Abstract
Gemtuzumab ozogamicin (GO, Mylotarg®) consists of a humanized CD33-targeted antibody-drug conjugated to a calicheamicin derivative. Growing evidence of GO efficacy in acute myeloid leukemia (AML), demonstrated by improved outcomes in CD33-positive AML patients across phase I to III clinical trials, led to the Food and Drug Administration (FDA) approval on 1 September 2017 in CD33-positive AML patients aged 2 years and older. Discrepancies in GO recipients outcome have raised significant efforts to characterize biomarkers predictive of GO response and have refined the subset of patients that may strongly benefit from GO. Among them, CD33 expression levels, favorable cytogenetics (t(8;21), inv(16)/t(16;16), t(15;17)) and molecular alterations, such as NPM1, FLT3-internal tandem duplications and other signaling mutations, represent well-known candidates. Additionally, in depth analyses including minimal residual disease monitoring, stemness expression (LSC17 score), mutations or single nucleotide polymorphisms in GO pathway genes (CD33, ABCB1) and molecular-derived scores, such as the recently set up CD33_PGx6_Score, represent promising markers to enhance GO response prediction and improve patient management.
1. Introduction
Standard of care for acute myeloid leukemia (AML) has long been based on chemotherapy combinations with or without hematopoietic stem cell transplantation (HSCT). Despite efforts in supportive care improvement, 5-year overall survival (OS) of adult patients with AML remains at 30–40% [1]. Over the past years, significant advances have been made in understanding the AML mutational landscape, identifying leukemic cells and characterizing their intrinsic properties leading to the development of new drugs, among which eight have been approved by the Food and Drug Administration (FDA) for the treatment of AML between 2017 and 2019. Notably, gemtuzumab ozogamicin (GO, CMA-676, Mylotarg®) is a humanized cluster of differentiation 33 (CD33)-targeted antibody-drug conjugated to a calicheamicin derivative, a natural antitumor antibiotic. CD33 antigen represents a hallmark of myeloid leukemic blasts, widely expressed in AML patients. Several clinical studies have highlighted the clinical benefit of GO on patient outcome. GO stands for the first antibody drug conjugate approved by the FDA. Enhanced knowledge about the GO metabolic pathway at both cellular and molecular levels has raised and improved understanding on GO response biomarkers.
After a brief review about the mechanism of action of GO and its efficacy across successive clinical trials, this review will discuss the biomarkers predicting GO response (Figure 1).
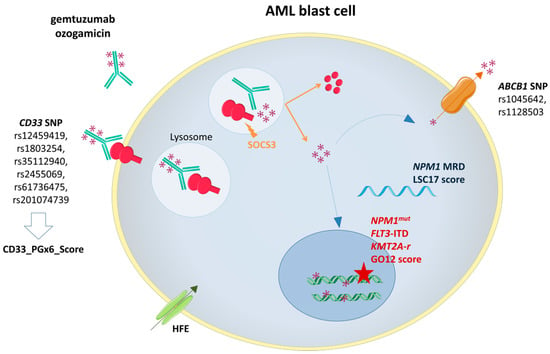
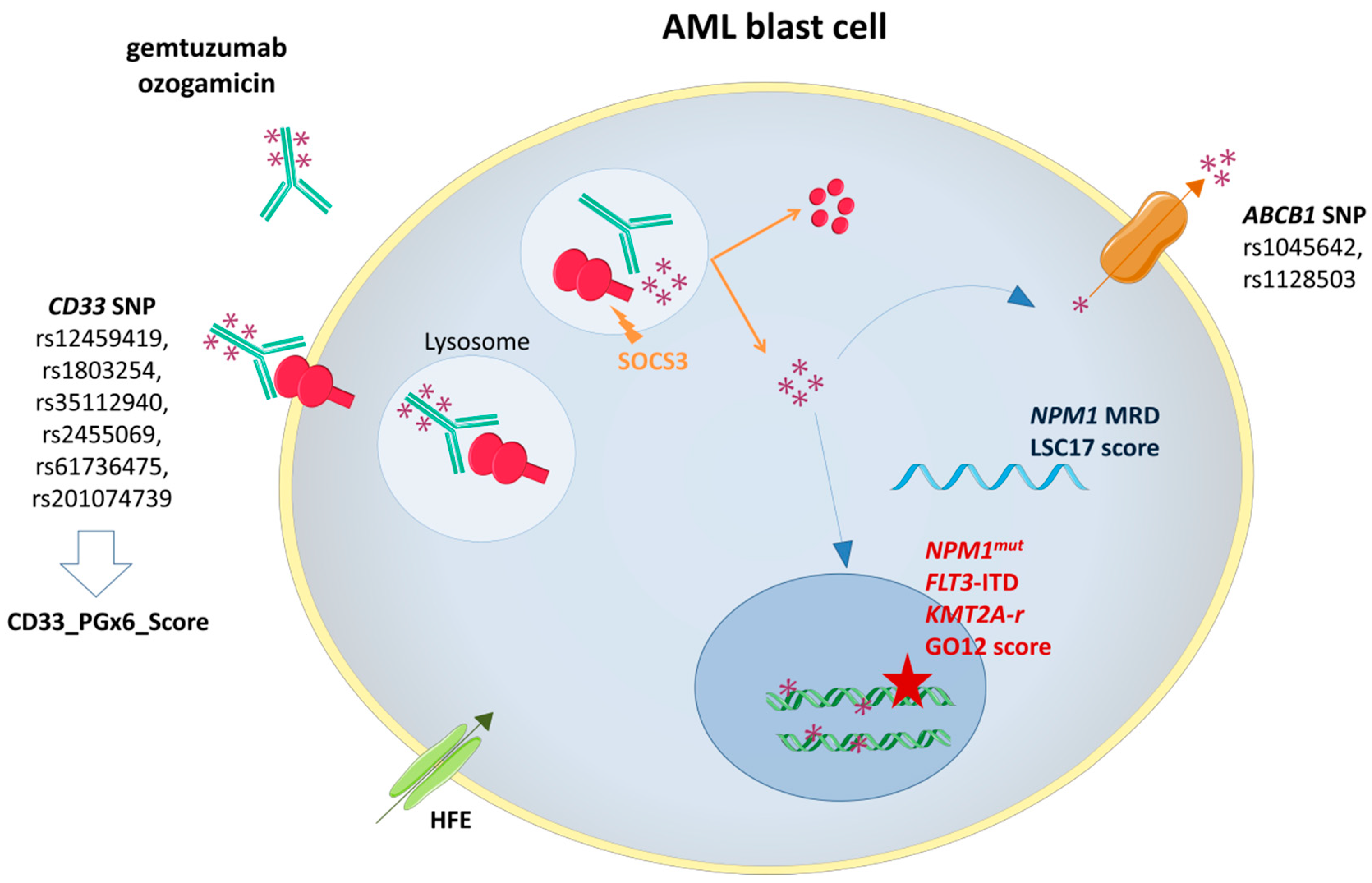
Figure 1.
Gemtuzumab ozogamicin (GO) mechanism of action and biomarkers of response. SOCS3: Suppressor Of Cytokine Signaling 3; ABCB1: ATP-binding cassette subfamily B member 1 gene; NPM1mut: Nucleophosmin 1 gene mutation; FLT3-ITD: FMS-Like Tyrosine Kinase 3 Internal Tandem Duplication; KMT2A-r: Lysine Methyltransferase 2A rearrangement.
2. Gemtuzumab Ozogamicin
2.1. CD33: The Target Antigen
The CD33 antigen is a 67 kD single chain transmembrane glycoprotein that belongs to the sialic-acid-binding immunoglobulin-like lectins family (Siglecs) [2]. The CD33 gene, located on chromosome 19q13.4, is composed of eight exons. Exons 1 and 2 encode for the amino-terminal V-set signal peptide, an immunoglobulin-like domain mediating the sialic-acid binding, exons 3 and 4 encode the C2-set domain, and exon 5 encodes the transmembrane domain. The intracytoplasmic domain, encoded by exons 6, 7a and 7b, comprises two tyrosine-based inhibitory signaling motifs (Y340 and Y358) which, upon phosphorylation, provide docking sites for the Src homology-2 domain-containing tyrosine phosphatases (SHP) and the suppressor of cytokine signaling 3 (SOCS3) [3,4,5]. In turn, SHP-1 and SHP-2 dephosphorylate CD33 and negatively regulate other surrounding receptors [3]. SOCS3 competes with SHP-1/2 for CD33 binding and recruits the Elongin B/C-Cul2/Cul5-SOCS-box protein E3 ubiquitin ligase leading to the proteasomal degradation of CD33 and SOCS3 [6].
CD33 is a differentiation antigen especially expressed among myeloid progenitors, while it is not expressed by normal hematopoietic stem cells [7]. AML originates from clonal evolution of driver and cooperative genetic alterations in multipotent CD34+/CD33− stem cells and/or in committed CD34+/CD33+ myeloid progenitors [8,9,10]. Previous studies have shown that CD33 was expressed on leukemic blasts in 85% to 90% of AML patients [11,12]. Collectively, these data raised a huge interest to consider CD33 as potent and selective therapeutic target in AML.
