p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation
Abstract
1. Introduction
2. Results
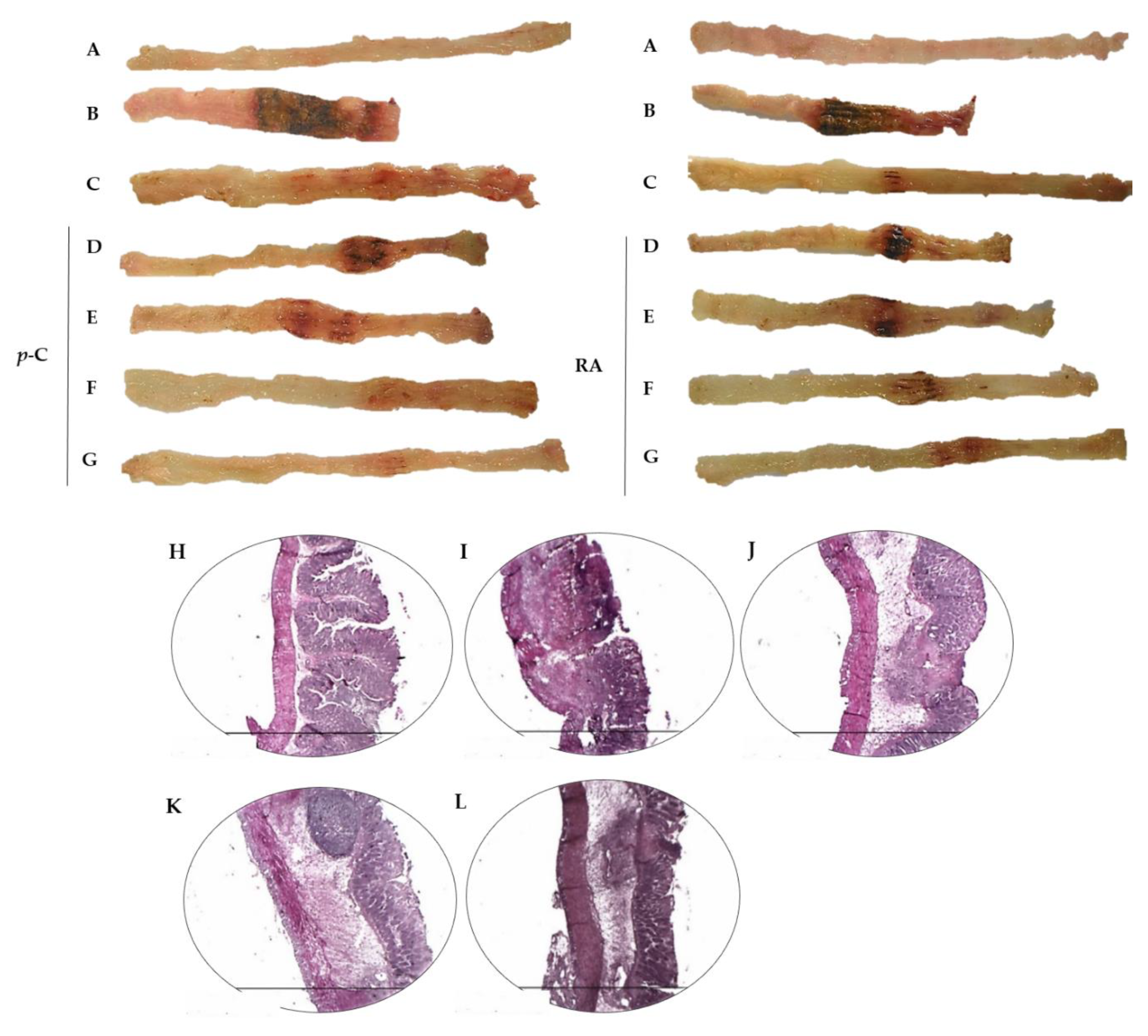
2.1. p-C and RA Decreased Intestinal Inflammation in a TNBS-Induced Colitis Model
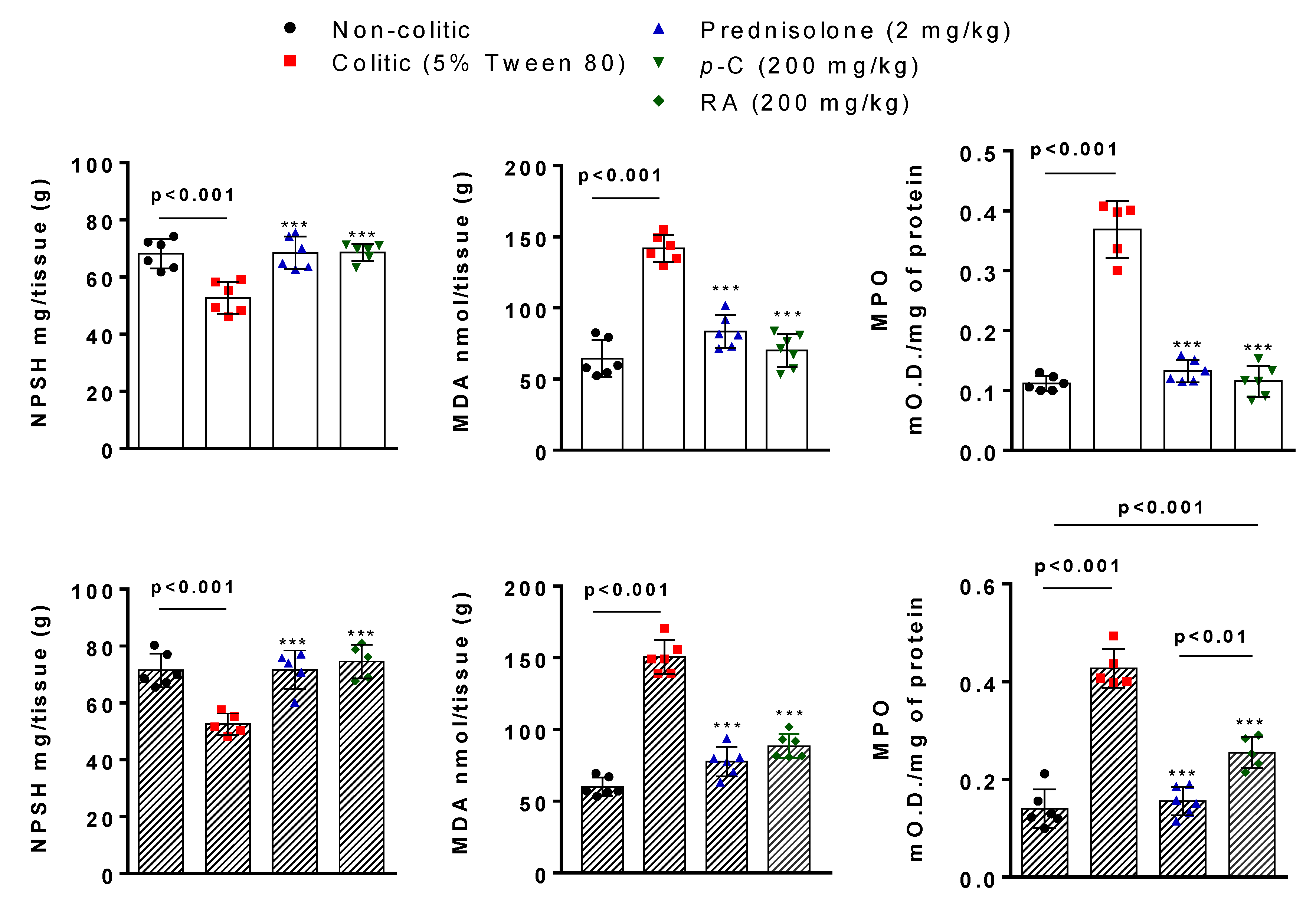
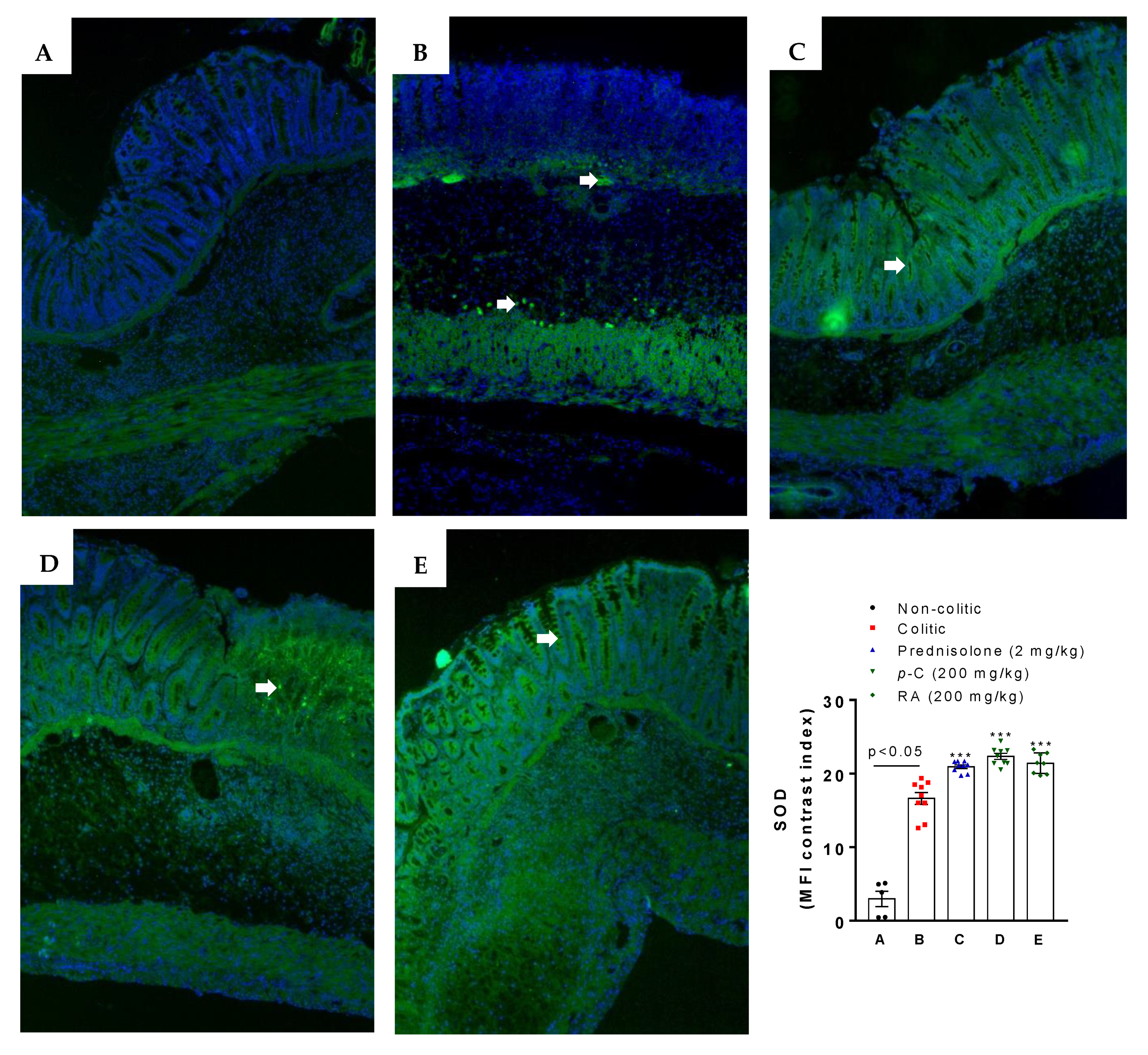
2.2. p-C and RA Increased Antioxidant Molecules (GSH and SOD) and Decreased Malondialdehyde (MDA) and Myeloperoxidase (MPO) Levels in a TNBS-Induced Colitis Model
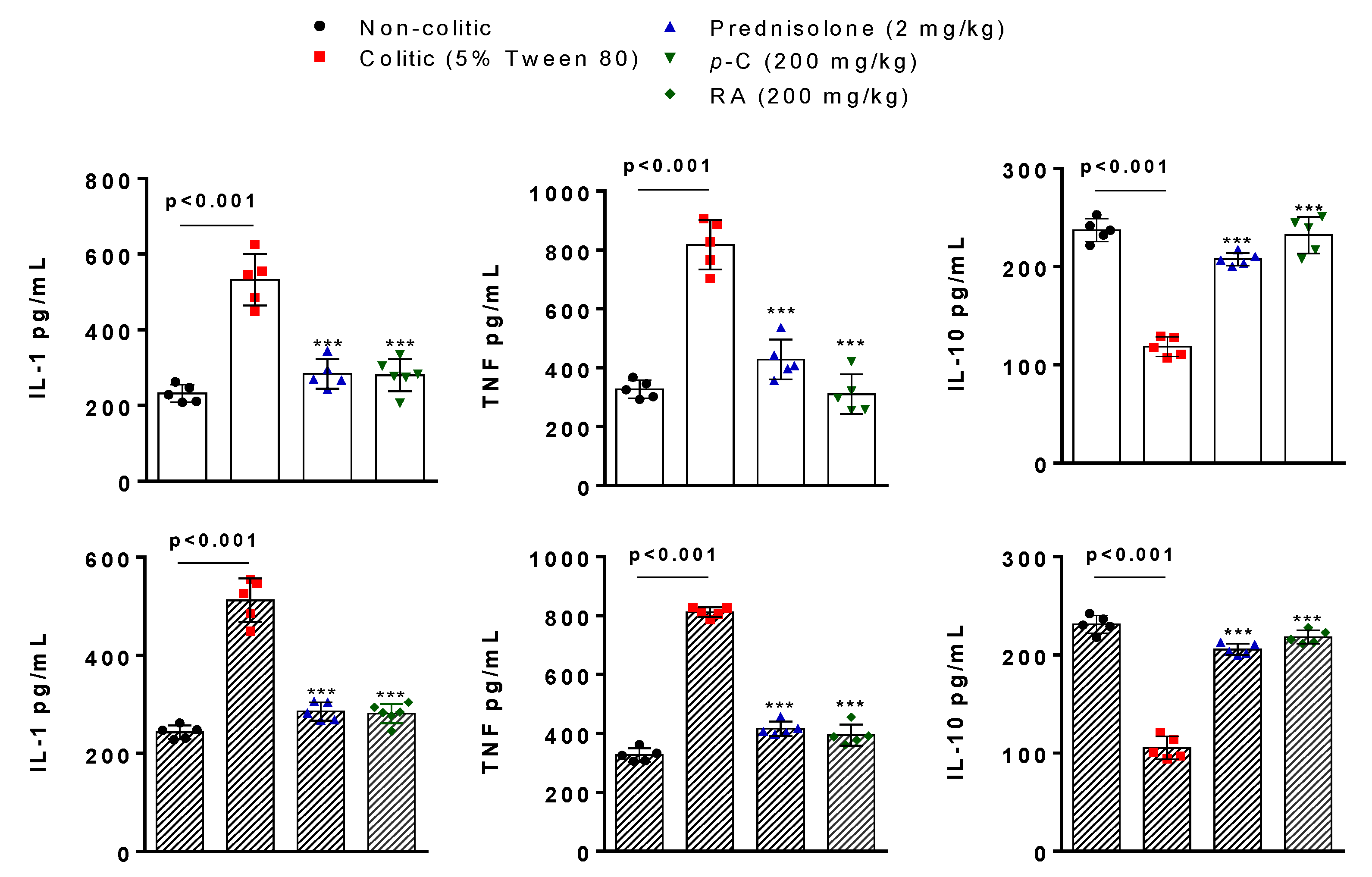
2.3. p-C and RA Decreased Pro-Inflammatory (IL-1β and TNF-α) and Increased Anti-Inflammatory (IL-10) Cytokines in a TNBS-Induced Colitis Model
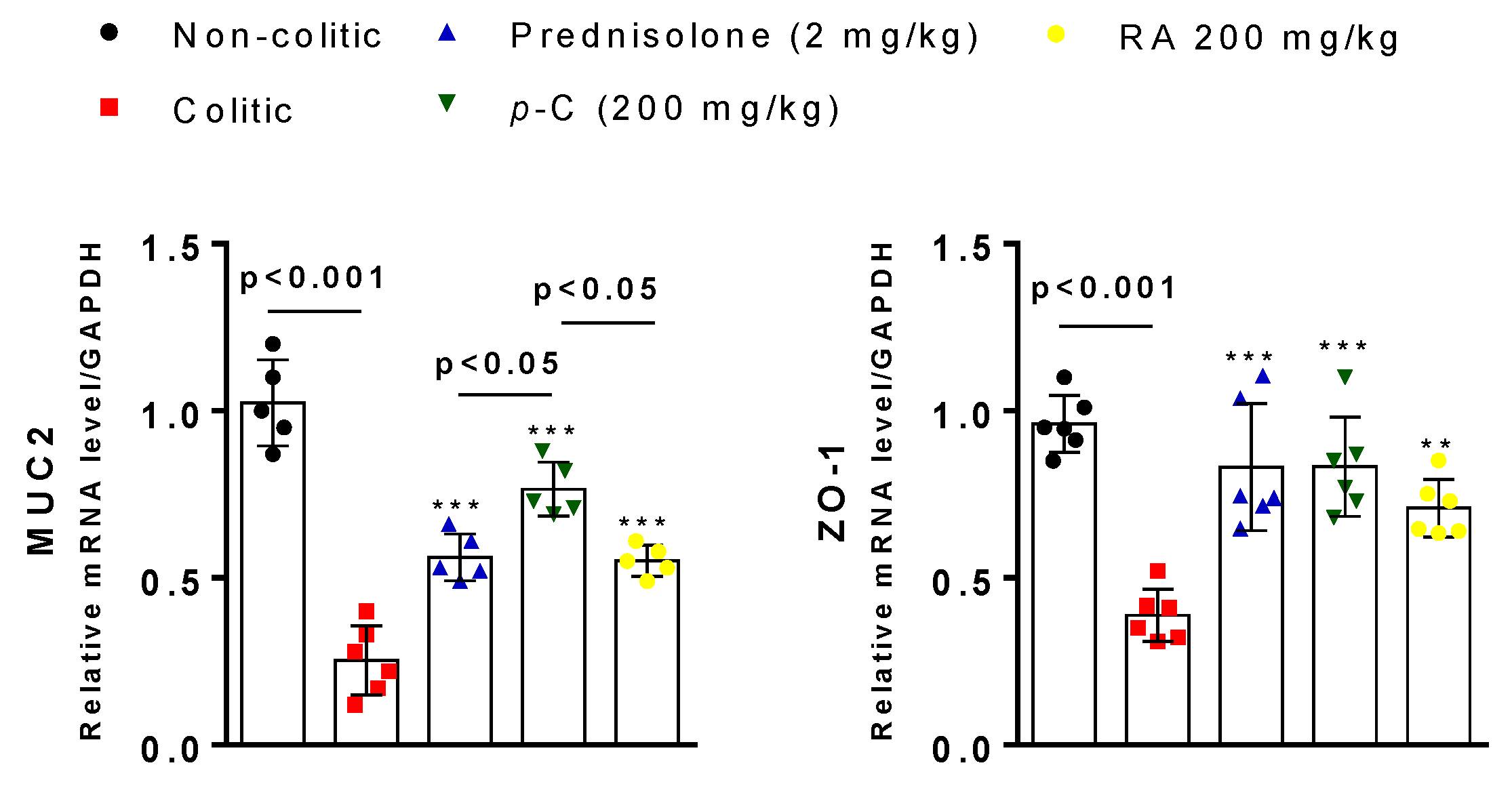
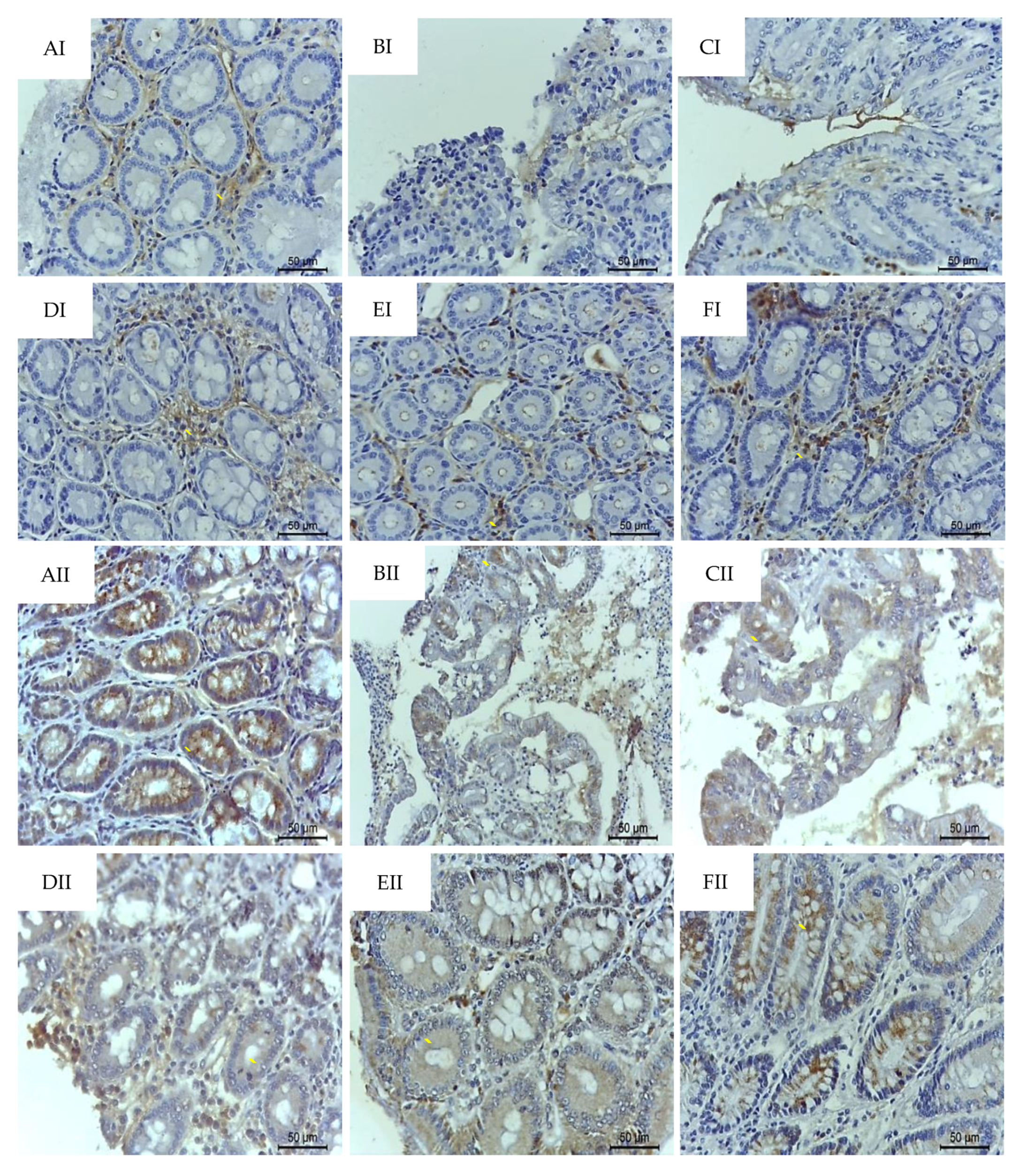
2.4. p-C and RA Maintained Constitutive Proteins of Intestinal Barrier (MUC-2 and ZO-1) in TNBS-Induced Colitis Model
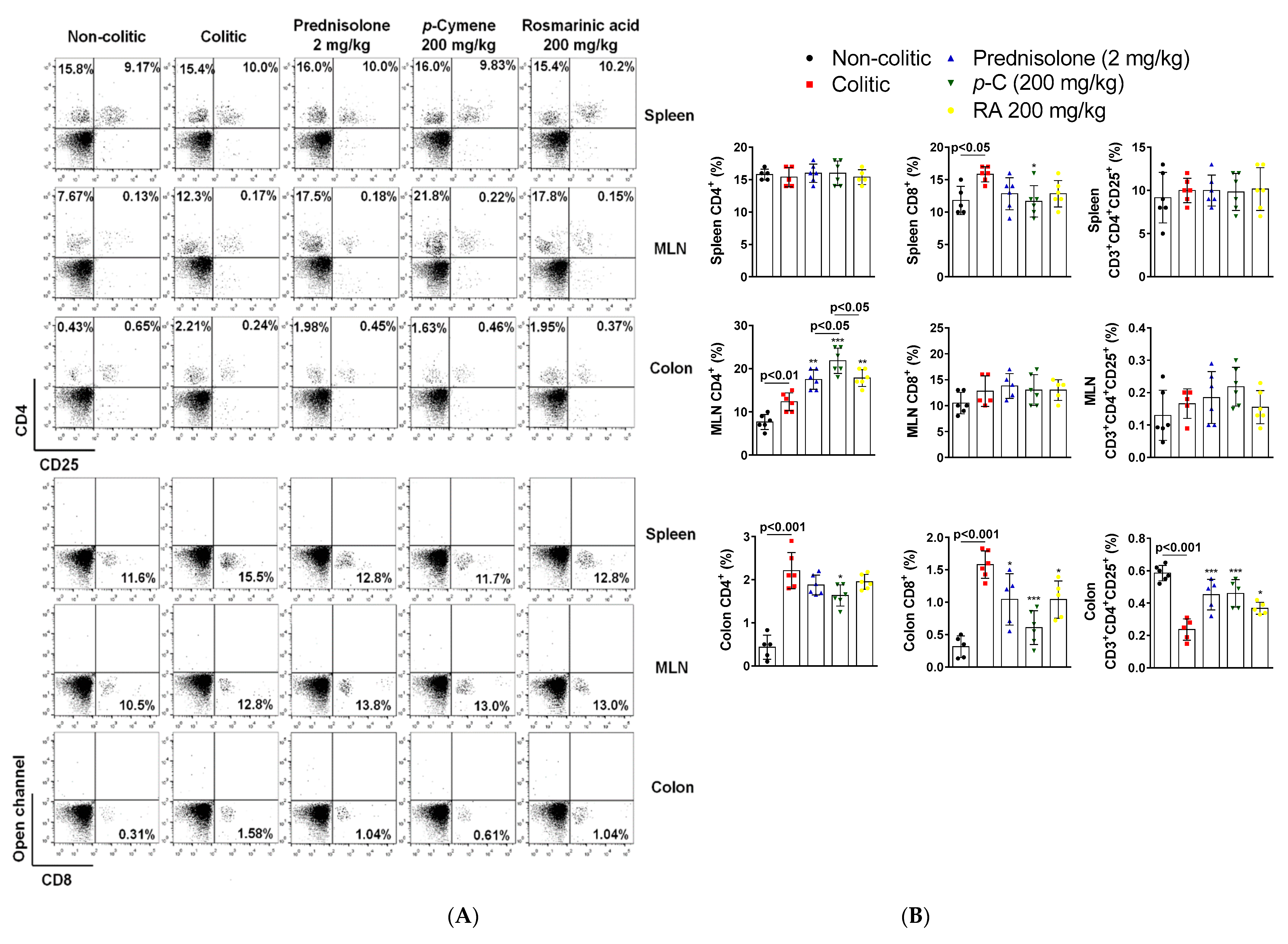
2.5. p-C and RA Modulated T Cell Populations in Colon, Spleen, and Mesenteric on Lymph Nodes in a TNBS-Induced Colitis Model
2.6. Effect of p-C and RA on the Gene Expression of COX-2, IFN-γ, iNOS, SOCS3, and NFκB-p65
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Trinitrobenzene Sulfonic Acid (TNBS)-Induced Intestinal Inflammation in Rats
4.4. Histological Analysis
4.5. Determination of Reduced Glutathione (GSH) Levels
4.6. Determination of Malondialdehyde (MDA) Levels
4.7. Determination of Myeloperoxidase (MPO) Activity
4.8. Determination of IL-1β, TNF-α, and IL-10 Levels
4.9. Immunofluorescence
4.10. Immunohistochemical Analysis
4.11. Flow Cytometry Analysis
4.12. Gene Expression by RT-PCR
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Allophycocyanin |
| BSA | Bovine serum albumin |
| CD25 | Cluster of differentiation 25 |
| CD3 | Cluster of differentiation 3 |
| CD4 | Cluster of differentiation 4 |
| CD8 | Cluster of differentiation 8 |
| cDNA | Complementary deoxyribonucleic acid |
| COX-2 | Cyclooxygenase-2; |
| DTNB | 5-5’-dithiobis-2-nitrobenzoic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| EI | Evacuation index |
| ERK | Extracellular signal-regulated kinases |
| FITC | Fluorescein isothiocyanate |
| GSH | Reduced glutathione |
| H2O2 | Hydrogen peroxide |
| HE | Hematoxylin-eosin |
| HTAB | Hexadecyltrimethylammonium bromide |
| IFN-γ | Interferon-gamma |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin 1 beta |
| iNOS | Inducible nitric oxide synthase |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| MUC2 | Mucin type 2 |
| NFκB-p65 | Factor nuclear kappa B subunit p65 |
| NPSH | Non-protein sulfhydryl |
| PBS | Phosphate-buffered saline |
| PE | Phycoerythrin |
