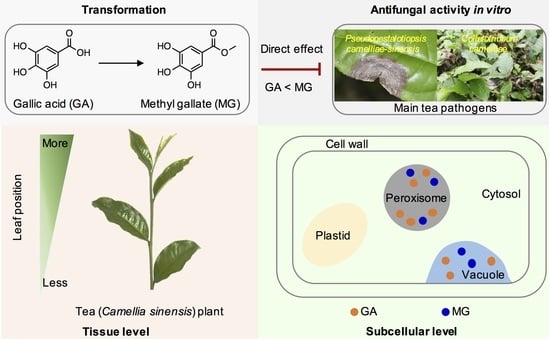
Metabolism of Gallic Acid and Its Distributions in Tea (Camellia sinensis) Plants at the Tissue and Subcellular Levels
Abstract
1. Introduction
2. Results and Discussion
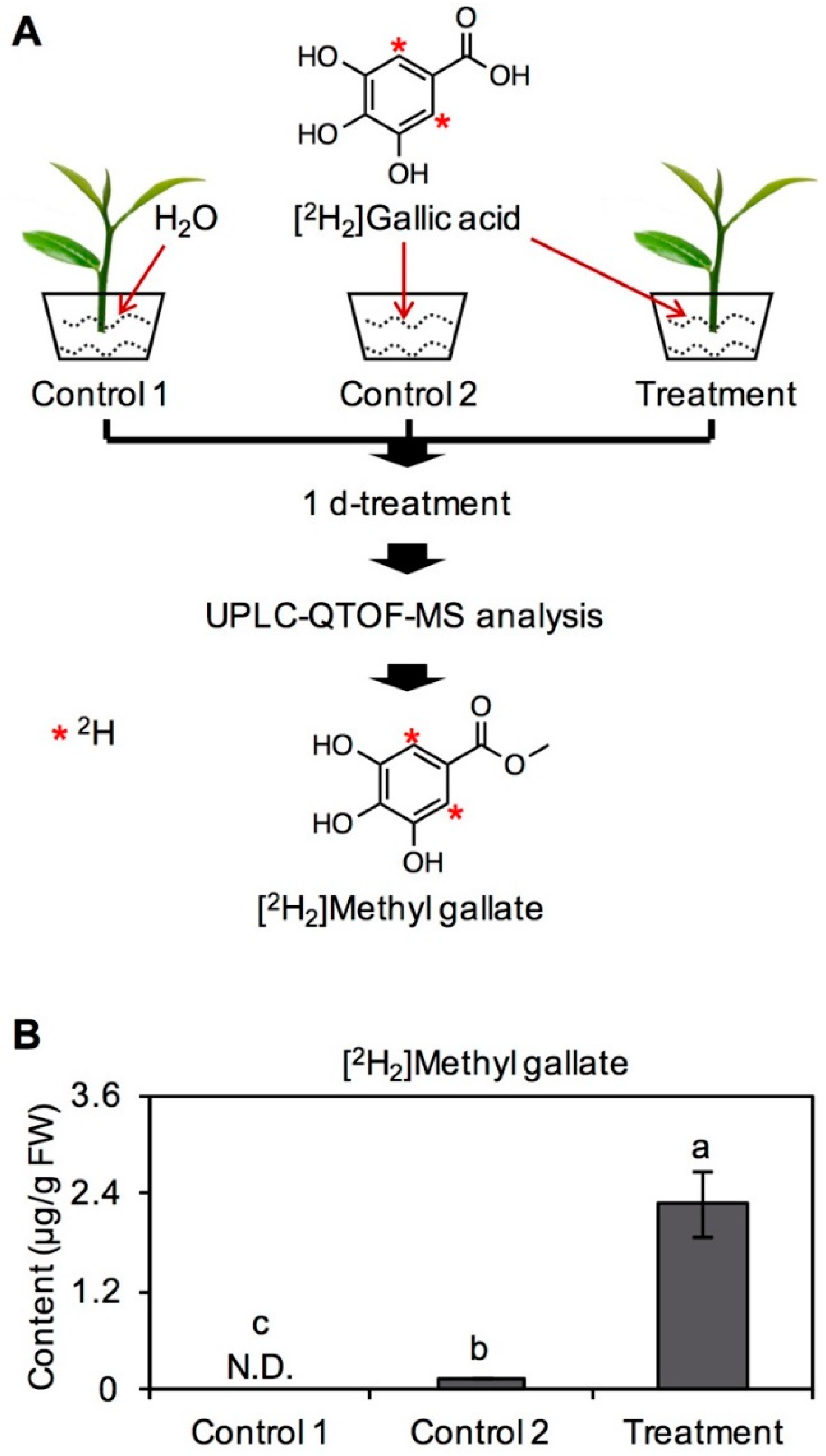
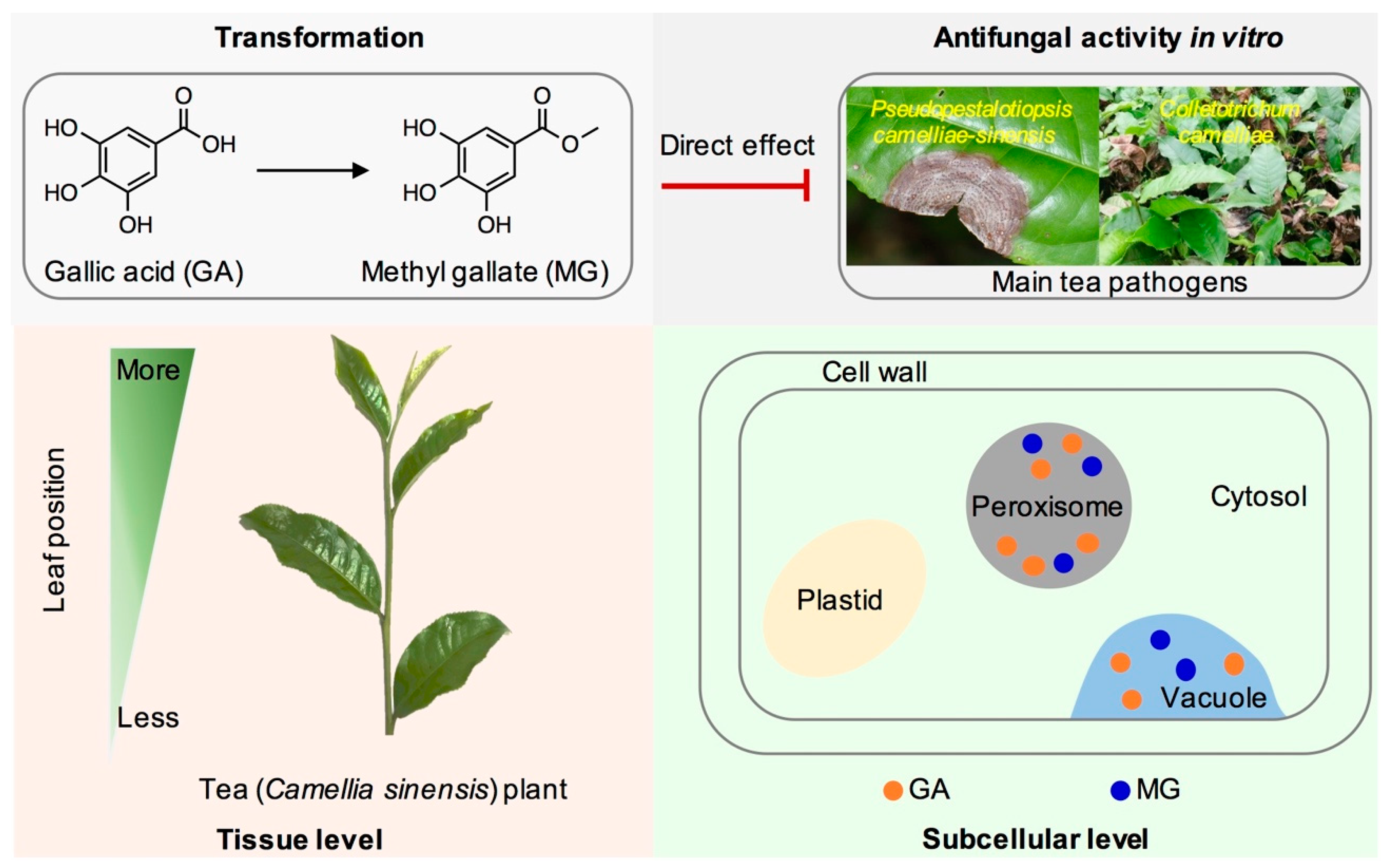
2.1. Transformation of GA into MG in Tea Leaves
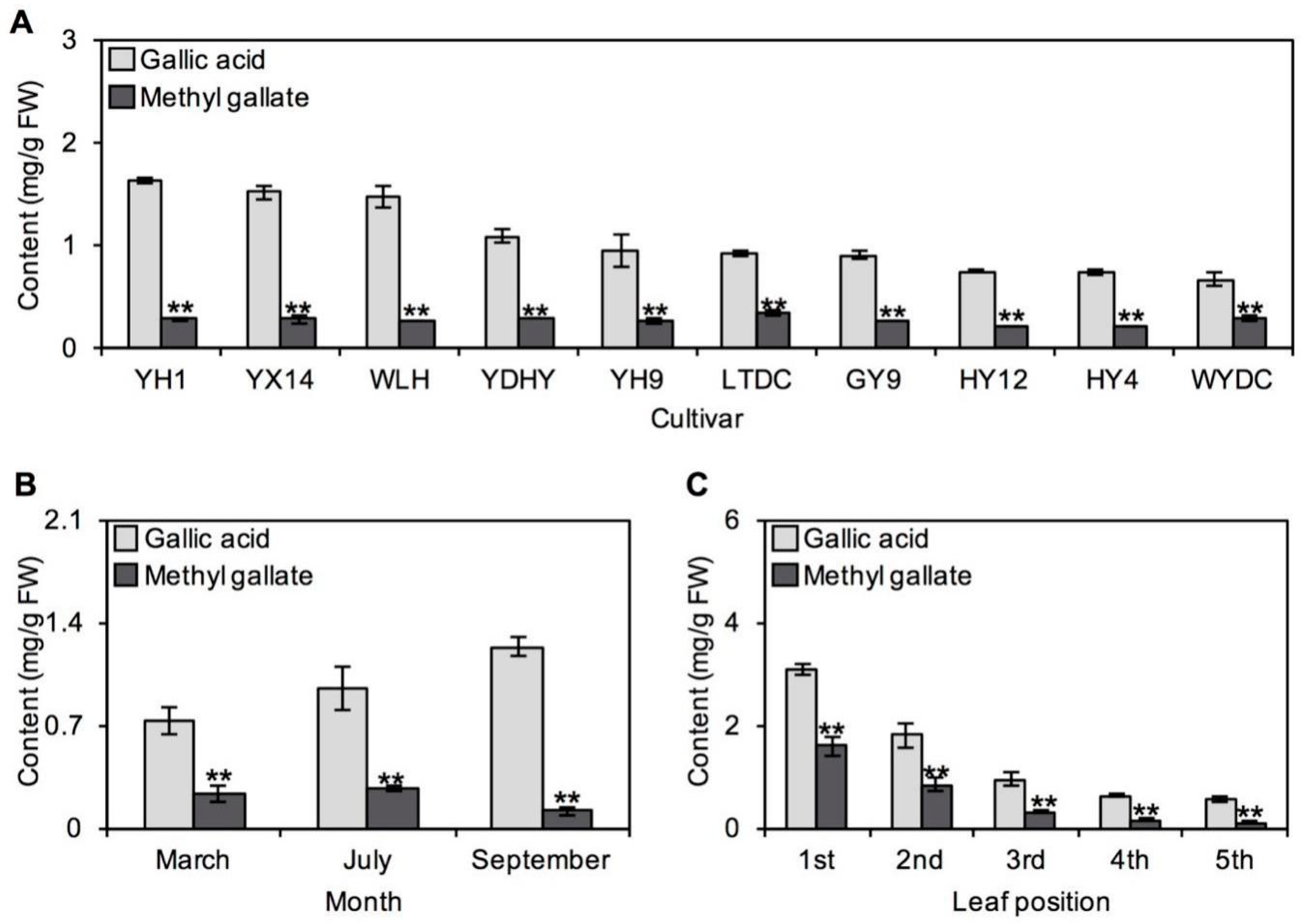
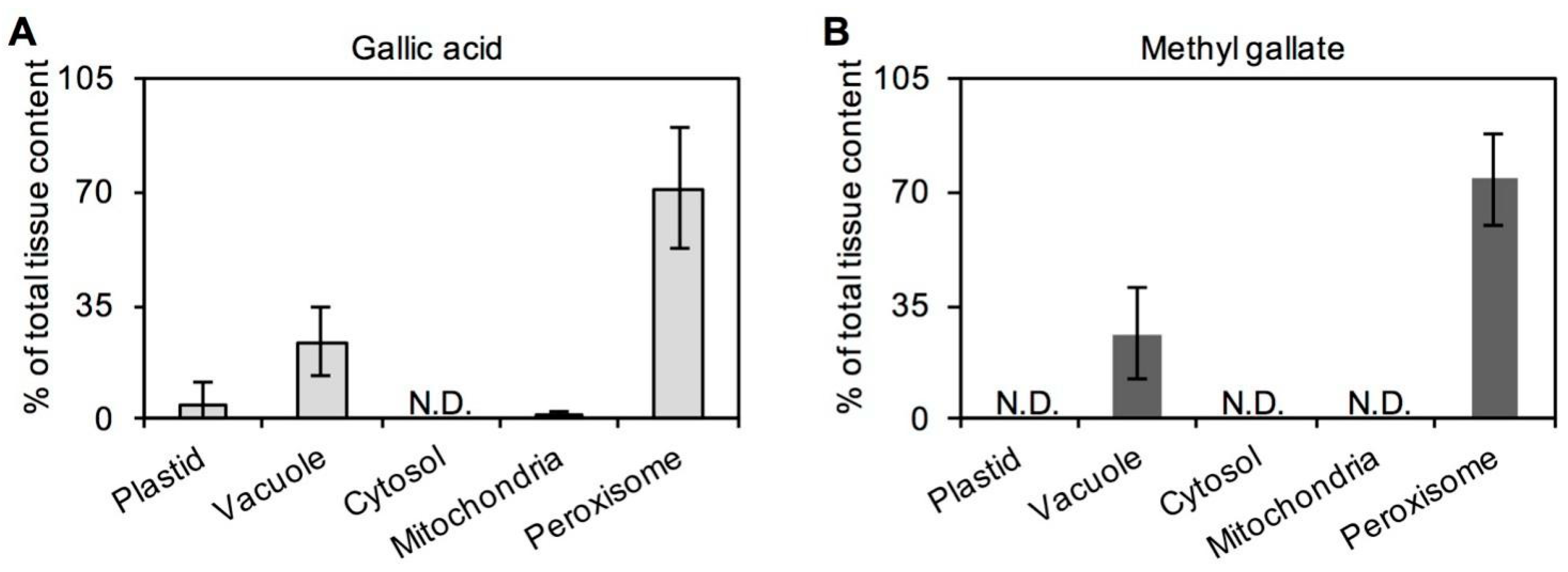
2.2. Occurrences of GA and MG in Tea Plants
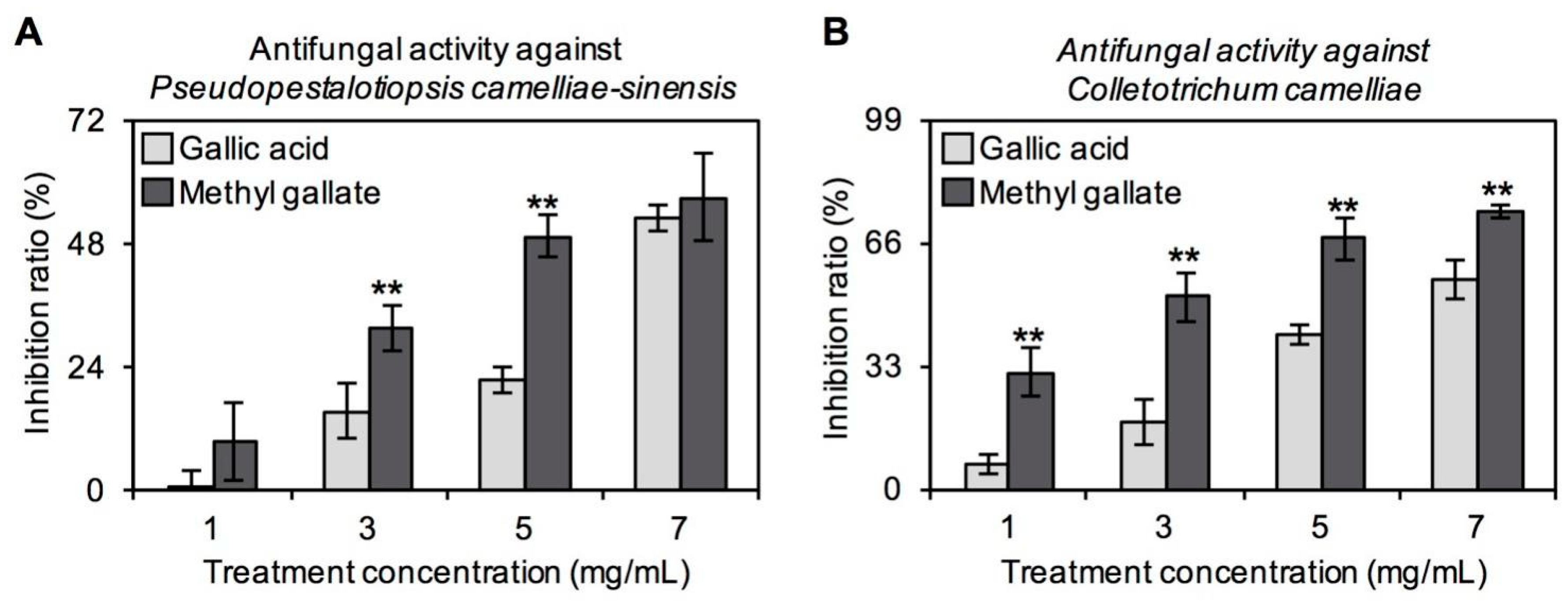
2.3. Antifungal Properties In Vitro of GA and MG against Main Tea Pathogens
3. Materials and Methods
3.1. Plant Materials
3.2. Feeding Experiment in Tea Leaves with [2H2]GA and Determination of [2H2]MG
3.3. Extraction and Analysis of GA and MG in Tea Leaves
3.4. Determination of Subcellular Distribution of GA and MG
3.5. In Vitro Antifungal Experiment
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FW | Fresh weight |
| GA | Gallic acid |
| MG | Methyl gallate |
| NAF | Nonaqueous fractionation |
| UPLC–QTOF–MS | Ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry |
| UHPLC | Ultra-high–performance liquid chromatography |
References
- Wan, X.C. Tea Biochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.C.; Xia, T. Secondary Metabolism of Tea Plant, 1st ed.; Science Press: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Huang, H.-L.; Lin, C.-C.; Jeng, K.-C.G.; Yao, P.-W.; Chuang, L.-T.; Kuo, S.-L.; Hou, C.