2,4-Diamino-Quinazoline, a Wnt Signaling Inhibitor, Suppresses Gastric Cancer Progression and Metastasis
Abstract
1. Introduction
2. Results
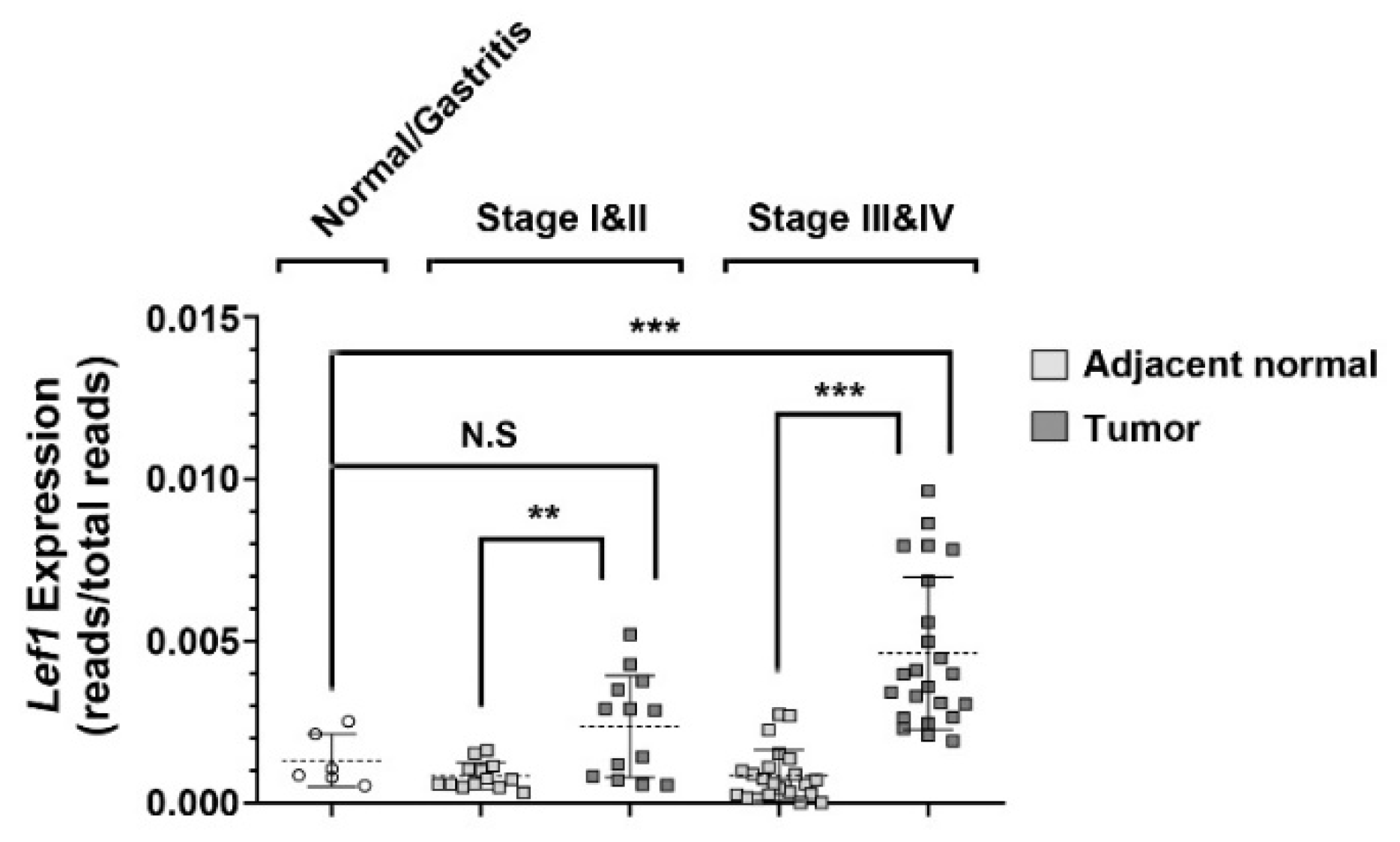
2.1. Aberrant Expression of Lef1 in Gastric Cancer
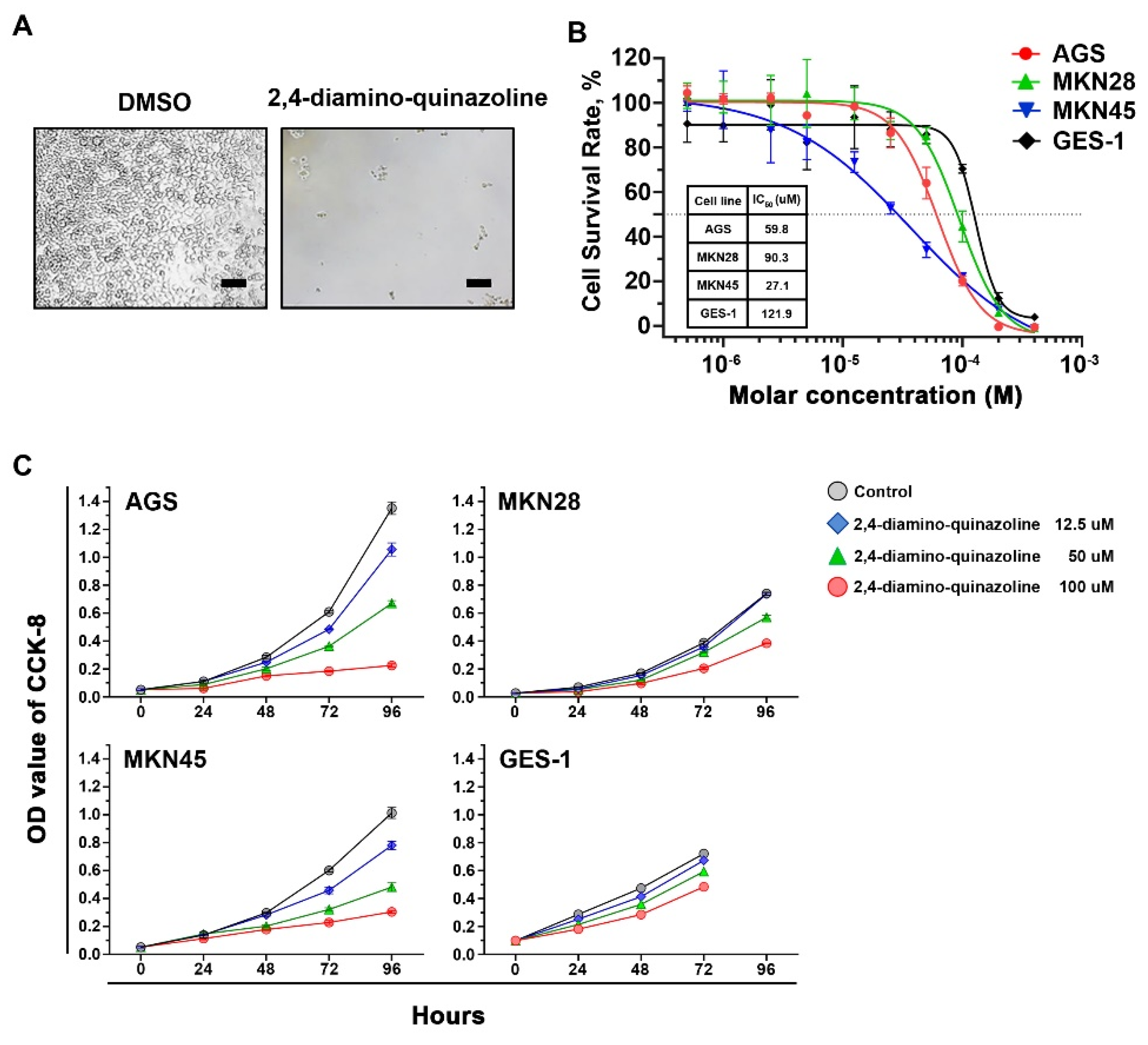
2.2. Inhibitory Effects of Wnt Signaling Inhibitors on Gastric Cancer Cells
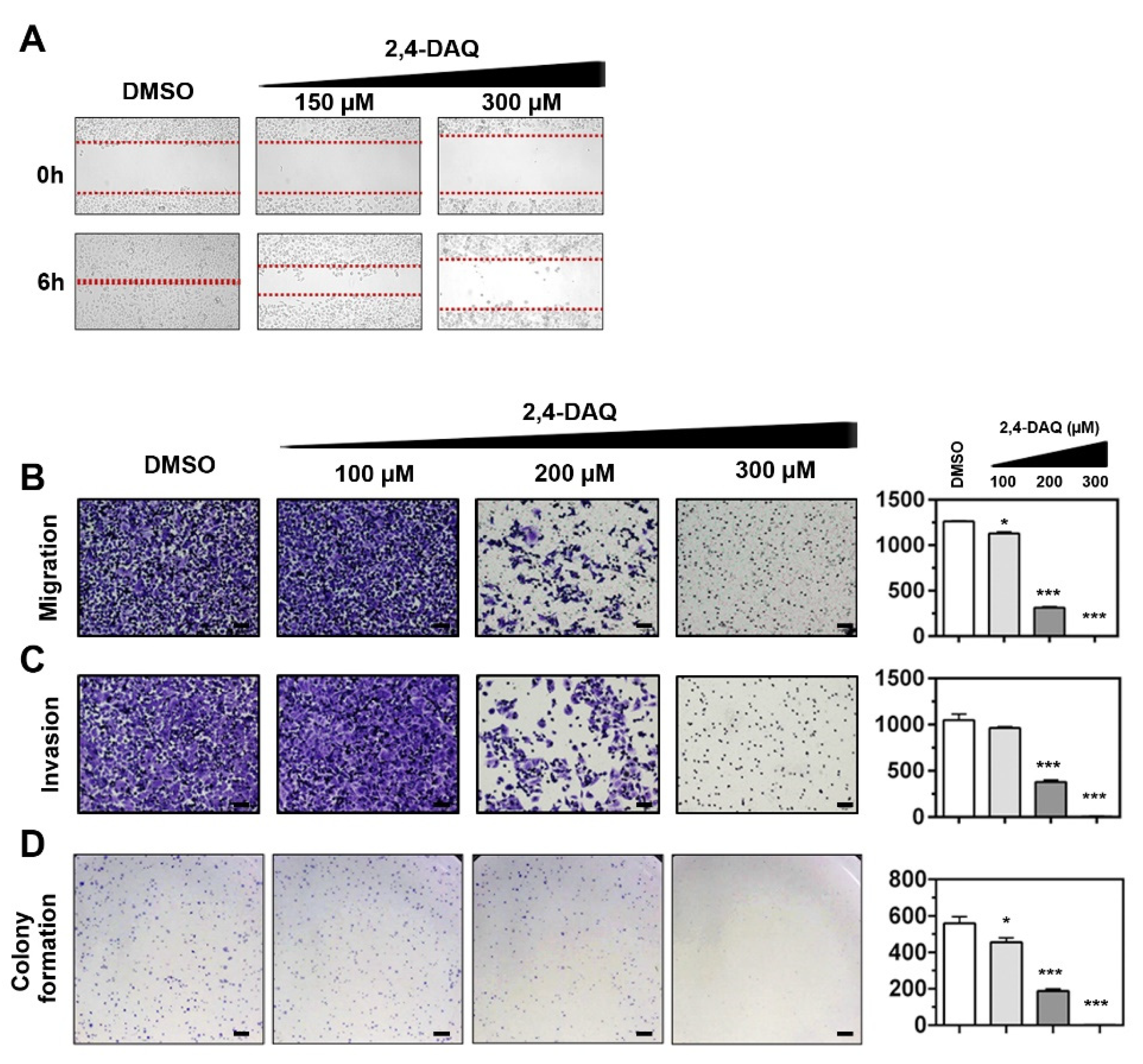
2.3. Effect of 2,4-DAQ on Colony Formation, Cell Migration and Invasion of Gastric Cancer Cells
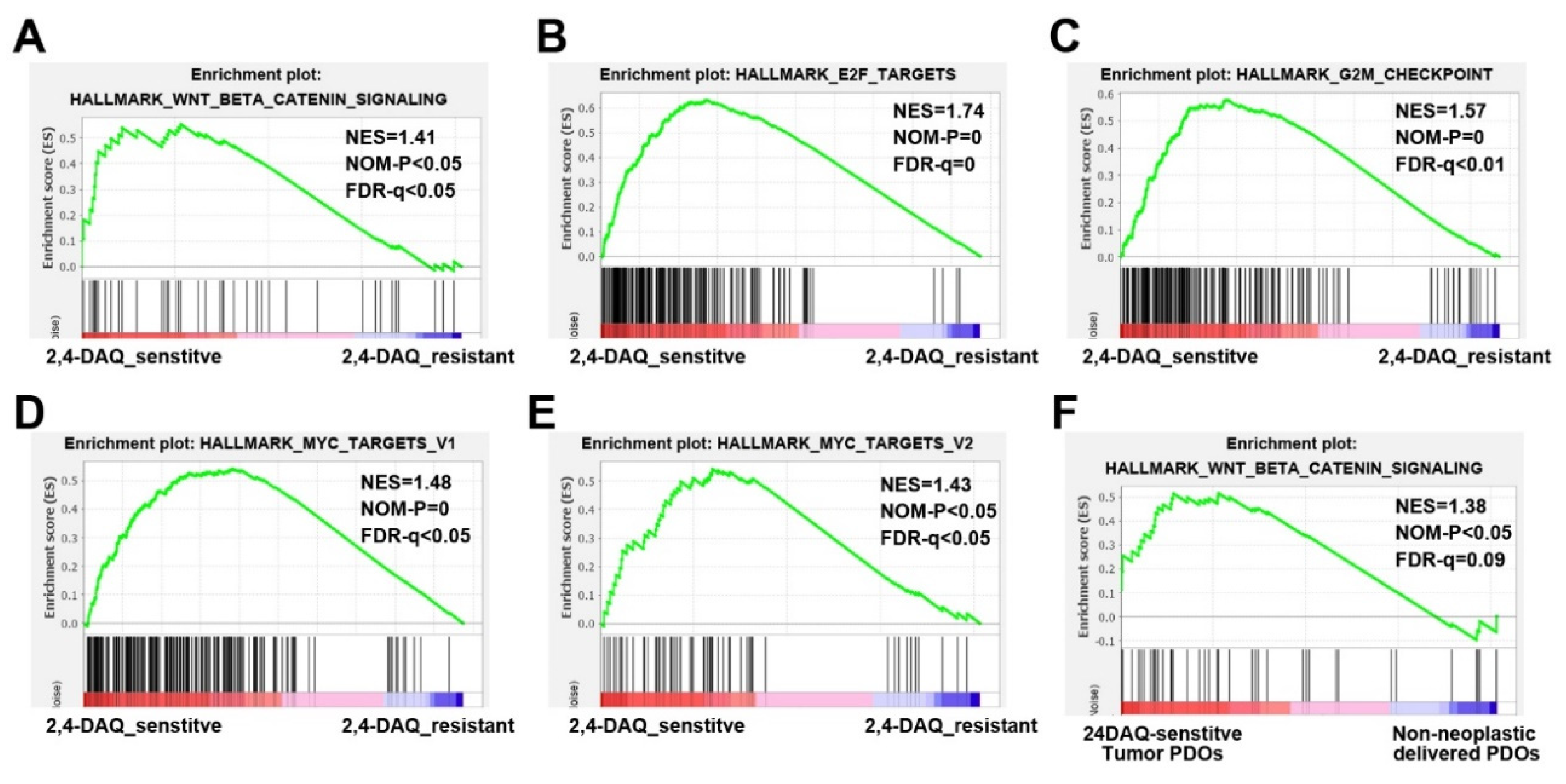
2.4. 2,4-DAQ Inhibits Xenograft Tumor Growth In Vivo
2.5. Inhibitory Effects of 2,4-DAQ on Patient-Derived Organoids
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Gastric Cancer Tissue
4.2. Targeted Sequencing and Transcriptome Sequencing
4.3. Cell Proliferation Assay (Cell Counting Kit-8)
4.4. Cell Proliferation Assay (alamarBlue)
4.5. Western Blot Analysis
4.6. Colony Formation Assay
4.7. Wound-Healing Assay
4.8. Cell Migration and Invasion Assay
4.9. Xenograft Studies
4.10. Generating Wnt3a and Wnt3a/R-Spondin 3/noggin Conditioned Medium
4.11. Organoid Culture
4.12. Gene Set Enrichment Analysis (GSEA)