| RNA | Ribonucleic acid |
| SOCS3 | Suppressor of cytokine signaling 3 |
| SOD | Superoxide dismutase |
| TNBS | 2,4,6-trinitrobenzene sulfonic acid |
| TNF-α | Tumoral necrosis factor alpha |
| UA | Ulcerative area |
| ZO-1 | Zonula occludens |
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Liverani, E. How to predict clinical relapse in inflammatory bowel disease patients. World J. Gastroenterol. 2016, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Proinflammatory Cytokines in the Pathogenesis of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1756–1767.e1. [Google Scholar] [CrossRef]
- Valatas, V.; Vakas, M.; Kolios, G. The value of experimental models of colitis in predicting efficacy of biological therapies for inflammatory bowel diseases. Am. J. Physiol. -Gastrointest. Liver Physiol. 2013, 305, G763–G785. [Google Scholar] [CrossRef]
- DeRoche, T.C.; Xiao, S.-Y.; Liu, X. Histological evaluation in ulcerative colitis. Gastroenterol. Rep. 2014, 2, 178–192. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Boucher, G.; Lees, C.W.; Franke, A.; D’Amato, M.; Taylor, K.D.; Lee, J.C.; Goyette, P.; Imielinski, M.; Latiano, A.; et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- De Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 103. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Mas-Moya, J.; Singhi, A.D. The gross pathology of inflammatory bowel disease. Diagn. Histopathol. 2015, 21, 261–266. [Google Scholar] [CrossRef]
- Triantafillidis, J. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des. Dev. Ther. 2011, 185–210. [Google Scholar] [CrossRef]
- Weisshof, R.; el Jurdi, K.; Zmeter, N.; Rubin, D.T. Emerging Therapies for Inflammatory Bowel Disease. Adv. Ther. 2018, 35, 1746–1762. [Google Scholar] [CrossRef]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar] [CrossRef]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 167–182. [Google Scholar] [CrossRef]
- Actis, G.C.; Pellicano, R. Inflammatory bowel disease: Efficient remission maintenance is crucial for cost containment. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Santos, F. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem. Toxicol. 2004, 42, 579–584. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.; de Sousa, D. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Shapiro, H.; Singer, P.; Halpern, Z.; Bruck, R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut 2007, 56, 426–436. [Google Scholar] [CrossRef]
- Baratta, M.T.; Dorman, H.J.D.; Deans, S.G.; Biondi, D.M.; Ruberto, G. Chemical Composition, Antimicrobial and Antioxidative Activity of Laurel, Sage, Rosemary, Oregano and Coriander Essential Oils. J. Essent. Oil Res. 1998, 10, 618–627. [Google Scholar] [CrossRef]
