Pyridoxine Deficiency Exacerbates Neuronal Damage after Ischemia by Increasing Oxidative Stress and Reduces Proliferating Cells and Neuroblasts in the Gerbil Hippocampus
Abstract
1. Introduction
2. Results
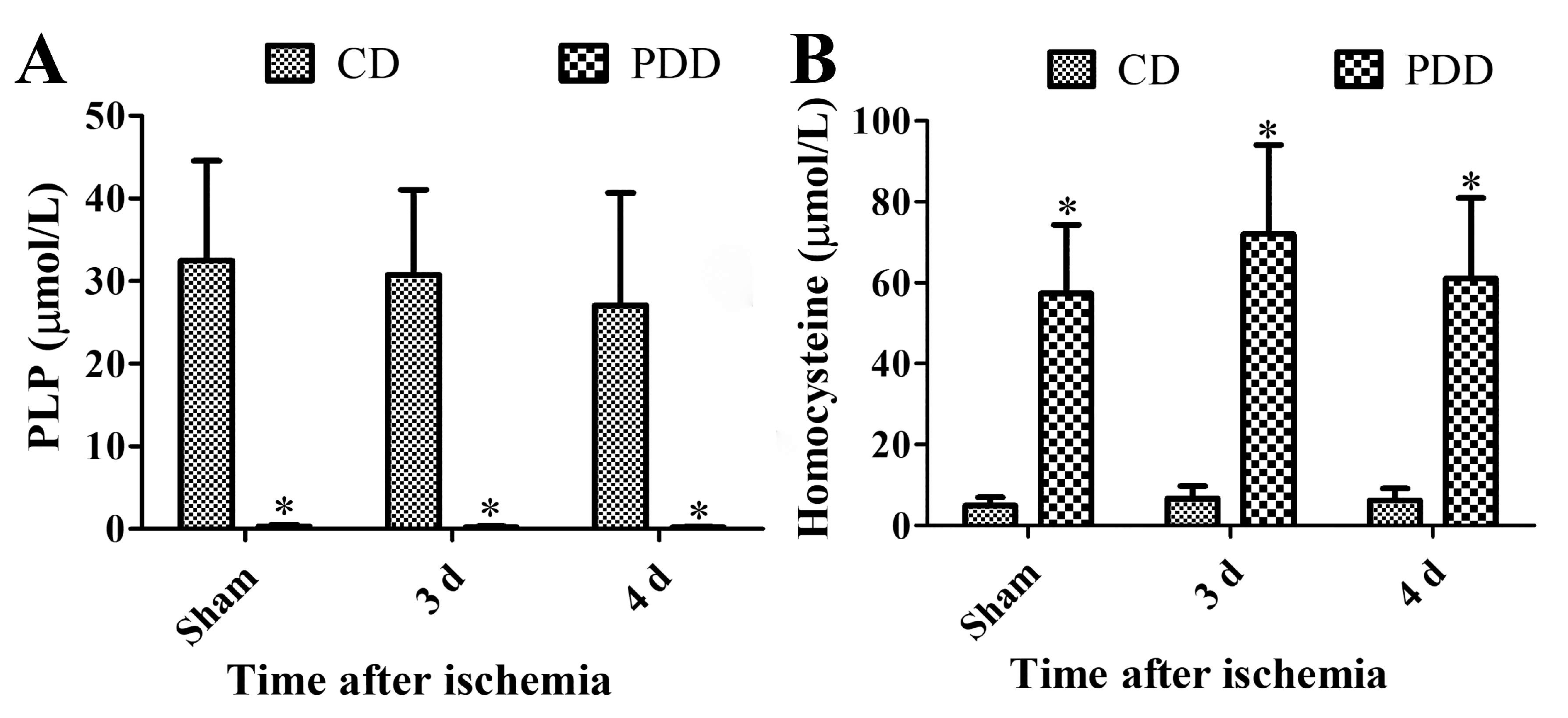
2.1. Pyridoxine Deficiency Decreases PLP and Increases Homocysteine Levels in Serum
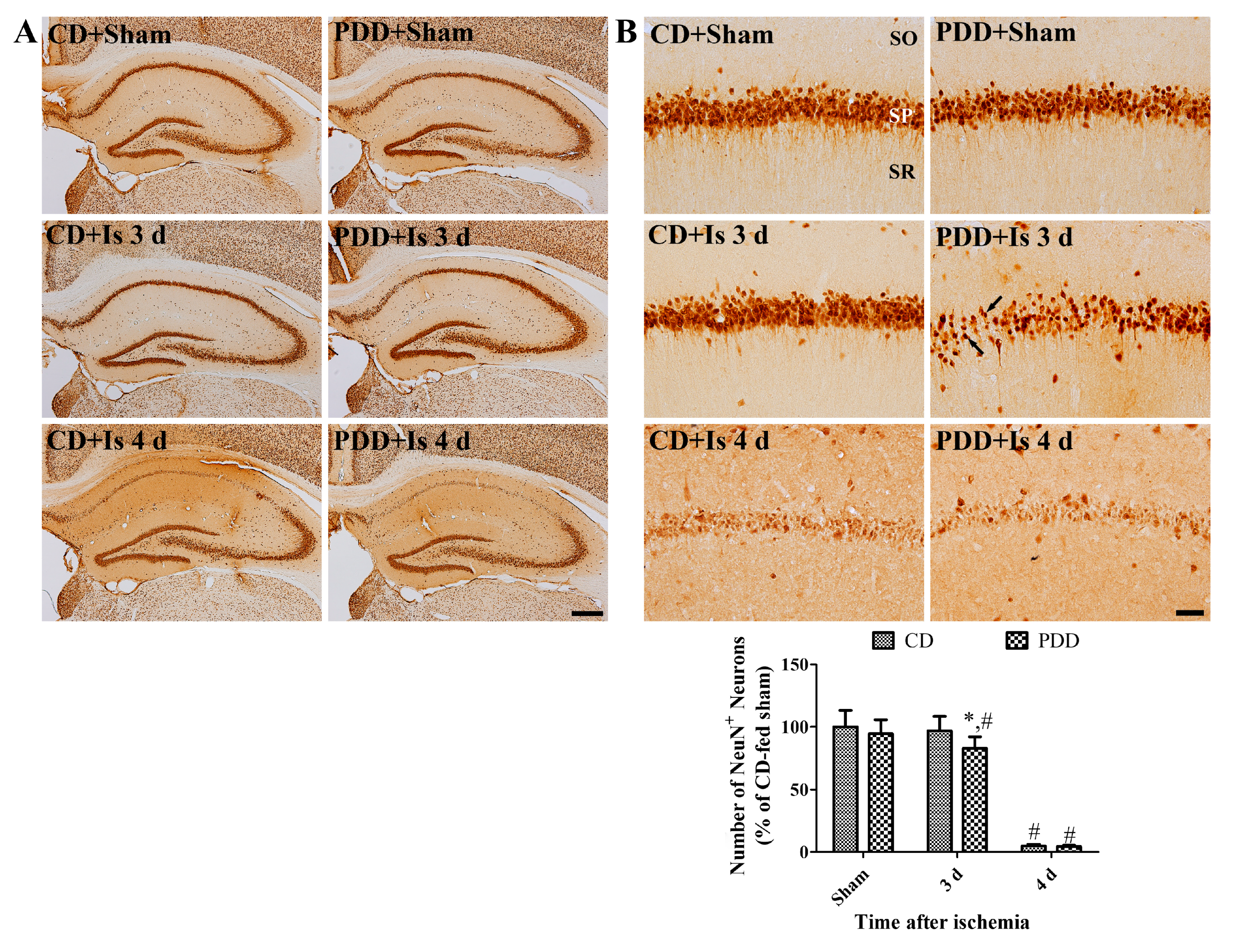
2.2. Pyridoxine Deficiency Causes Early Neuronal Death after Ischemia
2.3. Pyridoxine Deficiency Facilitates the Activation of Astrocytes and Microglia after Ischemia
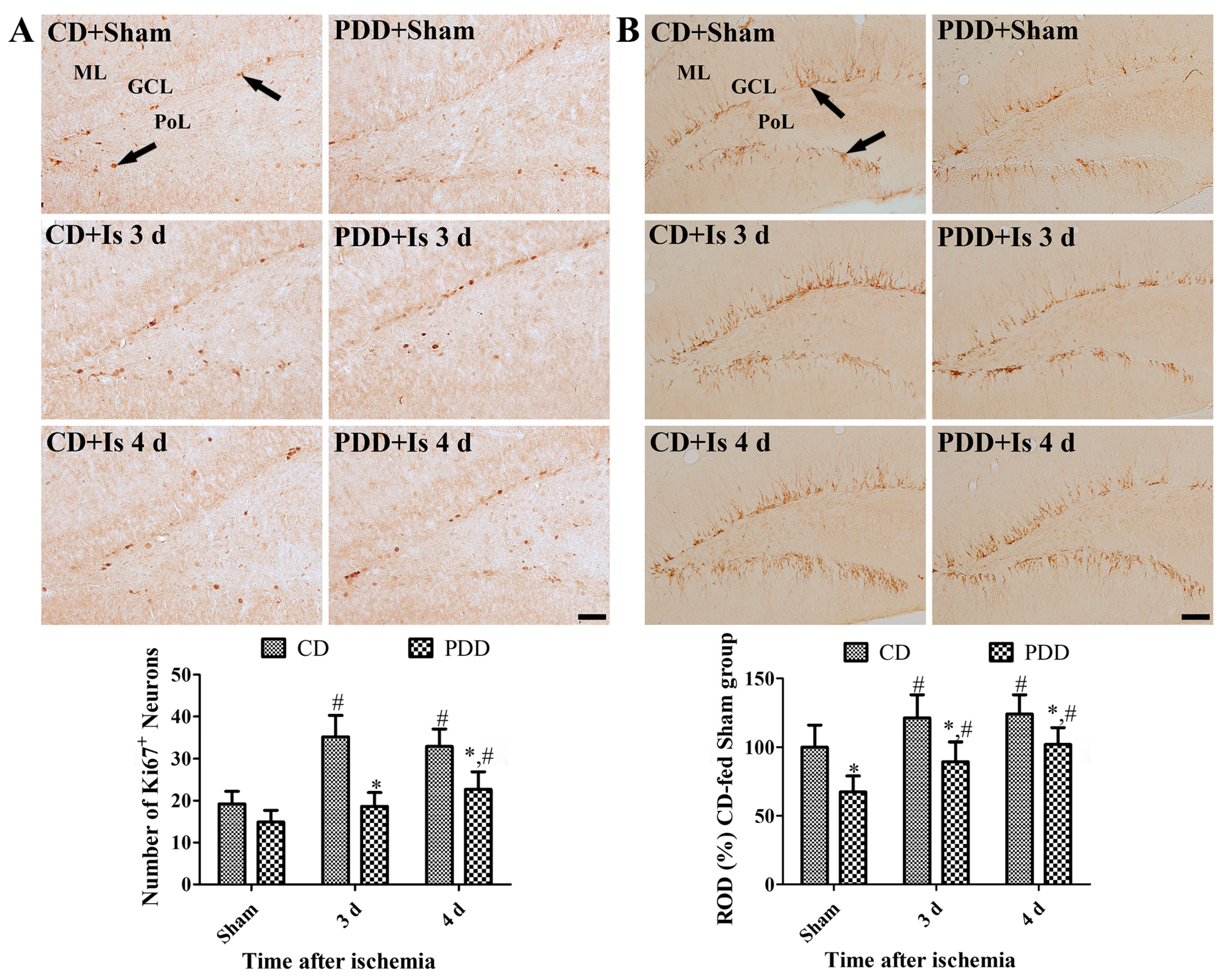
2.4. Pyridoxine Deficiency Decreases Ischemia-Induced Proliferating Cells and Neuroblasts
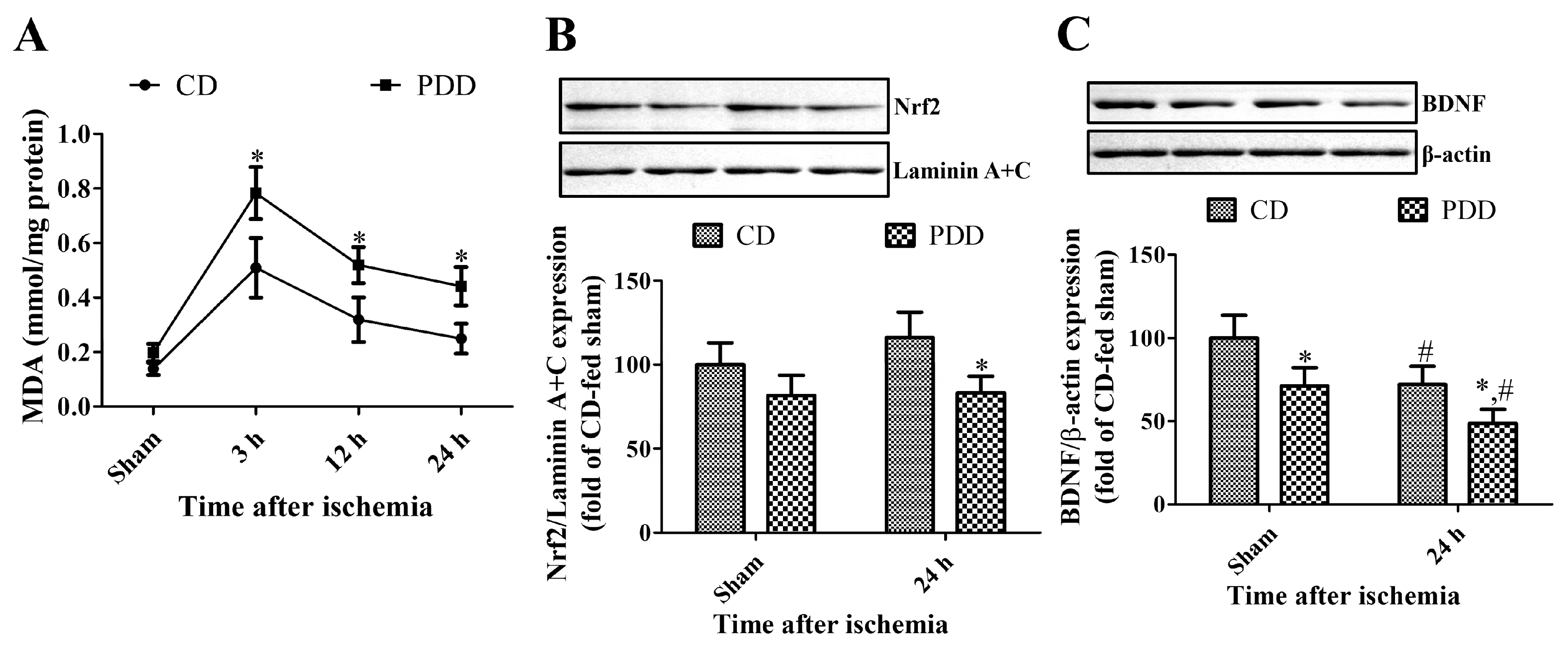
2.5. Pyridoxine Deficiency Increases Lipid Peroxidation and Decreases Nrf2 and BDNF Expression
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Ischemic Surgery
4.3. Immunohistochemistry
4.4. High-Performance Liquid Chromatography (HPLC) Analysis
4.5. Malondialdehyde Assay
4.6. Western Blot Analysis
4.7. Data Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Spector, R.; Johanson, C.E. Vitamin transport and homeostasis in mammalian brain: Focus on Vitamins B and E. J. Neurochem. 2007, 103, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem. Photobiol. 2000, 71, 129–134. [Google Scholar] [CrossRef]
- Hellmann, H.; Mooney, S. Vitamin B6: A molecule for human health? Molecules 2010, 15, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.A.; Khalifah, R.G.; Hudson, B.G. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: Comparison with aminoguanidine. Biochem. Biophys. Res. Commun. 1996, 220, 113–119. [Google Scholar] [CrossRef]