-W. Fresh green tea and gallic acid ameliorate oxidative stress in kainic acid-induced status epilepticus. J. Agric. Food Chem. 2012, 60, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Liu, L.; Yang, Q.; Lu, Z.; Nie, Z.; Wang, Y.; Xia, T. Purification and characterization of a novel galloyltransferase involved in catechin calloylation in the tea plant (Camellia sinensis). J. Biol. Chem. 2012, 287, 44406–44417. [Google Scholar] [CrossRef]
- Chokkathukalam, A.; Kim, D.-H.; Barrett, M.P.; Breitling, R.; Creek, D.J. Stable isotope-labeling studies in metabolomics: New insights into structure and dynamics of metabolic networks. Bioanalysis 2014, 6, 511–524. [Google Scholar] [CrossRef]
- Boatright, J.; Negre, F.; Chen, X.; Kish, C.M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in vivo benzenoid metabolism in petunia petal tissue1. Plant Physiol. 2004, 135, 1993–2011. [Google Scholar] [CrossRef]
- Eisenreich, W.; Bacher, A.; Arigoni, D.; Rohdich, F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell. Mol. Life Sci. 2004, 61, 1401–1426. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, Y.; Gui, J.; Fu, X.; Mei, X.; Zhen, Y.; Ye, T.; Du, B.; Dong, F.; Watanabe, N.; et al. Formation of volatile tea constituent indole during the oolong tea manufacturing process. J. Agric. Food Chem. 2016, 64, 5011–5019. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.; Wang, X.; Liao, Y.; Zeng, L.; Dong, F.; Yang, Z. Studies on the biochemical formation pathway of the amino acid L-theanine in tea (Camellia sinensis) and other plants. J. Agric. Food Chem. 2017, 65, 7210–7216. [Google Scholar] [CrossRef]
