S.; Dorostkar, R.; Yasemi, M. Development of T follicular helper cells and their role in disease and immune system. Biomed. Pharmacother. 2016, 84, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Haynes, N.M.; Allen, C.D.C.; Lesley, R.; Ansel, K.M.; Killeen, N.; Cyster, J.G. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 2007, 179, 5099–5108. [Google Scholar] [CrossRef]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent Antigen and Germinal Center B Cells Sustain T Follicular Helper Cell Responses and Phenotype. Immunity 2013, 38, 596–605. [Google Scholar] [CrossRef]
- Chen, X.; Ma, W.; Zhang, T.; Wu, L.; Qi, H. Phenotypic Tfh development promoted by CXCR5-controlled re-localization and IL-6 from radiation-resistant cells. Protein Cell. 2015, 6, 825–832. [Google Scholar] [CrossRef]
- Young, A.R.; Duarte, J.D.G.; Coulson, R.; O’Brien, M.; Deb, S.; Lopata, A.; Behren, A.; Mathivanan, S.; Lim, E.; Meeusen, E. Immunoprofiling of Breast Cancer Antigens Using Antibodies Derived from Local Lymph Nodes. Cancers 2019, 11, 682. [Google Scholar] [CrossRef]
- Schlosser, G.; Pocsfalvi, G.; Malorni, A.; Puerta, A.; De Frutos, M.; Vékey, K. Detection of immune complexes by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2741–2747. [Google Scholar] [CrossRef]
- Zhu, J.; Garrigues, L.; Van Den Toorn, H.; Stahl, B.; Heck, A.J.R. Discovery and Quantification of Nonhuman Proteins in Human Milk. J. Proteome Res. 2019, 18, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Coronella-Wood, J.A.; Hersh, E.M. Naturally occurring B-cell responses to breast cancer. Cancer Immunol. Immunother. 2003, 52, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Unger, S.; Seidl, M.; Schmitt-Graeff, A.; Böhm, J.; Schrenk, K.; Wehr, C.; Goldacker, S.; Dräger, R.; Gärtner, B.C.; Fisch, P.; et al. Ill-Defined Germinal Centers and Severely Reduced Plasma Cells are Histological Hallmarks of Lymphadenopathy in Patients with Common Variable Immunodeficiency. J. Clin. Immunol. 2014, 34, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Klocperk, A.; Unger, S.; Friedmann, D.; Seidl, M.; Zoldan, K.; Pfeiffer, J.; Hausmann, O.; Benes, V.; Andrieux, G.; Boettler, T.; et al. Exhausted phenotype of follicular CD8 T cells in CVID. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.-J.; Panel Members. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Aliseychik, M.; Patrikeev, A.; Gusev, F.; Grigorenko, A.; Andreeva, T.; Biragyn, A.; Rogaev, E. Dissection of the Human T-Cell Receptor γ Gene Repertoire in the Brain and Peripheral Blood Identifies Age- and Alzheimer’s Disease-Associated Clonotype Profiles. Front. Immunol. 2020, 11, 12. [Google Scholar] [CrossRef]
| n Patients | Mean | Std | Min | 25% | 50% | 75% | Max | |
|---|---|---|---|---|---|---|---|---|
| Age | 19 | 66.32 | 12.82 | 35.00 | 55.50 | 72.00 | 75.50 | 79.00 |
| Grading (G1-3) | 19 | 2.68 | 0.48 | 2.00 | 2.00 | 3.00 | 3.00 | 3.00 |