- Martínez, N.S.; Machado, J.M.; Pérez-Saad, H.; Coro-Antich, R.M.; Berlanga-Acosta, J.A.; Salgueiro, S.R.; Illera, G.G.; Alba, J.S.; del Barco, D.G. Global brain ischemia in Mongolian gerbils: Assessing the level of anastomosis in the cerebral circle of Willis. Acta Neurobiol. Exp. (Wars.) 2012, 72, 377–384. [Google Scholar]
- Atochin, D.N.; Chernysheva, G.A.; Aliev, O.I.; Smolyakova, V.I.; Osipenko, A.N.; Logvinov, S.V.; Zhdankina, A.A.; Plotnikova, T.M.; Plotnikov, M.B. An improved three-vessel occlusion model of global cerebral ischemia in rats. Brain Res. Bull. 2017, 132, 213–221. [Google Scholar] [CrossRef]
- Khodanovich, M.; Kisel, A.; Kudabaeva, M.; Chernysheva, G.; Smolyakova, V.; Krutenkova, E.; Wasserlauf, I.; Plotnikov, M.; Yarnykh, V. Effects of fluoxetine on hippocampal neurogenesis and neuroprotection in the model of global cerebral ischemia in rats. Int. J. Mol. Sci. 2018, 19, 162. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kisel’, A.A.; Chernysheva, G.A.; Smol’yakova, V.I.; Kudabaeva, M.S.; Krutenkova, E.P.; Tyumentseva, Y.A.; Plotnikov, M.B. p-Tyrosol enhances the production of new neurons in the hippocampal CA1 field after transient global cerebral ischemia in rats. Bull. Exp. Biol. Med. 2019, 168, 224–228. [Google Scholar] [CrossRef]
- Taguchi, N.; Nakayama, S.; Tanaka, M. Fluoxetine has neuroprotective effects after cardiac arrest and cardiopulmonary resuscitation in mouse. Resuscitation 2012, 83, 652–656. [Google Scholar] [CrossRef]
- Yamashima, T.; Zhao, L.; Wang, X.D.; Tsukada, T.; Tonchev, A.B. Neuroprotective effects of pyridoxal phosphate and pyridoxal against ischemia in monkeys. Nutr. Neurosci. 2001, 4, 389–397. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.Y.; Kim, D.H.; Lee, B.H.; Kwon, Y.G.; Won, M.H. Time course of changes in pyridoxal 5′-phosphate (vitamin B6 active form) and its neuroprotection in experimental ischemic damage. Exp. Neurol. 2007, 206, 114–125. [Google Scholar] [CrossRef]