2.2. Mechanism of Action
GO consists of a recombinant humanized immunoglobulin G4 kappa CD33-targeted antibody (hP67.6) covalently linked to the semi-synthetic antitumor antibiotic of the enediyne family, the N-acetyl gamma calicheamicin, via the acid-labile hybrid 4-(4′-acetylphenoxy)butanoic acid linker [13]. After binding to the CD33 antigen, the complex GO-CD33 is rapidly internalized [14]. In the cytoplasm, this complex is routed in the lysosome. Under the acidic environment of the lysosome, the butanoic acid linker is hydrolyzed, releasing the toxic moiety of the GO. The calicheamicin derivative is reduced by the glutathione into a highly reactive species which induces simple- and double-stranded DNA breaks, leading to DNA-damage [15,16,17]. Downstream, the DNA repair pathway is activated through the ataxia-telangiectasia mutated (ATM)/ataxia-telangiectasia and Rad3-related (ATR) and the DNA-dependent protein kinase pathways [18,19]. In turn, ATM and ATR proteins phosphorylate Chk1 and Chk2 proteins, which eventually results in G2/M cell cycle arrest. The DNA-dependent pathway activation mediates DNA repair through H2AX phosphorylation. Hence, cells defective in ATM, DNA-dependent protein kinase or genes coding for the non-homologous end joining repair are hypersensitive to calicheamicin [16,20]. However, the predominant downstream pathway following the ATM/ATR activation is the mitochondrial apoptotic pathway mediated by the B-cell lymphoma 2 (Bcl-2) family proteins Bax and Bak which releases the cytochrome-c and eventually activates caspases 9 and 3. This pro-apoptotic pathway acts independently of the tumor protein 53 (TP53) and Fas-Associated protein with Death Domain (FADD)-signaling pathways [21,22]. Data from a phase II trial suggest that Bcl-2 antisense (Oblimersen sodium) may enhance the pro-apoptotic pathway in patients treated concomitantly with GO [23].
2.3. Clinical Data
Successive clinical trials have demonstrated the anti-leukemic activity of GO and its clinical benefit on patient outcome (Table 1).

Table 1.
Overview of the main clinical trials evaluating GO efficacy
2.3.1. GO Administered as Monotherapy
In a phase I dose escalation trial, 40 adult patients with relapsed/refractory CD33-positive AML received GO until a maximum dose of 9 mg/m2 saturating almost all AML CD33-binding sites (92.2%) with an acceptable safety profile [24]. Similarly, a phase I trial undertaken in 29 children with relapsed/refractory AML, showed the tolerability and the efficacy of GO (overall response rate [ORR], 28%) [37].
Three phase II trials assessed the safety and the efficacy of GO as a single agent given at 9 mg/m2 on day 1 and day 14 in adult AML patients experiencing first relapse. A total of 30% of the patient population achieved complete remission (CR)/complete remission without platelet recovery (CRp) [25]. Based on these results, GO was granted FDA accelerated approval on 17 May 2000 as a monotherapy for CD33-positive AML patients older than 60 years of age, experiencing first relapse and unfit for intensive treatment [41]. Of note, final analysis revealed that this GO schedule was associated with frequent grade 3 and 4 hematological toxicities (profound neutropenia and thrombocytopenia) and liver toxicities (veno-occlusive disease) [42].
The European Organization for Research and Treatment of Cancer-Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (EORTC-GIMEMA) AML-19 trial is a sequential phase II/III trial that first determined the best GO induction regimen (GO administered as monotherapy, 6 mg/m2 on day 1 plus 3 mg/m2 on day 8 versus [vs.] GO 3 mg/m2 on days 1, 3 and 5) before undertaking the phase III trial comparing GO to best supportive care in previously untreated AML patients older than 61 years unfit for intensive chemotherapy. The GO schedule 6 mg/m2 on day 1 plus 3 mg/m2 on day 8 led to a higher rate of disease non-progression, defined as the rate of patients in CR/CRp or in stable disease at the end of induction course (63% vs. 38%), and was retained for the phase III [43]. In the subsequent phase III, first-line GO monotherapy significantly improved OS compared to best supportive care (1-year OS: 24.3% vs. 9.7%, hazard ratio [HR]: 0.69, 95% CI: 0.53–0.90, p = 0.005) [36].
2.3.2. GO Administered in Combination with Intensive Chemotherapy
A phase III randomized trial was undertaken by the Southwest Oncology Group (SWOG, S0106 trial) to further confirm the clinical benefit of the addition of GO (6 mg/m2 on day 4) to the standard ‘3 + 7’ induction regimen, associating daunorubicin and cytarabine. Patients allocated to the GO arm received lower dose of daunorubicin (45 mg/m2 vs. 60 mg/m2 in the control arm) in order to balance toxicities [29]. However, the interim analysis revealed higher rate of fatal induction toxicities in the GO arm (5.5% vs. 1.4%, p = 0.0062) leading to the premature end of the study and withdrawal from the market on 21 June 2010. Final data analysis of the S0106 trial failed to demonstrate any clinical benefit of the addition of GO neither on relapse-free survival (RFS) nor on OS (GO arm vs. non GO arm: 5-year RFS: 43% vs. 42%, p = 0.40; 5-year OS: 46% vs. 50%, p = 0.85).
In an attempt to reduce GO toxicities, a dose-finding trial assessed the addition of GO 3 mg/m2 vs. 6 mg/m2 to intensive chemotherapy regimens. The addition of GO 6 mg/m2 was not feasible due to hepatotoxicity and delayed hematopoietic recovery. However, 3 mg/m2 GO appeared effective and safe [44]. This study led to two randomized phase III trials, addressing the clinical benefit of adding GO 3 mg/m2 to the induction regimen in younger adults (Medical Research Council (MRC) AML15 trial) and in older patients (National Cancer Research Institute (NCRI) AML16 trial) [28,30]. In these two trials, the addition of GO showed an improved OS in older patients, and in younger adults with favorable-risk AML. Given the rapid re-expression on the CD33 antigen after GO exposure [14,17], a phase II trial addressed the efficacy of fractionated GO (3 mg/m2 on days 1, 4 and 7) in adults with relapsed AML [26]. Fractionated GO administered as monotherapy led to a CR/CRp rate of 33% with a good safety profile, in particular, no grade 3 or 4 liver toxicity. When combining fractionated GO to chemotherapy, 65% to 75% of patients achieved CR/CRp [27,45]. Based on these encouraging results of lowering and fractionating doses of GO, the randomized phase III trial Acute Leukemia French Association (ALFA)-0701 compared the clinical benefit of low fractionated doses of GO in addition to the standard intensive chemotherapy regimen in newly diagnosed CD33-positive AML patients, aged 50 to 70 years old [32]. In the experimental arm, patients were administered fractionated doses of GO 3 mg/m2, during induction (on days 1, 4 and 7) and consolidation courses (on day 1 of each course). Event-free survival (EFS) was remarkably improved in the GO arm (median EFS, assessed by the blinded independent review: 13.6 vs. 8.5 months, HR: 0.66, 95% CI: 0.49–0.89, p = 0.006) whilst median OS did not significantly differ between the two arms (HR: 0.81, 95% CI: 0.60–1.09, p = 0.16) [33]. These results led the FDA to approve GO for newly diagnosed CD33-positive AML in adults and for relapsed or refractory CD33-positive AML in patients aged 2 years and older on 1 September 2017. GO received the European Medicines Agency’s (EMA) marketing authorization on 19 April 2018 for the treatment of de novo CD33-positive AML patients aged 15 years and above as frontline therapy in combination with daunorubicin and cytarabine.
Corroborating these results, a meta-analysis including 3325 patients from five open-label randomized phase III controlled trials (MRC AML15, NCRI AML16, SWOG S0106, GOELAMS-AML 2006 IR and ALFA-0701 [28,29,30,31,32]) highlighted the benefit of the addition of GO on the risk of relapse (RR) and on OS (RR: OR: 0.81, 95% CI: 0.73–0.90, p = 0.0001; 5-year OS: OR: 0.90, 95% CI: 0.82–0.98, p = 0.01) [46].
More recently, a phase III trial, the NCRI AML17 trial, evaluated the impact of GO dosing 3 vs. 6 mg/m2 combined with intensive chemotherapy in previously untreated AML patients. Among the 788 included patients, increased GO dosing (6 mg/m2) did not improve neither response rate nor patient outcome (OS: HR: 1.10, 95% CI: 0.90–1.34, p = 0.3; RFS: HR: 1.11, 95% CI: 0.91–1.35, p = 0.3) [34].
In the pediatric population, phase II studies from the Children’s Oncology Group (COG, AAML00P2 and AAML03P1) demonstrated the benefit of the addition of GO to chemotherapy [38,39]. In the subsequent phase III trial AAML0531, AML patients aged 0 to 29 years with newly diagnosed AML were randomly assigned to a five-course chemotherapy regimen alone or combined with two doses of GO 3 mg/m2 (on day 6 during induction course 1, and on day 7 during intensification course 2). Among the 1022 evaluable patients, GO recipients experienced better EFS (3-year EFS: 53.1% vs. 46.9%, HR: 0.83, 95% CI: 0.70–0.99, p = 0.04) but no improved OS (3-year OS: 69.4% vs. 65.4%, HR: 0.91, 95% CI: 0.74–1.13, p = 0.39) [40]. Based on these results, the FDA extended the indication of GO to newly diagnosed CD33-positive AML patients aged one month and older, on June 16, 2020.
3. Biomarkers
3.1. CD33 Expression on AML Cells
As previously described, CD33 is widely expressed in AML patients (>80%, [25]). However, the CD33 expression level on leukemic cells is heterogeneous, varying more than 2 log-fold among AML patients [47,48]. In the pediatric AAML03P1 cohort, high expression of CD33 is associated with poor outcome in multivariate analysis [47,49]. In vitro studies demonstrated that GO-induced cytotoxicity was highly dependent on cell surface expression of CD33 and higher CD33 expression levels correlated with an increase of GO binding to CD33 antigenic sites and thus enhanced clearance of AML blasts [14,50]. Remarkably, good responders among GO recipients express higher mean of CD33 expression levels inversely correlated with a low ATP-binding cassette subfamily B-member 1 (ABCB1) expression which mediates drug efflux [51]. Among the 825 patients from the pediatric AAML0531 trial, GO improved EFS in patients with high CD33 expression (quartiles (Q), Q2-Q4, GO vs. no-GO, 5-year EFS: 53% vs. 41%, p = 0.005), whereas patients with low CD33 expression (Q1) did not benefit from the addition of GO to the induction chemotherapy (GO vs. no-GO, 5-year EFS: 53% vs. 58%, p = 0.456) [48]. By contrast, in the adult population no interaction on survival was observed between CD33 expression quartiles and GO in non-core binding factor (CBF)-AML patients [52]. However, GO reduced the RR of adult patients with Q4-CD33 expression (HR: 0.63, 95% CI: 0.35–1.12). Higher CD33 expression have been observed in patients displaying mutations in Nucleophosmin 1 (NPM1) gene or FMS-like tyrosine kinase 3 internal tandem duplications (FLT3-ITD) and is further detailed in the corresponding section [47,48,52,53,54,55]. In a retrospective analysis performed on 200 adult patients from the ALFA-0701 trial, and considering CD33 expression as a binary variable defined by a 70% cutoff, GO improved EFS and RFS of patients with high CD33 expression even after adjustment for cytogenetics and NPM1/FLT3-ITD mutations [55]. Thus CD33 expression appears, as expected, as an important pre-treatment biomarker for GO response.