| pT | 19 | 1.53 | 0.51 | 1.00 | 1.00 | 2.00 | 2.00 | 2.00 |
| pN | 19 | 0.37 | 0.76 | 0.00 | 0.00 | 0.00 | 0.50 | 3.00 |
| Number of LN Metastases | 19 | 0.74 | 2.28 | 0.00 | 0.00 | 0.00 | 0.50 | 10.00 |
| Total Number of LNs | 19 | 6.21 | 8.48 | 1.00 | 1.50 | 3.00 | 5.00 | 31.00 |
| Lymphangiosis (1 = yes, 0 = no) | 19 | 0.16 | 0.37 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 |
| HER2/neu IHC Score | 19 | 0.53 | 0.70 | 0.00 | 0.00 | 0.00 | 1.00 | 2.00 |
| MIB-1/Ki67 Proliferation Index | 15 | 56.13 | 22.59 | 17.00 | 40.00 | 50.00 | 75.00 | 90.00 |
| Event-Specific Overall Survival in Months | 15 | 26.73 | 20.68 | 5.00 | 14.50 | 18.00 | 32.00 | 72.00 |
| Overall Survival in Months | 19 | 36.26 | 26.31 | 5.00 | 15.50 | 26.00 | 71.00 | 72.00 |
| n Patients | Mean | Std | Min | 25% | 50% | 75% | Max | |
|---|---|---|---|---|---|---|---|---|
| CD8+ Interfollicular Density (cells per mm2) | 18 | 3228.61 | 1305.26 | 1267.61 | 2314.34 | 2746.34 | 4211.88 | 5714.43 |
| Interfollicular Area (mm2) | 18 | 49.62 | 22.83 | 15.17 | 32.06 | 45.51 | 66.35 | 97.87 |
| Mean Number of CD8+ Cells per GC | 19 | 17.67 | 19.00 | 1.24 | 2.75 | 13.91 | 21.18 | 74.38 |
| Median Number of CD8+ Cells per GC | 19 | 5.47 | 6.76 | 0.00 | 1.00 | 2.50 | 7.50 | 28.00 |
| Mean GC Density of CD8+ Cells (cells per µm2) | 19 | 641.97 | 664.70 | 97.10 | 189.75 | 406.79 | 810.49 | 2694.89 |
| Median GC Density of CD8+ Cells (cells per µm2) | 19 | 321.35 | 486.22 | 0.00 | 61.85 | 144.99 | 356.46 | 1810.47 |
| Mean Number of PD1+ Tfh per GC | 19 | 61.53 | 45.42 | 0.58 | 23.76 | 61.17 | 88.84 | 158.52 |
| Median Number of PD1+ Tfh per GC | 19 | 21.84 | 24.30 | 0.00 | 0.00 | 11.50 | 38.50 | 65.50 |
| Mean GC Density of PD1+ Tfh (cells per µm2) | 19 | 2475.33 | 1928.32 | 70.60 | 812.83 | 2539.88 | 4354.01 | 5598.64 |
| Median GC Density of PD1+ Tfh (cells per µm) | 19 | 1380.91 | 1749.04 | 0.00 | 0.00 | 849.68 | 2294.69 | 6089.25 |
| n Patients | Mean | Std | Min | 25% | 50% | 75% | Max | |
|---|---|---|---|---|---|---|---|---|
| Number of GCs | 19 | 44.89 | 35.77 | 7.00 | 18.00 | 40.00 | 65.50 | 125.00 |
| Sum of GC Areas in mm | 19 | 1.56 | 1.82 | 0.07 | 0.39 | 1.05 | 1.76 | 7.78 |
| Ratio: Sum of GC Areas/Whole LN area | 19 | 0.02 | 0.02 | 0.00 | 0.00 | 0.01 | 0.02 | 0.08 |
| Mean GC Area in µm2 | 19 | 29907.58 | 17232.53 | 9303.41 | 18429.66 | 23661.85 | 35912.72 | 80989.81 |
| Median GC Area in µm2 | 19 | 16521.21 | 6728.29 | 7755.20 | 11012.91 | 15450.05 | 21316.81 | 29980.66 |
| Mean Circularity Ratio (1 circular, >1 non-circular) | 19 | 1.24 | 0.12 | 1.08 | 1.15 | 1.20 | 1.31 | 1.54 |
| Median Circularity Ratio (1 circular, >1 non-circular) | 19 | 1.17 | 0.09 | 1.06 | 1.09 | 1.13 | 1.22 | 1.43 |
| n Patients | Mean | Std | Min | 25% | 50% | 75% | Max | |
|---|---|---|---|---|---|---|---|---|
| Identical TRG Sequences Between Tumor and LN: Sum of % Reads in Tumor | 14 | 3.67 | 4.42 | 0.00 | 0.43 | 2.34 | 5.08 | 14.37 |
