Immune-Driven Pathogenesis of Neurotoxicity after Exposure of Cancer Patients to Immune Checkpoint Inhibitors
Abstract
1. Introduction
2. Clinical Phenotype of NirAEs
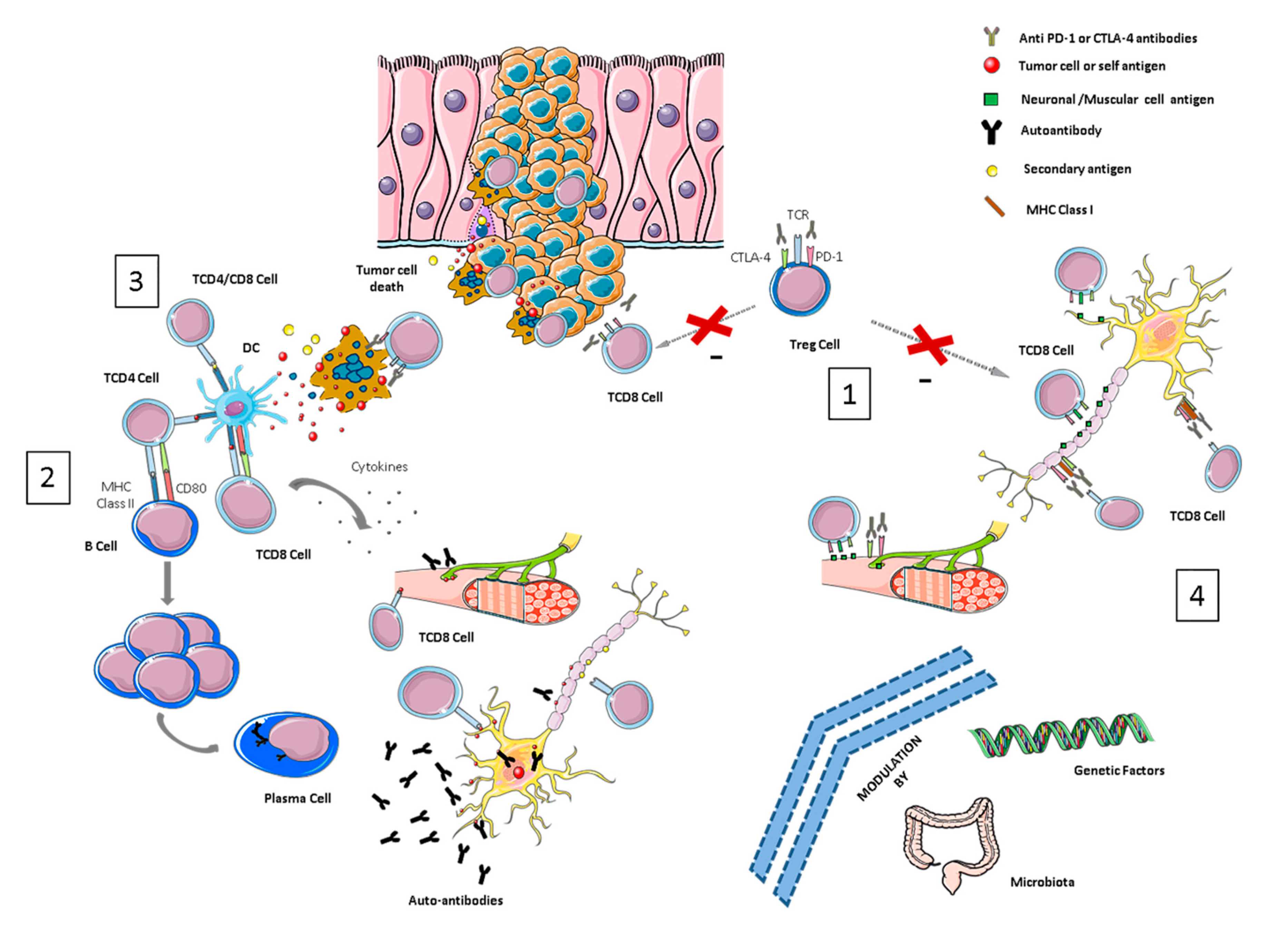
3. Mechanisms of NirAE Pathogenesis
3.1. Breaking Immune Tolerance
3.2. Molecular Mimicry and Cross-Reactivity Among Tumor- and Self-Tissue Antigens
3.3. Epitope Spreading
3.4. Recognition of Target Molecules (CTLA-4 and/or PD-1/PD-L1) in Nervous System Tissues
3.5. Exacerbation of Previous Autoimmune Reactions
3.6. Genetic and Microbiome-Related Factors
4. Clinical Implications in NirAE Management
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CTLA-4 | T-lymphocyte-associated protein 4 |
| PD-1 | Programmed cell death protein 1 |
| ICIs | Immune checkpoint inhibitors |
| irAEs | Immune related adverse events |
| NirAEs | Neurological immune-related adverse events |
| CNS | Central nervous system |
| Tregs | Regulatory T-cells |
| APCs | Antigen-presenting cells |
| PNS | Paraneoplastic syndrome |
| NMDAR | N-methyl-D-aspartate receptor |
| ES | Epitope spreading |
| NSCLC | Non-small cell lung cancer |
| Mab | Monoclonal antibodies |
References
- Bluestone, J.A. Mechanisms of tolerance. Immunol. Rev. 2011, 241, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Xu, M.; Nie, Y.; Yang, Y.; Lu, Y.T.; Su, Q. Risk of Neurological Toxicities Following the Use of Different Immune Checkpoint Inhibitor Regimens in Solid Tumors: A Systematic Review and Meta-analysis. Neurologist 2019, 24, 75–83. [Google Scholar] [CrossRef]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef]
- Psimaras, D.; Velasco, R.; Birzu, C.; Tamburin, S.; Lustberg, M.; Bruna, J.; Argyriou, A.A. Immune checkpoint inhibitors-induced neuromuscular toxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. 2), S74–S85. [Google Scholar] [CrossRef]
- Bruna, J.; Argyriou, A.A.; Anastopoulou, G.G.; Alemany, M.; Nadal, E.; Kalofonou, F.; Piulats, J.M.; Simó, M.; Velasco, R.; Kalofonos, H.P. Incidence and characteristics of neurotoxicity in immune checkpoint inhibitors with focus on neuromuscular events: Experience beyond the clinical trials. J. Peripher. Nerv. Syst. JPNS 2020, 25, 171–177. [Google Scholar] [CrossRef]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kieseier, B.C.; Hartung, H.P.; Wiendl, H. Immune circuitry in the peripheral nervous system. Curr. Opin. Neurol. 2006, 19, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Congdon, K.L.; Sanchez-Perez, L.A.; Sampson, J.H. Effective effectors: How T cells access and infiltrate the central nervous system. Pharmacol. Ther. 2019, 197, 52–60. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Sato, K.; Mano, T.; Iwata, A.; Toda, T. Neurological and related adverse events in immune checkpoint inhibitors: A pharmacovigilance study from the Japanese Adverse Drug Event Report database. J. Neuro Oncol. 2019, 145, 1–9. [Google Scholar] [CrossRef]