- Russo, M.; Galletti, G.C.; Bocchini, P.; Carnacini, A. Essential Oil Chemical Composition of Wild Populations of Italian Oregano Spice (Origanum v ulgare ssp. h irtum (Link) Ietswaart): A Preliminary Evaluation of Their Use in Chemotaxonomy by Cluster Analysis. 1. Inflorescences. J. Agric. Food Chem. 1998, 46, 3741–3746. [Google Scholar] [CrossRef]
- Bonjardim, L.R.; Cunha, E.S.; Guimarães, A.G.; Santana, M.F.; Oliveira, M.G.B.; Serafini, M.R.; Araújo, A.A.S.; Antoniolli, Â.R.; Cavalcanti, S.C.H.; Santos, M.R.V.; et al. Evaluation of the Anti-Inflammatory and Antinociceptive Properties of p-Cymene in Mice. Z. Für Nat. C. 2012, 67, 15–21. [Google Scholar] [CrossRef]
- De Oliveira, T.M.; de Carvalho, R.B.F.; da Costa, I.H.F.; de Oliveira, G.A.L.; de Souza, A.A.; de Lima, S.G.; de Freitas, R.M. Evaluation of p-cymene, a natural antioxidant. Pharm. Biol. 2015, 53, 423–428. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Braidy, N.; Matin, A.; Rossi, F.; Chinain, M.; Laurent, D.; Guillemin, G.J. Neuroprotective Effects of Rosmarinic Acid on Ciguatoxin in Primary Human Neurons. Neurotox. Res. 2014, 25, 226–234. [Google Scholar] [CrossRef]
- Lembo, S.; Balato, A.; di Caprio, R.; Cirillo, T.; Giannini, V.; Gasparri, F.; Monfrecola, G. The Modulatory Effect of Ellagic Acid and Rosmarinic Acid on Ultraviolet-B-Induced Cytokine/Chemokine Gene Expression in Skin Keratinocyte (HaCaT) Cells. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- OH, S.Y.; CHO, K.-A.; KANG, J.L.; KIM, K.H.; WOO, S.-Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS Colitis Models: Providing Insights Into Inflammatory Bowel Disease and Effects of Dietary Fat. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Joo, M.; Kim, H.S.; Kwon, T.H.; Palikhe, A.; Zaw, T.S.; Jeong, J.H.; Sohn, U.D. Anti-inflammatory Effects of Flavonoids on TNBS-induced Colitis of Rats. Korean J. Physiol. Pharmacol. 2015, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Minaiyan, M.; Ghannadi, A.R.; Afsharipour, M.; Mahzouni, P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res. Pharm. Sci. 2011, 6, 13–21. [Google Scholar] [PubMed]
- Bukovská, A.; Cikoš, Š.; Juhás, Š.; Il’ková, G.; Rehák, P.; Koppel, J. Effects of a Combination of Thyme and Oregano Essential Oils on TNBS-Induced Colitis in Mice. Mediat. Inflamm. 2007, 2007, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, I.; Tijjani, B.M.; Wani, H.; Hassan, N.S.; Khan, A.B.; Hussain, S. Biomarkers in the management of ulcerative colitis: A brief review. Ger. Med. Sci. 2011, 16, 1–7. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef]