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare Taiwan. Cause of Death Statistics. 2018. Available online: https://www.mohw.gov.tw/cp-4650-50697-2.html (accessed on 27 March 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Flejou, J.F. WHO Classification of digestive tumors: The fourth edition. Ann. Pathol. 2011, 31 (Suppl. 5), S27. [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Holland, J.D.; Klaus, A.; Garratt, A.N.; Birchmeier, W. Wnt signaling in stem and cancer stem cells. Curr. Opin. Cell Biol. 2013, 25, 254–264. [Google Scholar] [CrossRef]
- Clements, W.M.; Wang, J.; Sarnaik, A.; Kim, O.J.; MacDonald, J.; Fenoglio-Preiser, C.; Groden, J.; Lowy, A.M. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002, 62, 3503–3506. [Google Scholar]
- Chang, T.S.; Wei, K.L.; Lu, C.K.; Chen, Y.H.; Cheng, Y.T.; Tung, S.Y.; Wu, C.S.; Chiang, M.K. Inhibition of CCAR1, a Coactivator of beta-Catenin, Suppresses the Proliferation and Migration of Gastric Cancer Cells. Int. J. Mol. Sci. 2017, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Harb, J.; Lin, P.J.; Hao, J. Recent Development of Wnt Signaling Pathway Inhibitors for Cancer Therapeutics. Curr. Oncol. Rep. 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zheng, D.; Hu, P.; Zeng, Z.; Li, M.; Tucker, L.; Monahan, R.; Resnick, M.B.; Liu, M.; Ramratnam, B. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the beta-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 2014, 42, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Venkatesan, A.M.; Dehnhardt, C.M.; Dos Santos, O.; Delos Santos, E.; Ayral-Kaloustian, S.; Chen, L.; Geng, Y.; Arndt, K.T.; Lucas, J.; et al. 2,4-Diamino-quinazolines as inhibitors of beta-catenin/Tcf-4 pathway: Potential treatment for colorectal cancer. Bioorg. Med. Chem. Lett. 2009, 19, 4980–4983. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Takahashi-Yanaga, F.; Kahn, M. Targeting Wnt signaling: Can we safely eradicate cancer stem cells? Clin. Cancer Res. 2010, 16, 3153–3162. [Google Scholar] [CrossRef]
- Baba, A.; Kawamura, N.; Makino, H.; Ohta, Y.; Taketomi, S.; Sohda, T. Studies on disease-modifying antirheumatic drugs: Synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect. J. Med. Chem. 1996, 39, 5176–5182. [Google Scholar] [CrossRef]
- Aly, A.A. Synthesis of Novel Quinazoline Derivatives as Antimicrobial Agents. Chin. J. Chem. 2010, 21, 339–346. [Google Scholar] [CrossRef]
- Antipenko, L.; Karpenko, A.; Kovalenko, S.; Katsev, A.; Komarovska-Porokhnyavets, E.; Novikov, V.; Chekotilo, A. Synthesis of new 2-thio-[1,2,4]triazolo[1,5-c]quinazoline derivatives and its antimicrobial activity. Chem. Pharm. Bull. 2009, 57, 580–585. [Google Scholar] [CrossRef]