- Deng, W.-W.; Wang, R.; Yang, T.; Jiang, L.; Zhang, Z. Functional characterization of salicylic acid carboxyl methyltransferase from Camellia sinensis, providing the aroma compound of methyl salicylate during the withering process of white tea. J. Agric. Food Chem. 2017, 65, 11036–11045. [Google Scholar] [CrossRef]
- Ji, H.-G.; Lee, Y.-R.; Lee, M.-S.; Hwang, K.H.; Kim, E.-H.; Park, J.S.; Hong, Y.-S.; Hwang, K.H. Metabolic phenotyping of various tea (Camellia sinensis L.) cultivars and understanding of their intrinsic metabolism. Food Chem. 2017, 233, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ma, J.-Q.; Chen, J.-D.; Ma, C.-L.; Chen, W.; Yao, M.-Z.; Chen, L. Gene coexpression networks reveal key drivers of flavonoid variation in eleven tea cultivars (Camellia sinensis). J. Agric. Food Chem. 2019, 67, 9967–9978. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Song, Q.; Li, D.; Wan, X. Discrimination of the production season of Chinese green tea by chemical analysis in combination with supervised pattern recognition. J. Agric. Food Chem. 2012, 60, 7064–7070. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; He, Y.; Yan, X.; Zhao, S.; Zhu, J.; Wei, C. Unraveling a crosstalk regulatory network of temporal aroma accumulation in tea plant (Camellia sinensis) leaves by integration of metabolomics and transcriptomics. Environ. Exp. Bot. 2018, 149, 81–94. [Google Scholar] [CrossRef]
- Dai, X.; Liu, Y.; Zhuang, J.; Yao, S.; Liu, L.; Jiang, X.; Zhou, K.; Wang, Y.; Xie, D.-Y.; Bennetzen, J.L.; et al. Discovery and characterization of tannase genes in plants: Roles in hydrolysis of tannins. New Phytol. 2020, 226, 1104–1116. [Google Scholar] [CrossRef]