- Li, P.; Zhu, M.L.; Pan, G.P.; Lu, J.X.; Zhao, F.R.; Jian, X.; Liu, L.Y.; Wan, G.R.; Chen, Y.; Ping, S.; et al. Vitamin B6 prevents isocarbophos-induced vascular dementia in rats through N-methyl-D-aspartate receptor signaling. Clin. Exp. Hypertens. 2018, 40, 192–201. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, M.; Mei, M.; Wang, H.; Han, Z.; Chen, M.; Yao, H.; Song, N.; Ding, X.; Ding, J.; et al. Pyridoxine induces glutathione synthesis via PKM2-mediated Nrf2 transactivation and confers neuroprotection. Nat. Commun. 2020, 11, 941. [Google Scholar] [CrossRef]
- Kuypers, N.J.; Hoane, M.R. Pyridoxine administration improves behavioral and anatomical outcome after unilateral contusion injury in the rat. J. Neurotrauma. 2010, 27, 1275–1282. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Kim, W.; Kim, D.W.; Yoo, K.Y.; Chung, J.Y.; Youn, H.Y.; Yoon, Y.S.; Choi, S.Y.; Won, M.H.; Hwang, I.K. Pyridoxine enhances cell proliferation and neuroblast differentiation by upregulating the GABAergic system in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 713–721. [Google Scholar] [CrossRef]
- Page, J.H.; Ma, J.; Chiuve, S.E.; Stampfer, M.J.; Selhub, J.; Manson, J.E.; Rimm, E.B. Plasma vitamin B6 and risk of myocardial infarction in women. Circulation 2009, 120, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, J.; Iso, H.; Inoue, M.; Iwasaki, M.; Okada, K.; Kita, Y.; Kokubo, Y.; Okayama, A.; Tsugane, S.; JPHC Study Group. Intake of folate, vitamin B6 and vitamin B12 and the risk of CHD: The Japan Public Health Center-Based Prospective Study Cohort I. J. Am. Coll. Nutr. 2008, 27, 127–136. [Google Scholar] [CrossRef]
- Jeon, J.; Park, K. Dietary vitamin B6 intake associated with a decreased risk of cardiovascular disease: A prospective cohort study. Nutrients 2019, 11, 1484. [Google Scholar] [CrossRef]