3.2. Prognostic Impact of the Cytogenetic Alterations on GO Efficacy
The benefit of GO has been widely observed in the non-adverse cytogenetic-risk groups [28,32,36,46,56]. In a meta-analysis, the addition of GO was strongly associated with a survival benefit in patients from good (acute promyelocytic leukemia [APL] excluded) and intermediate cytogenetic groups (absolute survival benefit at 6 years: + 20.7%; OR: 0.47, 95% CI: 0.31–0.73, p = 0.0006; + 5.7%; OR: 0.84, 95% CI: 0.75–0.95, p = 0.005, respectively) [46]. Conversely, in the adverse cytogenetic group, in which the CD33 expression level is usually lower, GO failed to improve OS (absolute survival benefit at 6 years: + 2.2%, OR: 0.99, 95% CI: 0.83–1.18, p = 0.9).
CBF-AMLs correlate with a low CD33 expression [52]. It is assumed that the first event, t(8;21)/inv(16)/t(16.16), initiates very early in preleukemic CD33- cells [57]. By contrast, subsequent driver events, such as tyrosine kinase mutations leading to the proliferative AML phenotype occur when the cells express CD33 [58]. Hence, GO could eradicate the proliferative clone and spare CD33- preleukemic cells. Additionally, GO efficacy in CBF-AML may be explained by a high sensitivity of CBF-AML blasts to calicheamicin [9,59]. A phase II trial assessed the efficacy of the FLAG induction regimen, including fludarabine, cytarabine and filgrastim, combined with GO 3 mg/m2, at induction day 1, and post-remission courses 1 and 2 day 1 (FLAG-GO) as frontline therapy in adult CBF-AML patients. Among the 45 enrolled patients, the FLAG-GO regimen was associated with a high remission rate (95%), and 3-year OS and RFS of 78% and 85% respectively [60]. A recent study assessed the benefit of the FLAG-GO regimen compared to the association FLAG and idarubicin regimen (FLAG-Ida). The FLAG-GO regimen was associated with a higher molecular response rate (76% vs. 42%, p = 0.002) and an improved 5-year RFS (87% vs. 68%, p = 0.02), but not 5-year OS (p = 0.7) compared to the FLAG-Ida regimen [61].
The standard of care for APL treatment relies on all-trans retinoic acid (ATRA) plus or minus arsenic trioxide (ATO)-based regimen [62,63]. However, APL blast cells express the CD33 antigen in nearly 100% of APL patients along with a low ABCB1 expression and offer the opportunity of a new treatment option for APL patients [64,65,66]. Administered as a single-agent or combined with ATRA, GO improved response rate of APL patients [67,68,69,70]. Interestingly, in vitro studies have reported the efficacy of GO in ATRA- or arsenic trioxide (ATO)-resistant APL cell lines which translated into remission achievement in clinical trials [65,71]. The combination of ATRA plus ATO with or without GO appeared safe and effective, with a CR rate of 90%, and 81% in high-risk patients [72]. These results were further confirmed in the phase III UK-NCRI AML17 trial [70]. In high-risk APL patients treated with ATRA, the addition of GO plus ATO was as effective as the adjunction of idarubicine (5-year OS: 84% vs. 100%, p = 0.453) [73]. Recently, the SWOG assessed the efficacy of ATRA plus ATO with GO combination in high-risk APL patients in a phase II trial (SWOG S0535). Among the 70 evaluable patients, 86% achieved CR and the 3-year OS and 3-year RFS were 86% and 91%, respectively [74]. Hence, this chemotherapy-free combination appears as a relevant option in high-risk APL patient management.
11q23/lysine methyltransferase 2A (KMT2A) rearrangements are recurrent cytogenetic alterations in AML, more commonly identified in children. These rearrangements correlate with elevated CD33 expression levels on leukemic cells [48,75]. Several reports have highlighted the anti-leukemic effect of GO in relapsed/refractory KMT2A-rearranged AML [76,77]. In the COG AAML0531 trial, 215 patients harbored a 11q23/KMT2A rearrangement. In this patient population, patients treated with GO plus chemotherapy experienced a significantly higher EFS than those treated with chemotherapy alone (5-year EFS: 48% vs. 28%, p = 0.002), although 5-year OS did not reach significant difference across treatment arms (5-year OS: 64% vs. 53%, p = 0.053) [78].
3.3. Prognostic Impact of the Molecular Profile on GO Efficacy
Aside from favorable cytogenetic alterations, GO seems to benefit patients with NPM1 or FLT3 mutations.
NPM1 mutations are identified in 25% to 35% of AML patients and more frequently in cytogenetically normal AML (45%–60%) [79]. As previously mentioned, patients with NPM1 mutations display higher CD33 expression levels [53,54,55]. In the ALFA-0701 trial, the subset analyses pointed out the benefit of the addition of GO on 2-year EFS, RFS and OS in NPM1-mutated patients [32]. Recently, the prospective, randomized phase III trial Acute Myeloid Leukemia Study Group (AMLSG) 09-09 addressed the clinical benefit of the addition of GO to induction (3 mg/m2 on day 1) and consolidation chemotherapy (3 mg/m2 on day 1 of the first consolidation cycle) in adult patients with NPM1-mutated AML eligible to receive intensive chemotherapy [80]. Among the 588 included patients, 292 were assigned to the GO arm and 296 to the standard arm. GO did not improve 2-year EFS in this trial (HR: 0.83, 95% CI: 0.65–1.04, p = 0.10). In patients achieving CR/CR with incomplete hematologic recovery, GO significantly decreased the cumulative incidence of relapse (HR: 0.66, 95% CI: 0.49–0.88, p = 0.005). Interestingly, GO improved 2-year EFS of FLT3 wild-type, but not FLT3-ITD mutated patients (HR: 0.72, 95% CI: 0.56–0.95 vs. HR: 1.53, 95% CI: 0.95–2.48, respectively; interaction test, p = 0.002).
FLT3-ITD mutations are found in approximately 20% of AML patients, and are associated with high expression of CD33 and impaired outcome [79]. The addition of GO has demonstrated improved OS, EFS and RFS in adult AML patients with FLT3-ITD mutations [32,56]. In a retrospective analysis of FLT3-ITD mutated patients from the COG AAML03P1 and AAML0531 trials, the addition of GO was associated with a decreased RR (37% vs. 59%, p = 0.02) [81]. Among the subset of patients who underwent hematopoietic stem cell transplantation (HSCT) in first CR, this effect was even stronger, prior exposure to GO was associated with a reduced cumulative incidence of relapse (22% vs. 56%, p = 0.003). Patients displaying a high allelic ratio (> 0.4) experienced a lower RR of relapse when GO was administered prior to HSCT (15% vs. 53%, p = 0.007). By contrast, in the adult cohorts from the MRC AML15 and NCRI AML16 trials, GO did not improve clinical outcome of FLT3-ITD mutated AML patients. However, in these trials GO was administered as a single dose while fractionated doses of GO were administered in the ALFA-0701 and the COG AAML03P1 and AAML0531 trials.
Mutational profile of AML has been widely deciphered by high-throughput sequencing technologies over the past decade [82]. Mutations have become strong prognostic factors and have been integrated in the latest European LeukemiaNet (ELN) 2017 risk stratification [83]. A retrospective analysis from the ALFA-0701 showed a benefit of the addition of GO on EFS in patients from the ELN favorable-risk (HR: 0.54, 95% CI: 0.30–0.98, p = 0.04) and intermediate-risk groups (HR: 0.57, 95% CI: 0.33–1.00, p = 0.05), but not in patients from the ELN adverse-risk group (HR: 0.93, 95% CI: 0.61–1.43, p = 0.74) [84]. In particular, considering mutations by functional group as previously described [82], GO predominantly improved EFS of patients harboring signaling mutations, (HR: 0.43, 95% CI: 0.28–0.65) [84]. These mutations were associated with higher CD33 expression levels.
3.4. Prognostic Impact of Minimal Residual Disease (MRD)
AML prognosis highly depends on pretreatment markers such as cytogenetic and molecular alterations. These prognostic factors have been integrated in the latest ELN 2017 risk classification and guide HSCT decision [83]. Growing evidence has suggested the prognostic impact of persisting leukemic cells assessed by the minimal disease monitoring after induction even in patients achieving morphological CR [85,86,87,88,89,90,91]. Different cytometric or molecular markers have been evaluated to monitor MRD [92]. In the pediatric AML02 trial, patients were allocated to either chemotherapy alone, chemotherapy plus GO, or GO alone depending on MRD levels, assessed by flow cytometry [93]. Among patients with positive MRD, 13 out of 17 reached MRD negativity after GO administration alone, and 13 out of 29 had negative MRD after GO plus chemotherapy [94]. In the NCRI-AML16 trial, MRD measured by flow cytometry accounted for an independent prognostic factor for patient outcome. However, this trial failed to demonstrate a significantly higher proportion of MRD negativity measured by flow cytometry among patients receiving GO compared to the control arm (57% vs. 48%, p = 0.18) [85].
