Abstract
Ever since the 1970s, when profound immunosuppression caused by exogenous dioxin-like compounds was first observed, the involvement of the aryl hydrocarbon receptor (AHR) in immunomodulation has been the focus of considerable research interest. Today it is established that activation of this receptor by its high-affinity endogenous ligand, 6-formylindolo[3,2-b]carbazole (FICZ), plays important physiological roles in maintaining epithelial barriers. In the gut lumen, the small amounts of FICZ that are produced from L-tryptophan by microbes are normally degraded rapidly by the inducible cytochrome P4501A1 (CYP1A1) enzyme. This review describes how when the metabolic clearance of FICZ is attenuated by inhibition of CYP1A1, this compound passes through the intestinal epithelium to immune cells in the lamina propria. FICZ, the level of which is thus modulated by this autoregulatory loop involving FICZ itself, the AHR and CYP1A1, plays a central role in maintaining gut homeostasis by potently up-regulating the expression of interleukin 22 (IL-22) by group 3 innate lymphoid cells (ILC3s). IL-22 stimulates various epithelial cells to produce antimicrobial peptides and mucus, thereby both strengthening the epithelial barrier against pathogenic microbes and promoting colonization by beneficial bacteria. Dietary phytochemicals stimulate this process by inhibiting CYP1A1 and causing changes in the composition of the intestinal microbiota. The activity of CYP1A1 can be increased by other microbial products, including the short-chain fatty acids, thereby accelerating clearance of FICZ. In particular, butyrate enhances both the level of the AHR and CYP1A1 activity by stimulating histone acetylation, a process involved in the daily cycle of the FICZ/AHR/CYP1A1 feedback loop. It is now of key interest to examine the potential involvement of FICZ, a major physiological activator of the AHR, in inflammatory disorders and autoimmunity.
1. Introduction
Although the cytochrome P450 (CYP) family of enzymes was initially thought to catalyze the metabolism of xenobiotics and thereby be involved in chemical carcinogenesis, it has since become clear that many of these enzymes also play physiological roles in the metabolism of a variety of endogenous compounds [1]. One of these endogenous compounds is 6-formylindolo[3,2-b]carbazole (FICZ), which binds to the aryl hydrocarbon receptor with the highest affinity yet reported. In stark contrast to the anthropogenic 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and other well-characterized ligands for the AHR, FICZ is also an excellent substrate for CYP1A1, CYP1A2, and CYP1B1, all encoded by genes regulated by the AHR [1]. Accordingly, FICZ participates in an autoregulatory feedback loop, which maintains its own steady-state concentration, like that of many hormones, at a low level (reviewed in [2]).
FICZ was discovered serendipitously in connection with experiments designed to produce dimerized photoproducts of planar biomolecules for testing as ligands for AHR. Adenine was subjected to ultraviolet radiation in the presence of tryptophan (Trp), a photo-sensitizing molecule, and it turned out that similar irradiation of solutions containing Trp alone produced some compound(s) that could compete efficiently with TCDD for binding to the AHR. Two products with molecular weights of 284 and 312 Daltons were identified and immediately assumed to be endogenous signal substances, since they bind to this receptor with higher affinity than any other known compound, including TCDD [3,4].
Subsequently, FICZ was shown to be formed upon exposure of Trp to H2O2 alone, as well as via several enzyme-catalyzed pathways [5]. To date, FICZ has been detected by liquid chromatography-mass spectrometry in aged batches of Trp [3], cell culture media [6,7], cultured non-hematopoietic cells [8], hematopoietic cells [9], yeast cells [10], and extracts of human skin [10,11], as well as in mouse colorectal tissue [12]. In addition, sulfoconjugates of phenolic metabolites of FICZ are present in human urine [13]. Thus, ubiquitous formation/presence of FICZ, albeit at low levels, in most tissues under normal conditions is highly probable. Still, establishing FICZ as an important endogenous AHR agonist has been controversial since many studies have described the AHR to be highly promiscuous and suggested AHR ligand binding by a myriad of both endogenous and exogenous molecules.
Although the AHR is not essential for survival, this receptor is involved in several physiological processes, including regulation of homeostasis and immunity at epithelial barriers such as the one formed by intestinal epithelial cells (IECs) (reviewed by [14]). Since the most potent immune responses in the body occur in the gut, considerable focus is now being placed on elucidating the molecular mechanism(s) underlying the role of the AHR in cells in the intestinal mucosa—including the IECs and various immune cells, such as B cells, T cell receptor γδ T cells (TCRγδ), T helper 17 cells (Th17), regulatory T cells (Treg), type 1 regulatory T cells (Tr1), innate lymphoid cells (ILC), macrophages (MQ), intraepithelial lymphocytes (IEL), dendritic cells (DC), and neutrophils (reviewed by [15]).
CYP1A1 plays an essential role in the intestinal immune system, controlling related steady-state processes, as well as responses to pathogenic insults. In the present review, I discuss new perspectives on the role of FICZ produced by the microbiota in gut immunity, with particular focus on the key role played by CYP1A1 in the dynamic regulation of FICZ-stimulated production of interleukin 22 (IL-22) and the temporal pattern of AHR signaling in the gut.
2. Activators of the AHR Promote Intestinal Immune Responses
The enormous numbers and huge variety of bacteria in the intestine, as well as the pronounced diurnal oscillations in both the composition and function of the gut microbiome, require an efficient barrier for protection of the host. Normally, abundant secretion of mucus and a vigorous, but closely regulated immune system maintain this barrier, but perturbations of gut microbiota (termed dysbiosis) are associated with several pathological states, including inflammatory bowel disease (IBD), metabolic syndrome, and colorectal cancer (CRC) (reviewed in [16]).
Recently, several important investigations have established that the AHR is required both for regulation of the homeostasis of the intestinal epithelial and associated immune cells, as well as for mounting appropriate responses to epithelial damage and invading pathogens [17,18,19,20,21,22]. In addition, this receptor appears to be involved in peristaltic and secretory reflexes [23,24].
In mice raised in a conventional manner, CYP1A1 is expressed by epithelial cells in the duodenum, jejunum and ileum [25], with the most pronounced up-regulation by TCDD occurring in the proximal parts of the small intestine (SI) [26]. Mice that are germ-free (GF) or have been treated with an antibiotic and are thus exposed to lower levels of factors produced by the microbiota, express the AHR, AHRR, and CYP1A1 genes at lower levels in their SI [25,27,28,29]. The lack of functional AHR signaling in such animals may explain why they are more susceptible to colitis induced experimentally, e.g., by trinitrobenzene sulfonic acid (TNBS) or dextran sulfate sodium (DSS) [30,31]. Such findings highlight the involvement of commensal microbes in the intestinal immune system through their production of factors that activate the AHR.
2.1. Dietary Activators of the AHR
Even before Alan Poland described the AHR in 1976 [32], Lee Wattenberg and colleagues had reported an elevated level of CYP1A1 activity (at that time measured as benzo[a]pyrene (BaP) metabolism or aryl hydrocarbon hydroxylase (AHH) activity) in the liver and gastrointestinal tract (GI) of rodents fed standard chow [33]. They discovered subsequently that feeding rats and mice cereal-based chow and synthetic semi-purified diets fortified with phytochemicals isolated from the Brassicaceae family of plants, alfalfa or spinach resulted in a high basal level of highly inducible CYP1A1 activity in their gut, from the gastrointestinal epithelium of the SI to the colon (reviewed by [34]). This basal activity was most pronounced in the proximal region of the SI.
These findings, in combination with the early interest in phytochemicals as potential anti-carcinogens, motivated a number of investigations designed to test whether cruciferous vegetables induce AHH activity. For example, indole-3-carbinol (I3C), a hydrolysis product of glucobrassicin produced by myrosinase, was found to increase CYP1A1 activity in the liver and intestine of rodents [35,36]. I3C itself binds to the AHR with low affinity, but under acidic conditions can give rise to indolo[3,2-b]carbazole (ICZ), which has high affinity [37].
Investigations designed to unravel the mechanisms by which dietary phytochemicals induce CYP1A1 in the gut and also influence gut immunity and protect against inflammatory disorders within the GI often involve comparisons between conventional chow based on grain and purified diets (AIN-76, AIN-93, or similar) supplemented with I3C. It is now known that a wide range of phytochemicals—including flavonoids, alkaloids, stilbenes and curcuminoids—bind to this receptor with no or low affinity but still activate CYP1A1, and we have proposed that compounds that inhibit the expression and/or, activity of CYP1A1 may attenuate clearance of endogenous FICZ, leading to indirect activation of the AHR [38]. This may explain why this receptor in the intestine, systemically, is activated by many dietary phytochemicals that are potent inhibitors of CYP1A1 activity [39,40].
2.2. Microbial Activators of the AHR
The symbiosis between resident microbiota and the host is beneficial to both in many ways. Under the anaerobic conditions in the gut, the microbiota modify many small molecules present in the diet and these then enter the host circulation and influence immunity and other distal functions in a manner that contributes to the well-being of the host. For instance, gut bacteria give rise to essential vitamins (e.g., A, group B and K), as well as serotonin (5-HT), short-chain fatty acids (SCFAs), and some secondary bile acids [41,42].