- Zhang, J.; Dou, W.; Zhang, E.; Sun, A.; Ding, L.; Wei, X.; Chou, G.; Mani, S.; Wang, Z. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am. J. Physiol. -Gastrointest. Liver Physiol. 2014, 306, G27–G36. [Google Scholar] [CrossRef]
- Medicherla, K.; Sahu, B.D.; Kuncha, M.; Kumar, J.M.; Sudhakar, G.; Sistla, R. Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct. 2015, 6, 2984–2995. [Google Scholar] [CrossRef]
- Jin, B.-R.; Chung, K.-S.; Cheon, S.-Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.-J.; An, H.-J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 46, 46252. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, M.; Ates, I.; Kaplan, M.; Arikan, M.F.; Ozderin, Y.; Zeki, O.; Kilic, M.Y.; Topcuoglu, C.; Kayacetin, E. Is Oxidative Stress Associated with Activation and Pathogenesis of Inflammatory Bowel Disease? J. Med. Biochem. 2017, 36, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Ardite, E.; Sans, M.; Panés, J.; Romero, F.J.; Piqué, J.M.; Fernández-Checa, J.C. Replenishment of Glutathione Levels Improves Mucosal Function in Experimental Acute Colitis. Lab. Investig. 2000, 80, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.A.; de Andrade, K.Q.; dos Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef]
- Balmus, I.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3. [Google Scholar] [CrossRef]
- Yildiz, G.; Yildiz, Y.; Ulutas, P.; Yaylali, A.; Ural, M. Resveratrol Pretreatment Ameliorates TNBS Colitis in Rats. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 134–140. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef]
- Sands, B.E.; Kaplan, G.G. The Role of TNFα in Ulcerative Colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Vounotrypidis, P.; Kouklakis, G.; Anagnostopoulos, K.; Zezos, P.; Polychronidis, A.; Maltezos, E.; Efremidou, E.; Pitiakoudis, M.; Lyratzopoulos, N. Interleukin-1 associations in inflammatory bowel disease and the enteropathic seronegative spondylarthritis. Autoimmun. Highlights 2013, 4, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, A.; Mueller, C. The role of activated cytotoxic T cells in inflammatory bowel disease. Histol. Histopathol. 2000, 15, 167–172. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Shehab, K.W.; Ghishan, F.K.; Kiela, P.R. T Lymphocyte Dynamics in Inflammatory Bowel Diseases: Role of the Microbiome. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arora, Z.; Shen, B. Biological therapy for ulcerative colitis. Gastroenterol. Rep. 2015, 3, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Peyrin-Biroulet, L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Ther. Adv. Gastroenterol. 2015, 8, 66–82. [Google Scholar] [CrossRef]