- Sasmal, S.; Balasubrahmanyam, D.; Kanna Reddy, H.R.; Balaji, G.; Srinivas, G.; Cheera, S.; Abbineni, C.; Sasmal, P.K.; Khanna, I.; Sebastian, V.J.; et al. Design and optimization of quinazoline derivatives as melanin concentrating hormone receptor 1 (MCHR1) antagonists: Part 2. Bioorg. Med. Chem. Lett. 2012, 22, 3163–3167. [Google Scholar] [CrossRef] [PubMed]
- Malamas, M.S.; Millen, J. Quinazolineacetic acids and related analogues as aldose reductase inhibitors. J. Med. Chem. 1991, 34, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.J.; Cronin, T.H.; Scriabine, A. Antihypertensive 2-amino-4(3H)-quinazolinones. J. Med. Chem. 1968, 11, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ugale, V.G.; Bari, S.B. Quinazolines: New horizons in anticonvulsant therapy. Eur. J. Med. Chem. 2014, 80, 447–501. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; ElTahir, K.E.H. Design, synthesis and anticonvulsant evaluation of novel 8-substituted-4(3H)-quinazolines. Med. Chem. Res. 2011, 21, 3785–3796. [Google Scholar] [CrossRef]
- Alvarado, M.; Barcelo, M.; Carro, L.; Masaguer, C.F.; Ravina, E. Synthesis and biological evaluation of new quinazoline and cinnoline derivatives as potential atypical antipsychotics. Chem. Biodivers. 2006, 3, 106–117. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I. An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. Medchemcomm 2017, 8, 871–885. [Google Scholar] [CrossRef]