These microbiota also metabolize the essential amino acid Trp, present at high levels in protein-rich food, into a multitude of different indoles. Examples include indole itself, indole-3-acetaldehyde (IAAl), indole-3-acetic acid (IAA), indole-3-aldehyde (IAl), indole-3-lactic acid (IAL), indole-3-acrylic acid (IA), indole-3-propionic acid (IPA), indole-3-pyruvate (I3P), skatole, and tryptamine (Tra). Many of these activate AHR signaling (reviewed by [42,43,44,45]).
It is now evident that indole metabolites of Trp formed in the mammalian intestine play important roles in gut health. In particular, Trp metabolism by Lactobacillus species elicits an anti-microbial immune response including production of IL-22, which is vital for maintenance of mucosal barrier integrity within the SI. Numerous studies with GF rodents or animals treated with antibiotics have demonstrated that reduced microbial catabolism of dietary Trp results in higher concentrations of this amino acid in the serum and or feces and reduced levels of AHR-activating compounds [20,28,46,47,48].
Impaired Trp transport systems also have negative consequences for the health of the gut. For instance, mice lacking the angiotensin-converting enzyme 2 (Ace2) take up Trp from the diet poorly and also display an altered intestinal microbiome and enhanced susceptibility to the development of colitis [49]. Similarly, the intestinal microbiome in mice that lack the caspase recruitment domain family member 9 (CARD9) is altered and these animals fail to metabolize Trp into activators of the AHR, exhibit defective expression of IL-22 and antimicrobial peptides (AMPs) such as RegIIIβ and RegIIIγ in the colon, and are more susceptible to colitis [19]. Furthermore, defects in the LAT1-CD98 complex, which transports aromatic amino acids, in immune cells limit the intracellular level of FICZ due to lower access to Trp [9]. In contrast, the elevated levels of Trp resulting from the reduced metabolism of this amino acid in knock-out mice that do not express indoleamine 2,3-dioxygenase 1 (IDO1) enhances formation of microbial indoles that activate the AHR [20,48].
A recent update identified I3P as one of the microbial catabolites of Trp that activate the AHR most potently [50]. In a competitive binding assay, I3P displaced TCDD from the mouse AHR with an IC50 of 55 µM, i.e., more efficiently than IAl (IC50 > 1 mM), but much less efficiently than FICZ (IC50 2nM), which was included in this experiment as a positive control, but not considered to be a potential microbial catabolite. However, in another recent study, the levels of FICZ in colorectal mouse tissue were quantified and the results support the notion that this ligand is the most potent AHR-activating Trp catabolite in the intestine [12]. These highly preliminary data require confirmation in further studies.
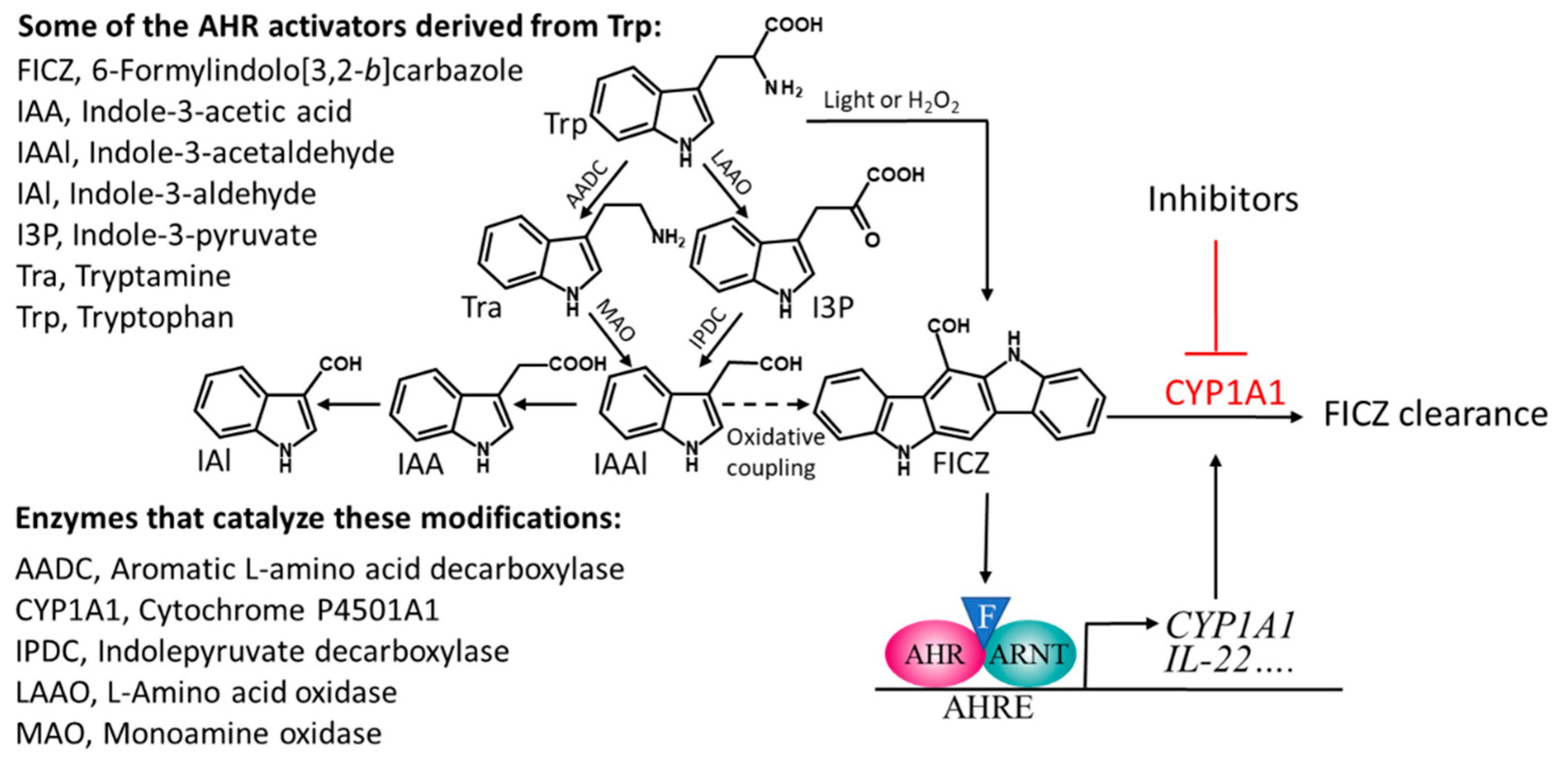
The species of commensal bacteria that have so far been shown to produce indoles that activate the AHR include certain strains of Lactobacillus, Allobaculum, Peptostreptococcus, and Propionibacterium [20,48,51,52]. However, many other such species are likely to be identified, since the common bacterial inhabitants of the gut, belonging to several different phyla, convert Trp into I3P, Tra, or IAAl [42,44,53], all of which are precursors of FICZ and other AHR-activating indoles as well (Figure 1).
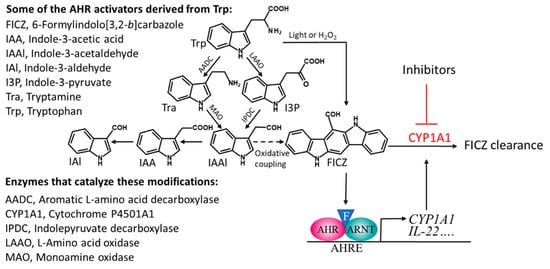
Figure 1.
A scheme describing the microbiota-mediated catabolism of tryptophan that leads to several compounds that can activate the aryl hydrocarbon receptor (AHR), including the high affinity ligand FICZ (illustrated by an F in a blue triangle). Stimulation with FICZ causes AHR to partner with the nuclear translocator of AHR (ARNT), bind to AHR response elements (AHREs), and stimulate expression of CYP1A1, which takes part in the metabolic clearance of FICZ. The oxidative coupling that generates FICZ from IAAl has been described in [5].
Another group of AHR activators produced by the gut microbiota encompass the SCFAs acetate, propionate and butyrate (BUT), of which the role of BUT in maintaining intestinal immune homeostasis is most well documented. BUT is derived from microbial fermentation of indigestible polysaccharides, in particular dietary fibers and resistant starch, which escape digestion by enzymes in the upper gut and are consequently present at relatively high levels (mM) in the lumen of the lower gut (reviewed in [54]). In addition to providing an important source of energy for colonocytes, BUT inhibits inflammation of the intestine and promotes the development and function of Tregs in a beneficial manner. Many of the anti-inflammatory properties of BUT reflect its inhibition of histone deacetylases (HDACs) and simultaneous activation of certain G-protein-coupled receptors on colonic epithelial cells, in particular GPR109A, which is expressed at very high levels by innate immune cells and on the epithelium [55,56,57,58].