NPM1 mutation and Wilms’ tumor 1 (WT1) gene overexpression represent valuable prognostic molecular markers to assess MRD by real-time quantitative polymerase chain reaction (RQ-PCR) [89,95,96,97,98,99,100,101]. In the ALFA-0701 study, the addition of GO to standard induction regimen increased NPM1 MRD negativity proportion both at the end of induction and at the end of treatment (39% vs. 7%, p = 0.006; and 91% vs. 61% p = 0.028, respectively) [97]. By contrast, no impact on WT1 MRD has been observed when adding GO to standard induction regimen neither after induction nor after end of treatment (MRD negativity: 75% vs. 65%, p = 0.29; 82% vs. 80%, p = 1, respectively). The lower sensitivity of WT1 MRD compared to NPM1 MRD may have accounted for this discrepancy [97].
These encouraging results of MRD monitoring in patients treated with GO gave rise to consider MRD as a surrogate endpoint for patient outcome. To date, several MRD-directed trials are currently investigating GO benefit [102].
3.5. GO and Stemness Signature
Different studies support the critical role of leukemic stem cells (LSC) in AML maintenance. LSC are characterized by intrinsic properties of cell cycle quiescence, self-renewal and increased drug efflux which confer chemotherapy resistance [8,103,104]. A recent study based on five independent AML cohorts (n = 908 patients) set up the LSC17 score, derived from a 17-gene expression signature for LSC [105]. This score stands for a strong prognostic factor in AML. Interestingly, in the ALFA-0701 trial, the addition of GO correlated with improved outcome in patients with low but not high LSC17 score (EFS: HR: 0.42, p = 0.001; RFS: HR: 0.53, p = 0.03). Hence, the LSC17 score appears as relevant biomarker to predict GO benefit in AML patients [105].
Patients harboring normal karyotype and NPM1 mutations without FLT3-ITD are assimilated to a low molecular risk (LMR). Interestingly, the GO12 score, derived from 12 GO pathway genes accurately identified LMR/LMR-like patients that may benefit from GO across 5 independent AML cohorts (n = 1188 patients; area under the curve: 80.8%) [106].
3.5.1. CD33 Single Nucleotide Polymorphisms
Recent studies have addressed the relationship between CD33 genotype and GO efficacy. A pivotal retrospective study from the St Jude (AML02 trial) found out a single nucleotide polymorphism (SNP) in the splice enhancer region of the CD33 gene exon 2, rs12459419 (C > T; Ala14Val) that affected response to GO, as measured by flow cytometric MRD [107]. This SNP resulted in CD33 exon 2 skipping, leading to a shorter CD33 isoform lacking the immunoglobulin-like V-set domain which is the epitope for GO and for the P67.6-CD33 antibody, used for CD33-expression determination by immunophenotyping [108,109]. In patients displaying TT genotype, median CD33 expression was significantly lower than those with CT or CC genotype (TT vs. CT vs. CC: 44.8% vs. 97.4% vs. 152.2%, p < 0.001) [110]. These first results were further confirmed in AML patients aged 0 to 29 years from the AAML0531 trial [111]. Among the 816 patients genotyped for the SNP rs12459419, 51%, 39% and 10% of the patients had CC, CT, TT genotype, respectively. A benefit of the addition of GO was demonstrated on both RR and RFS only in patient with CC genotype. A recent similar study undertaken in younger adults with AML (13–69 years) from the randomized MRC AML15 and NCRI AML17 trials showed a similar distribution of CC, CT and TT genotypes (47%, 44%, 9%, respectively). However, this study failed to demonstrate any benefit of GO on OS and on RFS in the different genotype subgroups [112]. Likewise, the prognostic value of the CD33 splice site genotype was evaluated in patients receiving an alternative CD33-targeting antibody-drug conjugate, the vadastuximab talirine (SGN-CD33A) administered as monotherapy or in combination with hypomethylating agents, in a cohort of 20 adult patients (mean age: 69.8 years, range: 27.5–82.6 years) with AML [113]. Genotyping analysis of CD33 SNP rs12459419 revealed the following distribution of the CC/CT/TT genotypes: 50%/40%/10%. Similarly to the previous study led in adult patients, CD33 splice site genotype did not impact patient outcome neither in OS (p = 0.923) nor in EFS (p = 0.683). Differences in trial designs including age range of inclusion and GO dosing may explain these discrepancies. The ABCB1-mediated drug efflux, which is higher in older patients, may have encountered for such differences between pediatric and adult populations [51,113,114,115].
Genotyping studies of CD33 SNPs have identified five other SNP such as rs1803254(G > C; 3′UTR), rs35112940(G > A; Arg304Gly), rs2455069(A > G; Arg69Gly), rs61736475(Ser305Pro) and rs201074739 (CCGG deletion) which may modulate GO anti-leukemic effect [116]. A reduced RR in GO recipients was observed in patients displaying the following genotypes: rs1803254 GG (p = 0.009), rs35112940 GG (p < 0.001), rs2455069 GG (p = 0.005), rs61736475 TT (p = 0.002), and rs201074739 CCGG/CCGG (p = 0.002).
Interestingly, the CD33_PGx6_Score—a composite score derived from six CD33 SNP of prognostic significance (rs12459419, rs2455069, rs201074739, rs35112940, rs61736475 and rs1803254)—has been recently set up to assess the impact of CD33 genotype on CD33 expression and GO response among 938 patients with de novo AML, aged 0–29 years [116]. CD33_PGx6_Score of 0 or higher was associated with higher CD33 expression levels and improved RFS and reduced RR in patients treated in GO arm (GO vs. no-GO arm, 5-year RFS: 62.5% vs. 46.8%, p = 0.008; 5-year RR: 28.3% vs. 49.9%, p < 0.001). By contrast, the addition of GO showed no improvement on patient outcome when the score was less than 0.
3.5.2. Prognostic Impact of ABCB1
Despite its pro-apoptotic effects, free calicheamicin may also be a substrate of the ABCB1 transporter (also known as permeability glycoprotein (Pgp) and multi-drug resistance protein (MDR1)) and to a lesser degree, the multidrug resistance-associated protein 1 (MRP1 or ABCC1), but not the breast cancer resistance protein (BCRP) [117,118,119]. Hence, ABCB1 and MRP1 may pump calicheamicin out of the cell before exerting its cytotoxic activity and ultimately compromise GO efficacy.
ABCB1 is expressed in 58% of AML patients and its expression on blasts cells varies from 19% to 75% [118,120]. ABCB1 expression strongly correlates with response to GO, and higher expression level of ABCB1 stands for an independent poor prognostic factor in OS and EFS [51,117,120,121]. Importantly, in a retrospective study from three phase II trials [25,42,117], the expression of CD33 was inversely correlated with the ABCB1 drug efflux activity. However, after adjusting for CD33 expression, ABCB1 was still associated with outcome (p < 0.001) [51].
Interestingly, a comprehensive analysis of ABCB1 demonstrated that ABCB1 expression was shown to correlate with low white blood cell count and high expression of the following genes: CD34, BAALC, CD7 and CD200 [120]. Additionally, ABCB1 activity seems to be linked to the absence of FLT3-ITD and NPM1 mutations.
A recent study has assessed the clinical impact of ABCB1 genotype among 942 patients from the COG-AAML0531 trial [122]. GO recipients displaying CT or TT genotype for the SNP rs1045642 (C > T; Ile1145Ile) had improved outcomes compared to those with CC genotype (CT or TT vs. CC, 5-year EFS: p = 0.022; 5-year RR: p = 0.007) as a result of an increased accumulation of calicheamicin.
3.5.3. SOCS3 Methylation
By binding to the CD33, SOCS3 induces the proteasomal degradation of the CD33-SOCS3 complex [6]. SOCS3 expression was suggested to modulate anti-CD33 antibodies response [123].
Analysis of the methylation status of the SOCS3 CpG islands was associated with a trend of improved response rate and OS in patients with SOCS3 hypermethylation (ORR: 86% vs. 56%, p = 0.17; OS: 25.1 months vs. 10.3 months; HR: 0.29%, 95% CI: 0.06–1.32, p = 0.09) [124].
3.5.4. HFE Mutations
HFE mutations are associated with higher risk of cancer. Interestingly, GO improved patient outcome among heterozygote HFE mutated patients compared to wild type patients, probably related to an impaired CD33 internalization [125].
4. Conclusions
Given its high expression on AML blasts, CD33 antigen represents an attractive target in AML. Different clinical trials have confirmed the anti-leukemic activity of GO in CD33-positive AML cells and have shown improved outcome in AML patients. Over the past years, flow cytometry, cytogenetics, and molecular approaches, including sequencing technologies, MRD monitoring, and genotyping studies of CD33 and ABCB1 SNPs have offered a comprehensive analysis of promising biomarkers for GO response. Collectively, these improvements have helped to refine the subset of patients that may benefit from GO and improve patient management. Increasing knowledge of the molecular alterations in AML paves the way to new combinatory regimens that may enhance GO efficacy. Hence, ongoing trials are evaluating the feasibility and the efficacy of combining GO to FLT3-ITD inhibitors (NCT03900949, NCT04385290, NCT04293562) and Bcl-2 inhibitors (NCT04070768, NCT04070768).
Author Contributions
L.F., E.F., M.C., T.B., F.G., S.C., N.B., J.L., H.D., C.P. and N.D. equally contributed to the manuscript writing and revision. All authors have read and agreed to the published version of the manuscript.