- Faehling, M.; Achenbach, J.; Staib, P.; Steffen, U.; Tessen, H.W.; Gaillard, V.E.; Brugger, W. Erlotinib in routine clinical practice for first-line maintenance therapy in patients with advanced non-small cell lung cancer (NSCLC). J. Cancer Res. Clin. Oncol. 2018, 144, 1375–1383. [Google Scholar] [CrossRef]
- Cohen, M.H.; Williams, G.A.; Sridhara, R.; Chen, G.; Pazdur, R. FDA drug approval summary: Gefitinib (ZD1839) (Iressa) tablets. Oncologist 2003, 8, 303–306. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Baldini, E.; Biricotti, M.; Ulisse, S.; Materazzi, G.; Miccoli, P.; Antonelli, A. The safety and efficacy of vandetanib in the treatment of progressive medullary thyroid cancer. Expert Rev. Anticancer Ther. 2016, 16, 1109–1118. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Marzaro, G.; Guiotto, A.; Chilin, A. Quinazoline derivatives as potential anticancer agents: A patent review (2007–2010). Expert Opin. Ther. Pat. 2012, 22, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Dehnhardt, C.M.; Venkatesan, A.M.; Chen, Z.; Ayral-Kaloustian, S.; Dos Santos, O.; Delos Santos, E.; Curran, K.; Follettie, M.T.; Diesl, V.; Lucas, J.; et al. Design and synthesis of novel diaminoquinazolines with in vivo efficacy for beta-catenin/T-cell transcriptional factor 4 pathway inhibition. J. Med. Chem. 2010, 53, 897–910. [Google Scholar]