Reports published as early as 1996 and 1999 demonstrated that both BUT and trichostatin A, another inhibitor of HDAC, successfully restore expression of the AHR in hepatoma cells deficient in induction of CYP1A1 mRNA, as well as de-repress CYP1A1 expression in fibroblasts non-responsive to ligands of the AHR [59,60]. Several subsequent investigations established that BUT alters expression of the CYP1A1, AHR, and AHRR genes and induces CYP1A1 activity both in different types of cells in vitro [61,62,63,64] and in experimental animals [28,65] by inhibiting HDAC activity. However, although Marinelli and colleagues could reproduce the HDAC-dependent activation of CYP1A1 by trichostatin A, they did not obtain the same effect with BUT, but instead proposed that this compound induced the transcription of AHR-dependent genes as an AHR ligand [66]. Moreover, two other reports documented an increase in the expression of CYP1A1 in cells exposed to BUT alone [62,63]. These latter findings could reflect the presence of FICZ or some other activator of the AHR in the cell culture medium, since, indeed, FICZ has been detected in cell culture media exposed to light [6].
3. The Mechanism(s) by Which FICZ, IL-22 and Butyrate Promote Gut Homeostasis
Although dietary and microbial indoles that activate the AHR and support colonization by commensal bacteria are necessary for intestinal health, a balance between tolerance to such beneficial bacteria and immunological responses to potential pathogenic species must be maintained [67,68]. In this context, accumulating evidence indicates that the intestinal epithelium requires a constant supply of moderate levels of IL-22, IL-17, and a granulocyte macrophage-colony stimulating factor (GM-CSF) to protect against undesirable microbial invasion [69].
In adult mammals, the group 3 ILCs (ILC3s) are involved in protecting against microbial pathogens, as well as in regulating the integrity of the intestinal barrier and the relative abundance of the populations of various commensal bacteria. At the same time, through their expression of the transcription factor RORγt and cytotoxicity receptor NKp46 (NKp46+RORγt+ ILC3s), these cells are considered to be the most important source of IL-22 in the SI lamina propria [69,70,71,72], where they respond to cues provided by the diet and the commensal microbiota, as well as to their own intrinsic circadian rhythm to produce IL-22 [73,74]. In this connection, a key observation was that the AHR is required for postnatal expansion of such intestinal ILCs [19,75,76]. Indeed, Qiu and colleagues have proposed that in mouse pups after weaning, the AHR responds to bacteria in the gut in a manner that leads to the development of RORγt+ ILCs [19].
Furthermore, it is well documented that under steady-state conditions, phytochemicals present in conventional rodent chow promote and sustain IL-22-producing RORγt+ ILCs [19,75,76,77]. Accordingly, the microbial flora, the AHR, and ligands of this receptor that stimulate IL-22 production by intestinal ILC3s are important innate effectors of intestinal health.
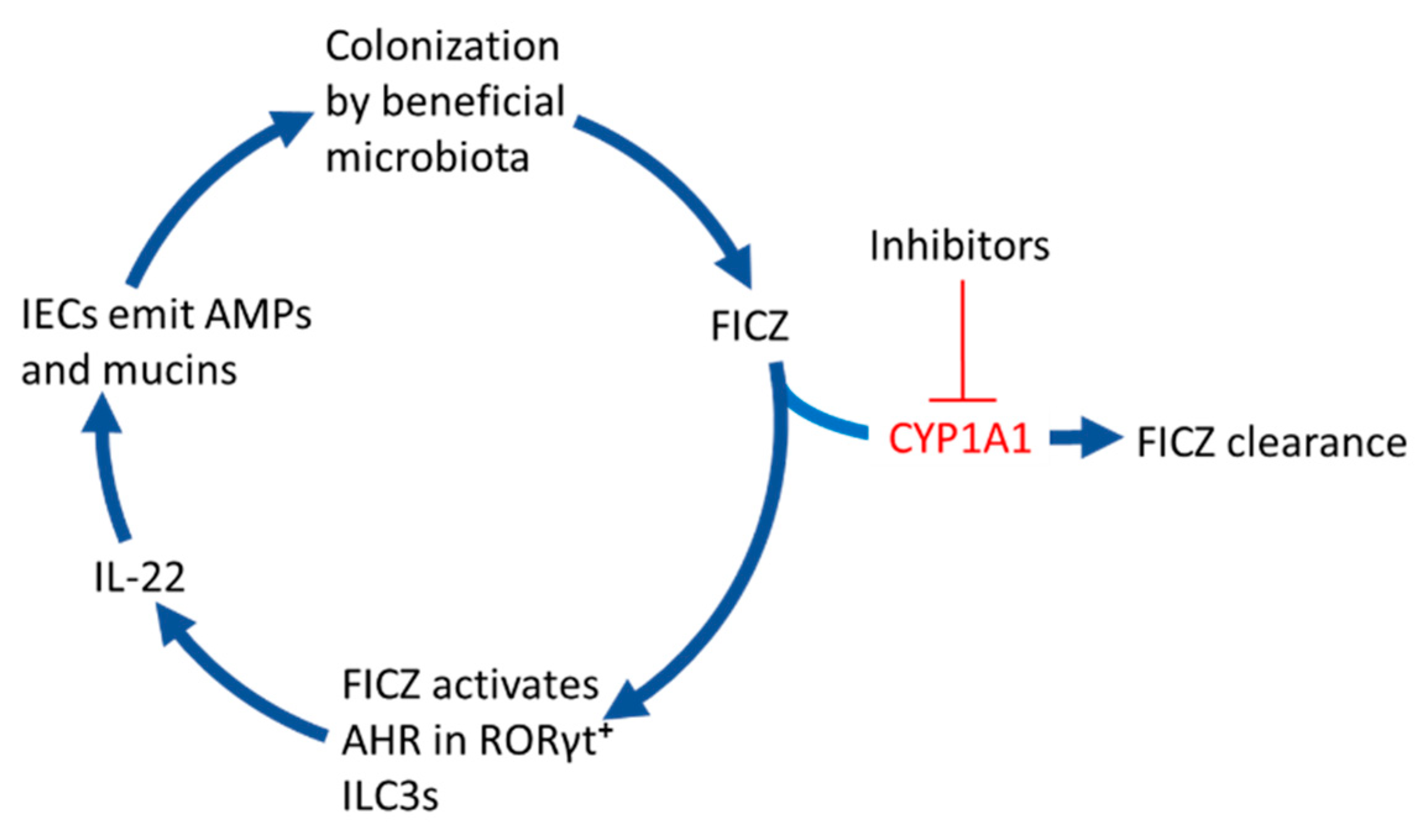
Figure 2 and the sections below describe how the FICZ that is not broken down in the IECs can support colonization of the gut by beneficial strains of bacteria.
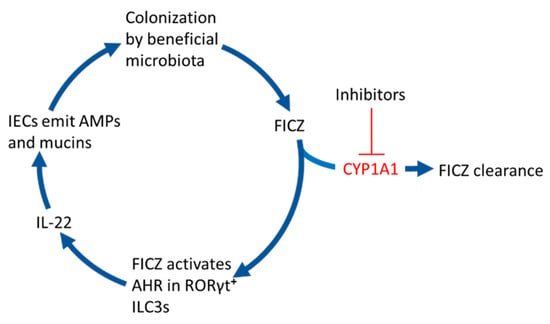
Figure 2.
6-formylindolo[3,2-b]carbazole (FICZ) stimulates gut colonization by beneficial microbiota. When the CYP1A1-mediated clearance of microbially produced FICZ in the intestinal epithelium is inhibited by for example dietary phytochemicals, drugs, or inflammatory mediators, this compound will bind to the aryl hydrocarbon receptor (AHR) in RORγt positive group 3 innate lymphoid cells (ILC3s). This stimulates them to secrete the interleukin IL-22 that signals to intestinal epithelial cells (IECs) to emit antimicrobial peptides (AMPs) and mucins, which promote colonization by commensal bacteria.
3.1. Repressors of CYP1A1 Prevent the Clearance of FICZ
The importance of a functional AHR-dependent pathway in the intestinal epithelium for induction of CYP1A1 expression by a factor originating in the gut was first demonstrated by the findings by Ito and colleagues in 2007 [78]. In mice (fed standard chow), where the ARNT gene was disrupted, predominantly in IECs, the levels of CYP1A1 mRNA and corresponding enzymatic activities were markedly elevated in almost all other tissues. In another study performed with mice deficient in CYP1A1/1A2/1B1, endogenous AHR ligands that escape breakdown in the epithelial cell lining activate this receptor, as demonstrated with a Cyp1a1 fate-reporter [21].