- Mohammadnia-Afrouzi, M.; Zavaran Hosseini, A.; Khalili, A.; Abediankenari, S.; Hosseini, V.; Maleki, I. Decrease of CD4 + CD25 + CD127 low FoxP3 + regulatory T cells with impaired suppressive function in untreated ulcerative colitis patients. Autoimmunity 2015, 48, 556–561. [Google Scholar] [CrossRef]
- Engelhardt, K.R.; Grimbacher, B. IL-10 in Humans: Lessons from the Gut, IL-10/IL-10 Receptor Deficiencies, and IL-10 Polymorphisms. Curr. Top. Microbiol. Immunol. 2014, 380, 1–18. [Google Scholar] [CrossRef]
- Li, Y.; de Haar, C.; Peppelenbosch, M.P.; van der Woude, C.J. SOCS3 in immune regulation of inflammatory bowel disease and inflammatory bowel disease-related cancer. Cytokine Growth Factor Rev. 2012, 23, 127–138. [Google Scholar] [CrossRef]
- Suzuki, A.; Hanada, T.; Mitsuyama, K.; Yoshida, T.; Kamizono, S.; Hoshino, T.; Kubo, M.; Yamashita, A.; Okabe, M.; Takeda, K.; et al. Cis3/Socs3/Ssi3 Plays a Negative Regulatory Role in Stat3 Activation and Intestinal Inflammation. J. Exp. Med. 2001, 193, 471–482. [Google Scholar] [CrossRef]
- Miyanaka, Y. Clinical significance of mucosal suppressors of cytokine signaling 3 expression in ulcerative colitis. World J. Gastroenterol. 2007, 13, 2939. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ray, A.; Jiang, X.; Wang, J.; Basu, S.; Liu, X.; Qian, T.; He, R.; Dittel, B.N.; Chu, Y. T regulatory cells and B cells cooperate to form a regulatory loop that maintains gut homeostasis and suppresses dextran sulfate sodium-induced colitis. Mucosal Immunol. 2015, 8, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, P.A.; Ostan, R.; Bisson, J.-F.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-Limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Lee, C.H.; Jang, S.-E.; Park, J.-W.; Lim, S.-J.; Kim, D.-H.; Bae, H.; Kim, H.-J.; Cha, J.M. Amelioration of trinitrobenzene sulfonic acid-induced colitis in mice by liquiritigenin. J. Gastroenterol. Hepatol. 2015, 30, 858–865. [Google Scholar] [CrossRef]
- Gkouskou, K.K.; Deligianni, C.; Tsatsanis, C.; Eliopoulos, A.G. The gut microbiota in mouse models of inflammatory bowel disease. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef]
- Antoni, L. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1165. [Google Scholar] [CrossRef]
- Shirazi, T. Mucins and inflammatory bowel disease. Postgrad. Med J. 2000, 76, 473–478. [Google Scholar] [CrossRef]