- Yshii, L.M.; Hohlfeld, R.; Liblau, R.S. Inflammatory CNS disease caused by immune checkpoint inhibitors: Status and perspectives. Nat. Rev. Neurol. 2017, 13, 755–763. [Google Scholar] [CrossRef]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef]
- Alissafi, T.; Hatzioannou, A.; Legaki, A.I.; Varveri, A.; Verginis, P. Balancing cancer immunotherapy and immune-related adverse events: The emerging role of regulatory T cells. J. Autoimmun. 2019, 104, 102310. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kohm, A.P.; Carpentier, P.A.; Anger, H.A.; Miller, S.D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002, 169, 4712–4716. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.T.; Paterson, A.M.; Lovitch, S.B.; Sharpe, A.H. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 2014, 41, 1026–1039. [Google Scholar] [CrossRef]
- Thibult, M.L.; Mamessier, E.; Gertner-Dardenne, J.; Pastor, S.; Just-Landi, S.; Xerri, L.; Chetaille, B.; Olive, D. PD-1 is a novel regulator of human B-cell activation. Int. Immunol. 2013, 25, 129–137. [Google Scholar] [CrossRef]
- de Moel, E.C.; Rozeman, E.A.; Kapiteijn, E.H.; Verdegaal, E.; Grummels, A.; Bakker, J.A.; Huizinga, T.; Haanen, J.B.; Toes, R.; van der Woude, D. Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2019, 7, 6–11. [Google Scholar] [CrossRef]
- Toomer, K.H.; Malek, T.R. Cytokine Signaling in the Development and Homeostasis of Regulatory T cells. Cold Spring Harb. Perspect. Biol. 2018, 10, a028597. [Google Scholar] [CrossRef]
- Das, R.; Verma, R.; Sznol, M.; Boddupalli, C.S.; Gettinger, S.N.; Kluger, H.; Callahan, M.; Wolchok, J.D.; Halaban, R.; Dhodapkar, M.V.; et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol. 2015, 194, 950–959. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carleton, M.; Zhou, M.; Chen, T.; Feng, Y.; Huang, S.P.; Walsh, A.M.; Baxi, V.; Pandya, D.; Baradet, T.; et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020, 26, 688–692. [Google Scholar] [CrossRef]
- Yuen, K.C.; Liu, L.F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E.; Koeppen, H., 3rd; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Lee, J.H.; Gide, T.N.; Menzies, A.M.; Guminski, A.; Carlino, M.S.; Breen, E.J.; Yang, J.; Ghazanfar, S.; Kefford, R.F.; et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Darnell, R.B.; Posner, J.B. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003, 349, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Haugh, A.M.; Probasco, J.C.; Johnson, D.B. Neurologic complications of immune checkpoint inhibitors. Expert Opin. Drug Saf. 2020, 19, 479–488. [Google Scholar] [CrossRef]