In addition to such genetic silencing, several types of agents that could prevent the CYP1A1-mediated breakdown of FICZ have been described. CYP1A1 activity can be inhibited by external factors consumed orally or formed by microbiota, including dietary phytochemicals such as α-naphthoflavone, β-naphthoflavone, galangin, chrysin, kaempferol, apigenin, baicalein, and quercetin [40], as well as I3C and its acid-condensation products 3,3-diindolylmethane (DIM) and ICZ [21]. In fact, FICZ can slow down its own metabolic degradation by inhibiting CYP1A1 activity [79]. Other inhibitors of CYP1A1 activity include a large number of anti-inflammatory, anti-depressant, anti-parasitic, anti-psoriatic, beta-blocking, and cytostatic drugs, and inhibitors of proton pumps [38,39] and several ubiquitous carcinogenic polycyclic aromatic hydrocarbons (PAHs), exemplified by BaP and 3-methylcholanthrene (with IC50 values in the nM range) [80]. In the studies referred to above, the ability to inhibit 7-ethoxyresorufin O-deethylation activity was used as a measure of CYP1A1 inhibition, and the inhibitors were mostly substrates that competed with 7-ethoxyresorufin for binding to the enzyme. Thus, the inhibitors of CYP1A1 that has been shown to attenuate metabolic clearance of FICZ include a wide range of phytochemicals [81], environmental pollutants [82], metals, and oxidants [38,83,84].
Moreover, signals released in connection with microbial-associated molecular patterns (MAMPs) or pathogen-associated microbial patterns (PAMPs) can interact with pattern recognition receptors (PRRs) on the surface of IECs, DCs and MQs to trigger downstream signaling cascades. This promotes the production of mediators of inflammation or infection, such as IL-1β, IL-6, TNFα, and IFNs, which also leads to inhibition of CYP1A1 expression and/or activity (reviewed in [85]).
Theoretically, several molecular mechanisms can be involved in the downregulation of the expression and/or activity of CYP1A1, e.g., competitive or mixed inhibition of the enzyme, alterations caused by reactive oxygen species, or chromatin remodeling in the promoter region of this gene. Regardless of the mechanism involved, lower CYP1A1 activity permits higher concentrations of FICZ to reach innate lymphoid cells that express IL-22.
3.2. FICZ Induces Expression of IL-22 by ILC3s
IL-22 binds to the heterodimeric receptor IL-22Rα1/IL-10Rβ on IECs and induces a downstream signaling cascade that leads ultimately to phosphorylation of the transcription factor STAT3. This signaling supports the epithelial barrier against bacterial infections by stimulating secretion of AMPs, e.g., RegIIIβ, RegIIIγ, and members of the S100 family of proteins by enterocytes and Paneth cells in the SI and enterocytes in the colon of mice [86,87]. Paneth cells sense commensal bacteria in the gut and aid in broad regulation of both commensal and pathogenic bacteria that maintain intestinal homeostasis [88,89]. Furthermore, IL-22 controls tissue regeneration and repair through direct action on epithelial stem cells [90], as well as inducing epithelial goblet cells to secrete more components of mucus to form a thick gel-like layer impenetrable to many commensal bacteria, thereby limiting their potential to cause inflammation [91].
As mentioned above, RORγt+ ILCs appear to be the principal source of IL-22 under steady-state conditions and their constitutive expression of this cytokine is apparently unaffected by the proinflammatory IL-23 cytokine, which is known to activate IL-22 production as part of the response to pathogens [92]. The strict dependence of the ILC3s and their secretion of IL-22 can be explained by the presence of AHR-responsive elements in the promoter region and intron 1 of the IL-22 gene [19]. Moreover, recruitment of the transcription factor RORγt to the IL-22 promoter is facilitated by the AHR [19,93]. Intriguingly, circadian regulation of the numbers of these cells and their expression of circadian clock genes, AHR and IL-22 was recently documented [73].
The fact that exposure of T cells to FICZ, under conditions that induce Th17-cells, potently up-regulates their level of IL-22 mRNA, was reported in 2008 [94,95]. Subsequently, expression of this same mRNA by differentiated mouse Th17 cells was found to be elevated in the presence of as little as 10 pM FICZ, and this induction was enhanced considerably by co-exposure to fluoranthene, pyrene, and phenanthrene, environmental PAHs that inhibit CYP1A1 [21,82].
It is now clear that FICZ stimulates the expression of IL-22 by a variety of different immune cells, including ILC3s, both in vitro [21,82,96,97,98], including human intestinal lamina propria mononuclear cells from IBD patients [18], and in experimental animals [18,20,29,98,99]. In this context, Monteleone and colleagues (2011) found that a remarkably low amount of FICZ ameliorates colitis induced experimentally in mice [18]. A single intraperitoneal administration of 1 µg per mouse (50 µg/kg) lowered the mortality, enhanced the level of IL-22 in colonic samples, and ameliorated colitis induced by TNBS or DSS. As proof-of-concept, they also demonstrated that a neutralizing antibody against IL-22 largely prevented these anti-inflammatory effects of FICZ.
Similar results were obtained in another study [20], and in their comparable investigation, Zelante and coworkers demonstrated that administration of IAl (18 mg/kg daily for 12 days) to mice ameliorated DSS-induced colitis [48]. In fact, there are nine more reports that FICZ protects against bacterial infections and colitis caused by T-cell transfer, DSS or TNBS [29,99,100,101,102,103,104,105,106], with only one study observing no protection against TNBS-induced colitis [12]. When Qiu and colleagues injected mice intraperitoneally with 0.5 µg FICZ for 6 days, IL-22-producing ILCs accumulated both in the large intestine (LI) and, especially, in the SI [19].
3.3. IL-22 Promotes Colonization by Commensal Bacteria
Normally, more than 1 × 1013 bacteria, predominantly of the Firmicutes and Bacteroidetes phyla, symbiotically colonize the mammalian intestine. Microbial density is lowest in the SI and the microbiota demonstrate diurnal rhythmicity with respect to both their localization and production of metabolites that depend on light exposure, the time of food consumption and the type of food [16,107,108,109].
IL-22 has been shown to be needed to promote the colonization of the GI by beneficial bacteria and gut homeostasis both in studies with mice lacking this cytokine and mice treated with antibodies against it [110]. Zenewich and co-workers found that healthy IL-22 KO mice have an altered colonic microbiome containing lower relative abundances of certain families of bacteria, including Lactobacillaceae, Bacteroidaceae, Clostridiaceae, and Peptococcaceae, and more of others. In addition, when colitis was induced experimentally into these animals, they developed more severe disease. Moreover, when their altered gut microbiota were transferred to wild-type (WT) mice in the same cage, these wild-type animals also exhibited enhanced susceptibility to experimental colitis [110].
Notably, bacteria that metabolize Trp are more abundant in the SI, where constitutive expression of IL-22 helps to shape and constrain the commensal community [20,111]. It has been proposed that colonization by bacteria that metabolize Trp and/or promote health in other ways is regulated by the availability of mucins, as well as by antimicrobial proteins that may increase the proportion of Lactobacillus [20,48,110].
In comparison to the diverse microbiota of mice reared on conventional grain-based chow, the immune phenotype of the microbiome of mice fed purified, phytochemical-free diets (often termed AHR ligand-free diets) is changed in a manner similar to that seen in AHR-deficient animals [28,112,113]. Like the AHR-deficient mice, animals fed purified, phytochemical-free diets exhibit enhanced susceptibility to severe colitis [113,114,115]. Furthermore, there are numerous reports that phytochemicals—including berberine [116], curcumin [117], galangin [118], resveratrol [119], and rutin [120]—can prevent microbial dysbiosis and stimulate colonization of the gut by beneficial anaerobic bacteria (reviewed by [121,122]). 13C, which is commonly used to activate the AHR and stimulates IL-22 expression, can also promote colonization by beneficial bacteria, both when administered in the diet [113] and injected intraperitoneally [123]. Accordingly, Schanz found that purified diets exert a profound negative impact on the composition of the microbiome of the murine SI and colon, lowering counts of Gram-positive bacteria belonging to the Firmicutes phylum, such as Clostridium butyricum, Faecalibacterium prausnitzii, Roseburia, and Lactobacillus species [113]. Importantly, this deleterious change could be reversed by addition of I3C to the diet. Interestingly, the beneficial effects of 13C even after intraperitoneal administration [123] exclude the possibility that DIM and ICZ, acid condensation products of this compound that are formed in the stomach [37], were the protective compounds.
Furthermore, BUT concentrations were higher in mice fed conventional chow diets than in mice fed purified diets [124], and butyrate-producing Roseburia spp. were more common in mice fed purified diets and administered I3C intraperitoneally [123], which illustrates the important role of phytochemicals in supporting the growth of BUT-producing bacteria. The lower portion of the SI (ileum) and colon, where microbial density is highest, contain large numbers of BUT-producing bacteria belonging to the Firmicutes phyla (e.g., the Bacteroides fragilis and Clostridium clusters IV and XIVa strains) [55,125]. Commensal bacteria that produce BUT and utilize mucins as an energy source are capable of penetrating the inner mucus layer and stimulating the underlying IECs to produce mucin peptides, as well as of supporting the production of different SCFAs. For example, acetate that contributes to the production of BUT must sometimes be supplied through co-colonization by primary fiber degraders that initiate the utilization of complex fibers [54].
The observations described above indicate that conventional chow and diets enriched in phytochemicals influence the composition of the intestinal microbiota in a manner that favors the production of both FICZ and BUT. As expected, this production of beneficial microbial metabolites is reduced in germ-free animals [20,28,46,47,48].