Funding
Laurène Fenwarth has received grants from Association Action Leucémies and Fondation Initiatives-Science–Innovation–Territoires–Economie, Université Lille Nord-Europe (I-SITE ULNE). This review was supported by the French National Cancer Institute (PRTK: TRANSLA10-060 and PHRC 2007/1911).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Abbreviations
| ABC | ATP-Binding Cassette |
| ABCB1 | ATP-Binding Cassette Subfamily B Member 1 |
| ALFA | Acute Leukemia French Association |
| AML | Acute Myeloid Leukemia |
| AMLSG | Acute Myeloid Leukemia Study Group |
| APL | Acute Promyelocytic Leukemia |
| ATM | Ataxia-Telangiectasia Mutated |
| ATR | Ataxia-Telangiectasia and Rad3-Related |
| ATRA | All-Trans Retinoic Acid |
| BCRP | Breast Cancer Resistance Protein |
| Bcl-2 | B-Cell Lymphoma 2 |
| BSC | Best Supportive Care |
| CBF | Core-Binding Factor |
| CD | Cluster Of Differentiation |
| COG | Children’s Oncology Group |
| CR | Complete Remission |
| CRp | Complete Remission Without Platelet Recovery |
| DA | Daunorubicin Plus Cytarabine |
| DAE | Cytarabine, Daunorubicin, And Etoposide |
| ECS | Elongin B/C-Cul2/Cul5-Socs-Box Protein |
| EFS | Event-Free Survival |
| ELN | European LeukemiaNet |
| EMA | European Medicines Agency |
| EORTC | European Organization For Research And Treatment Of Cancer |
| FADD | Fas-Associated Protein With Death Domain |
| FDA | Food And Drug Administration |
| FLAG-Ida | Fludarabine, Cytarabine, Granulocyte Colony-Stimulating Factor, and Idarubicin |
| FLT3-ITD | FMS-Like Tyrosine Kinase 3 Internal Tandem Duplication |
| GIMEMA | Gruppo Italiano Malattie Ematologiche Maligne Dell’adulto |
| GO | Gemtuzumab Ozogamicin |
| HR | Hazard Ratio |
| HSCT | Hematopoietic Stem Cell Transplantation |
| KMT2A | Lysine Methyltransferase 2A |
| KMT2A-r | Lysine Methyltransferase 2A Rearrangement |
| LMR | Low Molecular Risk |
| LSC score | Leukemic Stem Cell Score |
| MACE | Amsacrine, Cytarabine and Etoposide |
| MDS | Myelodysplastic Syndrome |
| MICE | Mitoxantrone, Cytarabine, and Etoposide |
| MidAC | Mitoxantrone and Cytarabine |
| MRC | Medical Research Council |
| MRD | Minimal Residual Disease |
| MRP1 | Multidrug Resistance-Associated Protein 1 |
| NCRI | National Cancer Research Institute |
| NPM1 | Nucleophosmin 1 gene |
| NS | Not Significant |
| ORR | Overall Response Rate |
| OS | Overall Survival |
| Pgp | Permeability Glycoprotein |
| Q | Quartile |
| RFS | Relapse-Free Survival |
| RQ-PCR | Real-Time Quantitative Polymerase Chain Reaction |
| RR | Risk Of Relapse |
| SHP | Src Homology-2 Domain-Containing Tyrosine Phosphatases |
| Siglec | Sialic-Acid-Binding Immunoglobulin-Like Lectins Family |
| SNP | Single Nucleotide Polymorphism |
| SOCS3 | Suppressor Of Cytokine Signaling 3 |
| SWOG | Southwest Oncology Group |
| TP53 | Tumor Protein 53 |
| Vs | Versus |
| WT1 | Wilms’ Tumor 1 Gene |
References
- Othus, M.; Kantarjian, H.; Petersdorf, S.; Ravandi, F.; Godwin, J.; Cortes, J.; Pierce, S.; Erba, H.; Faderl, S.; Appelbaum, F.R.; et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given “intense” induction regimens: A report from SWOG and MD anderson. Leukemia 2014, 28, 289–292. [Google Scholar] [CrossRef]
- Cowan, A.J.; Laszlo, G.S.; Estey, E.H.; Walter, R.B. Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin. Front. Biosci. 2013, 18, 1311–1334. [Google Scholar]
- Paul, S.P.; Taylor, L.S.; Stansbury, E.K.; McVicar, D.W. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 2000, 96, 483–490. [Google Scholar] [CrossRef]
- Godwin, C.D.; Gale, R.P.; Walter, R.B. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia 2017, 31, 1855–1868. [Google Scholar] [CrossRef]
- Taylor, V.C.; Buckley, C.D.; Douglas, M.; Cody, A.J.; Simmons, D.L.; Freeman, S.D. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J. Biol. Chem. 1999, 274, 11505–11512. [Google Scholar] [CrossRef]
- Orr, S.J.; Morgan, N.M.; Elliott, J.; Burrows, J.F.; Scott, C.J.; McVicar, D.W.; Johnston, J.A. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood 2007, 109, 1061–1068. [Google Scholar] [CrossRef]
- Andrews, R.G.; Takahashi, M.; Segal, G.M.; Powell, J.S.; Bernstein, I.D.; Singer, J.W. The L4F3 antigen is expressed by unipotent and multipotent colony-forming cells but not by their precursors. Blood 1986, 68, 1030–1035. [Google Scholar] [CrossRef]
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807. [Google Scholar] [CrossRef]
- Walter, R.B.; Appelbaum, F.R.; Estey, E.H.; Bernstein, I.D. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 2012, 119, 6198–6208. [Google Scholar] [CrossRef]
- Welch, J.S.; Ley, T.J.; Link, D.C.; Miller, C.A.; Larson, D.E.; Koboldt, D.C.; Wartman, L.D.; Lamprecht, T.L.; Liu, F.; Xia, J.; et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012, 150, 264–278. [Google Scholar] [CrossRef]
- Griffin, J.D.; Linch, D.; Sabbath, K.; Larcom, P.; Schlossman, S.F. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk. Res. 1984, 8, 521–534. [Google Scholar] [CrossRef]
- Dinndorf, P.A.; Andrews, R.G.; Benjamin, D.; Ridgway, D.; Wolff, L.; Bernstein, I.D. Expression of normal myeloid-associated antigens by acute leukemia cells. Blood 1986, 67, 1048–1053. [Google Scholar] [CrossRef]
- Hamann, P.R.; Hinman, L.M.; Hollander, I.; Beyer, C.F.; Lindh, D.; Holcomb, R.; Hallett, W.; Tsou, H.-R.; Upeslacis, J.; Shochat, D.; et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 2002, 13, 47–58. [Google Scholar] [CrossRef]
- van Der Velden, V.H.; te Marvelde, J.G.; Hoogeveen, P.G.; Bernstein, I.D.; Houtsmuller, A.B.; Berger, M.S.; van Dongen, J.J. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: In vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 2001, 97, 3197–3204. [Google Scholar] [CrossRef]
- Hamann, P.R.; Hinman, L.M.; Beyer, C.F.; Lindh, D.; Upeslacis, J.; Flowers, D.A.; Bernstein, I. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug. Chem. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Elmroth, K.; Nygren, J.; Mårtensson, S.; Ismail, I.H.; Hammarsten, O. Cleavage of cellular DNA by calicheamicin gamma1. DNA Repair 2003, 2, 363–374. [Google Scholar] [CrossRef]
- Linenberger, M.L. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: Progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia 2005, 19, 176–182. [Google Scholar] [CrossRef]
- Amico, D.; Barbui, A.M.; Erba, E.; Rambaldi, A.; Introna, M.; Golay, J. Differential response of human acute myeloid leukemia cells to gemtuzumab ozogamicin in vitro: Role of Chk1 and Chk2 phosphorylation and caspase 3. Blood 2003, 101, 4589–4597. [Google Scholar] [CrossRef]
- Mårtensson, S.; Nygren, J.; Osheroff, N.; Hammarsten, O. Activation of the DNA-dependent protein kinase by drug-induced and radiation-induced DNA strand breaks. Radiat. Res. 2003, 160, 291–301. [Google Scholar] [CrossRef]
- Sullivan, N.; Lyne, L. Sensitivity of fibroblasts derived from ataxia-telangiectasia patients to calicheamicin γ1I. Mutat. Res. Lett. 1990, 245, 171–175. [Google Scholar] [CrossRef]
- Prokop, A.; Wrasidlo, W.; Lode, H.; Herold, R.; Lang, F.; Henze, G.; Dörken, B.; Wieder, T.; Daniel, P.T. Induction of apoptosis by enediyne antibiotic calicheamicin thetaII proceeds through a caspase-mediated mitochondrial amplification loop in an entirely Bax-dependent manner. Oncogene 2003, 22, 9107–9120. [Google Scholar] [CrossRef]
- Haag, P.; Viktorsson, K.; Lindberg, M.L.; Kanter, L.; Lewensohn, R.; Stenke, L. Deficient activation of Bak and Bax confers resistance to gemtuzumab ozogamicin-induced apoptotic cell death in AML. Exp. Hematol. 2009, 37, 755–766. [Google Scholar] [CrossRef]
- Moore, J.; Seiter, K.; Kolitz, J.; Stock, W.; Giles, F.; Kalaycio, M.; Zenk, D.; Marcucci, G. A Phase II study of Bcl-2 antisense (oblimersen sodium) combined with gemtuzumab ozogamicin in older patients with acute myeloid leukemia in first relapse. Leuk. Res. 2006, 30, 777–783. [Google Scholar] [CrossRef]
- Sievers, E.L.; Appelbaum, F.R.; Spielberger, R.T.; Forman, S.J.; Flowers, D.; Smith, F.O.; Shannon-Dorcy, K.; Berger, M.S.; Bernstein, I.D. Selective Ablation of Acute Myeloid Leukemia Using Antibody-Targeted Chemotherapy: A Phase I Study of an Anti-CD33 Calicheamicin ImmunoconjugatePresented in part at the 1997 Annual Meeting of the American Society of Clinical Oncology, Denver, CO; the 1997 European Cancer Conference, Hamburg, Germany; and the 1997 Annual Meeting of the American Society of Hematology, San Diego, CA. Blood 1999, 93, 3678–3684. [Google Scholar]