| Identical TRG Sequences Between Tumor and LN: Sum of % Reads in LN | 14 | 4.10 | 5.95 | 0.00 | 0.12 | 2.16 | 4.82 | 21.22 |
| Identical TRG Sequences Between Tumor and GC: Sum of % Reads in Tumor | 10 | 0.59 | 1.03 | 0.00 | 0.00 | 0.04 | 0.56 | 2.54 |
| Identical TRG Sequences Between Tumor and GC: Sum of % Reads in GC | 10 | 19.07 | 38.65 | 0.00 | 0.00 | 0.04 | 3.60 | 92.62 |
| Identical TRG Sequences Between LN and GC: Sum of % Reads LN | 10 | 5.67 | 12.30 | 0.00 | 0.52 | 1.86 | 3.56 | 40.34 |
| Identical TRG Sequences Between LN and GC: Sum of % Reads GC | 10 | 44.19 | 40.79 | 0.12 | 15.71 | 19.98 | 90.13 | 94.24 |
| Number of Identical TRG Sequences Between Tumor and LN | 14 | 69.93 | 109.55 | 0.00 | 4.50 | 18.50 | 108.25 | 391.00 |
| Identical TRG sequences: Ratio of Sum % Reads Tumor/Sum % Reads LN | 11 | 2.48 | 2.81 | 0.11 | 0.58 | 1.38 | 3.51 | 9.01 |
| Number of identical TRG Sequences Between Tumor and GC | 10 | 14.20 | 33.07 | 0.00 | 0.00 | 1.50 | 7.75 | 107.00 |
| Identical TRG Sequences: Ratio of Sum % Reads Tumor/Sum % Reads GC | 5 | 0.43 | 0.72 | 0.02 | 0.03 | 0.03 | 0.42 | 1.68 |
| Number of Identical TRG Sequences Between LN and GC | 10 | 24.80 | 26.97 | 2.00 | 4.00 | 11.50 | 47.25 | 71.00 |
| Identical TRG Sequences Between LN and GC: Ratio of Sum % Reads LN/Sum % Reads GC | 10 | 0.33 | 0.82 | 0.00 | 0.01 | 0.06 | 0.14 | 2.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bronsert, P.; von Schoenfeld, A.; Villacorta Hidalgo, J.; Kraft, S.; Pfeiffer, J.; Erbes, T.; Werner, M.; Seidl, M. High Numbers and Densities of PD1+ T-Follicular Helper Cells in Triple-Negative Breast Cancer Draining Lymph Nodes Are Associated with Lower Survival. Int. J. Mol. Sci. 2020, 21, 5948. https://doi.org/10.3390/ijms21175948
Bronsert P, von Schoenfeld A, Villacorta Hidalgo J, Kraft S, Pfeiffer J, Erbes T, Werner M, Seidl M. High Numbers and Densities of PD1+ T-Follicular Helper Cells in Triple-Negative Breast Cancer Draining Lymph Nodes Are Associated with Lower Survival. International Journal of Molecular Sciences. 2020; 21(17):5948. https://doi.org/10.3390/ijms21175948
Chicago/Turabian StyleBronsert, Peter, Anna von Schoenfeld, Jose Villacorta Hidalgo, Stefan Kraft, Jens Pfeiffer, Thalia Erbes, Martin Werner, and Maximilian Seidl. 2020. "High Numbers and Densities of PD1+ T-Follicular Helper Cells in Triple-Negative Breast Cancer Draining Lymph Nodes Are Associated with Lower Survival" International Journal of Molecular Sciences 21, no. 17: 5948. https://doi.org/10.3390/ijms21175948
APA StyleBronsert, P., von Schoenfeld, A., Villacorta Hidalgo, J., Kraft, S., Pfeiffer, J., Erbes, T., Werner, M., & Seidl, M. (2020). High Numbers and Densities of PD1+ T-Follicular Helper Cells in Triple-Negative Breast Cancer Draining Lymph Nodes Are Associated with Lower Survival. International Journal of Molecular Sciences, 21(17), 5948. https://doi.org/10.3390/ijms21175948