- Marniemi, J.; Alanen, E.; Impivaara, O.; Seppänen, R.; Hakala, P.; Rajala, T.; Rönnemaa, T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 188–197. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, W.; Hahn, K.R.; Kwon, H.J.; Nam, S.M.; Chung, J.Y.; Yoon, Y.S.; Kim, D.W.; Yoo, D.Y.; Hwang, I.K. Effects of pyridoxine deficiency on hippocampal function and its possible association with V-type proton ATPase subunit B2 and heat shock cognate protein 70. Cells 2020, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Mikoda, N.; Kitazawa, M.; LaFerla, F.M. Treatment of Alzheimer’s disease with anti-homocysteic acid antibody in 3xTg-AD male mice. PLoS ONE 2010, 5, e8593. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Jacobs, R.L.; Stead, L.M.; Brosnan, M.E. Methylation demand: A key determinant of homocysteine metabolism. Acta Biochim. Pol. 2004, 51, 405–413. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Hyperhomocysteinemia and arteriosclerosis: Historical perspectives. Clin. Chem. Lab. Med. 2005, 43, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy-- Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. Cns Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Spinneker, A.; Sola, R.; Lemmen, V.; Castillo, M.J.; Pietrzik, K.; González-Gross, M. Vitamin B6 status, deficiency and its consequences--an overview. Nutr. Hosp. 2007, 22, 7–24. [Google Scholar]
- Hwang, I.K.; Yoo, K.Y.; Suh, H.W.; Kim, Y.S..; Kwon, D.Y.; Kwon, Y.G.; Yoo, J.H.; Won, M.H. Folic acid deficiency increases delayed neuronal death, DNA damage, platelet endothelial cell adhesion molecule-1 immunoreactivity, and gliosis in the hippocampus after transient cerebral ischemia. J. Neurosci. Res. 2008, 86, 2003–2015. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.J. Serum homocysteine levels are correlated with behavioral and psychological symptoms of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2014, 10, 1887–1896. [Google Scholar]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Stanger, O.; Fowler, B.; Piertzik, K.; Huemer, M.; Haschke-Becher, E.; Semmler, A.; Lorenzl, S.; Linnebank, M. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: Review and treatment recommendations. Expert Rev. Neurother. 2009, 9, 1393–1412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.E.; Wei, W.; Liu, Y.H.; Peng, J.H.; Tian, Q.; Liu, G.P.; Zhang, Y.; Wang, J.Z. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am. J. Pathol. 2009, 174, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ilango, K.; Singh, P.K.; Karmakar, D.; Singh, G.P.; Kumari, R.; Dubey, G.P. Age dependent levels of plasma homocysteine and cognitive performance. Behav. Brain Res. 2015, 283, 139–144. [Google Scholar]
- Lehotský, J.; Tothová, B.; Kovalská, M.; Dobrota, D.; Beňová, A.; Kalenská, D.; Kaplán, P. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front. Neurosci. 2016, 10, 538. [Google Scholar]
- Kumar, M.; Sandhir, R. Hydrogen sulfide suppresses homocysteine-induced glial activation and inflammatory response. Nitric Oxide 2019, 90, 15–28. [Google Scholar] [CrossRef]
- Beard, R.S., Jr.; Reynolds, J.J.; Bearden, S.E. Hyperhomocysteinemia increases permeability of the blood–brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood 2011, 118, 2007–2014. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef]