- Gerhardt, R.; Heldt, H.W. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984, 75, 542–547. [Google Scholar] [CrossRef]
- Farré, E.M.; Tiessen, A.; Roessner, U.; Geigenberger, P.; Trethewey, R.N.; Willmitzer, L. Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol. 2001, 127, 685–700. [Google Scholar] [CrossRef]
- Krueger, S.; Steinhauser, D.; Lisec, J.; Giavalisco, P. Analysis of subcellular metabolite distributions within Arabidopsis thaliana leaf tissue: A primer for subcellular metabolomics. In Arabidopsis Protocols; Sanchez-Serrano, J.J., Salinas, J., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 575–596. [Google Scholar]
- Fu, X.; Liao, Y.; Cheng, S.; Xu, X.; Grierson, D.; Yang, Z. Nonaqueous fractionation and overexpression of fluorescent-tagged enzymes reveals the subcellular sites of L-theanine biosynthesis in tea. Plant Biotechnol. J. 2020, in press. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Liu, P.; Shi, Y.; Zhu, L.; Pu, L.; Yuanyuan, S.; Zhu, L.-W. Genetic variation in resistance to Valsa canker is related to arbutin and gallic acid content in Pyrus bretschneideri. Hortic. Plant J. 2018, 4, 233–238. [Google Scholar] [CrossRef]
- Chillet, M.; Minier, J.; Hoarau, M.; Meile, J.-C. Potential use of thymol to control anthracnose development in mango. Eur. J. Plant Pathol. 2019, 155, 943–952. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kang, O.-H.; Lee, Y.-S.; Oh, Y.-C.; Kwon, D.-Y. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J. Microbiol. Biotechnol. 2008, 18, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-G.; Kang, O.