- Yshii, L.M.; Gebauer, C.M.; Pignolet, B.; Mauré, E.; Quériault, C.; Pierau, M.; Saito, H.; Suzuki, N.; Brunner-Weinzierl, M.; Bauer, J.; et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain J. Neurol. 2016, 139, 2923–2934. [Google Scholar] [CrossRef]
- Williams, T.J.; Benavides, D.R.; Patrice, K.A.; Dalmau, J.O.; de Ávila, A.L.; Le, D.T.; Lipson, E.J.; Probasco, J.C.; Mowry, E.M. Association of Autoimmune Encephalitis With Combined Immune Checkpoint Inhibitor Treatment for Metastatic Cancer. JAMA Neurol. 2016, 73, 928–933. [Google Scholar] [CrossRef]
- Wei, X.; Walia, V.; Lin, J.C.; Teer, J.K.; Prickett, T.D.; Gartner, J.; Davis, S.; NISC Comparative Sequencing Program; Stemke-Hale, K.; Davies, M.A.; et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat. Genet. 2011, 43, 442–446. [Google Scholar] [CrossRef]
- Schneiderbauer, R.; Schneiderbauer, M.; Wick, W.; Enk, A.H.; Haenssle, H.A.; Hassel, J.C. PD-1 Antibody-induced Guillain-Barré Syndrome in a Patient with Metastatic Melanoma. Acta Derm. Venereol. 2017, 97, 395–396. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Vogrig, A.; Fouret, M.; Joubert, B.; Picard, G.; Rogemond, V.; Pinto, A.L.; Muñiz-Castrillo, S.; Roger, M.; Raimbourg, J.; Dayen, C.; et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. (R) Neuroimmunol. Neuroinflammat. 2019, 6, e604. [Google Scholar] [CrossRef]
- Makarious, D.; Horwood, K.; Coward, J. Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. Eur. J. Cancer 2017, 82, 128–136. [Google Scholar] [CrossRef]
- Kwek, S.S.; Dao, V.; Roy, R.; Hou, Y.; Alajajian, D.; Simko, J.P.; Small, E.J.; Fong, L. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J. Immunol. 2012, 189, 3759–3766. [Google Scholar] [CrossRef]
- Memarnejadian, A.; Meilleur, C.E.; Shaler, C.R.; Khazaie, K.; Bennink, J.R.; Schell, T.D.; Haeryfar, S. PD-1 Blockade Promotes Epitope Spreading in Anticancer CD8+ T Cell Responses by Preventing Fratricidal Death of Subdominant Clones To Relieve Immunodomination. J. Immunol. 2017, 199, 3348–3359. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Mok, T.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V., Jr.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef]
- Karandikar, N.J.; Vanderlugt, C.L.; Walunas, T.L.; Miller, S.D.; Bluestone, J.A. CTLA-4: A negative regulator of autoimmune disease. J. Exp. Med. 1996, 184, 783–788. [Google Scholar] [CrossRef]
- Perrin, P.J.; Maldonado, J.H.; Davis, T.A.; June, C.H.; Racke, M.K. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J. Immunol. 1996, 157, 1333–1336. [Google Scholar]
- Gerdes, L.A.; Held, K.; Beltrán, E.; Berking, C.; Prinz, J.C.; Junker, A.; Tietze, J.K.; Ertl-Wagner, B.; Straube, A.; Kümpfel, T.; et al. CTLA4 as Immunological Checkpoint in the Development of Multiple Sclerosis. Ann. Neurol. 2016, 80, 294–300. [Google Scholar] [CrossRef]