- Won, M.H.; Kang, T.C.; Jeon, G.S.; Lee, J.C.; Kim, D.Y.; Choi, E.M.; Lee, K.H.; Choi, C.D.; Chung, M.H.; Cho, S.S. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999, 836, 70–78. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Mhadu, N.H.; Al-Dalain, S.M.; Martínez, G.; León, O.S. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci. Res. 2001, 41, 233–241. [Google Scholar] [CrossRef]
- Wang, Q.; Tompkins, K.D.; Simonyi, A.; Korthuis, R.J.; Sun, A.Y.; Sun, G.Y. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006, 1090, 182–189. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Cho, S.B.; Jung, H.Y.; Kim, W.; Lee, K.Y.; Kim, J.W.; Moon, S.M.; Won, M.H.; Choi, J.H.; Yoon, Y.S.; et al. Protein disulfide-isomerase A3 significantly reduces ischemia-induced damage by reducing oxidative and endoplasmic reticulum stress. Neurochem. Int. 2019, 122, 19–30. [Google Scholar] [CrossRef]
- Lovell, M.A.; Ehmann, W.D.; Mattson, M.P.; Markesbery, W.R. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol. Aging 1997, 18, 457–461. [Google Scholar] [CrossRef]
- Montine, K.S.; Olson, S.J.; Amarnath, V.; Whetsell, W.O., Jr.; Graham, D.G.; Montine, T.J. Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4. Am. J. Pathol. 1997, 150, 437–443. [Google Scholar]
- Sayre, L.M.; Zelasko, D.A.; Harris, P.L.; Perry, G.; Salomon, R.G.; Smith, M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997, 68, 2092–2097. [Google Scholar] [CrossRef]
- Ishrat, T.; Hoda, M.N.; Khan, M.B.; Yousuf, S.; Ahmad, M.; Khan, M.M.; Ahmad, A.; Islam, F. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT). Eur. Neuropsychopharmacol. 2009, 19, 636–647. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef]
- Narayanan, S.V.; Dave, K.R.; Saul, I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke 2015, 46, 1626–1632. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Waterhouse, E.G.; An, J.J.; Orefice, L.L.; Baydyuk, M.; Liao, G.Y.; Zheng, K.; Lu, B.; Xu, B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. Version 2. J. Neurosci. 2012, 32, 14318–14330. [Google Scholar] [CrossRef]