3.4. BUT Fine-Tunes IL-22 Signaling
BUT helps maintain immunological homeostasis in the gut by inducing the differentiation of IL-10-producing Treg cells [55] and Tr1 cells [126], in addition to its indispensable counteraction of the pro-inflammatory responses associated with the signaling pathways involving activated nuclear factor-kB (NF-kB) and IL-6/STAT3/IL-17 [127,128,129]. IL-10-producing Tregs play a particularly important role in limiting inflammatory responses and there are relatively high numbers of these cells in the lamina propria (LP) of the ileum and the colon, where the density of BUT-producing bacteria is also higher than in the SI [108]. BUT induces the differentiation and expansion of Tregs by inhibiting HDACs, which results in acetylation of the histones associated with the promoter region of the forkhead box P3 (FoxP3) gene [55]. Differentiation of FoxP3-negative Tr1 cells is enhanced by this same inhibition in combination with signaling through GPR109A [126].
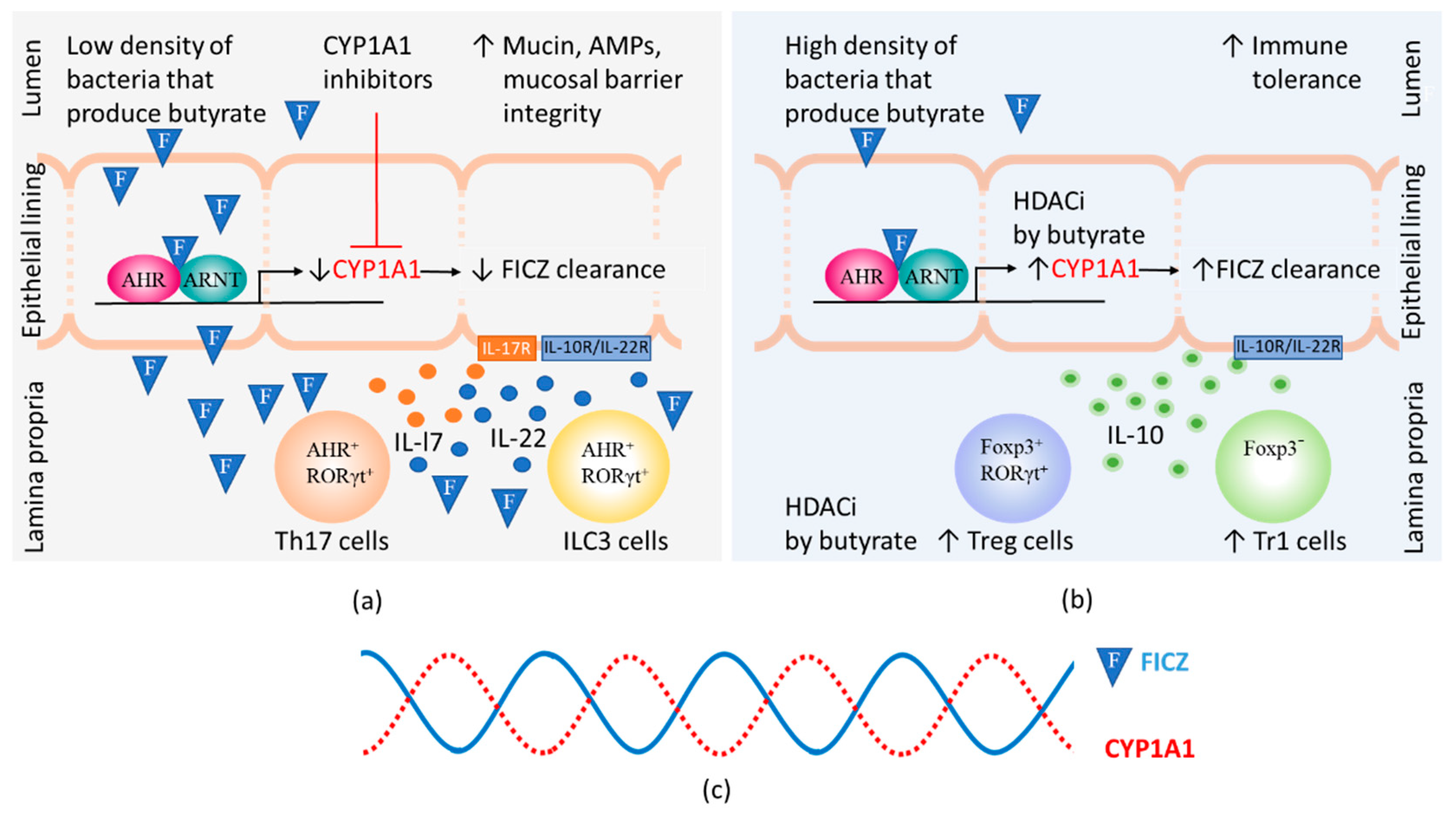
When the density of bacteria that produce BUT is low, inhibitors of CYP1A1 activity can elevate the steady-state level of FICZ to which the LP is exposed, thereby stimulating the production of IL-22 by RORγt+ ILC3s (Figure 3a). When BUT-producing bacteria are abundant, inhibition of HDAC by BUT enhances the expression and thereby activity of CYP1A1 (for references see Section 2.2). Crucially, this results in more extensive clearance of FICZ, preventing this substance from continuing to stimulate IL-22 production by ILC3s (Figure 3b).
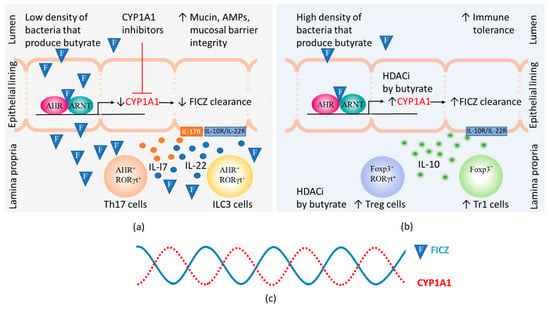
Figure 3.
The steady-state levels of 6-formylindolo[3,2-b]carbazole (FICZ) influences gut immunity. The amount of FICZ (illustrated by an F in a blue triangle) that reaches the lamina propria depends on the CYP1A1 activity in the epithelial lining, which is modified by the presence of CYP1A1 inhibitors. Butyrate can by virtue of its histone deacetylase inhibitory function (HDACi) contribute to increased numbers of Foxp3 positive and negative regulatory T cells (Treg and Tr1 cells) as well as increased CYP1A1 expression in intestinal epithelial cells. (a) At low levels of butyrate, heightened immunity is promoted by CYP1A1 inhibitors that cause FICZ to reach lamina propria and stimulate IL-22 and IL-17 expression in Th17 and ILC3 cells. Binding of IL-17 and IL-22 to their receptors on epithelial cells stimulate increased production of mucins and antimicrobial peptides (AMPs); (b) At high levels of butyrate, high CYP1A1 activity promotes the clearance of FICZ. Immunity is suppressed and Treg and Tr1 cells produce IL-10 that helps maintain tolerance to commensal bacteria by binding to receptors on epithelial cells; (c) Daily 24 h cycles in the CYP1A1 activity and the levels of FICZ.
4. Diurnal Rhythmicity in CYP1A1 Activity
The findings of Schiering and colleagues linked an absence of CYP1A1 activity in murine IECs to elevated numbers of ILC3s and Th17s in the colon, more IL-22 protein in cultures of colon explants, and an increased response to pathogens [21]. In contrast, the colon of mice whose IECs expressed CYP1A1 constitutively contained substantially fewer ILC3s and Th17s and less IL-22 protein, and these animals were more susceptible to enteric infection. These observations demonstrate that, at least in mice, CYP1A1 activity in IECs control gut levels of IL-22, and that to avoid inflammatory disorders, these levels must be regulated.
Interestingly, there are pronounced diurnal fluctuations in the composition of the intestinal microbiome [130], with as much as a 10-fold difference in the number of bacteria that adhere to the intestinal epithelium at night than day. Moreover, the amounts of SCFAs and other microbial products fluctuate in response to the nature and timing of the diet [130,131]. Consequently, microbial production of ligands derived from Trp and of BUT in the intestine varies during the 24-h day. Moreover, the level of mRNA encoding and activity of CYP1A1 in the liver and lungs of rodents also oscillate during the day [132,133], as do hepatic levels of AHR and ARNT mRNA and protein [133,134], as well as AHR mRNA in enteric ILC3s [73]. However, since few researchers in this field are aware of these fluctuations, few experimental studies include sampling at different times throughout the day.
In summary, a diurnal oscillation in the FICZ/AHR/CYP1A1 autoregulatory loop aids colonization of the gut by a symbiotic microbiome, helping to maintain tolerance to beneficial bacteria (Figure 3c).
5. When the Microbial Homeostasis in the Gut Is Disrupted
The fact that IL-22 production by ILC3s has both beneficial and deleterious effects has led several reviews to refer to IL-22 as a two-faced cytokine or a double-edged sword [67,69,74,111]. Under healthy conditions, IL-22 is constitutively expressed by ILC3s in the SI independently of IL-23 [92,111] and barely detectable in the colonic mucosa [91,104,135].