- Sievers, E.L.; Larson, R.A.; Stadtmauer, E.A.; Estey, E.; Löwenberg, B.; Dombret, H.; Karanes, C.; Theobald, M.; Bennett, J.M.; Sherman, M.L.; et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 3244–3254. [Google Scholar] [CrossRef]
- Taksin, A.-L.; Legrand, O.; Raffoux, E.; de Revel, T.; Thomas, X.; Contentin, N.; Bouabdallah, R.; Pautas, C.; Turlure, P.; Reman, O.; et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: A prospective study of the alfa group. Leukemia 2007, 21, 66–71. [Google Scholar] [CrossRef]
- Farhat, H.; Reman, O.; Raffoux, E.; Berthon, C.; Pautas, C.; Kammoun, L.; Chantepie, S.; Gardin, C.; Rousselot, P.; Chevret, S.; et al. Fractionated doses of gemtuzumab ozogamicin with escalated doses of daunorubicin and cytarabine as first acute myeloid leukemia salvage in patients aged 50–70-year old: A phase 1/2 study of the acute leukemia French association. Am. J. Hematol. 2012, 87, 62–65. [Google Scholar] [CrossRef]
- Burnett, A.K.; Hills, R.K.; Milligan, D.; Kjeldsen, L.; Kell, J.; Russell, N.H.; Yin, J.A.L.; Hunter, A.; Goldstone, A.H.; Wheatley, K. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 369–377. [Google Scholar] [CrossRef]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Kell, J.; Freeman, S.; Kjeldsen, L.; Hunter, A.E.; Yin, J.; Craddock, C.F.; Dufva, I.H.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3924–3931. [Google Scholar] [CrossRef]
- Delaunay, J.; Recher, C.; Pigneux, A.; Witz, F.; Vey, N.; Blanchet, O.; Lefebvre, P.; Luquet, I.; Guillerme, I.; Volteau, C.; et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. results of the GOELAMS AML 2006 IR study. Blood 2011, 118, 79. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet Lond. Engl. 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Burnett, A.; Cavenagh, J.; Russell, N.; Hills, R.; Kell, J.; Jones, G.; Nielsen, O.J.; Khwaja, A.; Thomas, I.; Clark, R.; et al. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: A comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 trial. Haematologica 2016, 101, 724–731. [Google Scholar] [CrossRef]
- Amadori, S.; Suciu, S.; Stasi, R.; Salih, H.R.; Selleslag, D.; Muus, P.; De Fabritiis, P.; Venditti, A.; Ho, A.D.; Lübbert, M.; et al. Sequential combination of gemtuzumab ozogamicin and standard chemotherapy in older patients with newly diagnosed acute myeloid leukemia: Results of a randomized phase III trial by the EORTC and GIMEMA consortium (AML-17). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 4424–4430. [Google Scholar] [CrossRef]
- Amadori, S.; Suciu, S.; Selleslag, D.; Aversa, F.; Gaidano, G.; Musso, M.; Annino, L.; Venditti, A.; Voso, M.T.; Mazzone, C.; et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC-GIMEMA AML-19 trial. J. Clin. Oncol. 2016, 34, 972–979. [Google Scholar] [CrossRef]
- Arceci, R.J.; Sande, J.; Lange, B.; Shannon, K.; Franklin, J.; Hutchinson, R.; Vik, T.A.; Flowers, D.; Aplenc, R.; Berger, M.S.; et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood 2005, 106, 1183–1188. [Google Scholar] [CrossRef]
- Aplenc, R.; Alonzo, T.A.; Gerbing, R.B.; Lange, B.J.; Hurwitz, C.A.; Wells, R.J.; Bernstein, I.; Buckley, P.; Krimmel, K.; Smith, F.O.; et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: A report from the children’s oncology group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2390–3295. [Google Scholar] [CrossRef]
- Cooper, T.M.; Franklin, J.; Gerbing, R.B.; Alonzo, T.A.; Hurwitz, C.; Raimondi, S.C.; Hirsch, B.; Smith, F.O.; Mathew, P.; Arceci, R.J.; et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the children’s oncology group. Cancer 2012, 118, 761–769. [Google Scholar] [CrossRef]
- Gamis, A.S.; Alonzo, T.A.; Meshinchi, S.; Sung, L.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Kahwash, S.B.; Heerema-McKenney, A.; Winter, L.; et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III children’s oncology group trial AAML0531. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3021–3032. [Google Scholar] [CrossRef]
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496. [Google Scholar] [PubMed]
- Larson, R.A.; Sievers, E.L.; Stadtmauer, E.A.; Löwenberg, B.; Estey, E.H.; Dombret, H.; Theobald, M.; Voliotis, D.; Bennett, J.M.; Richie, M.; et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005, 104, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Suciu, S.; Selleslag, D.; Stasi, R.; Alimena, G.; Baila, L.; Rizzoli, V.; Borlenghi, E.; Gaidano, G.; Magro, D.; et al. Randomized trial of two schedules of low-dose gemtuzumab ozogamicin as induction monotherapy for newly diagnosed acute myeloid leukaemia in older patients not considered candidates for intensive chemotherapy. A phase II study of the EORTC and GIMEMA leukaemia groups (AML-19). Br. J. Haematol. 2010, 149, 376–382. [Google Scholar] [PubMed]
- Kell, W.J.; Burnett, A.K.; Chopra, R.; Yin, J.A.L.; Clark, R.E.; Rohatiner, A.; Culligan, D.; Hunter, A.; Prentice, A.G.; Milligan, D.W. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood 2003, 102, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Pilorge, S.; Rigaudeau, S.; Rabian, F.; Sarkozy, C.; Taksin, A.L.; Farhat, H.; Merabet, F.; Ghez, S.; Raggueneau, V.; Terré, C.; et al. Fractionated gemtuzumab ozogamicin and standard dose cytarabine produced prolonged second remissions in patients over the age of 55 years with acute myeloid leukemia in late first relapse. Am. J. Hematol. 2014, 89, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Pollard, J.A.; Alonzo, T.A.; Loken, M.; Gerbing, R.B.; Ho, P.A.; Bernstein, I.D.; Raimondi, S.C.; Hirsch, B.; Franklin, J.; Walter, R.B.; et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood 2012, 119, 3705–3711. [Google Scholar] [CrossRef]
- Pollard, J.A.; Loken, M.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Aplenc, R.; Bernstein, I.D.; Gamis, A.S.; Alonzo, T.A.; Meshinchi, S. CD33 expression and its association with gemtuzumab ozogamicin response: Results from the randomized phase III children’s oncology group trial AAML0531. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 747–755. [Google Scholar] [CrossRef]
- Dinndorf, P.A.; Buckley, J.D.; Nesbit, M.E.; Lampkin, B.C.; Piomelli, S.; Feig, S.A.; Kersey, J.H.; Hammond, G.D.; Bernstein, I.D. Expression of myeloid differentiation antigens in acute nonlymphocytic leukemia: Increased concentration of CD33 antigen predicts poor outcome—A report from the childrens cancer study group. Med. Pediatr. Oncol. 1992, 20, 192–200. [Google Scholar] [CrossRef]
- Walter, R.B.; Raden, B.W.; Kamikura, D.M.; Cooper, J.A.; Bernstein, I.D. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood 2005, 105, 1295–1302. [Google Scholar] [CrossRef]
- Walter, R.B.; Gooley, T.A.; van der Velden, V.H.J.; Loken, M.R.; van Dongen, J.J.M.; Flowers, D.A.; Bernstein, I.D.; Appelbaum, F.R. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 2007, 109, 4168–4170. [Google Scholar] [CrossRef]
- Khan, N.; Hills, R.K.; Virgo, P.; Couzens, S.; Clark, N.; Gilkes, A.; Richardson, P.; Knapper, S.; Grimwade, D.; Russell, N.H.; et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia 2017, 31, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- De Propris, M.S.; Raponi, S.; Diverio, D.; Milani, M.L.; Meloni, G.; Falini, B.; Foà, R.; Guarini, A. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica 2011, 96, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, A.; Kramer, M.; Röllig, C.; Thiede, C.; Bornhäuser, M.; von Bonin, M.; Wermke, M.; Feldmann, A.; Bachmann, M.; Ehninger, G.; et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014, 4, e218. [Google Scholar] [CrossRef] [PubMed]
- Olombel, G.; Guerin, E.; Guy, J.; Perrot, J.-Y.; Dumezy, F.; de Labarthe, A.; Bastie, J.-N.; Legrand, O.; Raffoux, E.; Plesa, A.; et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Blood 2016, 127, 2157–2160. [Google Scholar] [CrossRef] [PubMed]
- Renneville, A.; Abdelali, R.B.; Chevret, S.; Nibourel, O.; Cheok, M.; Pautas, C.; Duléry, R.; Boyer, T.; Cayuela, J.-M.; Hayette, S.; et al. Clinical impact of gene mutations and lesions detected by SNP-array karyotyping in acute myeloid leukemia patients in the context of gemtuzumab ozogamicin treatment: Results of the ALFA-0701 trial. Oncotarget 2014, 5, 916–932. [Google Scholar] [CrossRef]