- Wang, B.; Chen, Q.; Cao, Y.; Ma, X.; Yin, C.; Jia, Y.; Zang, A.; Fan, W. LGR5 Is a Gastric Cancer Stem Cell Marker Associated with Stemness and the EMT Signature Genes NANOG, NANOGP8, PRRX1, TWIST1, and BMI1. PLoS ONE 2016, 11, e0168904. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Liu, Y.; Dong, Y.; Wang, Y.; Kassab, M.A.; Fan, W.; Yu, X.; Wu, C. LGR5 regulates gastric adenocarcinoma cell proliferation and invasion via activating Wnt signaling pathway. Oncogenesis 2018, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.H.M.; Amin, N.; Flanagan, D.J.; Johanson, T.M.; Phesse, T.J.; Vincan, E. Wnt is necessary for mesenchymal to epithelial transition in colorectal cancer cells. Dev. Dyn. 2018, 247, 521–530. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Li, M.; Izpisua Belmonte, J.C. Organoids—Preclinical Models of Human Disease. N. Engl. J. Med. 2019, 380, 569–579. [Google Scholar] [CrossRef]
- Tuveson, D.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, Y.; Qin, S.; Zhao, W.; Chu, Q.; Wu, K. Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp. Hematol. Oncol. 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Steele, N.G.; Chakrabarti, J.; Wang, J.; Biesiada, J.; Holokai, L.; Chang, J.; Nowacki, L.M.; Hawkins, J.; Mahe, M.; Sundaram, N.; et al. An Organoid-Based Preclinical Model of Human Gastric Cancer. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernandez-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.Y.; Tung, S.Y.; Pan, H.Y.; Yen, C.W.; Xu, H.W.; Deng, Y.F.; Lin, Y.J.; Hsu, W.T.; Wu, C.S.; Li, C. Upregulation of bone morphogenetic protein 1 is associated with poor prognosis of late-stage gastric Cancer patients. BMC Cancer 2018, 18, 508. [Google Scholar] [CrossRef] [PubMed]
- TruSeq® Targeted RNA Expression Reference Guide. Available online: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/samplepreps_truseq/truseqtargetedrna/truseq-targeted-rna-expression-reference-guide-15034665-01.pdf (accessed on 24 July 2018).
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R., 3rd; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Stappenbeck, T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 2013, 8, 2471–2482. [Google Scholar] [CrossRef]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]


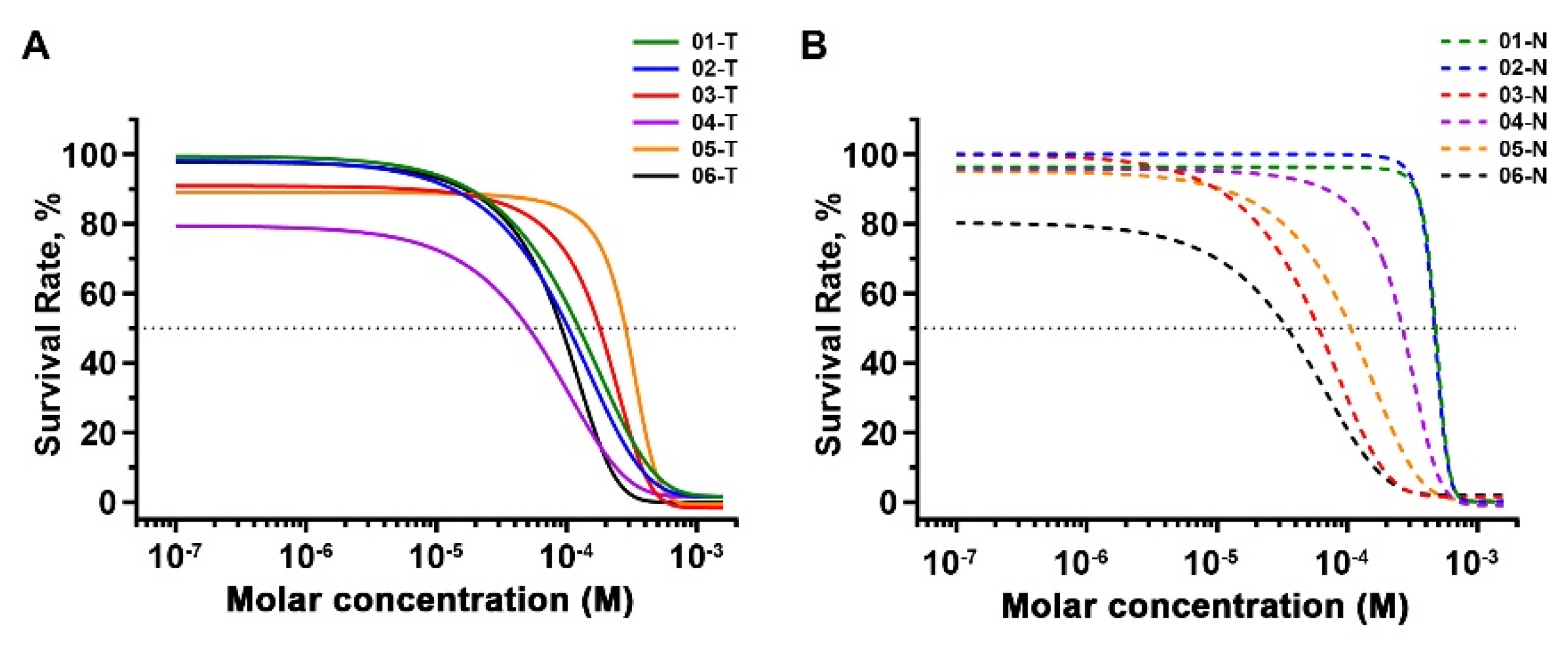
| Patient ID | IC50 (μM) | |
|---|---|---|
| Tumor | Non-Neoplastic | |
| 01 | 110.0 | 464.5 |
| 02 | 82.5 | 456.8 |
| 03 | 142.5 | 54.3 |
| 04 | 24.1 | 242.0 |
| 05 | 247.6 | 91.8 |