In order to keep pathogenic microbes such as Citrobacter rodentium under control in the colon, expression of AMPs by epithelial cells is induced via a process involving IL-23 signaling and early production of IL-22, with subsequent expression of IL-17 that acts synergistically with IL-22 [67,69,70,136]. Qiu and colleagues (2012) found that AHR-deficient ILCs lack the IL-23 receptor (IL-23R) and that AHR KO mice express IL-22 at reduced levels and, unlike wild-type mice, succumb to infection with C. rodentium. Administration of a plasmid encoding IL-22 protects such AHR KO animals from early mortality [19]. In line with this, ILC3s in the colon of patients suffering from IBD are dysregulated and express abnormally high levels of both IL-22 and IL-17 [69]. An important observation that has been repeatedly seen is that IBD patients display dramatically augmented microbial dysbiosis, in particular with a reduced abundance of bacteria that produce BUT [137,138,139]. A significant decrease of butyrate production has also been documented to occur in patients suffering from other autoimmune diseases such as type 2 diabetes [140], Behçet syndrome [141], and rheumatoid arthritis [65].
Together, the data documented in Table 1 suggest that, during infections, control of endogenous AHR signaling is required, because otherwise excessive and sustained production of IL-22 may exert deleterious effects on the colon that could lead to chronic inflammatory disorders and potential autoimmunity.

Table 1.
The potential influence of various conditions that regulate the level of CYP1A1 activity on the levels of FICZ and immunity in the intestine.
6. Conclusions
The observations described above indicate that dietary phytochemicals activate AHR-dependent immune processes, not as ligands to the AHR, but by influencing the composition of the intestinal microbiota in a manner that favors the production of both FICZ and BUT.
Unfortunately, the thousands of experimental studies on colitis that have been reported—involving the suffering and distress of large numbers of laboratory animals—had not yet led to effective treatment of patients with IBD. Novel strategies based on our knowledge concerning the involvement of FICZ in intestinal immunity may lead to more effective control of this disease, as well as of other autoimmune diseases, since FICZ can activate tolerogenic T cells (reviewed in [2]), in addition to the stimulation of systemic IL-22 signaling described in this review. However, as mentioned above, the biological functions of IL-22 are complex and therefore more mechanistic information is needed.
The most striking insight that comes from earlier work in our own laboratory in combination with the literature reviewed here is that rhythmic variations in the levels of FICZ and IL-22 are required for the maintenance of gut homeostasis.
- When CYP1A1 activity is too low (resulting in high levels of FICZ), defenses against commensal and pathogenic microbes are boosted.
- On the other hand, when CYP1A1 activity is too high (low FICZ levels), the host becomes susceptible to infections.
- Diurnal fluctuations in CYP1A1 activity fine-tune the activity of IL-22.
Therefore, a more detailed understanding of the diurnal regulation of crosstalk between FICZ and commensal bacteria that produce BUT, both when the intestinal barrier is functioning normally and during periods of infection, could pave the way for novel therapies for IBD, other autoimmune diseases, and possibly, for CRC as well.
Funding
This research received no external funding.
Acknowledgments
I wish to thank Ulf Ran nug for fruitful discussions and help in reviewing the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| AHR | aryl hydrocarbon receptor |
| AHRE | AHR response element |
| AHRR | AHR repressor |
| ARNT | nuclear translocator of AHR |
| AMP | antimicrobial peptides |
| BUT | butyrate |
| CRC | colorectal cancer |
| DC | dendritic cell |
| DIM | 3,3-Diindolylmethane |
| DSS | dextran sulfate sodium |
| FICZ | 6-Formylindolo[3,2-b]carbazole |
| GF | germ-free |
| GPR109a | G-protein coupled receptor 109a |
| HDAC | Histone deacetylase |
| IA | indole-3-acrylic acid |
| IAA | indole-3-acetic acid |
| IAAl | indole-3-acetaldehyde |
| IAl | indole-3-aldehyde |
| IAL | indole-3-lactic acid |
| I3C | indole-3-carbinol |
| I3P | indole-3-pyruvate |
| IBD | inflammatory bowel disease |
| IC50 | half maximal inhibitory concentration |
| ICZ | indolo[3,2-b]carbazole |
| IEC | intestinal epithelial cells |
| IEL | intraepithelial lymphocytes |
| IL | interleukin |
| ILC3 | group 3 innate lymphoid cells |
| IPA | indole-3-propionic acid |
| KO | knock out |
| LI | large intestine |
| LP | lamina propria |
| MQ | macrophages |
| RORγt | RAR-related orphan receptor γt |
| SCFA | short chain fatty acid |
| SI | small intestine |
| STAT | signal transducers and activator of transcription |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TCR | T cell receptor |
| TGF-β | tumor growth factor beta |
| TNBS | trinitrobenzene sulfonic acid |
| Tra | tryptamine |
| Tr1 | type 1 regulatory T cells |
| Treg | regulatory T cells |
| Trp | tryptophan |
| UC | Ulcerative colitis |
| WT | wild type |
References
- Nebert, D.W. Proposed role of drug-metabolizing enzymes: Regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Mol. Endocrinol. 1991, 5, 1203–1214. [Google Scholar] [CrossRef]
- Rannug, A.; Rannug, U. The tryptophan derivative 6-formylindolo3,2-bcarbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation. Crit. Rev. Toxicol. 2018, 48, 555–574. [Google Scholar] [CrossRef]
- Rannug, A.; Rannug, U.; Rosenkranz, H.; Winqvist, L.; Westerholm, R.; Agurell, E.; Grafstrom, A.-K. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J. Biol. Chem. 1987, 262, 15422–15427. [Google Scholar] [PubMed]
- Rannug, U.; Rannug, A.; Sjöberg, U.; Li, H.; Westerholm, R.; Bergman, J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem. Biol. 1995, 2, 841–845. [Google Scholar] [CrossRef]
- Smirnova, A.; Wincent, E.; Vikström Bergander, L.; Alsberg, T.; Bergman, J.; Rannug, A.; Rannug, U. Evidence for New Light-Independent Pathways for Generation of the Endogenous Aryl Hydrocarbon Receptor Agonist FICZ. Chem. Res. Toxicol. 2016, 29, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Oberg, M.; Bergander, L.; Håkansson, H.; Rannug, U.; Rannug, A. Identification of the tryptophan photoproduct 6-formylindolo3,2-bcarbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol. Sci. 2005, 85, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Diani-Moore, S.; Labitzke, E.; Brown, R.; Garvin, A.; Wong, L.; Rifkind, A.B. Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and In Vivo. Toxicol. Sci. 2006, 90, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, E.; Schäfer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hubenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; Saiz, M.L.; de la Fuente, H.; Sanchez-Diaz, R.; Moreno-Gonzalo, O.; Jorge, I.; Ferrarini, A.; Vazquez, J.; Punzon, C.; Fresno, M.; et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 2016, 17, 985–996. [Google Scholar] [CrossRef]
- Magiatis, P.; Pappas, P.; Gaitanis, G.; Mexia, N.; Melliou, E.; Galanou, M.; Blachos, C.; Stathopoulou, K.; Skaltsounis, A.L.; Marselos, M.; et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J. Investig. Dermatol. 2013, 133, 2023–2030. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Salem, M.A.E.; Gibbons, N.C.J.; Maitland, D.J.; Marsch, E.; Elwary, S.M.A.; Healey, A.R. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 2: Epidermal H2O2/ONOO(-)-mediated stress in vitiligo hampers indoleamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immune response signaling. FASEB J. 2012, 26, 2471–2485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, H.; Tan, S.; Tang, Q.; Liu, X.; Zuo, D.; Liao, Y.; Nan, Z.; Tan, C. The Chinese medicinal herb decoction QRZSLXF enhances anti-inflammatory effect in TNBS-induced colitis via balancing Th17/Tregs differentiation. J. Ethnopharmacol. 2020, 251, 112549. [Google Scholar] [CrossRef] [PubMed]
- Wincent, E.; Amini, N.; Luecke, S.; Glatt, H.; Bergman, J.; Crescenzi, C.; Rannug, A.; Rannug, U. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo3,2-bcarbazole is present in humans. J. Biol. Chem. 2009, 284, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef] [PubMed]