-H.; Lee, Y.-S.; Oh, Y.-C.; Chae, H.-S.; Jang, H.-J.; Shin, D.-W.; Kwon, D.-Y. Antibacterial activity of methyl gallate isolated from galla rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules 2009, 14, 1773–1780. [Google Scholar] [CrossRef]
- Kimura, Y.; Kouge, A.; Nakamura, K.; Koshino, H.; Uzawa, J.; Fujioka, S.; Kawano, T. Pesthetoxin, a new phytotoxin produced by the gray blight fungus, Pestalotiopsis theae. Biosci. Biotechnol. Biochem. 1998, 62, 1624–1626. [Google Scholar] [CrossRef][Green Version]
- Guo, M.; Pan, Y.M.; Dai, Y.L.; Gao, Z.M. First report of brown blight disease caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui Province, China. Plant Dis. 2014, 98, 284. [Google Scholar] [CrossRef]
- Chen, Z.M.; Sun, X.L. Concise Identity Handbook of Major Plant Diseases and Insect Pests of Tea, 1st ed.; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.; Wang, Y.; Liu, B.; Wang, M.; Zhang, M.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 2015, 35, 63–86. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, W.; Zeng, L.; Shen, D.; Liu, Z.; Wang, X.; Tong, H. Characterization, pathogenicity, and phylogenetic analyses of Colletotrichum species associated with brown blight disease on Camellia sinensis in China. Plant Dis. 2017, 101, 1022–1028. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Thirugnanasambantham, K.; Mandal, A. Suppressive subtractive hybridization approach revealed differential expression of hypersensitive response and reactive oxygen species production genes in tea (Camellia sinensis (L.) O. Kuntze) leaves during Pestalotiopsis thea infection. Appl. Biochem. Biotechnol. 