- Alipour, M.; Zaidi, D.; Valcheva, R.; Jovel, J.; Martínez, I.; Sergi, C.; Walter, J.; Mason, A.L.; Wong, G.K.-S.; Dieleman, L.A.; et al. Mucosal Barrier Depletion and Loss of Bacterial Diversity are Primary Abnormalities in Paediatric Ulcerative Colitis. J. Crohn’s Colitis 2016, 10, 462–471. [Google Scholar] [CrossRef]
- Amit-Romach, E.; Reifen, R.; Uni, Z. Mucosal function in rat jejunum and ileum is altered by induction of colitis. Int. J. Mol. Med. 2006. [Google Scholar] [CrossRef]
- Renes, I.B.; Boshuizen, J.A.; van Nispen, D.J.P.M.; Bulsing, N.P.; Büller, H.A.; Dekker, J.; Einerhand, A.W.C. Alterations in Muc2 biosynthesis and secretion during dextran sulfate sodium-induced colitis. Am. J. Physiol. -Gastrointest. Liver Physiol. 2002, 282, G382–G389. [Google Scholar] [CrossRef]
- Van der Sluis, M.; de Koning, B.A.E.; de Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; van Goudoever, J.B.; Büller, H.A.; Dekker, J.; van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.F.; Heneghan, A.F.; Feliciano, R.P.; Shanmuganayagam, D.; Roenneburg, D.A.; Krueger, C.G.; Reed, J.D.; Kudsk, K.A. Cranberry Proanthocyanidins Improve the Gut Mucous Layer Morphology and Function in Mice Receiving Elemental Enteral Nutrition. J. Parenter. Enter. Nutr. 2013, 37, 401–409. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Taira, T.; Yamaguchi, S.; Takahashi, A.; Okazaki, Y.; Yamaguchi, A.; Sakaguchi, H.; Chiji, H. Dietary polyphenols increase fecal mucin and immunoglobulin A and ameliorate the disturbance in gut microbiota caused by a high fat diet. J. Clin. Biochem. Nutr. 2015, 57, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef] [PubMed]
- Oh-oka, K.; Kono, H.; Ishimaru, K.; Miyake, K.; Kubota, T.; Ogawa, H.; Okumura, K.; Shibata, S.; Nakao, A. Expressions of Tight Junction Proteins Occludin and Claudin-1 Are under the Circadian Control in the Mouse Large Intestine: Implications in Intestinal Permeability and Susceptibility to Colitis. PLoS ONE 2014, 9, e98016. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Choi, W.; Buckley, A.; Shashikanth, N.; Joseph, N.E.; Wang, Y.; Warren, M.H.; Buschmann, M.M.; Pavlyuk, R.; Hildebrand, J.; et al. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J. Cell Sci. 2017, 130, 243–259. [Google Scholar] [CrossRef]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. ZO-1 and ZO-2 Independently Determine Where Claudins Are Polymerized in Tight-Junction Strand Formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef]
- Mankertz, J.; Tavalali, S.; Schmitz, H.; Mankertz, A.; Riecken, E.O.; Fromm, M.; Schulzke, J.D. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J. Cell Sci. 2000, 113, 2085–2090. [Google Scholar]