- Nobs, S.P.; Tuganbaev, T.; Elinav, E. Microbiome diurnal rhythmicity and its impact on host physiology and disease risk. EMBO Rep. 2019, 20, e47129. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.J.M.; Rosser, E.C.; Oleinika, K.; Nistala, K.; Krausgruber, T.; Rendeiro, A.F.; Banos, A.; Drozdov, I.; Villa, M.; Thomson, S.; et al. Aryl Hydrocarbon Receptor Contributes to the Transcriptional Program of IL-10-Producing Regulatory B Cells. Cell Rep. 2019, 29, 1878–1892.e7. [Google Scholar] [CrossRef]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; Macdonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248. [Google Scholar] [CrossRef]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.-M.E.; Fish, K.; Fu, Y.-X.; Zhou, L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Schiering, C.; Wincent, E.; Metidji, A.; Iseppon, A.; Li, Y.; Potocnik, A.J.; Omenetti, S.; Henderson, C.J.; Wolf, C.R.; Nebert, D.W.; et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Moura-Alves, P.; Puyskens, A.; Stinn, A.; Klemm, M.; Guhlich-Bornhof, U.; Dorhoi, A.; Furkert, J.; Kreuchwig, A.; Protze, J.; Lozza, L.; et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science 2017, 366. [Google Scholar] [CrossRef] [PubMed]
- Manzella, C.R.; Ackerman, M.; Singhal, M.; Ticho, A.L.; Ceh, J.; Alrefai, W.A.; Saksena, S.; Dudeja, P.K.; Gill, R.K. Serotonin Modulates AhR Activation by Interfering with CYP1A1-Mediated Clearance of AhR Ligands. Cell Physiol. Biochem. 2020, 54, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Castano, A.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; Gomez de Aguero, M.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Fu, Z.D.; Selwyn, F.P.; Cui, J.Y.; Klaassen, C.D. RNA-Seq Profiling of Intestinal Expression of Xenobiotic Processing Genes in Germ-Free Mice. Drug Metab. Dispos. 2017, 45, 1225–1238. [Google Scholar] [CrossRef]
- Uno, S.; Dragin, N.; Miller, M.L.; Dalton, T.P.; Gonzalez, F.J.; Nebert, D.W. Basal and inducible CYP1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract. Free Radic. Biol. Med. 2008, 44, 570–583. [Google Scholar] [CrossRef]
- Ikuta, T.; Kobayashi, Y.; Kitazawa, M.; Shiizaki, K.; Itano, N.; Noda, T.; Pettersson, S.; Poellinger, L.; Fujii-Kuriyama, Y.; Taniguchi, S.; et al. ASC-associated inflammation promotes cecal tumorigenesis in aryl hydrocarbon receptor-deficient mice. Carcinogenesis 2013, 34, 1620–1627. [Google Scholar] [CrossRef]
- Korecka, A.; Dona, A.; Lahiri, S.; Tett, A.J.; Al-Asmakh, M.; Braniste, V.; D’Arienzo, R.; Abbaspour, A.; Reichardt, N.; Fujii-Kuriyama, Y.; et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes 2016, 2, 16014. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Tian, H.; Tian, F.; Zhang, Y.; Zhang, L.; Gao, X.; Wang, X. Aryl hydrocarbon receptor/IL-22/Stat3 signaling pathway is involved in the modulation of intestinal mucosa antimicrobial molecules by commensal microbiota in mice. Innate Immun. 2018, 24, 297–306. [Google Scholar] [CrossRef]
- Kitajima, S.; Morimoto, M.; Sagara, E.; Shimizu, C.; Ikeda, Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp. Anim. 2001, 50, 387–395. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946. [Google Scholar] [PubMed]
- Wattenberg, L.W.; Leong, J.L.; Strand, P.J. Benzpyrene hydroxylase activity in the gastrointestinal tract. Cancer Res. 1962, 22, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.W. Dietary modulation of cytochrome P450 in the small intestinal epithelium. Pharmacology 1991, 43, 36–46. [Google Scholar] [CrossRef]
- Loub, W.D.; Wattenberg, L.W.; Davis, D.W. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J. Natl. Cancer Inst. 1975, 54, 985–988. [Google Scholar]
- Park, J.Y.; Bjeldanes, L.F. Organ-selective induction of cytochrome P-450-dependent activities by indole-3-carbinol-derived products: Influence on covalent binding of benzoapyrene to hepatic and pulmonary DNA in the rat. Chem. Biol. Interact. 1992, 83, 235–247. [Google Scholar] [CrossRef]
- Bjeldanes, L.F.; Kim, J.Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol In Vitro and In Vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar] [CrossRef]
- Wincent, E.; Bengtsson, J.; Mohammadi Bardbori, A.; Alsberg, T.; Luecke, S.; Rannug, U.; Rannug, A. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4479–4484. [Google Scholar] [CrossRef]
- Westerink, W.M.; Stevenson, J.C.; Schoonen, W.G. Pharmacologic profiling of human and rat cytochrome P450 1A1 and 1A2 induction and competition. Arch. Toxicol. 2008, 82, 909–921. [Google Scholar] [CrossRef]
- Shimada, T.; Tanaka, K.; Takenaka, S.; Murayama, N.; Martin, M.V.; Foroozesh, M.K.; Yamazaki, H.; Guengerich, F.P.; Komori, M. Structure-function relationships of inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 flavonoid derivatives. Chem. Res. Toxicol. 2010, 23, 1921–1935. [Google Scholar] [CrossRef]
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J. Immunol. 2017, 198, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Zheng, X.; Xie, G.; Zhao, A.; Zhao, L.; Yao, C.; Chiu, N.H.; Zhou, Z.; Bao, Y.; Jia, W.; Nicholson, J.K.; et al. The footprints of gut microbial-mammalian co-metabolism. J. Proteome. Res. 2011, 10, 5512–5522. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Vyhlídalová, B.; Krasulová, K.; Pečinková, P.; Marcalíková, A.; Vrzal, R.; Zemánková, L.; Vančo, J.; Trávníček, Z.; Vondráček, J.; Karasová, M.; et al. Gut Microbial Catabolites of Tryptophan Are Ligands and Agonists of the Aryl Hydrocarbon Receptor: A Detailed Characterization. Int. J. Mol. Sci. 2020, 21, 2614. [Google Scholar] [CrossRef]
- Fukumoto, S.; Toshimitsu, T.; Matsuoka, S.; Maruyama, A.; Oh-Oka, K.; Takamura, T.; Nakamura, Y.; Ishimaru, K.; Fujii-Kuriyama, Y.; Ikegami, S.; et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. 2014, 92, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Zhang, J.; Watson, A.J.; Probst, M.R.; Minehart, E.; Hankinson, O. Basis for the loss of aryl hydrocarbon receptor gene expression in clones of a mouse hepatoma cell line. Mol. Pharmacol. 1996, 50, 1454–1462. [Google Scholar]
- Gradin, K.; Toftgârd, R.; Poellinger, L.; Berghard, A. Repression of dioxin signal transduction in fibroblasts. Identification Of a putative repressor associated with Arnt. J. Biol. Chem. 1999, 274, 13511–13518. [Google Scholar] [CrossRef]
- Garrison, P.M.; Rogers, J.M.; Brackney, W.R.; Denison, M.S. Effects of histone deacetylase inhibitors on the Ah receptor gene promoter. Arch. Biochem. Biophys. 2000, 374, 161–171. [Google Scholar] [CrossRef]
- Haarmann-Stemmann, T.; Bothe, H.; Kohli, A.; Sydlik, U.; Abel, J.; Fritsche, E. Analysis of the transcriptional regulation and molecular function of the aryl hydrocarbon receptor repressor in human cell lines. Drug Metab. Dispos. 2007, 35, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Cheng, Y.; Park, H.; Davidson, L.A.; Callaway, E.S.; Chapkin, R.S.; Jayaraman, A.; Asante, A.; Allred, C.; Weaver, E.A.; et al. Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci. Rep. 2017, 7, 10163. [Google Scholar] [CrossRef] [PubMed]
- Zapletal, O.; Tylichová, Z.; Neča, J.; Kohoutek, J.; Machala, M.; Milcová, A.; Pokorná, M.; Topinka, J.; Moyer, M.P.; Hofmanová, J.; et al. Butyrate alters expression of cytochrome P450 1A1 and metabolism of benzoapyrene via its histone deacetylase activity in colon epithelial cell models. Arch. Toxicol. 2017, 91, 2135–2150. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851.e10. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Zeng, B.; Shi, S.; Ashworth, G.; Dong, C.; Liu, J.; Xing, F. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 2019, 10, 315. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Vosshenrich, C.A.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–970. [Google Scholar] [CrossRef]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- De Agüero, M.G.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Silva, C.; Domingues, R.G.; Rendas, M.; Raposo, B.; Ribeiro, H.; da Silva, J.A.; Vieira, A.; Costa, R.M.; Barbosa-Morais, N.L.; Carvalho, T.; et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature 2019, 574, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.G.; Hepworth, M.R. Immunoregulatory Sensory Circuits in Group 3 Innate Lymphoid Cell (ILC3) Function and Tissue Homeostasis. Front. Immunol. 2020, 11, 116. [Google Scholar] [CrossRef]
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, 1561–1565. [Google Scholar] [CrossRef]
- Lee, J.S.; Cella, M.; Colonna, M. AHR and the Transcriptional Regulation of Type-17/22 ILC. Front. Immunol. 2012, 3, 10. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, X.; Chen, Z.M.; He, L.; Sonnenberg, G.F.; Artis, D.; Fu, Y.X.; Zhou, L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 2013, 39, 386–399. [Google Scholar] [CrossRef]
- Ito, S.; Chen, C.; Satoh, J.; Yim, S.; Gonzalez, F.J. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J. Clin. Investig. 2007, 117, 1940–1950. [Google Scholar] [CrossRef]