- von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018, 373, 729–741. [Google Scholar] [CrossRef]
- Kärkkäinen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.L.; Yamamoto, M.; et al. Nrf2 regulates neurogenesis and protects neural progenitor cells against Aβ toxicity. Stem Cells 2014, 32, 1904–1916. [Google Scholar]
- Cecchini, M.S.; Bourckhardt, G.F.; Jaramillo, M.L.; Ammar, D.; Müller, Y.M.R.; Nazari, E.M. Exposure to homocysteine leads to cell cycle damage and reactive gliosis in the developing brain. Reprod. Toxicol. 2019, 87, 60–69. [Google Scholar] [CrossRef]
- Nemirovich-Danchenko, N.M.; Khodanovich, M.Y. New neurons in the post-ischemic and injured brain: Migrating or resident? Front. Neurosci. 2019, 13, 588. [Google Scholar] [CrossRef]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef]
- Brown, M.J.; Beier, K. StatPearls: Vitamin B6 Deficiency (Pyridoxine); StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, T.H.; Kim, J.W.; Yoo, D.Y.; Kim, D.W.; Choi, J.H.; et al. Cuprizone Affects Hypothermia-Induced Neuroprotection and Enhanced Neuroblast Differentiation in the Gerbil Hippocampus after Ischemia. Cells 2020, 9, 1438. [Google Scholar] [CrossRef]
- Radtke-Schuller, S.; Schuller, G.; Angenstein, F.; Grosser, O.S.; Goldschmidt, J.; Budinger, E. Brain atlas of the Mongolian gerbil (Meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Struct. Funct. 2016, 221 (Suppl. 1), 1–272. [Google Scholar] [CrossRef]