- Wiemels, J.L.; Xiao, Z.; Buffler, P.A.; Maia, A.T.; Ma, X.; Dicks, B.M.; Smith, M.T.; Zhang, L.; Feusner, J.; Wiencke, J.; et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood 2002, 99, 3801–3805. [Google Scholar] [CrossRef]
- Jourdan, E.; Boissel, N.; Chevret, S.; Delabesse, E.; Renneville, A.; Cornillet, P.; Blanchet, O.; Cayuela, J.-M.; Recher, C.; Raffoux, E.; et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 2013, 121, 2213–2223. [Google Scholar] [CrossRef]
- Appelbaum, F.R.; Bernstein, I.D. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood 2017, 130, 2373–2376. [Google Scholar] [CrossRef]
- Borthakur, G.; Cortes, J.E.; Estey, E.E.; Jabbour, E.; Faderl, S.; O′Brien, S.; Garcia-Manero, G.; Kadia, T.M.; Wang, X.; Patel, K.; et al. Gemtuzumab ozogamicin with fludarabine, cytarabine, and granulocyte colony stimulating factor (FLAG-GO) as front-line regimen in patients with core binding factor acute myelogenous leukemia. Am. J. Hematol. 2014, 89, 964–968. [Google Scholar] [CrossRef]
- Borthakur, G.M.; Cortes, J.E.; Ravandi, F.; Garcia-Manero, G.; Kadia, T.M.; Jabbour, E.; Patel, K.; Issa, G.C.; Daver, N.G.; Ohanian, M.N.; et al. Fludarabine, cytarabine, G-CSF and gemtuzumab ozogamicin (FLAG-GO) regimen results in better molecular response and relapse-free survival in core binding factor acute myeloid leukemia than FLAG and idarubicin (FLAG-Ida). Blood 2019, 134, 290. [Google Scholar] [CrossRef]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.-J.; et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European leukemianet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef] [PubMed]
- Michieli, M.; Damiani, D.; Ermacora, A.; Geromin, A.; Michelutti, A.; Masolini, P.; Baccarani, M. P-glycoprotein (PGP), lung resistance-related protein (LRP) and multidrug resistance-associated protein (MRP) expression in acute promyelocytic leukaemia. Br. J. Haematol. 2000, 108, 703–709. [Google Scholar] [CrossRef]
- Takeshita, A.; Shinjo, K.; Naito, K.; Matsui, H.; Sahara, N.; Shigeno, K.; Horii, T.; Shirai, N.; Maekawa, M.; Ohnishi, K.; et al. Efficacy of gemtuzumab ozogamicin on ATRA- and arsenic-resistant acute promyelocytic leukemia (APL) cells. Leukemia 2005, 19, 1306–1311. [Google Scholar] [CrossRef]
- Breccia, M.; Lo-Coco, F. Gemtuzumab ozogamicin for the treatment of acute promyelocytic leukemia: Mechanisms of action and resistance, safety and efficacy. Expert Opin. Biol. Ther. 2011, 11, 225–234. [Google Scholar] [CrossRef]
- Estey, E.H.; Giles, F.J.; Beran, M.; O’Brien, S.; Pierce, S.A.; Faderl, S.H.; Cortes, J.E.; Kantarjian, H.M. Experience with gemtuzumab ozogamycin (“mylotarg”) and all-trans retinoic acid in untreated acute promyelocytic leukemia. Blood 2002, 99, 4222–4224. [Google Scholar] [CrossRef]
- Lo-Coco, F.; Cimino, G.; Breccia, M.; Noguera, N.I.; Diverio, D.; Finolezzi, E.; Pogliani, E.M.; Di Bona, E.; Micalizzi, C.; Kropp, M.; et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood 2004, 104, 1995–1999. [Google Scholar] [CrossRef]
- Breccia, M.; Cimino, G.; Diverio, D.; Gentilini, F.; Mandelli, F.; Lo Coco, F. Sustained molecular remission after low dose gemtuzumab-ozogamicin in elderly patients with advanced acute promyelocytic leukemia. Haematologica 2007, 92, 1273–1274. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Bowen, D.; Kell, J.; Knapper, S.; Morgan, Y.G.; Lok, J.; Grech, A.; Jones, G.; et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): Results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 1295–1305. [Google Scholar] [CrossRef]
- Takeshita, A.; Shinjo, K.; Naito, K.; Matsui, H.; Sahara, N.; Shigeno, K.; Suzumura, T.; Horii, T.; Shirai, N.; Maekawa, M.; et al. Two patients with all-trans retinoic acid-resistant acute promyelocytic leukemia treated successfully with gemtuzumab ozogamicin as a single agent. Int. J. Hematol. 2005, 82, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Estey, E.; Jones, D.; Faderl, S.; O′Brien, S.; Fiorentino, J.; Pierce, S.; Blamble, D.; Estrov, Z.; Wierda, W.; et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Abaza, Y.; Kantarjian, H.; Garcia-Manero, G.; Estey, E.; Borthakur, G.; Jabbour, E.; Faderl, S.; O′Brien, S.; Wierda, W.; Pierce, S.; et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 2017, 129, 1275–1283. [Google Scholar] [CrossRef]
- Lancet, J.E.; Moseley, A.B.; Coutre, S.E.; DeAngelo, D.J.; Othus, M.; Tallman, M.S.; Litzow, M.R.; Komrokji, R.S.; Erba, H.P.; Appelbaum, F.R. A phase 2 study of ATRA, arsenic trioxide, and gemtuzumab ozogamicin in patients with high-risk APL (SWOG 0535). Blood Adv. 2020, 4, 1683–1689. [Google Scholar] [CrossRef]
- Muñoz, L.; Nomdedéu, J.F.; Villamor, N.; Guardia, R.; Colomer, D.; Ribera, J.M.; Torres, J.P.; Berlanga, J.J.; Fernández, C.; Llorente, A.; et al. Acute myeloid leukemia with MLL rearrangements: Clinicobiological features, prognostic impact and value of flow cytometry in the detection of residual leukemic cells. Leukemia 2003, 17, 76–82. [Google Scholar] [CrossRef]
- Tamai, H.; Shioi, Y.; Yamaguchi, H.; Okabe, M.; Wakita, S.; Mizuki, T.; Nakayama, K.; Inokuchi, K.; Tajika, K.; Dan, K. Treatment of relapsed acute myeloid leukemia with MLL/AF6 fusion after allogeneic hematopoietic stem cell transplantation with gemtuzumab ozogamicin with a long interval followed by donor lymphocyte infusion. Leukemia 2008, 22, 1273–1274. [Google Scholar] [CrossRef][Green Version]
- Asano, H.; Yamamoto, G.; Hosoi, M.; Takahashi, T.; Hangaishi, A.; Kurokawa, M. Complete molecular remission in refractory acute myeloid leukemia with MLL/AF9 treated with gemtuzumab ozogamicin. Leuk. Res. 2010, 34, e152–e153. [Google Scholar] [CrossRef]
- Pollard, J.; Alonzo, T.A.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Sung, L.; Aplenc, R.; Guest, E.M.; Bernstein, I.D.; Loken, M.R.; et al. Treatment of 11q23/MLL + AML with gemtuzumab ozogamicin: Results from the randomized phase III children’s oncology group trial AAML0531. Blood 2015, 126, 799. [Google Scholar] [CrossRef]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Paschka, P.; Krzykalla, J.; Weber, D.; Kapp-Schwoerer, S.; Gaidzik, V.I.; Leis, C.; Fiedler, W.; Kindler, T.; Schroeder, T.; et al. Gemtuzumab ozogamicin in NPM1-mutated acute myeloid leukemia: Early results from the prospective randomized AMLSG 09-09 phase III study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 623–632. [Google Scholar] [CrossRef]
- Tarlock, K.; Alonzo, T.A.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Sung, L.; Pollard, J.A.; Aplenc, R.; Loken, M.R.; Gamis, A.S.; et al. Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: A report from the children’s oncology group. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Duployez, N.; Ducourneau, B.; Raffoux, E.; Turlure, P.; Caillot, D.; Thomas, X.; Marceau-Renaut, A.; Chantepie, S.; Malfuson, J.-V.; et al. Mutational profile and benefit of gemtuzumab ozogamicin in acute myeloid leukemia. Blood 2020, 135, 542–546. [Google Scholar] [CrossRef]
- Freeman, S.D.; Virgo, P.; Couzens, S.; Grimwade, D.; Russell, N.; Hills, R.K.; Burnett, A.K. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 4123–4131. [Google Scholar] [CrossRef]
- Freeman, S.D.; Hills, R.K.; Virgo, P.; Khan, N.; Couzens, S.; Dillon, R.; Gilkes, A.; Upton, L.; Nielsen, O.J.; Cavenagh, J.D.; et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J. Clin. Oncol. 2018, 36, 1486–1497. [Google Scholar] [CrossRef]
- Chen, X.; Xie, H.; Wood, B.L.; Walter, R.B.; Pagel, J.M.; Becker, P.S.; Sandhu, V.K.; Abkowitz, J.L.; Appelbaum, F.R.; Estey, E.H. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J. Clin. Oncol. 2015, 33, 1258–1264. [Google Scholar] [CrossRef]
- Walter, R.B.; Gyurkocza, B.; Storer, B.E.; Godwin, C.D.; Pagel, J.M.; Buckley, S.A.; Sorror, M.L.; Wood, B.L.; Storb, R.; Appelbaum, F.R.; et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia 2015, 29, 137–144. [Google Scholar] [CrossRef]
- Balsat, M.; Renneville, A.; Thomas, X.; de Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.-P.; Nibourel, O.; Berthon, C. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: A study by the acute leukemia french association group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular minimal residual disease in acute myeloid leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European leukemianet MRD working party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Rubnitz, J.E.; Inaba, H.; Dahl, G.; Ribeiro, R.C.; Bowman, W.P.; Taub, J.; Pounds, S.; Razzouk, B.I.; Lacayo, N.J.; Cao, X.; et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010, 11, 543–552. [Google Scholar] [CrossRef]