| 06 | 81.9 | 17.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-S.; Lu, C.-K.; Hsieh, Y.-Y.; Wei, K.-L.; Chen, W.-M.; Tung, S.-Y.; Wu, C.-S.; Chan, M.W.Y.; Chiang, M.-K. 2,4-Diamino-Quinazoline, a Wnt Signaling Inhibitor, Suppresses Gastric Cancer Progression and Metastasis. Int. J. Mol. Sci. 2020, 21, 5901. https://doi.org/10.3390/ijms21165901
Chang T-S, Lu C-K, Hsieh Y-Y, Wei K-L, Chen W-M, Tung S-Y, Wu C-S, Chan MWY, Chiang M-K. 2,4-Diamino-Quinazoline, a Wnt Signaling Inhibitor, Suppresses Gastric Cancer Progression and Metastasis. International Journal of Molecular Sciences. 2020; 21(16):5901. https://doi.org/10.3390/ijms21165901
Chicago/Turabian StyleChang, Te-Sheng, Chung-Kuang Lu, Yung-Yu Hsieh, Kuo-Liang Wei, Wei-Ming Chen, Sui-Yi Tung, Cheng-Shyong Wu, Michael W. Y. Chan, and Ming-Ko Chiang. 2020. "2,4-Diamino-Quinazoline, a Wnt Signaling Inhibitor, Suppresses Gastric Cancer Progression and Metastasis" International Journal of Molecular Sciences 21, no. 16: 5901. https://doi.org/10.3390/ijms21165901
APA StyleChang, T.-S., Lu, C.-K., Hsieh, Y.-Y., Wei, K.-L., Chen, W.-M., Tung, S.-Y., Wu, C.-S., Chan, M. W. Y., & Chiang, M.-K. (2020). 2,4-Diamino-Quinazoline, a Wnt Signaling Inhibitor, Suppresses Gastric Cancer Progression and Metastasis. International Journal of Molecular Sciences, 21(16), 5901. https://doi.org/10.3390/ijms21165901