- Rochfort, K.D.; Cummins, P.M. Cytokine-mediated dysregulation of zonula occludens-1 properties in human brain microvascular endothelium. Microvasc. Res. 2015, 100, 48–53. [Google Scholar] [CrossRef]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-γ and Tumor Necrosis Factor-α Synergize to Induce Intestinal Epithelial Barrier Dysfunction by Up-Regulating Myosin Light Chain Kinase Expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Morales, P.; Gotteland, M. Polyphenols Protect the Epithelial Barrier Function of Caco-2 Cells Exposed to Indomethacin through the Modulation of Occludin and Zonula Occludens-1 Expression. J. Agric. Food Chem. 2013, 61, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.; Conlon, M.; Topping, D. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Bell, C.J.; Gall, D.G.; Wallace, J.L. Disruption of colonic electrolyte transport in experimental colitis. Am. J. Physiol. -Gastrointest. Liver Physiol. 1995, 268, G622–G630. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.-L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems; Birkhäuser Basel: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. [42] Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Meth. Enzymol. 1990, 186, 407–421. [Google Scholar]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]

| Groups | Dose (mg/kg) | Ulcerative Area (mm2) | % Lesion Inhibition | Lesion Score | Weight/Length (mg/cm) | Evacuation Index (EI) |
|---|---|---|---|---|---|---|
| Non-colitic | - | ND | - | ND | 101.5 ± 16.04 | 370 |
| Colitis (Tween) | - | 318.6 ± 38.41 ### | 0 | 7.5 (6–9) ### | 191.2 ± 33.77 ### | 751 ### |
| Prednisolone | 2 | 91.5 ± 42.19 *** | 70.0 | 3 (2–5) *** | 131.4 ± 18.44 *** | 412 *** |
| p-Cymene | 25 | 255.3 ± 44.53 **,abcd | 19.7 | 5 (3–6) * | 150.9 ± 16.22 * | 578 ***,ad |
| 50 | 144.0 ± 43.91 ***,d | 54.7 | 4.5 (3–5) ** | 137.9 ± 31.33 ** | 509 ***,ad | |
| 100 | 111.2 ± 15.74 ***,d | 65.0 | 4 (2–5) ** | 130.0 ± 24.41 ** | 450 ***,d | |
| 200 | 58.53 ± 14.16 *** | 81.6 | 2.5 (1–4) *** | 119.3 ± 17.03 *** | 388 *** | |
| Non-colitic | - | ND | ND | ND | 117.1 ± 24.78 | 381 |
| Colitis (NaCl) | - | 341.1 ± 42.99 ### | 0 | 8 (7–10) ### | 182.5 ± 12.29 ### | 773 ### |
| Prednisolone | 2 | 114.3 ± 17.04 *** | 66.6 | 3 (2–6) *** | 122.3 ± 11.38 *** | 420 *** |
| Rosmarinic acid | 25 | 262.5 ± 23.92 **,acd | 19.1 | 6 (3–7) * | 155.0 ± 30.04 * | 613 ***,a.d |
| 50 | 184.7 ± 12.21 ***,ad | 44.1 | 5.5 (4–8) * | 146.6 ± 21.36 ** | 553 ***,ad | |
| 100 | 155.1 ± 39.02 *** | 54.2 | 5 (3–7) ** | 144.7 ± 11.14 ** | 488 *** | |
| 200 | 112.0 ± 15.26 *** | 67.2 | 4 (2–6) *** | 133.4 ± 9.89 *** | 470 *** |