- O′Hear, C.; Inaba, H.; Pounds, S.; Shi, L.; Dahl, G.; Bowman, W.P.; Taub, J.W.; Pui, C.-H.; Ribeiro, R.C.; Coustan-Smith, E.; et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer 2013, 119, 4036–4043. [Google Scholar] [CrossRef]
- Lapillonne, H.; Renneville, A.; Auvrignon, A.; Flamant, C.; Blaise, A.; Perot, C.; Lai, J.-L.; Ballerini, P.; Mazingue, F.; Fasola, S.; et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 1507–1515. [Google Scholar] [CrossRef]
- Cilloni, D.; Renneville, A.; Hermitte, F.; Hills, R.K.; Daly, S.; Jovanovic, J.V.; Gottardi, E.; Fava, M.; Schnittger, S.; Weiss, T.; et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: A European Leukemianet study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5195–5201. [Google Scholar] [CrossRef]
- Lambert, J.; Lambert, J.; Nibourel, O.; Pautas, C.; Hayette, S.; Cayuela, J.-M.; Terré, C.; Rousselot, P.; Dombret, H.; Chevret, S.; et al. MRD assessed by WT1 and NPM1 transcript levels identifies distinct outcomes in AML patients and is influenced by gemtuzumab ozogamicin. Oncotarget 2014, 5, 6280–6288. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of minimal residual disease in standard-risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef]
- Kayser, S.; Benner, A.; Thiede, C.; Martens, U.; Huber, J.; Stadtherr, P.; Janssen, J.W.G.; Röllig, C.; Uppenkamp, M.J.; Bochtler, T.; et al. Pretransplant NPM1 MRD levels predict outcome after allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia. Blood Cancer J. 2016, 6, e449. [Google Scholar] [CrossRef]
- Candoni, A.; De Marchi, F.; Zannier, M.E.; Lazzarotto, D.; Filì, C.; Dubbini, M.V.; Rabassi, N.; Toffoletti, E.; Lau, B.W.; Fanin, R. High prognostic value of pre-allogeneic stem cell transplantation minimal residual disease detection by WT1 gene expression in AML transplanted in cytologic complete remission. Leuk. Res. 2017, 63, 22–27. [Google Scholar] [CrossRef]
- Dillon, R.; Hills, R.; Freeman, S.; Potter, N.; Jovanovic, J.; Ivey, A.; Kanda, A.S.; Runglall, M.; Foot, N.; Valganon, M.; et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood 2020, 135, 680–688. [Google Scholar] [CrossRef]
- Ball, B.; Stein, E.M. Which are the most promising targets for minimal residual disease-directed therapy in acute myeloid leukemia prior to allogeneic stem cell transplant? Haematologica 2019, 104, 1521–1531. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Thomas, D.; Majeti, R. Biology and relevance of human acute myeloid leukemia stem cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Erwin, S.E.; Mitchell, A.; Minden, M.D.; Bullinger, L.; Döhner, H.; Dombret, H.; Preudhomme, C.; Cheok, M.; Dick, J.E.; et al. A novel predictor of response to gemtuzumab ozogamicin therapy in AML provides strategies for sensitization of leukemia stem cells in individual patients. Blood 2018, 132, 2765. [Google Scholar] [CrossRef]
- Lamba, J.K.; Pounds, S.; Cao, X.; Downing, J.R.; Campana, D.; Ribeiro, R.C.; Pui, C.-H.; Rubnitz, J.E. Coding polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML: A pilot study. Leukemia 2009, 23, 402–404. [Google Scholar] [CrossRef]
- Raj, T.; Ryan, K.J.; Replogle, J.M.; Chibnik, L.B.; Rosenkrantz, L.; Tang, A.; Rothamel, K.; Stranger, B.E.; Bennett, D.A.; Evans, D.A.; et al. CD33: Increased inclusion of exon 2 implicates the Ig V-set domain in alzheimer’s disease susceptibility. Hum. Mol. Genet. 2014, 23, 2729–2736. [Google Scholar] [CrossRef]
- Malik, M.; Chiles, J.; Xi, H.S.; Medway, C.; Simpson, J.; Potluri, S.; Howard, D.; Liang, Y.; Paumi, C.M.; Mukherjee, S.; et al. Genetics of CD33 in alzheimer’s disease and acute myeloid leukemia. Hum. Mol. Genet. 2015, 24, 3557–3570. [Google Scholar] [CrossRef]
- Mortland, L.; Alonzo, T.A.; Walter, R.B.; Gerbing, R.B.; Mitra, A.K.; Pollard, J.A.; Loken, M.R.; Hirsch, B.; Raimondi, S.; Franklin, J.; et al. Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 1620–1627. [Google Scholar] [CrossRef]
- Lamba, J.K.; Chauhan, L.; Shin, M.; Loken, M.R.; Pollard, J.A.; Wang, Y.-C.; Ries, R.E.; Aplenc, R.; Hirsch, B.A.; Raimondi, S.C.; et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: Report from randomized phase III children’s oncology group trial AAML0531. J. Clin. Oncol. 2017, 35, 2674–2682. [Google Scholar] [CrossRef]
- Gale, R.E.; Popa, T.; Wright, M.; Khan, N.; Freeman, S.D.; Burnett, A.K.; Russell, N.H.; Hills, R.K.; Linch, D.C. No evidence that CD33 splicing SNP impacts the response to GO in younger adults with AML treated on UK MRC/NCRI trials. Blood 2018, 131, 468–471. [Google Scholar] [CrossRef]
- Stanchina, M.; Pastore, A.; Devlin, S.; Famulare, C.; Stein, E.; Taylor, J. CD33 splice site genotype was not associated with outcomes of patients receiving the anti-CD33 drug conjugate SGN-CD33A. J. Hematol. Oncol. 2019, 12, 85. [Google Scholar] [CrossRef]
- Leith, C.P.; Kopecky, K.J.; Chen, I.M.; Eijdems, L.; Slovak, M.L.; McConnell, T.S.; Head, D.R.; Weick, J.; Grever, M.R.; Appelbaum, F.R.; et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: A southwest oncology group study. Blood 1999, 94, 1086–1099. [Google Scholar]
- Laszlo, G.S.; Beddoe, M.E.; Godwin, C.D.; Bates, O.M.; Gudgeon, C.J.; Harrington, K.H.; Walter, R.B. Relationship between CD33 expression, splicing polymorphism, and in vitro cytotoxicity of gemtuzumab ozogamicin and the CD33/CD3 BiTE ® AMG 330. Haematologica 2019, 104, e59–e62. [Google Scholar] [CrossRef]
- Chauhan, L.; Shin, M.; Wang, Y.-C.; Loken, M.; Pollard, J.; Aplenc, R.; Hirsch, B.A.; Raimondi, S.; Ries, R.E.; Bernstein, I.D. CD33_PGx6_score predicts gemtuzumab ozogamicin response in childhood acute myeloid leukemia: A report from the children’s oncology group. JCO Precis. Oncol. 2019, 3, 1–15. [Google Scholar] [CrossRef]
- Linenberger, M.L.; Hong, T.; Flowers, D.; Sievers, E.L.; Gooley, T.A.; Bennett, J.M.; Berger, M.S.; Leopold, L.H.; Appelbaum, F.R.; Bernstein, I.D. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood 2001, 98, 988–994. [Google Scholar] [CrossRef]
- Walter, R.B.; Raden, B.W.; Hong, T.C.; Flowers, D.A.; Bernstein, I.D.; Linenberger, M.L. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood 2003, 102, 1466–1473. [Google Scholar] [CrossRef]
- Walter, R.B.; Raden, B.W.; Thompson, J.; Flowers, D.A.; Kiem, H.-P.; Bernstein, I.D.; Linenberger, M.L. Breast cancer resistance protein (BCRP/ABCG2) does not confer resistance to gemtuzumab ozogamicin and calicheamicin-gamma1 in acute myeloid leukemia cells. Leukemia 2004, 18, 1914–1917. [Google Scholar] [CrossRef]
- Boyer, T.; Gonzales, F.; Barthélémy, A.; Marceau-Renaut, A.; Peyrouze, P.; Guihard, S.; Lepelley, P.; Plesa, A.; Nibourel, O.; Delattre, C.; et al. Clinical significance of ABCB1 in acute myeloid leukemia: A comprehensive study. Cancers 2019, 11, 1323. [Google Scholar] [CrossRef]
- Van Den Heuvel-Eibrink, M.M.; Van Der Holt, B.; Te Boekhorst, P.A.; Pieters, R.; Schoester, M.; Löwenberg, B.; Sonneveld, P. MDR 1 expression is an independent prognostic factor for response and survival in de novo acute myeloid leukaemia. Br. J. Haematol. 1997, 99, 76–83. [Google Scholar] [CrossRef]
- Rafiee, R.; Chauhan, L.; Alonzo, T.A.; Wang, Y.-C.; Elmasry, A.; Loken, M.R.; Pollard, J.; Aplenc, R.; Raimondi, S.; Hirsch, B.A.; et al. ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin: A report from children’s oncology group AAML0531 trial. Blood Cancer J. 2019, 9, 51. [Google Scholar] [CrossRef]
- Ball, E.D. Pairing SOCS with CD33. Blood 2007, 109, 852. [Google Scholar] [CrossRef]
- Middeldorf, I.; Galm, O.; Osieka, R.; Jost, E.; Herman, J.G.; Wilop, S. Sequence of administration and methylation of SOCS3 may govern response to gemtuzumab ozogamicin in combination with conventional chemotherapy in patients with refractory or relapsed acute myelogenous leukemia (AML). Am. J. Hematol. 2010, 85, 477–481. [Google Scholar] [CrossRef]
- Paubelle, E.; Marceau, A.; Zylbersztejn, F.; Dussiot, M.; Moura, I.C.; Cornillet-Lefebvre, P.; Delaunay, J.; Burnett, A.K.; Castaigne, S.; Guardiola, P.; et al. HFE gene mutation status predicts response to gemtuzumab ozogamicin in AML. Blood 2015, 126, 1307. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).