- Lau, K.H.; Kumar, A.; Yang, I.H.; Nowak, R.J. Exacerbation of myasthenia gravis in a patient with melanoma treated with pembrolizumab. Muscle Nerve 2016, 54, 157–161. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y. Myasthenia gravis exacerbation associated with pembrolizumab. Muscle Nerve 2016, 54, 506–507. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Lühder, F.; Höglund, P.; Allison, J.P.; Benoist, C.; Mathis, D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J. Exp. Med. 1998, 187, 427–432. [Google Scholar] [CrossRef]
- Lowe, J.R.; Perry, D.J.; Salama, A.K.; Mathews, C.E.; Moss, L.G.; Hanks, B.A. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J. Immunother Cancer 2016, 4, 89. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Schadendorf, D.; de Pril, V.; Heller, K.; Chen, T.T.; Ibrahim, R.; et al. Ipilimumab efficacy and safety in patients with advanced melanoma: A retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010, 10, 9. [Google Scholar]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H.; Calabrese, L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Spain, L.; Tippu, Z.; Larkin, J.M.; Carr, A.; Turajlic, S. How we treat neurological toxicity from immune checkpoint inhibitors. ESMO Open 2019, 4 (Suppl. 4), e000540. [Google Scholar] [CrossRef]
- Martins, F.; Sykiotis, G.P.; Maillard, M.; Fraga, M.; Ribi, C.; Kuntzer, T.; Michielin, O.; Peters, S.; Coukos, G.; Spertini, F.; et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019, 20, e54–e64. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilariño, N.; Bruna, J.; Kalofonou, F.; Anastopoulou, G.G.; Argyriou, A.A. Immune-Driven Pathogenesis of Neurotoxicity after Exposure of Cancer Patients to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 5774. https://doi.org/10.3390/ijms21165774
Vilariño N, Bruna J, Kalofonou F, Anastopoulou GG, Argyriou AA. Immune-Driven Pathogenesis of Neurotoxicity after Exposure of Cancer Patients to Immune Checkpoint Inhibitors. International Journal of Molecular Sciences. 2020; 21(16):5774. https://doi.org/10.3390/ijms21165774
Chicago/Turabian StyleVilariño, Noelia, Jordi Bruna, Foteini Kalofonou, Garifallia G. Anastopoulou, and Andreas A. Argyriou. 2020. "Immune-Driven Pathogenesis of Neurotoxicity after Exposure of Cancer Patients to Immune Checkpoint Inhibitors" International Journal of Molecular Sciences 21, no. 16: 5774. https://doi.org/10.3390/ijms21165774
APA StyleVilariño, N., Bruna, J., Kalofonou, F., Anastopoulou, G. G., & Argyriou, A. A. (2020). Immune-Driven Pathogenesis of Neurotoxicity after Exposure of Cancer Patients to Immune Checkpoint Inhibitors. International Journal of Molecular Sciences, 21(16), 5774. https://doi.org/10.3390/ijms21165774