| Treatments | Dose | MUC-2 (μm2) | ZO-1 (μm2) |
|---|---|---|---|
| Non-colitic | - | 2130 (332.1–2536.3) | 725 (136.4–1020.3) |
| 5% Tween 80 | 10 mL/kg | 745 (222.3–993.0) ### | 305 (90.5–489.1) ### |
| 0.9% NaCl | 10 mL/kg | 690 (221.4–823.4) ### | 295 (120.4–401.0) ### |
| Prednisolone | 2 mg/kg | 1700 (789.0–1944.1) *** | 675 (156.5–903.3) *** |
| p-Cymene | 200 mg/kg | 1903 (866.0–2531.5) *** | 685 (488.1–789.3) *** |
| Rosmarinic acid | 200 mg/kg | 1675 (659.3–1722.1) *** | 550 (222.4–697.5) *** |
| Gene | Primer Sequences |
|---|---|
| MUC-2 ZO-1 | Fw: GATAGGTGGCAGACAGGAGA Rv: GCTGACGAGTGGTTGGTGATTG Fw: GGGGCCTACACTGATCAAGA Rv: TGGAGATGAGGCTTCTGCTT |
| COX-2 | Fw: CGCTTCTCCCTGAAACCTTAC Rv: GTAGAGGGCTTTCAACTCTGCA |
| IFN-γ iNOS | Fw: ATGAGTGCTACACGCCGCGTCTTGG Rv: GAGTTCATTGACAGCTTTGTGCTGG Fw: GCTACACTTCCAACGCAACA Rv: GTGGGAGGGGTAGTGAT |
| SOCS3 | Fw: CCTCCAGCATCTTTGTCGGAAGAC Rv: TACTGGTCCAGGAACTCCCGAATG |
| NFκB (p65) | Fw: CTGGCAGCTCTTCTCAAAGC Rv: CCAGGTCATAGAGAGGCTCAA |
| GAPDH | Fw: CCATCACCATCTTCCAGGAG Rv: CCTGCTTCACCACCTTCTTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formiga, R.d.O.; Alves Júnior, E.B.; Vasconcelos, R.C.; Guerra, G.C.B.; Antunes de Araújo, A.; Carvalho, T.G.d.; Garcia, V.B.; de Araújo Junior, R.F.; Gadelha, F.A.A.F.; Vieira, G.C.; et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. https://doi.org/10.3390/ijms21165870
Formiga RdO, Alves Júnior EB, Vasconcelos RC, Guerra GCB, Antunes de Araújo A, Carvalho TGd, Garcia VB, de Araújo Junior RF, Gadelha FAAF, Vieira GC, et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. International Journal of Molecular Sciences. 2020; 21(16):5870. https://doi.org/10.3390/ijms21165870
Chicago/Turabian StyleFormiga, Rodrigo de Oliveira, Edvaldo Balbino Alves Júnior, Roseane Carvalho Vasconcelos, Gerlane Coelho Bernardo Guerra, Aurigena Antunes de Araújo, Thaís Gomes de Carvalho, Vinícius Barreto Garcia, Raimundo Fernandes de Araújo Junior, Francisco Allysson Assis Ferreira Gadelha, Giciane Carvalho Vieira, and et al. 2020. "p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation" International Journal of Molecular Sciences 21, no. 16: 5870. https://doi.org/10.3390/ijms21165870
APA StyleFormiga, R. d. O., Alves Júnior, E. B., Vasconcelos, R. C., Guerra, G. C. B., Antunes de Araújo, A., Carvalho, T. G. d., Garcia, V. B., de Araújo Junior, R. F., Gadelha, F. A. A. F., Vieira, G. C., Sobral, M. V., Barbosa Filho, J. M., Spiller, F., & Batista, L. M. (2020). p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. International Journal of Molecular Sciences, 21(16), 5870. https://doi.org/10.3390/ijms21165870