- Wei, Y.D.; Helleberg, H.; Rannug, U.; Rannug, A. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo3,2-bcarbazole. Chem. Biol. Interact. 1998, 2, 39–55. [Google Scholar] [CrossRef]
- Shimada, T.; Guengerich, F.P. Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2006, 19, 288–294. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Schiering, C.; Vonk, A.; Das, S.; Stockinger, B.; Wincent, E. Cytochrome P4501-inhibiting chemicals amplify aryl hydrocarbon receptor activation and IL-22 production in T helper 17 cells. Biochem. Pharmacol. 2018, 151, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Luecke, S.; Wincent, E.; Backlund, M.; Rannug, U.; Rannug, A. Cytochrome P450 1A1 gene regulation by UVB involves crosstalk between the aryl hydrocarbon receptor and nuclear factor kappaB. Chem. Biol. Interact. 2010, 184, 466–473. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Bergander, L.V.; Rannug, U.; Rannug, A. NADPH Oxidase-Dependent Mechanism Explains How Arsenic and Other Oxidants Can Activate Aryl Hydrocarbon Receptor Signaling. Chem. Res. Toxicol. 2015, 28, 2278–2286. [Google Scholar] [CrossRef]
- Gerbal-Chaloin, S.; Iankova, I.; Maurel, P.; Daujat-Chavanieu, M. Nuclear receptors in the cross-talk of drug metabolism and inflammation. Drug Metab. Rev. 2013, 45, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; de Sauvage, F.J.; et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Killig, M.; Glatzer, T.; Romagnani, C. Recognition strategies of group 3 innate lymphoid cells. Front. Immunol. 2014, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Yeste, A.; Mascanfroni, I.D.; Nadeau, M.; Burns, E.J.; Tukpah, A.M.; Santiago, A.; Wu, C.; Patel, B.; Kumar, D.; Quintana, F.J. IL-21 induces IL-22 production in CD4+ T cells. Nat. Commun. 2014, 5, 3753. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.C.; Stockinger, B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Trifari, S.; Kaplan, C.D.; Tran, E.H.; Crellin, N.K.; Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 2009, 10, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.M.; Brembilla, N.C.; Sorg, O.; Chicheportiche, R.; Matthes, T.; Dayer, J.M.; Saurat, J.H.; Roosnek, E.; Chizzolini, C. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur. J. Immunol. 2010, 40, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Kado, S.Y.; Hoeper, C.; Harel, S.; Vogel, C.F.A. Role of NF-kB RelB in Aryl Hydrocarbon Receptor-Mediated Ligand Specific Effects. Int. J. Mol. Sci. 2019, 20, 2652. [Google Scholar] [CrossRef]
- Martin, B.; Hirota, K.; Cua, D.J.; Stockinger, B.; Veldhoen, M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009, 31, 321–330. [Google Scholar] [CrossRef]
- Kimura, A.; Abe, H.; Tsuruta, S.; Chiba, S.; Fujii-Kuriyama, Y.; Sekiya, T.; Morita, R.; Yoshimura, A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2014, 26, 209–220. [Google Scholar] [CrossRef]
- Ji, T.; Xu, C.; Sun, L.; Yu, M.; Peng, K.; Qiu, Y.; Xiao, W.; Yang, H. Aryl Hydrocarbon Receptor Activation Down-Regulates IL-7 and Reduces Inflammation in a Mouse Model of DSS-Induced Colitis. Dig. Dis. Sci. 2015, 60, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Zorzi, F.; Marafini, I.; Di Fusco, D.; Dinallo, V.; Caruso, R.; Izzo, R.; Franzè, E.; Colantoni, A.; Pallone, F.; et al. Aryl hydrocarbon receptor-driven signals inhibit collagen synthesis in the gut. Eur. J. Immunol. 2016, 46, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pu, A.; Sheng, B.; Zhang, Z.; Li, L.; Liu, Z.; Wang, Q.; Li, X.; Ma, Y.; Yu, M.; et al. Aryl hydrocarbon receptor activation modulates CD8αα. Biomed Pharmacother 2017, 87, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Q.; Yu, K.; Fan, X.; Xiao, W.; Cai, Y.; Xu, P.; Yu, M.; Yang, H. 6-Formylindolo(3,2-b)carbazole induced aryl hydrocarbon receptor activation prevents intestinal barrier dysfunction through regulation of claudin-2 expression. Chem. Biol. Interact. 2018, 288, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, K.; Zhou, C.; Xu, P.; Xiao, W.; Yang, H. Aryl hydrocarbon receptor activation alleviates dextran sodium sulfate-induced colitis through enhancing the differentiation of goblet cells. Biochem. Biophys. Res. Commun. 2019, 514, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yin, X.; Wang, G.; Elinav, E.; Hao, L.; Zhao, L.; Flavell, R.A. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 2013, 190, 5306–5312. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Brawner, K.M.; Yeramilli, V.A.; Duck, L.W.; Van Der Pol, W.; Smythies, L.E.; Morrow, C.D.; Elson, C.O.; Martin, C.A. Depletion of dietary aryl hydrocarbon receptor ligands alters microbiota composition and function. Sci. Rep. 2019, 9, 14724. [Google Scholar] [CrossRef] [PubMed]
- Schanz, O.; Chijiiwa, R.; Cengiz, S.C.; Majlesain, Y.; Weighardt, H.; Takeyama, H.; Förster, I. Dietary AhR Ligands Regulate AhRR Expression in Intestinal Immune Cells and Intestinal Microbiota Composition. Int. J. Mol. Sci. 2020, 21, 3189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Innocentin, S.; Withers, D.R.; Roberts, N.A.; Gallagher, A.R.; Grigorieva, E.F.; Wilhelm, C.; Veldhoen, M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 2011, 147, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Boussenna, A.; Goncalves-Mendes, N.; Joubert-Zakeyh, J.; Pereira, B.; Fraisse, D.; Vasson, M.P.; Texier, O.; Felgines, C. Impact of basal diet on dextran sodium sulphate (DSS)-induced colitis in rats. Eur. J. Nutr. 2015, 54, 1217–1227. [Google Scholar] [CrossRef]
- Cui, H.; Cai, Y.; Wang, L.; Jia, B.; Li, J.; Zhao, S.; C hu, X.; Lin, J.; Zhang, X.; Bian, Y.; et al. Berberine Regulates Treg/Th17 Balance to Treat Ulcerative Colitis Through Modulating the Gut Microbiota in the Colon. Front. Pharmacol. 2018, 9, 571. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef]
- Xuan, H.; Ou, A.; Hao, S.; Shi, J.; Jin, X. Galangin Protects against Symptoms of Dextran Sodium Sulfate-induced Acute Colitis by Activating Autophagy and Modulating the Gut Microbiota. Nutrients 2020, 12, 347. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef]
- Power, K.A.; Lu, J.T.; Monk, J.M.; Lepp, D.; Wu, W.; Zhang, C.; Liu, R.; Tsao, R.; Robinson, L.E.; Wood, G.A.; et al. Purified rutin and rutin-rich asparagus attenuates disease severity and tissue damage following dextran sodium sulfate-induced colitis. Mol. Nutr. Food Res. 2016, 60, 2396–2412. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Nabavi, S.M.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Menzel, L.; Alrafas, H.R.; Dopkins, N.; Becker, W.; Miranda, K.; Tang, C.; Chatterjee, S.; Singh, U.; Nagarkatti, M.; et al. Indole-3-; carbinol prevents colitis and associated microbial dysbiosis in an IL-22-dependent manner. JCI Insight. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Takemura, N.; Ogasawara, T.; Sasajima, N.; Watanabe, J.; Ito, H.; Morita, T.; Sonoyama, K. Effects of fructo-oligosaccharide on DSS-induced colitis differ in mice fed nonpurified and purified diets. J. Nutr. 2010, 140, 2121–2127. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Kaisar, M.M.M.; Pelgrom, L.R.; van der Ham, A.J.; Yazdanbakhsh, M.; Everts, B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells. Front Immunol. 2017, 8, 1429. [Google Scholar] [CrossRef]
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel. Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Tredger, J.M.; Chhabra, R.S. Circadian variations in microsomal drug-metabolizing enzyme activities in rat and rabbit tissues. Xenobiotica 1977, 7, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Ceccatelli, S.; Rannug, A. A study on diurnal mRNA expression of CYP1A1, AHR, ARNT, and PER2 in rat pituitary and liver. Environ. Toxicol. Pharmacol. 2002, 11, 119–126. [Google Scholar] [CrossRef]
- Richardson, V.M.; Santostefano, M.J.; Birnbaum, L.S. Daily cycle of bHLH-PAS proteins, Ah receptor and Arnt, in multiple tissues of female Sprague-Dawley rats. Biochem. Biophys. Res. Commun. 1998, 252, 225–231. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008, 29, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Arancibia-Cárcamo, C.V.; Fleming, M.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Packey, C.D.; Sartor, R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr. Opin. Infect. Dis. 2009, 22, 292–301. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Consolandi, C.; Turroni, S.; Emmi, G.; Severgnini, M.; Fiori, J.; Peano, C.; Biagi, E.; Grassi, A.; Rampelli, S.; Silvestri, E.; et al. Behçet’s syndrome patients exhibit specific microbiome signature. Autoimmun. Rev. 2015, 14, 269–276. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).