- Moon, S.M.; Choi, G.M.; Yoo, D.Y.; Jung, H.Y.; Yim, H.S.; Kim, D.W.; Hwang, I.K.; Cho, B.M.; Chang, I.B.; Cho, S.M.; et al. Differential Effects of Pioglitazone in the Hippocampal CA1 Region Following Transient Forebrain Ischemia in Low- and High-Fat Diet-Fed Gerbils. Neurochem. Res. 2015, 40, 1063–1073. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.Y.; Kim, W.; Hahn, K.R.; Kang, M.S.; Kim, T.H.; Kwon, H.J.; Nam, S.M.; Chung, J.Y.; Choi, J.H.; Yoon, Y.S.; et al. Pyridoxine Deficiency Exacerbates Neuronal Damage after Ischemia by Increasing Oxidative Stress and Reduces Proliferating Cells and Neuroblasts in the Gerbil Hippocampus. Int. J. Mol. Sci. 2020, 21, 5551. https://doi.org/10.3390/ijms21155551
Jung HY, Kim W, Hahn KR, Kang MS, Kim TH, Kwon HJ, Nam SM, Chung JY, Choi JH, Yoon YS, et al. Pyridoxine Deficiency Exacerbates Neuronal Damage after Ischemia by Increasing Oxidative Stress and Reduces Proliferating Cells and Neuroblasts in the Gerbil Hippocampus. International Journal of Molecular Sciences. 2020; 21(15):5551. https://doi.org/10.3390/ijms21155551
Chicago/Turabian StyleJung, Hyo Young, Woosuk Kim, Kyu Ri Hahn, Min Soo Kang, Tae Hyeong Kim, Hyun Jung Kwon, Sung Min Nam, Jin Young Chung, Jung Hoon Choi, Yeo Sung Yoon, and et al. 2020. "Pyridoxine Deficiency Exacerbates Neuronal Damage after Ischemia by Increasing Oxidative Stress and Reduces Proliferating Cells and Neuroblasts in the Gerbil Hippocampus" International Journal of Molecular Sciences 21, no. 15: 5551. https://doi.org/10.3390/ijms21155551
APA StyleJung, H. Y., Kim, W., Hahn, K. R., Kang, M. S., Kim, T. H., Kwon, H. J., Nam, S. M., Chung, J. Y., Choi, J. H., Yoon, Y. S., Kim, D. W., Yoo, D. Y., & Hwang, I. K. (2020). Pyridoxine Deficiency Exacerbates Neuronal Damage after Ischemia by Increasing Oxidative Stress and Reduces Proliferating Cells and Neuroblasts in the Gerbil Hippocampus. International Journal of Molecular Sciences, 21(15), 5551. https://doi.org/10.3390/ijms21155551