2012, 168, 1917–1927. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Lu, Q.; Wang, L.; Qian, W.; Li, N.; Ding, C.; Wang, X.-C.; Yang, Y.-J. Transcriptional analysis and histochemistry reveal that hypersensitive cell death and H2O2 have crucial roles in the resistance of tea plant (Camellia sinensis (L.) O. Kuntze) to anthracnose. Hortic. Res. 2018, 5, 18. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Tongchusak, S.; Boonhok, R.; Chaiyaroj, S.C.; Junyaprasert, V.B.; Buajeeb, W.; Akanimanee, J.; Raksasuk, T.; Suddhasthira, T.; Satayavivad, J. In vitro antifungal activities of longan (Dimocarpus longan Lour.) seed extract. Fitoterapia 2012, 83, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dang, W.; Xie, J.; Zhu, R.; Sun, M.; Jia, F.; Zhao, Y.; An, X.; Qiu, S.; Li, X.; et al. Antimicrobial peptide protonectin disturbs the membrane integrity and induces ROS production in yeast cells. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Heredia, N.L.; Camacho-Corona, M.D.R.; Garcia-Alvarado, J.S.; Del Rayo, C.-C.M. Isolation, characterization and mode of antimicrobial action against Vibrio cholera of methyl gallate isolated from Acacia farnesiana. J. Appl. Microbiol. 2013, 115, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Farhoosh, R.; Sharif, A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014, 159, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Lilley, R.M.; Gerhardt, R.; Heldt, H.W. Metabolite levels in specific cells and subcellular compartments of plant leaves. In Methods in Enzymology; Academic Press: New York, NY, USA, 1989; Volume 174, pp. 518–552. [Google Scholar]
- Chen, Y.; Zeng, L.; Shu, N.; Jiang, M.; Wang, H.; Huang, Y.; Tong, H.; Huang, Y. Pestalotiopsis-like species causing gray blight disease on Camellia sinensis in China. Plant Dis. 2018, 102, 98–106. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zeng, L.; Chen, Y.; Wang, X.; Liao, Y.; Xiao, Y.; Fu, X.; Yang, Z. Metabolism of Gallic Acid and Its Distributions in Tea (Camellia sinensis) Plants at the Tissue and Subcellular Levels. Int. J. Mol. Sci. 2020, 21, 5684. https://doi.org/10.3390/ijms21165684
Zhou X, Zeng L, Chen Y, Wang X, Liao Y, Xiao Y, Fu X, Yang Z. Metabolism of Gallic Acid and Its Distributions in Tea (Camellia sinensis) Plants at the Tissue and Subcellular Levels. International Journal of Molecular Sciences. 2020; 21(16):5684. https://doi.org/10.3390/ijms21165684
Chicago/Turabian StyleZhou, Xiaochen, Lanting Zeng, Yingjuan Chen, Xuewen Wang, Yinyin Liao, Yangyang Xiao, Xiumin Fu, and Ziyin Yang. 2020. "Metabolism of Gallic Acid and Its Distributions in Tea (Camellia sinensis) Plants at the Tissue and Subcellular Levels" International Journal of Molecular Sciences 21, no. 16: 5684. https://doi.org/10.3390/ijms21165684
APA StyleZhou, X., Zeng, L., Chen, Y., Wang, X., Liao, Y., Xiao, Y., Fu, X., & Yang, Z. (2020). Metabolism of Gallic Acid and Its Distributions in Tea (Camellia sinensis) Plants at the Tissue and Subcellular Levels. International Journal of Molecular Sciences, 21(16), 5684. https://doi.org/10.3390/ijms21165684